Abstract
Human activity is rapidly transforming most of Earth’s natural systems. How this transformation is impacting human health, whose health is at greatest risk, and the magnitude of the associated disease burden are relatively new subjects within the field of environmental health. We discuss what is known about the human health implications of changes in the structure and function of natural systems and propose that these changes are affecting human health in a variety of important ways. We identify several gaps and limitations in the research that has been done to date and propose a more systematic and comprehensive approach to applied research in this field. Such efforts could lead to a more robust understanding of the human health impacts of accelerating environmental change and inform decision making in the land-use planning, environmental conservation, and public health policy realms.
Keywords: global change, ecosystem services, ecology, planetary boundaries, ecological footprint
At least since Hippocrates wrote On Airs, Waters, and Places, the natural environment has been viewed as an important determinant of human health. However, over the last century, the field of environmental health has focused increasingly on quantifying exposure–response relationships for toxins encountered in the human-dominated environment: from an initial focus on workplace exposures, to a population-level focus on radiation, heavy metals, air and water pollution, and more recently, to exposure to endocrine-disrupting chemicals. Over this period, relatively little attention has been paid to how changes in the structure and function of Earth’s natural systems might affect human health. Growing evidence that changes in these natural systems can affect human health in a variety of important ways and the increasing pace and extent of these changes has prompted this Perspective. In it, we review current understanding of this field, identify some of its gaps and limitations, and suggest an approach to expanding our understanding.
Human activity is transforming nearly all of Earth’s natural systems. With the human population now exceeding 7 billion people and rapid growth in per capita consumption of goods and services, humanity’s growing ecological footprint is altering the planet’s land cover, rivers and oceans, climate system, biogeochemical cycles, and the functioning of its ecosystems (1). This suite of changes has given rise to the definition of a new geological epoch: the Anthropocene (2).
The arrival of the Anthropocene presents an uncertain future, not only for the biosphere, but for humanity itself. There is widespread debate about the ability of an altered global environment to meet the needs of a growing and prospering human population. Health is one dimension of human well-being that has received particular attention in this discussion. In 2005, for example, 1,360 experts from 95 countries produced The Millennium Ecosystem Assessment (MA), a consensus document evaluating the state of the planet’s ecosystems. The authors concluded that “any progress achieved in addressing the Millennium Development Goals of poverty and hunger eradication, improved health, and environmental sustainability is unlikely to be sustained if most of the ecosystem services on which humanity relies continue to be degraded” (3). At the same time, the Director-General of the World Health Organization underscored that “Nature’s goods and services are the ultimate foundations of life and health” (4).
Despite the intuitive importance of natural systems to human health, the empirical evidence to support these claims has been relatively thin. On one hand, natural systems provide a suite of “ecosystem services” including nutrition, purification of water, protection from natural hazards, and reduction of some infectious diseases (3). On the other hand, extensive human alteration of the natural world has coincided with large improvements in most health indices globally.
Here we explore our current understanding of the human health impacts of alterations in the structure and functioning of Earth’s natural systems. Our goals are to (i) illustrate what is currently known, (ii) identify gaps and limitations that can be addressed by future research efforts, (iii) address the scale of the health burden associated with changes to natural systems, and (iv) propose a research approach that strengthens the practice of both public health and environmental conservation.
Highlights of the Recent Literature
Other reviews have laid out a more complete summary of the existing literature than we intend here (5). As these reviewers have noted, the literature exploring connections between human health and ecological alteration includes multiple studies scattered across a variety of disciplines that leave many of the most important relationships incompletely characterized. Despite its patchy nature, in aggregate, this work is convincing that there are significant linkages between the structure and function of natural systems and a variety of human health outcomes. Here, we provide an overview of the types of relationships that have been well studied and established as a prelude to exploring the gaps, limitations, and areas requiring further study.
With roughly half the temperate and tropical forests cut down, nearly half the ice-free, desert-free terrestrial landscape converted to croplands or pasture, and more than 800,000 dams impeding the flow through more than 60% of the world’s rivers, alterations to our planet’s land use and land cover represent some of the most pervasive changes humanity has made to Earth’s natural systems (1). Some of these changes have clearly been associated with public health benefits. Early efforts to reduce malaria in the Tennessee Valley (6) and countries in sub-Saharan Africa including Nigeria (7) by draining swamps that were habitat for mosquito vectors, for example, proved very successful. The primary motivation for deforestation, dams, and irrigation projects in many parts of the world has been to increase the supply of food and clean energy—critical building blocks for public health.
However, some of the negative impacts of land-use change have become clear more recently. Dams and irrigation projects cause very large increases in the prevalence of schistosomiasis (8–10) and malaria (11, 12) in parts of Africa and South Asia. They also increase exposure to other vector-borne diseases associated with significant morbidity and mortality including Rift Valley fever, filariasis, leishmaniasis, dracunculosis, onchocerciasis, and Japanese encephalitis (13–16). Deforestation increases exposure to malaria in Africa (17–21) and South America (20–26) but has less predictable impacts in Asia (27–31) where there are many more Anopheles vectors with less generalizable responses to reduced forest cover. In parts of Africa, forest cutting also alters the composition and density of aquatic snail species in a manner that favors transmission of schistosomiasis (32).
Some land-use changes affect disease exposure less directly. In Belize, for example, nutrient enrichment with nitrogen and phosphorus from agricultural runoff hundreds of miles upstream causes a change in the vegetation pattern of lowland wetlands that favors the more efficient malaria vector Anopheles vestipennis over the less efficient vector Anopheles albimanus, leading to increased malaria exposure among coastal populations (33).
Land-use changes that alter human–wildlife interactions can be an important source of zoonotic disease. Human encroachment into wildlife habitat (34) and the hunting and consumption of wild meat (35) can provide public health benefits as they provide new land for agriculture and, in the case of bushmeat, rich sources of nutrients, but these activities also create the potential for zoonotic infections to move from animal to human populations. There is compelling evidence that these mechanisms played the central role in initial outbreaks of HIV and Ebola virus, as well as several lesser-known zoonoses (36). The power of these shifts in animal–human interactions to affect disease transmission helps to explain the fact that roughly 75% of emerging infectious diseases are zoonoses (37).
Some types of land-use change involve the destruction of entire ecological systems and the services that they provide. One example is the loss of coastal barrier systems including coastal mangroves, coral reefs, vegetated dunes, and coastal wetlands. These systems can lessen storm surge and attenuate wave energy, thereby reducing morbidity and mortality from coastal storms or tsunamis (38–41). Their global destruction puts in harm’s way roughly a third of humanity who live within 100 km of the shore and at less than 50 m above sea level (42) at the same time that sea level rise and more extreme tropical storms increase the threat of storm surge and coastal flooding. Forest cover may reduce flooding and land slide activity during extreme storms (43). Wetlands and forest watersheds can filter pollutants and pathogens from surface water supplies (44, 45); and, to some extent, forests filter particulates from the air (46–48). Most such health-related ecosystem services remain poorly characterized.
One of the most dynamic areas of research into health impacts of ecosystem changes is disease ecology. Disease ecology explores comprehensively how changes in a whole suite of factors such as population dynamics, movement, physiological state, species richness, and relative abundance of species within an ecological community can alter risks of exposure to infectious diseases (49, 50). Recently, disease ecologists have shown that the risk of West Nile virus exposure in the United States rises as avian biodiversity falls (51, 52), and, similarly, Lyme disease exposure increases with falling mammalian diversity (53–55). In field experiments, exposure to hantavirus increases when mammalian diversity falls (56). For Chagas disease in Panama and the Brazilian Amazon, risk of human exposure is positively correlated with reduced mammalian species diversity (57, 58). This suite of findings has led to the proposal of a general principle of disease ecology—the “dilution effect”—whereby a greater diversity of intermediate hosts can dilute the pool of hosts that amplify transmission (competent hosts), resulting in decreased exposure to vector-borne disease (59, 60). Recent modeling work suggests the dilution effect may account for reduced malaria transmission in diverse regions of the Brazilian Amazon as well (61).
For such an effect to be generalizable across different diseases and ecosystems, it must often be the case that hosts that are more effective at transmitting pathogens (competent hosts) tend to persist and less competent ones disappear as diversity declines. Some recent research suggests that immunological tolerance, and hence high host competence, tends to characterize the species that are intrinsically more likely to persist when diversity declines (62). Related research has indicated that hosts with a high intrinsic rate of increase are both more ecologically resilient and reservoir competent (58). Other work has emphasized the idiosyncratic nature of particular disease/ecology relationships (63), and still other efforts have concluded that generalizations about the effect of biodiversity on disease transmission have been premature (64). Clarifying the impacts of disruption of natural systems on transmission of vector-borne disease and the extent to which such relationships can be generalized across diseases and ecosystems remains an important area for future research.
Reductions in biodiversity or the population sizes of species can have other important human health impacts unrelated to infectious disease. Losses of potential sources of pharmaceuticals, experimental models for studying disease, and wild relatives of important food crops can all have far-reaching health consequences (65). Crop pollination (by insects, birds, and bats inhabiting natural ecosystems) is critical in the production of a significant fraction of both nutrients and calories in the human diet (66, 67). A relatively new area of research indicates that aquatic and terrestrial wildlife populations are an important dietary source of both micro- and macronutrients. For example, if households in a population in Madagascar were unable to harvest wild meat for consumption, their children would experience a 30% higher risk of iron deficiency anemia—a condition that increases the risk for sickness and death from infectious disease, reduces IQ and learning, and reduces lifelong capacity for physical activity (68). There is growing awareness that dwindling populations of marine and terrestrial wildlife may represent a nutritional crisis for many people who cannot readily replace these foods with domesticated species or fortified foods.
Reduced access to fuel and water will also have health impacts, although they may be mediated through less direct causal chains. Forest clearing in many parts of the world has led to reduced access to fuel for cooking fires, creating a disproportionate burden on women and girls who are often responsible for gathering such fuel wood. Water scarcity can have direct impacts on health through reduced water quality and access to sanitation, as well as by creating an additional work burden on those who have to exert more energy carrying or pumping water from its source. The direct impacts of these types of scarcity on the health of individuals and families require better characterization.
The social and psychological impacts of ecological degradation are also described in the literature. A loss of a “sense of place” or identity, depression, and emotional stress have all been documented in people experiencing degradation of the natural environment around them (69). On the positive side, experience in nature, as well as outdoor exercise, have been associated with increased mental and physical well-being, as well as enhanced cognitive functioning (70). Reduced postoperative recovery times and lower analgesic requirements in hospitalized patients with access to a natural view through their room window point to a deeply seeded relationship between the natural world and the psycho-physiological dimensions of human health (71).
There is a growing literature exploring the human health impacts of altering another natural system—our planet’s atmosphere and its climate. Human health impacts of anthropogenic climatic disruption include changes in exposure to heat stress, air pollution, respiratory allergens, infectious disease, and natural hazards, as well as increased water scarcity, food insecurity, and population displacement. Geographic distributions of some infectious diseases (particularly vector-borne diseases like malaria) are shifting in response to changes in temperature and precipitation (72–74). Outbreaks of water-borne and food-borne disease are linked to extreme precipitation and heat events (75–77). Allergenic plants grown at elevated carbon dioxide produce more pollen over a longer pollen season than those grown at ambient levels (78). Modeling studies caution that warmer days will lead to higher concentrations of ground-level ozone and smog, which cause sickness and death from respiratory and cardiac disease (79). Natural hazards including droughts, floods, tropical cyclones, forest fires, and heat waves are projected to become more frequent (80). Studies predict multiple impacts of climatic disruption on food production and food security, as well as impacts on access to freshwater (81–83). Several reports have predicted sharp rises in the number of people displaced by combinations of these factors (84, 85). All of these outcomes represent significant public health threats, with impacts that are likely to be exacerbated by their interactions in some regions.
Limitations
We highlight four important limitations in the literature to date. First, much of the existing research on the human health impacts of alterations in natural systems focuses narrowly on a single health outcome—a particular infectious disease, for example—rather than focusing on the impacts of changes to a natural system across several dimensions of human health. The degradation of a particular ecosystem can result in multiple simultaneous impacts on health (e.g., deforestation leading to increased malaria exposure and loss of access to wild foods). Equally important, ecosystem degradation can lead to significant health improvements for local communities. Indeed, improved health is often the motivation for converting natural ecosystems (e.g., expanding agriculture or building dams to improve access to food and water). Assessing the net health effects of such changes would have more utility for conservation and public health policy and practice than the study of only one dimension of health at a time.
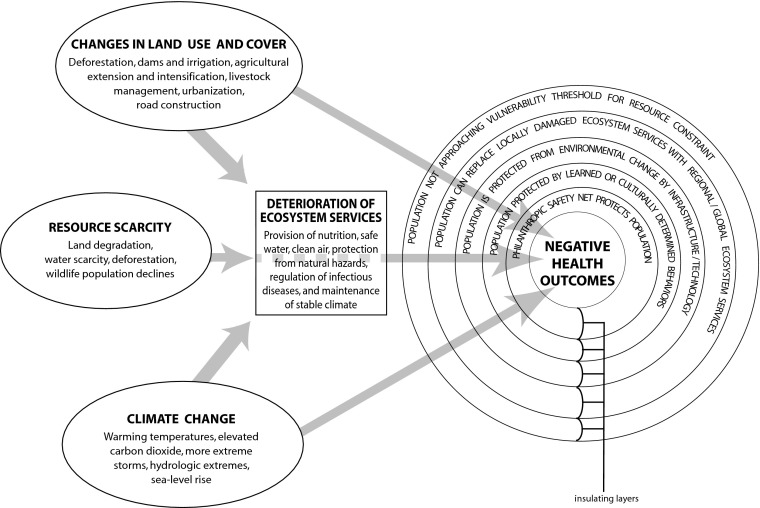
A related challenge is the need to evaluate the health consequences of the complex interplay of multiple contemporaneous environmental changes. In many parts of the world, land-use change, resource scarcities, and climate change effects are likely to interact to alter exposure to infectious diseases, access to food and water, protection from natural hazards, and even population displacement with its additional associated health impacts. Although these interactions can be represented schematically (Fig. 1), investigating how they will interact in particular places among particular people may require different research methodologies than those which have been applied to date.
Fig. 1.
Schematic of the complex relationships between altered environmental conditions and human health. Drivers of global environmental change (e.g., land-use change, resource scarcity, or climate change) can directly pose health risks or impair ecosystem services that subsequently influence health. Population level vulnerability, however, will be modified by multiple layers of social or infrastructure barriers that can buffer or eliminate risks associated with these exposures. Together, all components must be considered to achieve realistic assessments of population vulnerability.
A third limitation is that we have inadequately explored how human adaptations to ecosystem change may mediate the resulting health impacts. If a change in a natural system leads to an altered risk of exposure to disease, how will local populations respond to this new risk? If wildlife or fisheries are no longer sufficient to support harvest for human nutrition, what and how will people substitute for this loss and what will be the nutritional value of this substitution? If deforestation leads to increased malaria exposure, will local populations have access to bed nets or antimalarial medications? Understanding these and other human responses is particularly important in relation to climate change, where multiple threats may increase concurrently and the capacity for local adaptation may be the most important determinant of the ultimate health impacts.
Finally, and perhaps most important, the literature on ecological change and human health needs to be more specific about whose health is in question. A particular ecosystem alteration may provide health benefits for one segment of a population while incurring health costs for another. A dam project, for example, may provide a source of clean energy or increased agricultural productivity for some while increasing the risk of malaria and schistosomiasis for others. Perhaps economic development in general is improving health for most of the world’s people while the poorest populations disproportionately experience the negative impacts of degraded natural systems—a dynamic that would be invisible if looking only at aggregated data. A corollary to this point is that future generations may also bear a larger share of the burden of degraded natural systems. In the case of climate change, for example, fossil fuel consumption may be associated with large public health benefits as individuals become less dependent on biomass burning as a source of heat and cooking fuel, whereas the large health costs associated with climatic disruption will accrue primarily to future generations.
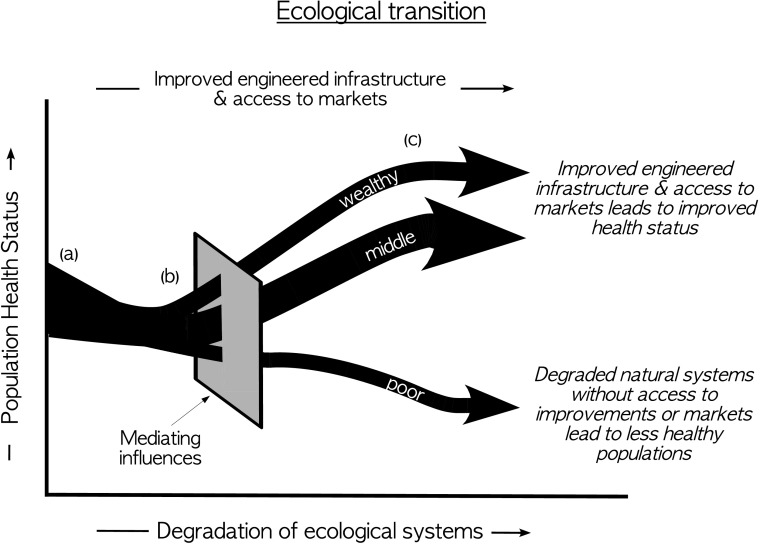
One way to think about this disparity in the way populations experience health impacts from alterations in natural systems is to consider a refinement of Smith and Ezzati’s “environmental risk transition” (86). Smith and Ezzati make a compelling case that, in aggregate, household level environmental risks (e.g., poor sanitation, indoor particulate exposures, and unsafe drinking water) are reduced concurrent with economic development. However, their characterization masks the winners and losers in this process. Over the course of economic development, people replace complex, natural systems with engineered infrastructure and markets as the source of food, water purification, shelter, fuel, clothing, and protection from natural hazards and infectious disease. A majority of people are able to make this transition and reap the benefits noted by Smith and Ezzati. However, the poorest and least entitled may fail to make the transition successfully, unable to access either the engineered infrastructure or markets (Fig. 2). They are left with degraded natural systems but little with which to replace them.
Fig. 2.
Hypothetical ecological transition for a fictional population. In this schematic, the population moves from a state (a) in which people rely primarily on natural systems for health-related ecosystem services to a state (c) where they become reliant on engineered infrastructure and markets for these services while ecological systems get degraded over time. Over the course of this transition (b), there are numerous society-level mediating influences that are likely to change the differential vulnerabilities and health status of members of the population. These include equity of income distribution, type and strength of governance, philanthropic safety nets, characteristics of the natural environment etc. It is also likely that the health implications of such a transition will be different for different dimensions of health.
In urban areas, these are slum dwellers without access to municipal water and sewage services, safe housing, health care, or other critical services. In rural areas, they are the remote and poor who are challenged with, for example, dwindling marine and terrestrial wildlife populations and other wild species relied on for food. They experience degraded soils, fresh water resources, and forests and generally diminished ecosystem services from the natural systems within which they live. We suspect that this population of roughly a billion people living in absolute poverty with little access to improved water or sanitation and frequently experiencing undernutrition are most susceptible to the health impacts of the quiet erosion of natural infrastructure and that research into the health effects of ecosystem alteration should be targeted at them.
Another way to conceptualize these health impacts is illustrated in Fig. 1. A combination of anthropogenic environmental changes including changes in land use and cover, resource availability, and climate interact to alter the quality of ecosystem services available to local populations. The vulnerability of a population to these changes depends on the extent to which they are relying on these services, how close they are to thresholds whereby further reductions in services have immediate impacts on their well-being, and their ability to replace these services with engineered infrastructure, markets, or philanthropy. The people who are most impacted by changes in the function of natural systems, therefore, are those who are geographically located in areas of greatest change and who have the fewest resources to insulate themselves from these changes through markets or infrastructure. As a result, they follow the lower arrow in Fig. 2, whereas others navigate the transition with more success.
That said, people in wealthier societies will not be immune to changes in exposure to infectious disease, the impacts of sea level rise, more extreme storms, and other natural hazards associated with climate change or the indirect effects of large-scale population displacement that are likely to follow the breakdown of ecosystem services.
How Big a Public Health Problem Is the Degradation of Natural Systems?
In public health, this sort of question is best answered using burden of disease assessment, which relies, for its metric, on the disability adjusted life-year (DALY). By estimating, for a particular health threat, the amount of associated morbidity and mortality for different demographic groups within a population and adjusting for the level of disability associated with nonfatal outcomes, it is possible to represent the burden of any public health threat using this metric. The advantage of such an approach is that it allows comparison across widely different health threats (e.g., alcoholism vs. measles) to assess their relative importance for a particular population or globally.
One widely publicized assessment of the global burden of disease associated with changes in the environment concluded that roughly one quarter of the global burden of disease can be attributed to environmental changes (87). This effort and many associated efforts to quantify disease burdens associated with environmental risk factors have, however, had a very different focus than that of this paper. The environment of concern in these efforts has primarily been the household environment with unsafe water, sanitation, and hygiene, indoor air pollution, and outdoor air pollution comprising the bulk of the risks. In addition, they included some of the classic elements of environmental health: exposures associated with occupations, radiation, or heavy metals. Such analyses are enormously valuable in helping public health practitioners determine the relative gains that can be achieved in the overall health of a population by modifying these aspects of the environment. However, they explicitly excluded “natural environments or ecosystems that cannot reasonably be modified” as beyond their scope and do not, therefore, provide us guidance on the relative importance of ecosystem change to human health (87).
Quantifying the disease burden associated with changes in the structure and function of natural systems presents several challenges. The first is that our understanding of the health impacts remains incomplete. As outlined above, there are many systems and dimensions of health where much more work needs to be done before we will fully understand the health implications of ecosystem change. A second challenge is that most of the health impacts associated with these types of environmental change are not distinct health outcomes but contributions to health outcomes whose causes are multifactorial (88). We must ask: how much of the malaria in the Brazilian Amazon is a result of deforestation, how much of the iron deficiency in Madagascar is due to wildlife population declines, and how much of the cardiopulmonary disease in Singapore is due to forest fires in Indonesia? These are tractable questions, but challenging, and they have not yet been answered.
A third challenge is that many of the relationships we are interested in involve the loss of a protective service as opposed to the presence of a risk. Viewed through a burden of disease lens, the burden is, paradoxically, generated by the loss of a protection not the presence of an exposure. As a result, we are concerned with calculating the “disease averted” that is associated with a natural system in a particular state. For example, as overfishing continues around the world, we expect to see additional burdens of disease from malnutrition among poor coastal peoples. The question is not just the degree of protein, calorie, and micronutrient deficiencies caused today, but how much will be seen in the future if sustainable fisheries management remains largely elusive? As we expressed earlier, the burden of ecosystem alteration may be disproportionately experienced by future generations. Recent evidence that global per capita burden of disease is in decline along with death and disability in children under five is encouraging (89); our concern is that growing resource scarcity, changes in land use and cover, climate change effects, and alterations to a range of ecosystem services are in danger of countering or even reversing these trends in the future.
Finally, the interactions between environmental changes at the population level and the mediating effect of human responses are enormously complex. In sub-Saharan Africa, for example, how will the combination of altered land cover, water scarcity, wildlife population declines, and climate change effects interact to affect the quality and quantity of food and water available? How will climatic changes and altered access to food and water contribute to population displacement? How will population displacement alter the incidence of infectious disease and malnutrition among the displaced, and to what extent will people moving into places where they are not welcome generate civil strife? These types of interactions will contribute to future burdens of disease but, at our current level of understanding, are impossible to quantify.
We are confident, however, that changes in the structure and function of natural systems are likely to broadly impact many of the most important public health risks we face globally. These changes are significant drivers in the emergence, distribution, and transmission of numerous infectious diseases. Recent work, for example, highlights the large burden associated with zoonotic diseases and the significant role that land-use change and resource scarcity play in driving the emergence and transmission of these diseases (90). A second example is the recent finding that Cryptosporidium infection is the second leading cause of moderate to severe diarrhea in infants in the developing world (91). Diarrheal disease accounts for 1 in 10 deaths in children under the age of five and causes 800,000 deaths annually (91). This pathogen is found ubiquitously in cattle (92), and outbreaks have been associated with both land-use change and extreme precipitation (76, 93). Other causes of childhood diarrhea are also likely to be sensitive to changes in land use and precipitation patterns.
Changes in the state of natural systems are also likely to exacerbate both undernutrition and micronutrient deficiencies that remain large contributors to the global burden of disease. Wildlife population declines are impacting both macro- and micronutrient nutrition for many populations, whereas climate change is expected to reduce the iron, zinc, and protein content of grains that are a critical source of these nutrients for large segments of the human population (94–97). In combination, iron and zinc deficiency have been estimated by the World Health Organization to cause 63 million life-years to be lost annually (98, 99). The combination of arable land degradation, increasing water scarcity, and climatic disruption is expected to reduce agricultural yields at the same time that humanity will need to roughly double global food production to keep up with demand (100, 101). These environmental headwinds are almost certain to produce regional, if not global, food shortages.
Even heart disease, which makes up an increasing share of the global burden of disease (89), is susceptible to changes in the functioning of natural systems. For example, large percentages of the global production of nutrients like folic acid and vitamin E come from crops dependent on animal pollinators, and dietary intake of these nutrients is associated with reduced risk of heart disease (66). At the same time, rising concentrations of atmospheric CO2 have been shown to lower the protein content in the major grains (97), and the resulting substitution of dietary carbohydrate for dietary protein has been shown to increase the risk of coronary heart disease (102).
Changes to Earth’s natural systems affect the quality of the water we drink and the air we breathe by multiple mechanisms and impact our vulnerability and exposure to natural hazards. We know that these changes are pervasive, affecting nearly every natural system on Earth and that they are accelerating. They impact both directly and indirectly most of the diseases that make up the majority of the global burden of disease. For the reasons outlined above, it is not yet possible to quantify the burden of disease associated with the disruption of these natural systems to the same degree that has been accomplished with other types of environmental health risks. We suspect, however, that the health burden associated with ecosystem alterations will be on a par with those other types of environmental health risks which were found to account for roughly one quarter of the global burden of disease (87).
Moving Forward
Environmental health has always been a pragmatic discipline focused on identifying and quantifying threats to human health in the environment so that these threats can be addressed. Removing lead from gasoline and the Clean Air Act and Clean Water Act in the United States are prominent examples. In the relatively new branch of environmental health focused on the health impacts of changes in the structure and function of natural systems, we believe that we need a similarly pragmatic focus. Although our understanding of the health impacts of ecosystem alteration in some areas is already adequate to inform policy decisions—the need to anticipate changes in vector-borne disease exposure associated with dams and irrigation projects, for example—our understanding remains too patchy to meaningfully inform policy or resource management decisions in many systems. We propose the following steps to generate a more robust and useful understanding of these relationships.
Fill the Gaps.
Many of the relationships that have been explored in the studies outlined above remain incompletely characterized. Snapshots of particular disease/land cover relationships in specific locations (e.g., deforestation and schistosomiasis in Cameroon) are compelling evidence that land cover changes can alter infectious disease transmission, but they fall short of allowing resource managers and policy makers to anticipate the full health implications of their decisions. We need to understand how these changes might be expected to affect transmission of other burdensome diseases and how generalizable these relationships are across different spatial scales. Applying methods that are already developed, investigators could characterize these relationships more completely, with the resulting knowledge base informing natural resource management much more meaningfully.
In disease ecology, much of the foundational work has been done using infectious disease systems that are associated with relatively small burdens of disease. We would benefit from greater understanding of the complex ecology of infectious diseases associated with very large global burdens such as malaria, diarrhea, influenza, schistosomiasis, dengue, Chagas disease, and leishmaniasis. In addition, as outlined above, there is important work to do to characterize whether there are differences between those organisms that are first removed from a community during disturbance and those which are left behind with respect to their ability to transmit infectious diseases. Such differences might help to explain whether there are general principles like the dilution effect that can be expected to hold up across different systems and diseases. Finally, the field would benefit from more studies that look at the impacts of disturbance or management interventions on the ecology of multiple diseases simultaneously. For example, recent work in Uganda shows how forest fragmentation and disturbance alter exposure among both humans and apes to a variety of different infectious disease agents (103).
We still have little understanding of the role that marine and terrestrial wildlife species play in providing both macro- and micronutrients for the many people who, for numerous reasons, do not have access to these nutrients through alternative sources. In a few populations, careful work comparing dietary survey data, biomarkers of nutrition, food composition analysis, and anthropometry is allowing quantification of the nutritional importance of access to wildlife in the diet (68). This work might fruitfully be extended to other systems and populations to assess how wildlife management, including marine conservation (104), impacts the nutritional status of local populations dependent upon these species for key nutrients.
Beyond the nutritional provisioning service of wildlife in the diet, there is a great deal of work to be done to characterize the health values of other ecosystem services and the impacts on health of changes in the condition of these systems. For example, pollination services have been shown to underpin the production of roughly one third of global calories produced for human consumption (67) but more specific work would allow us to quantify the importance of pollination services for both micro- and macronutrition for particular populations around the world and to model the health implications of pollinator declines in those locations.
There is much to learn about how different animal husbandry practices, land uses, and incursions into wildlife habitat affect the development and transmission of new zoonotic infections (105). This understanding will be important to the development of more effective surveillance approaches. In the area of climate change, there are many open questions that should be a priority, including the numerous and interacting impacts of climate change on food production and availability and the impacts of climatic disruption on the timing, quantity, and quality of water available for different populations. Determining the effects of climate change on the geographic distribution of infectious diseases is another important area of active research. Of course, this is not an exhaustive list but is meant to be illustrative.
Address the Limitations.
As we set about filling the gaps in our understanding, we also need to address the limitations outlined above. Research efforts that explore multiple health outcomes from a particular ecosystem alteration may generate more useful insights for policy and practice than studies focused on a single health outcome. New approaches that allow us to anticipate the health impacts of multiple, interacting environmental changes including resource scarcity, land-use change, and climate change should, in many instances, provide more realistic estimates than analyses that look at a particular change in isolation. Research in the Brazilian Amazon exploring multiple health outcomes associated with a combination of climate change and deforestation is one example of such work (106). Expanding research to factor in human responses and adaptations to environmental changes will also provide more realistic estimates of real world impacts of ecosystem alterations on human health, and some work has been achieved in this area (107, 108). Finally, we will reach more clarity by being very specific about whose health is being studied within a population or by explicitly disaggregating different segments of a population to look at differential impacts.
In addition to filling gaps in our understanding and addressing several limitations, we propose two additional approaches that could help to guide future work.
Respond to specific policy needs.
Much of the existing research appears to have been driven by scientific curiosity more than pragmatic questions about how to manage natural systems to optimize health and environmental outcomes. Only after obtaining results do scientific teams typically work to apply their findings to policy. We propose increased emphasis on policy-driven research. For example, what are the likely impacts of recent changes in Brazil's forest code on malaria rates in Amazonian states (109, 110)? The answer to this exciting research question, which would require field experiments and modeling to unravel, would almost inevitably inform refinements of this policy. Scientific efforts in biodiversity conservation, ecosystem services, and many other policy-relevant fields also tend to proceed from basic research to policy application, and we believe that a basic reversal of approach would increase the value of research results to society. Accomplishing this shift from curiosity-driven to policy-driven research will require a parallel culture shift in the scientific disciplines such that incentives in the form of promotions, funding, and success in publishing are consistent with this emphasis.
Build production functions.
In wide use in the industrial and agricultural sciences, production functions relate the amounts and qualities of inputs to the amounts of particular outputs in a system. Adapting this approach would allow us to functionally define the key components of natural systems that support human health and to predict the health impacts of incremental changes in those components. For example, the use of fire as a land-clearing approach in Southeast Asia is a major threat to biodiversity in the region and is also responsible for significant particulate air pollution exposures for downwind populations in Indonesia, Singapore, and Malaysia. Estimating the emissions associated with these fires and modeling their chemical evolution as they are transported through space and time to reach downwind populations will allow policy makers to understand how fires associated with particular geographies, land cover types, or land uses are affecting morbidity and mortality (primarily from cardiorespiratory disease) for regional populations. Such work will allow land-use planners to factor in the public health implications of the decisions they are making in a way that has been elusive to date. This careful quantification of specific health impacts as a function of changes in environmental condition is critical to moving away from an abstract discussion about the importance of natural systems to human health, and toward concrete tools that allow policy makers and resource managers to quantify the health implications of the decisions they make.
Conclusion
Human activity is transforming Earth’s natural systems in ways that are profound, pervasive, and accelerating. This transformation is generating a suite of health impacts that remain, in many instances, poorly characterized. However, ample evidence exists that nearly every dimension of human health is being affected, and it is likely that the disease burden associated with these aggregate ecosystem alterations is large and growing. We propose a more systematic and comprehensive approach to understanding the health impacts of ecosystem alteration to better inform decision making in the land-use planning, environmental conservation, and public health policy realms.
Acknowledgments
We acknowledge support from the entire Health & Ecosystems: Analysis of Linkages (HEAL) consortium in framing many of the issues discussed in this article. In particular, we thank the following individuals for valuable feedback on earlier versions of this manuscript: Stacy Jupiter, Carter Ingram, Arlyne Johnson, Mike Mascia, David Wilkie, and Ilana Brito. We also thank Damien Joly for assistance in preparing Fig. 2 and Kellie Nault for assistance in preparing Fig. 1. This work was supported by The Gordon and Betty Moore Foundation and The Rockefeller Foundation as part of the Health & Ecosystems: Analysis of Linkages (HEAL) program. Support was also received from the Bill and Melinda Gates Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Foley JA, et al. Global consequences of land use. Science. 2005;309(5734):570–574. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- 2.Schrag D. Geobiology of the Anthropocene. In: Knoll A, Canfield D, Konhauser K, editors. Fundamentals of Geobiology. Chichester, UK: John Wiley & Sons; 2012. pp. 425–436. [Google Scholar]
- 3.Millennium Ecosystem Assessment Program . Ecosystems and Human Well-Being: Synthesis. Washington, DC: Island Press; 2005. p. 2. [Google Scholar]
- 4. Corvalán C, Hales S, McMichael AJ, Millennium Ecosystem Assessment (Program), and World Health Organization (2005) Ecosystems and Human Well-Being: Health Synthesis (World Health Organization, Geneva), p 53.
- 5.Myers SS, Patz J. Emerging threats to human health from global environmental change. Annu Rev Environ Resour. 2009;34:223–252. [Google Scholar]
- 6.Shiff CJ. Integrated approach to malaria control. Clin Microbiol Rev. 2002;15(2):278–293. doi: 10.1128/CMR.15.2.278-293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keiser J, Singer BH, Utzinger J. Reducing the burden of malaria in different eco-epidemiological settings with environmental management: A systematic review. Lancet Infect Dis. 2005;5(11):695–708. doi: 10.1016/S1473-3099(05)70268-1. [DOI] [PubMed] [Google Scholar]
- 8.Cline BL, et al. 1983 Nile Delta schistosomiasis survey: 48 years after Scott. Am J Trop Med Hyg. 1989;41(1):56–62. [PubMed] [Google Scholar]
- 9.Malek EA. Effect of the Aswan High Dam on prevalence of schistosomiasis in Egypt. Trop Geogr Med. 1975;27(4):359–364. [PubMed] [Google Scholar]
- 10.Mutero CM. The Changing Face of Irrigation in Kenya: Opportunities for Anticipating Change in Eastern and Southern Africa. Colombo, Sri Lanka: International Water Management Institute; 2002. Health impact assessment of increased irrigation in the Tana River Basin, Kenya. [Google Scholar]
- 11.Ghebreyesus TA, et al. Incidence of malaria among children living near dams in northern Ethiopia: Community based incidence survey. BMJ. 1999;319(7211):663–666. doi: 10.1136/bmj.319.7211.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma VP. Re-emergence of malaria in India. Indian J Med Res. 1996;103:26–45. [PubMed] [Google Scholar]
- 13.Appawu MA, Dadzie SK, Baffoe-Wilmot A, Wilson MD. Lymphatic filariasis in Ghana: Entomological investigation of transmission dynamics and intensity in communities served by irrigation systems in the Upper East Region of Ghana. Trop Med Int Health. 2001;6(7):511–516. doi: 10.1046/j.1365-3156.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 14.Harb M, et al. The resurgence of lymphatic filariasis in the Nile delta. Bull World Health Organ. 1993;71(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- 15.Jobin W. Dams and Disease: Ecological Design and Health Impacts of Large Dams, Canals, and Irrigation Systems. London: E&FN Spon; 1999. p. 580. [Google Scholar]
- 16.Patz JA, Confalonieri UEC. Human health: Ecosystem regulation of infectious diseases. In: Hassan RM, Scholes R, Ash N, editors. Ecosystems and Human Well-Being: Current State and Trends: Findings of the Condition and Trends Working Group of the Millennium Ecosystem Assessment. Washington, DC: Island Press; 2005. pp. 391–415. [Google Scholar]
- 17.Cohuet A, et al. High malaria transmission intensity due to Anopheles funestus (Diptera: Culicidae) in a village of savannah-forest transition area in Cameroon. J Med Entomol. 2004;41(5):901–905. doi: 10.1603/0022-2585-41.5.901. [DOI] [PubMed] [Google Scholar]
- 18.Coluzzi M. Heterogeneities of the malaria vectorial system in tropical Africa and their significance in malaria epidemiology and control. Bull World Health Organ. 1984;62(Suppl):107–113. [PMC free article] [PubMed] [Google Scholar]
- 19.Coluzzi M. Malaria and the Afrotropical ecosystems: Impact of man-made environmental changes. Parassitologia. 1994;36(1-2):223–227. [PubMed] [Google Scholar]
- 20.Guerra CA, Snow RW, Hay SI. A global assessment of closed forests, deforestation and malaria risk. Ann Trop Med Parasitol. 2006;100(3):189–204. doi: 10.1179/136485906X91512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasuoka J, Levins R. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am J Trop Med Hyg. 2007;76(3):450–460. [PubMed] [Google Scholar]
- 22.de Castro MC, Monte-Mór RL, Sawyer DO, Singer BH. Malaria risk on the Amazon frontier. Proc Natl Acad Sci USA. 2006;103(7):2452–2457. doi: 10.1073/pnas.0510576103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer B, de Castro MC. Enhancement and suppression of malaria in the Amazon. Am J Trop Med Hyg. 2006;74(1):1–2. [PubMed] [Google Scholar]
- 24.Tadei WP, et al. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am J Trop Med Hyg. 1998;59(2):325–335. doi: 10.4269/ajtmh.1998.59.325. [DOI] [PubMed] [Google Scholar]
- 25.Olson SH, Gangnon R, Silveira GA, Patz JA. Deforestation and malaria in Mâncio Lima County, Brazil. Emerg Infect Dis. 2010;16(7):1108–1115. doi: 10.3201/eid1607.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wayant NM, Maldonado D, Rojas de Arias A, Cousiño B, Goodin DG. Correlation between normalized difference vegetation index and malaria in a subtropical rain forest undergoing rapid anthropogenic alteration. Geospat Health. 2010;4(2):179–190. doi: 10.4081/gh.2010.199. [DOI] [PubMed] [Google Scholar]
- 27.Amerasinghe FP, Ariyasena TG. Larval survey of surface water-breeding mosquitoes during irrigation development in the Mahaweli Project, Sri Lanka. J Med Entomol. 1990;27(5):789–802. doi: 10.1093/jmedent/27.5.789. [DOI] [PubMed] [Google Scholar]
- 28. Karla NL (1991) Forest malaria vectors in India: Ecological characteristics and epidemiological implications. Forest Malaria in Southeast Asia, eds Sharma VP, Kondrashin AV (World Health Organization/Medical Research Council, New Delhi), pp 93–114.
- 29. Konradsen F, Amerasinghe FP, van der Hoek W, Amerasinghe PH (1990) Malaria in Sri Lanka, Current Knowledge on Transmission and Control (International Water Management Institute, Battaramulla, Sri Lanka)
- 30.Molyneux D, Ostfeld RS, Bernstein A, Chivian E. Ecosystem disturbance, biodiversity loss, and human infectious disease. In: Chivian E, Bernstein A, editors. Sustaining Life: How Human Health Depends on Biodiversity. New York: Oxford Univ Press; 2008. pp. 287–323. [Google Scholar]
- 31.Taylor D. Seeing the forests for the more than the trees. Environ Health Perspect. 1997;105(11):1186–1191. doi: 10.1289/ehp.971051186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Southgate VR, van Wijk HB, Wright CA. Schistosomiasis at Loum, Cameroun; Schistosoma haematobium, S. intercalatum and their natural hybrid. Z Parasitenkd. 1976;49(2):145–159. doi: 10.1007/BF00382422. [DOI] [PubMed] [Google Scholar]
- 33. Rejmankova E, et al. (2006) Freshwater community interactions and malaria. Disease Ecology, eds Collinge SK, Ray C (Oxford Univ Press, Oxford), p 227.
- 34. Goldberg TL, Gillespie TR, Rwego IB (2008) Health and disease in the people, primates, and domestic animals of Kibale National Park: Implications for conservation. Science and Conservation in African Forests: The Benefits of Long-Term Research, eds Wrangham R, Ross E (Cambridge Univ Press, Cambridge, UK)
- 35.Wolfe ND, et al. Naturally acquired simian retrovirus infections in central African hunters. Lancet. 2004;363(9413):932–937. doi: 10.1016/S0140-6736(04)15787-5. [DOI] [PubMed] [Google Scholar]
- 36.Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: Scientific and public health implications. Science. 2000;287(5453):607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- 37.Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356(1411):983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahdouh-Guebas F, et al. How effective were mangroves as a defence against the recent tsunami? Curr Biol. 2005;15(12):R443–R447. doi: 10.1016/j.cub.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Danielsen F, et al. The Asian tsunami: A protective role for coastal vegetation. Science. 2005;310(5748):643. doi: 10.1126/science.1118387. [DOI] [PubMed] [Google Scholar]
- 40.Kunkel CM, Hallberg RW, Oppenheimer M. Coral reefs reduce tsunami impact in model simulations. Geophys Res Lett. 2006 33(23):L23612. [Google Scholar]
- 41.Marris E. Tsunami damage was enhanced by coral theft. Nature. 2005;436(7054):1071. doi: 10.1038/4361071a. [DOI] [PubMed] [Google Scholar]
- 42.Barbier EB, et al. Coastal ecosystem-based management with nonlinear ecological functions and values. Science. 2008;319(5861):321–323. doi: 10.1126/science.1150349. [DOI] [PubMed] [Google Scholar]
- 43.Cockburn A, St Clair J, Silverstein K. The politics of “natural” disaster: Who made Mitch so bad? Int J Health Serv. 1999;29(2):459–462. doi: 10.2190/BC4C-Y1T9-23P8-U991. [DOI] [PubMed] [Google Scholar]
- 44.Brauman KA, Daily GC, Duarte TKE, Mooney HA. The nature and value of ecosystem services: An overview highlighting hydrologic services. Annu Rev Environ Resour. 2007;32(1):67–98. [Google Scholar]
- 45.Pattanayak SK, Wendland KJ. Nature’s care: Diarrhea, watershed protection, and biodiversity conservation in Flores, Indonesia. Biodivers Conserv. 2007;16(10):2801–2819. [Google Scholar]
- 46.Beckett KP, Freer-Smith PH, Taylor G. Particulate pollution capture by urban trees: Effect of species and windspeed. Glob Change Biol. 2000;6(8):995–1003. [Google Scholar]
- 47.Freer-Smith PH, El-Khatib AA, Taylor G. Capture of particulate pollution by trees: A comparison of species typical of semi-arid areas (Ficus nitida and Eucalyptus globulus) with European and North American species. Water Air Soil Pollut. 2004;155(1-4):173–187. [Google Scholar]
- 48.Powe NA, Willis KG. Mortality and morbidity benefits of air pollution (SO2 and PM10) absorption attributable to woodland in Britain. J Environ Manage. 2004;70(2):119–128. doi: 10.1016/j.jenvman.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 49. Collinge SK,Ray C, eds (2006) Disease Ecology: Community Structure and Pathogen Dynamics (Oxford Univ Press, Oxford)
- 50. Ostfeld R, Keesing F, Eviner VT, eds (2008) Infectious Disease Ecology: The Effects of Ecosystems on Disease and of Disease on Ecosystems (Princeton Univ Press, Princeton)
- 51.Allan BF, et al. Ecological correlates of risk and incidence of West Nile virus in the United States. Oecologia. 2009;158(4):699–708. doi: 10.1007/s00442-008-1169-9. [DOI] [PubMed] [Google Scholar]
- 52.Swaddle JP, Calos SE. Increased avian diversity is associated with lower incidence of human West Nile infection: Observation of the dilution effect. PLoS ONE. 2008;3(6):e2488. doi: 10.1371/journal.pone.0002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9(4):485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 54.LoGiudice K, et al. Impact of host community composition on Lyme disease risk. Ecology. 2008;89(10):2841–2849. doi: 10.1890/07-1047.1. [DOI] [PubMed] [Google Scholar]
- 55.Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F. Climate, deer, rodents, and acorns as determinants of variation in lyme-disease risk. PLoS Biol. 2006;4(6):e145. doi: 10.1371/journal.pbio.0040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzán G, et al. Experimental evidence for reduced rodent diversity causing increased hantavirus prevalence. PLoS ONE. 2009;4(5):e5461. doi: 10.1371/journal.pone.0005461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xavier SC, et al. Lower richness of small wild mammal species and chagas disease risk. PLoS Negl Trop Dis. 2012;6(5):e1647. doi: 10.1371/journal.pntd.0001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gottdenker NL, Chaves LF, Calzada JE, Saldaña A, Carroll CR. Host life history strategy, species diversity, and habitat influence Trypanosoma cruzi vector infection in Changing landscapes. PLoS Negl Trop Dis. 2012;6(11):e1884. doi: 10.1371/journal.pntd.0001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keesing F, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468(7324):647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt KA, Ostfeld RS. Biodiversity and the dilution effect in disease ecology. Ecology. 2001;82(3):609–619. [Google Scholar]
- 61.Laporta GZ, Lopez de Prado PI, Kraenkel RA, Coutinho RM, Sallum MAM. Biodiversity can help prevent malaria outbreaks in tropical forests. PLoS Negl Trop Dis. 2013;7(3):e2139. doi: 10.1371/journal.pntd.0002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Previtali MA, et al. Relationship between pace of life and immune responses in wild rodents. Oikos. 2012;121(9):1483–1492. [Google Scholar]
- 63.Young H, Griffin RH, Wood CL, Nunn CL. Does habitat disturbance increase infectious disease risk for primates? Ecol Lett. 2013;16(5):656–663. doi: 10.1111/ele.12094. [DOI] [PubMed] [Google Scholar]
- 64.Wood CL, Lafferty KD. Biodiversity and disease: A synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol Evol. 2013;28(4):239–247. doi: 10.1016/j.tree.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 65.Chivian E, Bernstein A. Sustaining Life: How Human Health Depends on Biodiversity. New York: Oxford Univ Press; 2008. [Google Scholar]
- 66.Eilers EJ, Kremen C, Smith Greenleaf S, Garber AK, Klein A-M. Contribution of pollinator-mediated crops to nutrients in the human food supply. PLoS ONE. 2011;6(6):e21363. doi: 10.1371/journal.pone.0021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klein A-M, et al. Importance of pollinators in changing landscapes for world crops. Proc Biol Sci. 2007;274(1608):303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Golden CD, Fernald LC, Brashares JS, Rasolofoniaina BJ, Kremen C. Benefits of wildlife consumption to child nutrition in a biodiversity hotspot. Proc Natl Acad Sci USA. 2011;108(49):19653–19656. doi: 10.1073/pnas.1112586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Speldewinde PC, Cook A, Davies P, Weinstein P. A relationship between environmental degradation and mental health in rural Western Australia. Health Place. 2009;15(3):865–872. doi: 10.1016/j.healthplace.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 70.Bratman GN, Hamilton JP, Daily GC. Ostfeld R, Schlesinger W, editors. (2012) The impacts of nature experience on human cognitive function and mental health. Ann NY Acad Sci 1249:118–136. [DOI] [PubMed]
- 71.Depledge MH, Stone RJ, Bird WJ. Can natural and virtual environments be used to promote improved human health and wellbeing? Environ Sci Technol. 2011;45(11):4660–4665. doi: 10.1021/es103907m. [DOI] [PubMed] [Google Scholar]
- 72.Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. Climate change and infectious diseases: From evidence to a predictive framework. Science. 2013;341(6145):514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- 73.Ostfeld RS. Climate change and the distribution and intensity of infectious diseases. Ecology. 2009;90(4):903–905. doi: 10.1890/08-0659.1. [DOI] [PubMed] [Google Scholar]
- 74.Pascual M, Ahumada JA, Chaves LF, Rodó X, Bouma M. Malaria resurgence in the East African highlands: Temperature trends revisited. Proc Natl Acad Sci USA. 2006;103(15):5829–5834. doi: 10.1073/pnas.0508929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bentham G, Langford IH. Climate change and the incidence of food poisoning in England and Wales. Int J Biometeorol. 1995;39(2):81–86. doi: 10.1007/BF01212585. [DOI] [PubMed] [Google Scholar]
- 76.Curriero FC, Patz JA, Rose JB, Lele S. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948-1994. Am J Public Health. 2001;91(8):1194–1199. doi: 10.2105/ajph.91.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rose JB, et al. Climate variability and change in the United States: Potential impacts on water- and foodborne diseases caused by microbiologic agents. Environ Health Perspect. 2001;109(Suppl 2):211–221. doi: 10.1289/ehp.01109s2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wayne P, Foster S, Connolly J, Bazzaz F, Epstein P. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atmospheres. Ann Allergy Asthma Immunol. 2002;88(3):279–282. doi: 10.1016/S1081-1206(10)62009-1. [DOI] [PubMed] [Google Scholar]
- 79.Bell ML, et al. Climate change, ambient ozone, and health in 50 U.S. cities. Clim Change. 2007;82(1-2):61–76. [Google Scholar]
- 80. Schneider SH, Semenov S, Patwardhan A (2007) Assessing key vulnerabilities and the risk from climate change. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, eds Parry ML, Canziani OF, Palutikof JP, Van der Linden PJ, Hanson CE (Cambridge Univ Press, Cambridge, UK), pp 779–810.
- 81.Battisti DS, Naylor RL. Historical warnings of future food insecurity with unprecedented seasonal heat. Science. 2009;323(5911):240–244. doi: 10.1126/science.1164363. [DOI] [PubMed] [Google Scholar]
- 82.Lobell DB, et al. Prioritizing climate change adaptation needs for food security in 2030. Science. 2008;319(5863):607–610. doi: 10.1126/science.1152339. [DOI] [PubMed] [Google Scholar]
- 83.Schlenker W, Roberts MJ. Nonlinear temperature effects indicate severe damages to U.S. crop yields under climate change. Proc Natl Acad Sci USA. 2009;106(37):15594–15598. doi: 10.1073/pnas.0906865106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Johnstone LC (2008) Planning for the inevitable, the humanitarian consequences of climate change. Linking Climate Change Negotiations and Disaster Risk Reduction Conference, November 12–13, 2008, Copenhagen. Available at www.unhcr.org/49256c492.html. Accessed October 28, 2013.
- 85.Sachs JD. Climate change refugees. Sci Am. 2007;296(6):43. doi: 10.1038/scientificamerican0607-43. [DOI] [PubMed] [Google Scholar]
- 86.Smith KR, Ezzati M. How environmental health risks change with development: The epidemiologic and environmental risk transitions revisited. Annu Rev Environ Resour. 2005;30(1):291–333. [Google Scholar]
- 87.Prüss-Ustün A, Bonjour S, Corvalán C. The impact of the environment on health by country: A meta-synthesis. Environ Health. 2008;7:7. doi: 10.1186/1476-069X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McMichael A, Woodruff R. Detecting the health effects of environmental change: Scientific and political challenge. EcoHealth. 2005;2(1):1–3. [Google Scholar]
- 89.Murray CJL, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 90.Karesh WB, et al. Ecology of zoonoses: Natural and unnatural histories. Lancet. 2012;380(9857):1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kotloff KL, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet. 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 92.Graczyk TK, Evans BM, Shiff CJ, Karreman HJ, Patz JA. Environmental and geographical factors contributing to watershed contamination with Cryptosporidium parvum oocysts. Environ Res. 2000;82(3):263–271. doi: 10.1006/enrs.1999.4022. [DOI] [PubMed] [Google Scholar]
- 93.Mac Kenzie WR, et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331(3):161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 94.Högy P, Fangmeier A. Effects of elevated atmospheric CO2 on grain quality of wheat. J Cereal Sci. 2008;48:580–591. [Google Scholar]
- 95.Loladze I. Rising atmospheric CO2 and human nutrition: Toward globally imbalanced plant stoichiometry? Trends Ecol Evol. 2002;17(10):457–461. [Google Scholar]
- 96.Prior SA, Runion GB, Rogers HH, Torbert HA. Effects of atmospheric CO2 enrichment on crop nutrient dynamics under no-till conditions. J Plant Nutr. 2008;31(4):758–773. [Google Scholar]
- 97.Taub DR, Miller B, Allen H. Effects of elevated CO2 on the protein concentration of food crops: A meta-analysis. Glob Change Biol. 2008;14(3):565–575. [Google Scholar]
- 98. Caulfield LE, Black RE (2004) Zinc deficiency. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attribution to Selected Major Risk Factors, eds Ezzati M, Lopez AD, Rodgers A, Murray CJL (World Health Organization, Geneva), Vol 1, pp 257–280.
- 99. Stoltzfus RJ, Mullany L, Black RE (2004) Iron deficiency anaemia. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attribution to Selected Major Risk Factors, eds Ezzati M, Lopez AD, Rodgers A, Murray CJL (World Health Organization, Geneva), Vol 1, pp 163–210.
- 100.Alexandratos N. World food and agriculture: Outlook for the medium and longer term. Proc Natl Acad Sci USA. 1999;96(11):5908–5914. doi: 10.1073/pnas.96.11.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Myers SS, Bernstein A. The coming health crisis: Indirect health effects of global climate change. F1000 Biol Rep. 2011;3(1):3. doi: 10.3410/B3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Appel LJ, et al. OmniHeart Collaborative Research Group Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 103. Goldberg TL, Paige S, Chapman C (2012) The Kibale EcoHealth Project: Exploring connections among human health, animal health, and landscape dynamics in Western Uganda. New Directions in Conservation Medicine: Applied Cases of Ecological Health, eds Aguirre A, Ostfeld R, Daszak P (Oxford Univ Press, New York), pp 452–465.
- 104.Hall SJ, Hilborn R, Andrew NL, Allison EH. Innovations in capture fisheries are an imperative for nutrition security in the developing world. Proc Natl Acad Sci USA. 2013;110(21):8393–8398. doi: 10.1073/pnas.1208067110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones BA, et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci USA. 2013;110(21):8399–8404. doi: 10.1073/pnas.1208059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pattanayak SK, et al. Climate change and conservation in Brazil: CGE evaluation of health and wealth impacts. BE J Econ Anal Pol. 2009;9(2):42. [Google Scholar]
- 107.Pattanayak S, Yasuoka J. Deforestation and malaria: Revisiting the human ecology perspective. In: Colfer CJP, editor. Forests, People and Health: A Global Interdisciplinary Overview. Earthscan Publishers, Oxford; 2008. pp. 197–217. [Google Scholar]
- 108.Pattanayak SK, Pfaff A. Behavior, environment and health in developing countries: Evaluation and valuation. Annu Rev Resource Econ. 2009;1(1):183–222. [Google Scholar]
- 109.Vittor AY, et al. Linking deforestation to malaria in the Amazon: characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am J Trop Med Hyg. 2009;81(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- 110.Metzger JP, et al. Brazilian law: Full speed in reverse? Science. 2010;329(5989):276–277. doi: 10.1126/science.329.5989.276-b. [DOI] [PubMed] [Google Scholar]