Significance
The Drosophila master sex-determination switch gene, Sex-lethal (Sxl), was thought to elicit all aspects of female-specific somatic differentiation other than size dimorphism by acting on the gene transformer (tra) alone. Here we show that at least one aspect of Drosophila female-specific behavior, ovulation, is controlled by Sxl acting developmentally on some target(s) other than tra in a small subset of neurons in which, surprisingly, the tra target gene fruitless may function also. This minor branch in the sex-determination pathway should be useful for understanding how genes control behavior and perhaps also how, during evolution, Sxl managed to usurp the role of tra as the master regulator of sex determination relatively quickly in Drosophila's insect ancestors.
Abstract
The switch gene Sex-lethal (Sxl) was thought to elicit all aspects of Drosophila female somatic differentiation other than size dimorphism by controlling only the switch gene transformer (tra). Here we show instead that Sxl controls an aspect of female sexual behavior by acting on a target other than or in addition to tra. We inferred the existence of this unknown Sxl target from the observation that a constitutively feminizing tra transgene that restores fertility to tra− females failed to restore fertility to Sxl-mutant females that were adult viable but functionally tra−. The sterility of these mutant females was caused by an ovulation failure. Because tra expression is not sufficient to render these Sxl-mutant females fertile, we refer to this pathway as the tra-insufficient feminization (TIF) branch of the sex-determination regulatory pathway. Using a transgene that conditionally expresses two Sxl feminizing isoforms, we find that the TIF branch is required developmentally for neurons that also sex-specifically express fruitless, a tra gene target controlling sexual behavior. Thus, in a subset of fruitless neurons, targets of the TIF and tra pathways appear to collaborate to control ovulation. In most insects, Sxl has no sex-specific functions, and tra, rather than Sxl, is both the target of the primary sex signal and the gene that maintains the female developmental commitment via positive autoregulation. The TIF pathway may represent an ancestral female-specific function acquired by Sxl in an early evolutionary step toward its becoming the regulator of tra in Drosophila.
Understanding the regulatory gene pathway that controls sexual dimorphism in Drosophila melanogaster began with the discovery that diplo-X individuals develop as females, and haplo-X individuals develop as males (1). Subsequently, regulatory genes were identified that generate the X-chromosome dose signal, and switch genes were found that respond to that signal, either directly or indirectly, to elicit sexually dimorphic development (reviewed in refs. 2–9). Here we describe a surprising feature of the functional relationship between two key switch genes, Sex-lethal (Sxl) and transformer (tra), in this heavily studied sex-determination pathway with implications for development and possibly evolution as well.
Sxl is the feminizing switch gene that is activated directly by the diplo-X sex signal. It is activated soon after fertilization but stays active thereafter independent of this initiating signal by directing female-specific alternative splicing of its own transcripts to produce a set of feminizing RNA-binding proteins, hereafter abbreviated as “Sxl-F” (reviewed in ref. 8). Sxl-F controls sexual development and maintains a rate of X-chromosome dosage compensation appropriate for diplo-X cells. Sxl-F controls sexual differentiation by directing the pre-mRNA splicing of tra transcripts to produce the feminizing RNA-binding protein Tra-F. Tra-F in turn controls sex-specific alternative splicing of transcripts from its regulatory gene targets, which include the transcription factor-encoding switch genes doublesex (dsx) and fruitless (fru) (reviewed in refs. 4 and 7). Unlike Sxl, tra and its downstream switch-gene targets do not control the vital process of X-chromosome dosage compensation and hence are neither essential for female viability nor necessarily lethal when expressed in a sexually inappropriate fashion.
The regulatory relationship between Sxl and tra in the genus Drosophila proved to be an exception among insects (reviewed in refs. 10 and 11). More commonly, the tra ortholog appears to be the most immediate gene target of the primary sex-determination signal and the gene that maintains the female developmentally determined state thereafter by positive autoregulation. Remarkably, tra is the only gene other than Sxl found to maintain developmental fate via a pre-mRNA splicing positive-feedback loop. Although Sxl is easily identifiable in these other insect species, it has no apparent sex-specific role.
The relatively rapid evolutionary ascent of Sxl over tra to the position of master autoregulating sex-determination gene in a Drosophila ancestor is of obvious interest (12). A hypothesis for how functional redundancy in positive autoregulatory circuits between Sxl and tra might have led to the two genes changing places in a regulatory hierarchy followed from the discovery in Drosophila of unambiguous vestiges of functional redundancy in Sxl positive-feedback circuits (13). The first step in this hypothesized evolutionary route to the switch between tra and Sxl was for Sxl to come under the control of tra. Implicit in that first step was the prior acquisition by Sxl of a female-specific function that would make control by tra advantageous. Here we present evidence for a relatively limited feminizing function of Sxl-F that could reflect that ancestral first step toward Sxl becoming the master sex-switch gene. The potential relevance of this particular feminizing function to that ancestral first step stems from the fact that this function belongs to a regulatory branch in the sex-determination pathway that leads from Sxl but does not go through tra.
We call this minor branch in the Drosophila sex-determination pathway “tra-insufficient feminization” (TIF) to distinguish it from the established “tra-sufficient feminization” (TSF) major pathway. We use the term “insufficient” because we currently are limited to studying this aspect of Sxl female functioning in situations where Tra-F is provided constitutively. Consequently, we cannot yet distinguish between a feminizing Sxl-F function that is truly independent of Tra-F vs. one that requires both Tra-F and an unknown Sxl-F gene target.
We discovered TIF serendipitously using a more effective constitutively feminizing Tra-F transgene. Although the original Tra-F transgene was instrumental in demonstrating that tra is a feminizing switch gene controlled by Sxl (14), it and another subsequently generated transgene (15) were of limited utility because neither rescued the null tra phenotype enough to restore fertility to tra-mutant females. Females carrying the transgene but lacking endogenous tra+ gene function made eggs and mated but were sterile, at least in part because they failed to lay their eggs. Our Tra-F transgene driven by a U2af50 promoter overcame this limitation (16). U2af50 is a ubiquitously expressed RNA-splicing housekeeping gene. Because this transgene restored fertility even to tra− females, we anticipated being able to use it to study the meiotic effects of hypomorphic Sxl alleles that provided sufficient dosage compensation function to allow some mutant females to survive to the adult stage but not enough tra regulatory function to allow them to be rescued to fertility by the previously available Tra-F transgenes (see ref. 17).
To our surprise, some such adult-viable Sxl-mutant females carrying the U2af-traF transgene made normal-looking eggs and mated but nevertheless were sterile because they failed to lay their eggs—a phenotype resembling that for incomplete tra− rescue by the earlier transgenes. However, because tra− females carrying U2af-traF do lay their eggs, we speculated that, rather than reflecting incomplete tra rescue, the egg-laying defect of these Sxl-mutant females was caused by misregulation of some unknown Sxl-F protein target—the TIF target. Here we establish the validity of the TIF hypothesis by showing that this Sxl female-sterile phenotype must have a different cause than the female-sterile phenotype seen with incomplete rescue of the tra-null condition, which resembles it only superficially. We rule out the alternative possibilities that TIF-mutant female sterility arises from interference by the mutant Sxl alleles with the effectiveness of the U2af-traF transgene or from misregulation of the dosage-compensation pathway controlled by its master switch gene, the Sxl target msl2. Using an improved Sxl-F conditional-expression construct, we show the time and place at which TIF function is needed, with results that point to a surprisingly close functional relationship between presumed target of the TIF pathway and the TSF pathway target, fru.
Results
Ovulation-Defective Sxl-Mutant Females Reveal a TIF Branch of the Sex-Determination Regulatory Pathway.
The high viability (73% at eclosion; Table 1, cross A) and longevity of Sxlf7,M1/SxlM1,fΔ33-mutant females made this study possible and distinguished these Sxl adults from equally masculinized Sxl-mutant adult females described previously. The viability of those previously described females—Sxlf7,M1/SxlM1,f3 and Sxlf7,M1/Sxlf7,M1—was only 10% and 11% at eclosion, respectively, and nearly all died within a day (18). Like those two genotypes, Sxlf7,M1/SxlM1,fΔ33 masculinizes females more thoroughly than complete loss of tra+ in that the diplo-X Sxl-mutant pseudomales resemble true (haplo-X) males not only in external and internal somatic morphology but also in body size. In contrast, tra− pseudomales are larger, nearly the size of tra+ females. The only hints of somatic femininity for all three genotypes of Sxl-mutant females are a few small sixth-sternite bristles and ∼25% fewer sexcomb teeth than seen in true males. As expected, adding a constitutive Tra-F transgene to these genotypes feminizes without increasing their size.
Table 1.
The TIF− phenotype is recessive and not caused by upsets in msl-based dosage compensation
| Cross generating females* | Relevant genotype of females with P{U2af-traF}/+ (mated unless otherwise specified) | Relative viability, %† | Egg-laying parameters‡ |
|||
| % laying females§ | No. tested | Eggs laid⋅laying female−1⋅d−1 ± SEM¶ | Maximum no. eggs laid in 1 d|| | |||
| A | Sxlf7,M1/ SxlM1,fΔ33 | 73 | 0 | 39 | – | – |
| A | Sxlf7,M1/ SxlM1,fΔ33; Dp(Sxl+) | ref (n = 77) | 100 | 23 | 70.4 ± 3.4 | 59–142 |
| A | Dp(Sxl+) but not mated | 90 | 30 | 6.8 ± 1.3 | 3–57 | |
| B | Sxlf7,M1/ SxlM1,fΔ33; msl-2 | 52 | 4 | 78 | 0.3 ± 0.1 | 1–3 |
| B | +/Sxlf7,M1; msl-2 | ref (n = 272) | 100 | 34 | 60.6 ± 3.8 | 42–120 |
| C | P{SxlΔPm} Sxlf18,f32/Sxl f18,f32 | 93 | 89 | 37 | 7.3 ± 1.5 | 1–56 |
| C | P{SxlΔPm} Sxlf18,f32/Sxl f18,f32; Dp(Sxl+) | ref (n = 207) | 100 | 10 | 73.7 ± 5.2 | 68–126 |
| C | Dp(Sxl+) but not mated | 100 | 11 | 10.7 ± 2.0 | 1–74 | |
Full genotype of crosses: A. y w SxlM1,fΔ33 ct6 sn3/Binsinscy; P{U2af-traF w+mW.hs}2B/+ ☿☿ × ♂♂ w cm Sxlf7,M1 ct6 v/Y; Dp(1;3)sn13a1, cm+Sxl+ct+/+; B. y w SxlM1,fΔ33/Binsinscy; msl-21 P{U2af-traF w+mW.hs}2B/msl-21☿☿ × ♂♂ w cm Sxlf7,M1ct6 v/Y; msl-21/CyO; and C. y w cm P{w+mC SxlΔPm} Sxlf18, f32ct6/Binsinscy; P{U2af-traF w+mW.hs}2B/+ ☿☿ × ♂♂ y w Sxlf18, f32 ct6/Y; Dp(1;3)sn13a1, cm+Sxl+ct+/+.
% viability relative to the reference class (ref) indicated (no. individuals in that reference class), based on adult eclosion. U2af-traF had no effect on Sxlf7,M1/ SxlM1,fΔ33 viability. Only Sxl-mutant females that survived the entire 5-d laying test were included in the egg-laying calculations.
Virgin females were mated with four or five virgin Ore-R males 1–2 d after eclosion and then were allowed to lay for five successive days, with transfers to fresh food each day.
At least one egg laid over the 5-d test period qualified a female as “laying.”
Calculation based on pooled data for all 5 d of egg collection.
For individual laying females.
Longevity, which was essential for the present study on egg-laying ability, was improved: 36% of the U2af-traF/+-feminized adult Sxlf7,M1/ SxlM1,fΔ33 animals in Table 1 were alive at the end of the egg-laying test, 6–7 d after eclosion. Increased activity of mutant females caused by protracted courtship attempts by males during the egg-laying test seemed likely to be responsible for much of the posteclosion premature lethality, because in the absence of courting males 92% (24/26) of the females survived the test. Reluctance of the mutant females to mate led to extended courtship (see below).
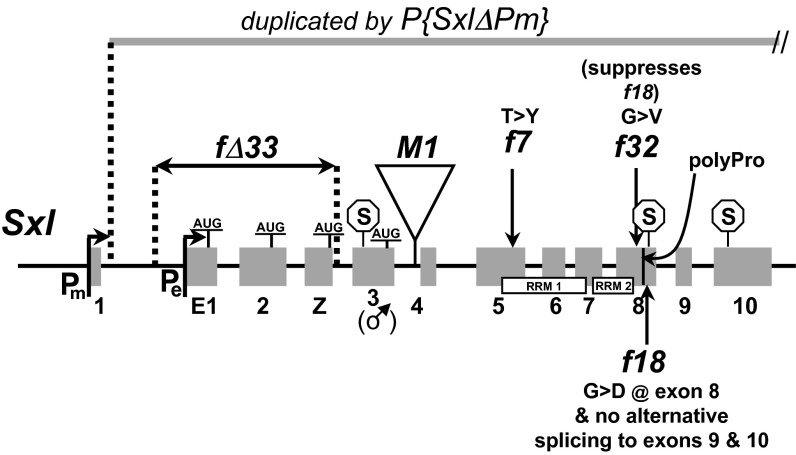
Fig. 1 illustrates the molecular nature of the Sxl-mutant lesions mentioned in the present study. SxlfΔ33 is an intragenic deletion eliminating all known wild-type translation start sites in Sxl mRNAs. Translation of mutant mRNAs likely initiates in exons 4 or 5 (19). Sxlf7,M1/SxlM1,fΔ33; U2af-traF/+ females have large ovaries full of mature eggs, but they fail to lay (Table 1, first row). Because adding an Sxl+ allele to this genotype restored full fertility (Table 1, second row), their failure to lay seems to reflect loss of some normal Sxl-F function for which Tra-F cannot substitute rather than the gain of some disruptive activity that the U2af-traF transgene could not be expected to counteract.
Fig. 1.
Lesions in partial-loss-of-function alleles that are central to the present study: Sxlf7,M1, SxlM1,fΔ33, and Sxlf18,f32. Putative translation starts (AUG) and stops (S) are indicated in the context of the establishment promoter (Pe) that transiently responds very early to the primary sex-determination signal, the maintenance promoter (Pm) that operates thereafter, and the various exons, among which are the male-specific translation-terminating exon 3, which is responsible for the gene's sex-specific functioning, and exons 9 and 10 that encode alternative C-terminal isoforms important for germ-line but not somatic functioning. The two RNA-binding domains (RRM) are shown, as is the location of a proline-rich domain (black line) essential for germ-line activity that is just proximal to the site of alternative splicing out of exon 8 that is blocked in the female-sterile allele, Sxlf18. M1 is a transposon insertion that, by itself, leads to semiconstitutive feminizing expression of Sxl and hence dominant, male-specific lethality. Sxlf7,M1 and SxlM1,fΔ33 were selected for suppression of that male-specific lethality, and Sxlf18,f32 was selected for suppression of Sxlf18 sterility. SxlfΔ33 is an intragenic deletion that interferes only partially with female functioning, whereas P{SxlΔPm} is a duplication of the entire gene minus the Pm region. It provides wild-type Pe function but nothing more. Details regarding mutations that are not referenced in the text are given in Materials and Methods.
Dissection of Sxlf7,M1/SxlM1,fΔ33; U2af-traF/+ females at the end of the egg-laying test period revealed that only 10% carried motile sperm. Another 8% may have mated, because they carried what appeared to be seminal fluid (immotile fibrous material) but no motile sperm. Although these females are defective in mating and/or storing sperm, these particular defects are unlikely to account for their failure to lay, because over the same test period, 90% of their virgin Sxl+ sisters did lay, some depositing nearly as many eggs as some of their mated Sxl+ sisters (Table 1, third row). Because there appear to be mating and/or sperm-storage defects even in some tra− females rescued by the U2af-traF transgene (16) (although this transgene has allowed the maintenance of a homozygous tra− line for many years), it is not yet possible to know the extent to which these phenotypes with Sxl-mutant alleles reflect a purely TIF-mutant defect.
Although Sxlf7,M1/SxlM1,fΔ33; U2af-traF/+ viability and longevity are remarkably high, the fact that neither parameter is wild type indicates that X-chromosome dosage compensation is somewhat impaired. Consequently, we explored the possibility that their egg-laying defect might be caused by incomplete repression by Sxl of the dosage-compensation switch gene, male-specific-lethal-2 (msl-2) in females. msl-2 hyperactivates X-chromosome gene expression in males to compensate for the fact that males have only a single copy of their X-linked genes, whereas females have two (reviewed in refs. 6 and 9). Sxl-F keeps msl-2 functionally silent in females to avoid the upset in dosage compensation that msl-2 expression otherwise would cause. Eliminating msl-2+ failed to raise the rate of egg laying above minimal levels (Table 1, cross B). Hence misregulation of the MSL dosage compensation pathway cannot be responsible for the egg-laying defect in these Sxl-mutant females. The fact that some of the few eggs laid by these msl2-mutant females developed into fertile adults showed that Sxlf7,M1/ SxlM1,fΔ33 animals feminized by U2af-traF can mate and make eggs that are capable of supporting normal development.
A TIF Defect Blocks Egg Transit Earlier than a TSF Defect.
Because the inability of earlier traF transgenes to rescue the tra-null phenotype fully also was manifested as an egg-laying defect, we were concerned that the egg-laying defect of Sxlf7,M1/SxlM1,fΔ33 chromosomal females feminized by U2af-traF might be caused by some inexplicable influence of these particular mutant Sxl alleles on the effectiveness of the U2af-traF transgene. A difference in the step at which transit of the egg is blocked in these two different situations rendered this possibility unlikely.
For the incomplete rescue of the tra-null phenotype by earlier traF transgenes, we found that passage of eggs in 6-d-old virgin females was invariably blocked after ovulation (n = 20). Ovulation is the step during which eggs are released from the ovary and pass into the lateral oviducts. From there they pass through the common oviduct to the uterus where they mature and may be fertilized. Mature eggs ultimately are passed into the environment in a process known as oviposition. A similar postovulation block had been reported for females mutant for dissatisfaction, a gene believed to be in a pathway controlled by tra that regulates egg laying (20). In contrast, the egg-transit block for virgin Sxlf7,M1/ SxlM1,Δf33 U2af-traF/+ females of the same age occurred earlier: They all failed to ovulate (n = 20).
Sxlf18,f32; U2af-traF/+ Mutant Females, Whether Kick-Started by Dp(1;1)SxlΔPm or Not, Are TIF Defective but Are Less so than Sxlf7,M1/SxlM1,fΔ33; U2af-traF/+ Females.
The TIF phenotype is not a peculiarity of one particular mutant Sxl genotype but also is evident in females homozygous for a very different adult-viable masculinizing mutant allele, Sxlf18,f32. Because TIF-defective Sxlf18,f32 diplo-X adults can be made even more viable and long-lived than Sxlf7,M1/SxlM1,fΔ33 diplo-X adults, they were the genotype of choice for the genetic screen described below that unequivocally established the TIF vs. TSF distinction.
This double-mutant allele, Sxlf18,f32, was derived from Sxlf18. Females homozygous for the parental allele, Sxlf18, are fully viable but sterile because of a germ-line–autonomous block in oogenesis (17, 21). The Sxlf18 point mutation blocks the alternative splicing necessary to generate exon-10–encoded C-terminal versions of Sxl-F and substitutes aspartic acid for glycine in the exon-8–encoded C-terminal isoforms (Fig. 1) (19, 21). Sxlf18 is suppressed in cis by Sxlf32, a transversion that substitutes valine for an evolutionarily invariant glycine three residues C-terminal to the second RNA-binding domain, a region common to all Sxl-F isoforms. Remarkably, although Sxlf18,f32/Sxlf18 females are fully viable and fertile, suppression of the oogenesis defect of Sxlf18 by Sxlf32 occurs at the expense of the allele's sex-determination function: It is unable to regulate tra in the soma. Thus, without U2af-traF to feminize them, homozygous adult Sxlf18,f32 females are as thoroughly masculinized as Sxlf7,M1/SxlM1,fΔ33 females.
Feminization of Sxlf18,f32/Sxlf18,f32 animals by U2af-traF revealed a TIF defect that was weaker than that for feminized Sxlf7,M1/SxlM1,fΔ33 animals (Table 1). Over the egg-laying test period, 93% (37/40) of the Sxlf18,f32; U2af-traF/+ adult females survived, but 11% of those survivors failed to lay. Three of the four nonlayers carried motile sperm, but, like Sxlf7,M1/SxlM1,fΔ33;U2af-traF/+ females, none ovulated. Moreover, the egg-laying rate of the females that did lay was an order of magnitude less than that of their mated Sxl+ control siblings and indeed was even lower than that of virgin control siblings. Interestingly, 58% of the mutant females that laid were sterile, even though at least 47% of those sterile layers had mated, as evidenced by the presence of sperm at the end of the test period. Even for fertile egg layers, only 45% of their eggs seemed to have been fertilized, as judged by the number of adults generated from egg collections made after the first daily collection that yielded progeny (signaling a successful mating had taken place) and by the unhatched eggs remaining white. Although all 10 control siblings had mated by the end of the first day of egg collection, only 7% of their ultimately fertile TIF-mutant sisters had mated at that time, and that number increased only to 57% by the end of the second day. This leakier ovulation phenotype revealed defects in female behavior besides ovulation that also were suggested in the previously described TIF-mutant females. However, because the female behavior of even tra− U2af-traF/+ females is not truly wild type (16) (notwithstanding the relative ease of maintaining tra− U2af-traF/+ lines), further work is needed to determine whether the TIF pathway branch is involved in more than just ovulation or whether instead the defects in mating behavior and sperm utilization are a consequence of incomplete rescue of TSF pathway defects.
Viability of Sxlf18,f32 females in Table 1 was artificially boosted to nearly 100% by the addition of Dp(1;1)SxlΔPm (Fig. 1), a 5′-truncated chromosomal duplication of SxlM1 that lacks SxlPm but has SxlPe and provides full SxlPe function, i.e., the transient burst of Sxl-F generated before the blastoderm stage in response to a female sex-determination signal. The Sxl-F burst from Dp(1;1)SxlΔPm helps stably engage the Sxl positive-feedback loop for mutant Sxl alleles with lowered but nonzero autoregulatory activity. Lacking SxlPm, the duplication provides no Sxl function after the early blastoderm stage. The ability of this transient early Sxl-F expression to engage stably the feedback loop for mutant Sxl alleles that otherwise could not engage stably shows that for Sxl, as for phage lambda (reviewed in ref. 22), greater autoregulatory activity is required to engage the Sxl positive-feedback loop initially than to maintain it. Without this truncated duplication, viability of homozygous Sxlf18,f32 females varied unpredictably between 15–75% as a function of undefined aspects of genetic background.
A Constitutively Feminizing Mutant Allele of the Endogenous tra Locus Provides the Most Definitive Evidence for TIF.
Differences between TIF- and TSF-mutant females in their egg-laying block argued against the possibility that what we had designated a TIF-mutant phenotype instead might be a TSF-mutant phenotype caused by an inability of the U2af-traF transgene in some mutant Sxl backgrounds to produce an adequate level of Tra-F to rescue a functionally null tra-mutant phenotype. Nevertheless, the importance of excluding this possibility led us to consider whether we could observe the TIF phenotype in a situation in which Tra-F was provided constitutively in a considerably more “natural” way than from the U2af-traF transgene. In part for this reason, we designed a forward genetic screen for a constitutively feminizing point-mutant allele of the endogenous tra+ locus (Fig. 2). If such a fully constitutive tra allele expressing Tra-F from the gene's native promoter in its native chromosomal location also failed to overcome the ovulation defect, the TIF explanation would be on even more solid ground. Analysis of tra had shown that mutations preventing use of the non–sex-specific splice-acceptor site in tra pre-mRNA cause the female-specific alternative splice-acceptor site to be used constitutively, thereby mimicking the effect of Sxl-F on tra (23). We anticipated that recovery of such a mutation in a random mutagenesis would be exceedingly infrequent, but in the screen we designed any animal carrying the desired constitutive endogenous tra allele would stand out in a sea of pseudomales by virtue of its female phenotype. According to our TIF hypothesis, such a female should make oocytes that she could ovulate only poorly, if at all. We used Sxlf18,f32 rather than Sxlf7,M1/ SxlM1,Δf33 females for this screen both because Sxlf18,f32 females are more viable and their weaker egg-laying block might allow a newly induced constitutive traF-mutant allele to be recovered and because the screen would test a straightforward hypothesis explaining why Sxlf32 suppression of the Sxlf18 germ-line defect is at the expense of tra regulation (SI Results).
Fig. 2.
A powerful genetic screen used to generate a constitutively feminizing tra allele for a test of the TIF/TSF distinction and to explore the basis for cis-dominant suppression of Sxlf18 sterility by Sxlf32. Among 300,000 somatically masculinized chromosomal daughters (Ψ males) from mutagenized fathers that were screened, one mosaic and one nonmosaic phenotypic female were recovered. Because only Ψ males normally survive this screen, rare phenotypic females arising by either intergenic or intragenic suppression were obvious. Dominant temperature-sensitive–lethal balancers (whose derivation is described in Materials and Methods) were used in conjunction with the recessive temperature-sensitive–lethal shibirets to facilitate generation of the large numbers of parents needed and to eliminate the superfluous classes of progeny.
From the screen in Fig. 2, no phenotypic females were recovered among 1.8 × 105 pseudomale progeny of gamma-irradiated males, but one phenotypic female and one sexually mosaic female were recovered among 1.2 × 105 pseudomale progeny of ethyl methanesulfonate (EMS)-treated males. Both were sterile, but the nonmosaic female looked normal inside and out. Although she had two large ovaries full of mature eggs and was provided with wild-type mates for a 12-d test period, she failed to ovulate. When she was killed and her tra alleles were sequenced, she proved to be unambiguously heterozygous for a G-to-A transition (characteristic of EMS) at the 3′ splice site AG dinucleotide of the non–sex-specific intron of tra, precisely the kind of constitutively feminizing tra mutation we hoped to generate. Thus, even with this constitutively feminizing mutant endogenous tra allele that mimicked the effect of Sxl-F on tra as closely as one could arrange, the Sxlf18,f32-mutant female remained TIF defective. The molecular explanation for the phenotypically female tissue in the sexually mosaic fly recovered in the same screen remains a mystery, because sequencing tra and Sxl revealed no newly induced lesions.
The TIF Pathway Functions Developmentally in a Subset of Presumably TSF Pathway Neurons.
We used the Gal4/upstream activation sequence (GAL4/UAS) expression system (24) to determine the nature of the cells in which TIF is required and whether TIF is needed for the development or functioning of those cells. For this purpose we constructed UAS-Sxlalt5-C8, a transgene designed to produce the two exon-8 C-terminal Sxl-F isoforms that derive from the highly conserved alternative splicing into exon 5. We assessed both the extent of rescue of the TIF egg-laying defect and the effect of Sxl-F expression on male viability and fertility (Table 2 and Table S1). This comparison between the sexes indicated how specific a given driver was for TIF-pathway cells, because expression of Sxl-F in males has the potential to kill or sterilize by upsetting male X-chromosome dosage compensation and to sterilize by inappropriately feminizing sexually dimorphic cells required for male reproduction.
Table 2.
GAL4/UAS-driven Sxlalt5-C8 expression that rescues the TIF− mutant ovulation phenotype reveals a neuronal basis for TIF
| P{UAS-Sxlalt5-C8} and GAL4 indicated driver* |
SxlM1,fΔ33/Sxlf7,M1;P{U2af-traF}/+ females† |
SxlM1,fΔ33/Y male brothers‡ |
||
| % females laying (no. tested) | No. eggs/d ± SEM for laying females | Relative viability vs. control siblings, % | % fertile (no. tested) | |
| αTub84B | 91 (43) | 43.5 ± 2.4 | 0 | — |
| Control§ | 0 (9) | – | (n = 44) | n.d. |
| elav | 89 (27) | 28.6 ± 3.4 | 27 | 0 (13) |
| Control§ | 14 (21) | 6.0 ± 3.8 | (n = 33) | 92 (26) |
| fruP1 | 82 (17) | 17.0 ± 4.7 | 92 | 0 (12) |
| Control§ | 0 (19) | – | (n = 49) | 100 (7) |
| OK233 | 95 (20) | 29.8 ± 0.6 | 71 | 56 (17) |
| Control§ | 20% (5) | 1.5 ± 1.3 | (n = 43) | 71 (9) |
Cross to make tested animals: y w SxlM1,fΔ33/Binsinscy, y w sn B; P{U2af-traF w+mW.hs}2B/+; P{UAS- Sxlalt5-C8, w+mC}A1/TM3,Sb Ser ♀♀ × ♂♂ w cm Sxlf7,M1 ct v/Y; GAL4/Ki for all but OK233; y w SxlM1,fΔ33/Binsinscy, y w sn B; P{U2af-traF w+mW.hs}2B/+; P{UAS- Sxlalt5-C8, w+mC}A1/TM3,Sb Ser ♀♀ × ♂♂ w cm Sxlf7,M1 ct v/Y; Gal4/CyO or Sco for OK233.
Virgin females were individually mated with four or five virgin Ore-R males 1–2 d after eclosion and then were allowed to lay for 2 d.
Single virgin males were mated with four or five Ore-R virgin females who were given 5 d to lay.
No Gal4 driver.
Ubiquitous Sxl-F expression driven indirectly by the alphaTubulin84B (tub) promoter (25) rescued the TIF egg-laying defect well and, as expected, killed all males. The previously available UAS-Sxl-F construct (26) that generates only a single Sxl-F C-terminal isoform was lethal even to females when driven by tub::GAL4 (0 with the driver vs. 210 sisters without). The embryonic lethal, abnormal vision-GAL4 (elav-GAL4) driver is active only in all neurons and their precursors. Our UAS-Sxl-F construct driven by this pan-neuronal driver (27) also rescued egg laying well, with 89% of the females laying an average of 29 eggs/d. In this case 27% of the males survived. The survivors were all sterile, as expected, because Sxl-F (via Tra-F) interferes with male-specific splicing from the fruP1 promoter in neurons, thereby disrupting male courtship behavior (reviewed in ref. 7).
As mentioned, transcripts from the fruP1 promoter whose male-specific pre-mRNA splicing is blocked by Tra-F, control many aspects of male sexual behavior. Surprisingly, when this TSF-pathway promoter was used to drive neuronal expression (28) of Sxl-F for the purpose of rescuing the TIF ovulation phenotype, significant rescue was observed, although not as much as with the pan-neural elav driver. Males expressing Sxl-F in the fruP1 pattern survived well but were sterile, as would be expected for the reasons given above for elav-GAL4. The most TIF-specific driver we tested (in the sense of giving robust recue of females while having the least deleterious effects in males) was OK233, a driver selected for expression in the embryonic nervous system. All OK233 females that laid eggs were fertile. Because the specific expression pattern of this driver (a gift from C. J. O'Kane, Department of Genetics, University of Cambridge, Cambridge, England; its construction is referenced in ref. 29) at various stages has not been determined, we include the result here not to define further the developmental focus for TIF functioning but simply to show that drivers do exist that can induce Sxl-F in females to rescue the ovulation block without the expression driven in males eliminating male viability or fertility. This result bodes well for further analysis along this line.
If the neuronal requirement for TIF is developmental, rescue of the TIF-mutant phenotype by Sxl-F should be possible only by expression induced before the relevant neuronal precursors differentiate. If, instead, Sxl-F is required for TIF only after development is complete, we should be able to rescue by providing Sxl-F in the adult. To distinguish between these alternatives, we exploited a heat-sensitive GAL80 (GAL80ts) to regulate the GAL4/UAS system negatively as a function of culture temperature (30). GAL80ts blocks the GAL4/UAS expression system at 18 °C but not at 30 °C. Hence with respect to GAL4-induced expression of a construct such as our UAS-Sxlalt5-C8, 18 °C is the nonpermissive temperature, and 30 °C the permissive temperature. Because the GAL80ts system is more effective in inducing the protein products of a GAL4 target following an upshift (18–30 °C) than it is in eliminating them following a downshift (30–18 °C), we restricted our analysis to upshifts.
For the purpose of studying TIF, we had to depart from the published single temperature-shift regimen from 18–30 °C because Sxlf7,M1and SxlM1,Δf33 failed to complement for female viability if exposed to 18 °C during early embryogenesis or if exposed to 30 °C during the pupal period. Instead, the heteroallelic mutant females were raised at 25 °C for the first day after fertilization; then all except the permissive control were downshifted to the nonpermissive temperature (18 °C). Moreover, subsequent upshifts for the test of TIF rescue timing were to a permissive temperature of 25 °C rather than 30 °C. As the unshifted controls in Table 3 show, the GAL80ts system worked well for our purposes even with these modifications. With respect to the egg-laying defect for females carrying a tub-GAL4 (ubiquitous) driver, the unshifted animals were all TIF− at 18 °C (Sxl-F off) and TIF+ at 25 °C (Sxl-F on). For females shifted from 18 °C (Sxl-F off) to 25 °C (Sxl-F on), effectiveness of rescue began to drop off for shifts to the permissive temperature during mid to late third larval instar (2–1 d before puparium formation). Shifts after the first 15% of pupal development (>1 d after puparium formation) essentially failed to rescue the ovulation defect. We conclude that Sxl-F is required for TIF during neuronal development.
Table 3.
Temperature-sensitive GAL4/UAS-driven Sxlalt5-C8 expression timing shows that Sxl-F is required developmentally to rescue the TIF− ovulation phenotype [SxlM1,fΔ33/Sxlf7,M1; P{U2af-traF}/P{tub-GAL80ts}; P{tub-GAL4}/P{UAS-Sxlalt5-C8} females*]
| Culture temperature | % females laying (no. tested) | Eggs/d ± SEM for laying females |
| Unshifted On | 100 (36) | 60 ± 2.5 |
| Off > On @ 3–2 d BPF | 100 (13) | 56 ± 5.8 |
| Off > On @ 2–1 d BPF | 93 (27) | 39 ± 3.5 |
| Off > On @ 1–0 d BPF | 79 (43) | 21 ± 2.4 |
| Off > On @ 0–1 d APF | 35 (60) | 11 ± 2.4 |
| Off > On @ 1–2 d APF | 21 (19) | 1.1 ± 0.3 |
| Off > On @ 2–3 d APF | 15 (26) | 2.1 ± 1.2 |
| Unshifted Off | 2 (60) | 1.8 ± 0.4 |
Females were collected 0–1 d after eclosion, were aged 1 more day, and then were individually mated with four or five Ore-R 4- to 8-d-old males. Eggs were collected for 2 d, and any female laying at least 1 egg during this period was classified as laying. APF, days @ 18 °C after puparium formation before shift up; BPF, days @ 25 °C before puparium formation after shift up; Off, 18 °C; On, 25 °C.
Full genotype of the parental cross and the relevant daughters: y w SxlM1,fΔ33/ w cm Sxlf7,M1ct v; P{U2af-traF w+mC}2B/P{tub-Gal80ts w+mC}; P{tub-Gal4, w+mC}/ P{UAS-Sxl w+mC}A1 daughters from the cross y w SxlM1,fΔ33/Binsinscy, y w sn B; P{U2af-traF w+mC }2B/+; P{UAS-Sxl w+mC}A1/TM3,Sb Ser ♀♀ × ♂♂ w cm Sxlf7,M1ct v/Y; P{tub-Gal80ts w+mC}/CyO or Sco; P{tub-Gal4, w+mC}/TM6, Hu.
Discussion
Developmental regulatory pathways are rarely as simple as they first appear, but as the twist to the Drosophila sex-determination pathway we report here suggests, complications can provide clues to evolution. We show that Sxl, the rapidly evolved target of the Drosophila primary sex-determination signal, no longer can be regarded as transmitting all its feminizing orders other than size dimorphism to the soma exclusively through its well-known switch-gene target tra. Instead, as illustrated in Fig. 3C, one must distinguish between a major pathway branch, TSF, in which tra is sufficient to dictate feminization, and a minor branch, TIF, in which it is not.
Fig. 3.
Possible origin of the TIF-pathway branch in the current Drosophila sex-determination regulatory gene pathway from a time when tra was the master autoregulating sex-determination gene with Sxl under its sex-specific control. (A) It is proposed that an early step in the switch from tra to Sxl as the positively autoregulating target of the female sex-determination signal was the acquisition by Sxl of a gene target with a female-specific function, with Sxl then becoming a regulatory target of tra. (B) Siera and Cline (13) suggested that redundancy in positive feedbacks involving tra and Sxl might have led to the present-day regulatory arrangement with tra downstream rather than upstream of Sxl. After the switch from A to B, female-specific targets of Sxl that originally were part of the TSF pathway would stand out as part of a TIF pathway. (C) Redrawing of B that presents the same regulatory relationships in a more conventional way.
Evidence for an Additional Branch in the Drosophila Sex-Determination Gene Hierarchy.
Evidence for the TIF branch derives from female-viable but masculinizing combinations of partial-loss-of-function Sxl alleles that fail to induce either TSF or TIF in diplo-X individuals, so that when TSF-branch activity is restored by our constitutively feminizing transgene U2af-traF or, even more definitively, by a constitutively feminizing mutant endogenous tra allele, mutant females remain TIF defective and hence sterile. Although TIF-mutant sterility superficially resembles sterility in TSF-mutant transgenics, in that both phenotypes include a failure to lay eggs, the TIF-mutant block to egg laying occurs at ovulation, whereas that in TSF-defective transgenics occurs later at oviposition.
The possibility that the kind of branch in the TIF pathway that we report here might exist was suggested first in a previous paper reporting the behavior of some U2af-traF–feminized gynandromorphs (coarse-grained XX//XO mosaics) in which the failure of Sxl to activate what we now know to be the TIF pathway was a consequence of the absence of a female primary sex-determination signal in Tra-F–feminized Sxl+ XO cells (16) rather than a consequence of Sxl mutations in Tra-F–feminized XX cells. Because 38% of the feminized egg-producing gynandromorphs failed to lay their eggs, we concluded that there must be some functionally Sxl− XO somatic cells that cannot substitute for the XX somatic cells required for egg laying, even when feminized by Tra-F. Although gynandromorphs are not nearly as convenient as Sxl-mutant females for studying TIF, they do strengthen the argument that TIF-defective sterility is not caused either by a upset in dosage compensation or by some idiosyncrasy of U2af-traF in Sxl-mutant females.
Strong evidence is necessary to legitimize the TIF claim because of our surprising finding that Sxl-F functioning in the TIF pathway takes place in a subset of neurons that sex-specifically express fru mRNAs. Because fru sex-specific splicing is controlled entirely by Tra-F (reviewed in refs. 5 and 7), the simplest model would suggest that any deficiency in the sex-specific functioning of these neurons reflects a TSF defect. Of course, just because fru is sex-specifically regulated in these neurons does not require that fru be solely or even partially responsible for their feminization in every case.
At this point the “I” in TIF necessarily stands for “insufficient” rather than “independent.” Because conditions under which the TIF phenotype was studied were all ones in which Tra-F activity for the TSF pathway was provided at a level sufficient to rescue the sterility of tra− females, no evidence for or against independence could be generated. If, as the fru neuron results might suggest, tra works with one or more unknown Sxl targets to achieve full feminization in some neurons, the name ultimately might have to be changed to something like “tra-partnered feminization.” Discovering the identity of the Sxl TIF-gene targets and the specific neurons in which they are required would provide the tools necessary to resolve this question about the relationship between TSF and TIF. The recent availability of an enormous panel of well-characterized neuronal GAL4 drivers (31) should be a great help in this connection, particularly in view of our finding that GAL4-driven Sxl-F expression can rescue the TIF-mutant phenotype in females while causing little damage to males. The gene female-specific-independent-of-transformer seemed to be a promising candidate TIF-pathway target until we showed (SI Results and Fig. S1) that, contrary to the report by Fujii and Amrein (32), it is firmly in the TSF pathway (and hence is in need of renaming).
The ovulation block should be particularly amenable to future genetic and developmental analyses designed to identify targets of the TIF because it is particularly suited to positive genetic selection in a suppression screen. Arguing for the potential of such a suppression screen is the fact mentioned above that fertility could be restored to TIF-defective females by a GAL4 driver/Sxl-F target combination that had relatively little adverse effect on male viability or fertility. Such sex specificity suggests that the set of neurons responsible for the TIF ovulation defect may not be very large and that disruption of their normal controls is unlikely to disrupt non–sex-specific aspects of development.
Tools for Studying Sxl.
This report introduces several genetic tools, among which the GAL4 target UAS-Sxlalt5-C8 is perhaps the most broadly useful. That this transgene, which conditionally generates both exon-5 alternative Sxl-F isoforms, provides relatively strong Sxl+ function while having no adverse effect on females indicates that the adverse effect on females caused by the Sxl GAL4 target previously reported (26), a transgene that encodes only a single exon-5 isoform, may not reflect a normal activity of Sxl-F protein. Another useful tool is Dp(1;1)SxlΔPm, which can expand the utility of various partial-loss-of-function Sxl alleles. This tool is a chromosomal duplication of Sxl truncated at its 5′ end so that it lacks the gene's maintenance promoter but retains an intact establishment promoter and all the activities that transiently active promoter elicits. The response of this truncated Sxl allele to the female X-chromosome dose signal, a response that ends during the early blastoderm stage, can facilitate engagement of the Sxl positive-feedback loop for various Sxl-mutant alleles without otherwise influencing their Sxl-mutant phenotype. For example, Dp(1;1)SxlΔPm is particularly useful in combination with the intriguing double mutant Sxlf18,f32 because together they can generate thoroughly masculinized Sxl-mutant adult females (pseudomales) with far higher viability and longevity than any previously described masculinizing Sxl genotype. Last, two dominant temperature-sensitive lethal balancers that were introduced in this study should be generally useful, because they allow crosses to be designed so that daughters with one combination of a maternal and paternal X chromosome of choice are the only progeny to survive.
Evolutionary Implications of the TIF Sex-Determination Pathway Branch.
Sex determination for flies in the family Drosophilidae is unlike that for most other higher insects in many fundamental respects, including having Sxl rather than tra as the target of their primary sex-determination signal and having Sxl rather than tra as the gene whose positive-feedback loop on its own pre-mRNA splicing maintains the female developmental pathway commitment (reviewed in refs. 10 and 11). Although the TIF branch could be a recent addition to the Drosophila sex-determination pathway made well after Sxl had taken over tra's role as the master feminizing gene, a more intriguing possibility is that TIF instead may reflect an ancestral function that Sxl acquired in the earliest step on its evolutionary path toward usurping tra's role as master sex switch (Fig. 3A). Because both TIF and TSF function in neurons that sex-specifically express fru, perhaps the first female-specific function that Sxl acquired was to modify the developmental functioning of fru in some neurons. Initially this function may have been achieved without the need for a sex-specific Sxl product, with sex-specific products coming only later as fine-tuning of that particular function under the control of tra. As Fig. 3B illustrates, the switch from tra as a regulator of Sxl to Sxl as a regulator of tra—a switch that could have been facilitated by the development of redundancy in the positive-feedback circuits for the two genes (13)—would make any female-specific gene target of Sxl that existed before the switch be independent of tra regulation today if its control by Sxl persisted.
Of course there are many important questions about the remarkable path taken by Sxl functional evolution and the forces that drove those changes for which an understanding of the TIF pathway might not be relevant. How did Sxl come to respond to an X-chromosome dose signal? How did it come to control X-chromosome dosage compensation? Why is Sxl's control of germ-line sex determination so different from its control of sex determination in the soma (reviewed in ref. 3; also see refs. 33 and 34)? On the other hand, because we know next to nothing about any of these questions, it is hard to predict where clues might lead regarding an early female-specific Sxl function that the TIF pathway might help reveal. Regardless of whether the TIF pathway is ancestral or recent, further analysis leading to the discovery of the Sxl-F targets in this regulatory branch undoubtedly will advance our understanding how genes control behavior and how Sxl-F proteins control RNA functioning.
Materials and Methods
Drosophila Culture and Genetics.
Flies were raised in uncrowded conditions on a standard cornmeal, yeast, sucrose, and molasses medium. Growth and all egg-laying and fertility tests were at 25 °C unless otherwise stated. Markers, balancers, and transgenes not mentioned below are described at http://flybase.bio.indiana.edu.
Sxl Mutants Not Previously Described.
SxlM1,fΔ33 was derived from SxlM1,PlacW-A by imprecise excision in males of a PlacW transposon at +6,217 in exon 2 (for all numbering, 0 corresponds to the SxlPm transcription start site). The PlacW insertion suppressed SxlM1-dominant male-specific lethality. Imprecise excisions were identified by loss of the transposon marker w+mC without loss of SxlM1 suppression. Complementation tests identified derivatives such as SxlM1,fΔ33 that lacked SxlPe function but retained some SxlPm function. SxlfΔ33 lacks PlacW and all Sxl DNA from +3,410 to +7,947. SxlM1,fΔ33 transcripts from SxlPm are constitutively spliced from exons 1–4, with translation start sites likely in exon 4 (19). SxlM1,fΔ33 supports wild-type oogenesis in homozygous mutant germ-line clones (Table S2) and fully complements female-sterile mutant Sxl alleles f4, f5, and f18, showing that the relatively recently evolved SXL N terminus required for tra+ regulation (35) is not required for Sxl germ-line functions.
Sxlf32, a G-to-T transversion at +13,355, was selected as a gamma ray–induced dominant intragenic suppressor of recessive Sxlf18 female sterility by the “reversion” scheme described by Sun and Cline (17). The screen yielded only one suppressor but four true revertants.
P{SxlΔPm} is a 5′-truncated duplication of SxlM1 located immediately centromere distal to Sxl's nearest neighbor, carmine (cm). It was generated by mobilization of a PlacW in SxlM1 at +820–823 (just downstream of exon 1) in the germ line of an otherwise sterile SxlM1,PlacW-B/Sxlf4 female. This PlacW hop brought with it a 5′ truncated duplicated version of SxlM1 (wild type for Sxlf4), extending from the original site of the PlacW insertion to beyond the most distal Sxl 3′ end, leaving an intact SxlM1 allele behind in cis to complement Sxlf4. Functionality of the 5′ truncated SxlM1 duplication was assessed by complementation after the intact SxlM1 was replaced in cis with the deletion allele, Sxlf7BO. Complementation tests showed that the truncated duplication provides full SxlPe function but no SxlPm function and fails to complement the female-sterile Sxl alleles f4, f5, and f18.
Dominant Temperature-Sensitive Lethal Balancers and the Mutant Screen in Fig. 2.
P{hs-hid}:=/Y and Binsinscy, let P{hs-hid} were generated by mobilization of P{w+mC hs-hid}4 on CyO to insert in either a y w f:=/Y-attached X chromosome or a Binsinscy, y w snx2 B balancer carrying a spontaneous recessive lethal mutation. Neither chromosome has any dominant lethal effect even at 29 °C, but both display fully penetrant dominant lethality when embryos older than 7 h are subjected to a 1-h heat shock by immersion of culture vials into a 37 °C water bath. The stock producing virgins for the screen was Binsinscy, y w sn B l (1) P{w+mC hs-hid}/ y w cm P{w+mC SxlΔPm} Sxlf18, f32ct6 shits ☿☿ × ♂♂ y w cm P{w+mC SxlΔPm} Sxlf18, f32ct6 shits/Y and that producing males to be mutagenized was y w f P{w+mC hs-hid}:=/Y ☿☿ × ♂♂ w Sxlf18, f32 sn3/Y. Three- to five-day-old virgin males whose sperm was to be mutagenized were exposed to either 3,500 rad of gamma radiation from a 137Cs sealed source or to 50 μM EMS in a 1% (wt/vol) aqueous sucrose solution, according to a standard protocol (36).
Sxl-F Expression Constructs.
The GAL4/UAS target transgene UAS-Sxlalt5-C8 that conditionally produces the two exon-8 C-terminal Sxl-F isoforms generated by the use of highly conserved alternative exon-5 splice acceptors was constructed using a BamH1 hybrid Sxl cDNA/genomic DNA fusion fragment inserted into the multiple cloning site of the UASp vector (37), 33 bp downstream of the P-element transposase basal promoter. The 5′ part of the hybrid fragment was from the female-specific cDNA cF1 (38) and runs from +28 in exon 1 (with an artificial BamH1 site appended on the 5′ end) through +10,449 in exon 4. The genomic part that follows runs from +10,450 to +16,546, well beyond the longest exon-8 3′ UTR. Before making this construct, we had determined that a transgene with the same cDNA/genomic fusion fragment (including the artificial 5′ BamH1 site) but expressed using Sxl's own 5′ UTR and promoter (to −2,360) was much more effective at rescuing Sxl mutants than any previously available Sxl transgene, all of which generated only one of the two exon-5, exon-8 C-terminal isoforms.
Supplementary Material
Acknowledgments
We thank Louise Sefton and Bryan Soper for the isolation and initial molecular characterization of SxlfΔ33, Melissa Burns for technical assistance with the screen in Fig. 2, Jennifer Stichman for assistance with screening GAL4 drivers for egg-laying rescue, and Barbara J. Meyer for helpful comments on the manuscript. This work was supported by National Institutes of Health Grant GM23468 (to T.W.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319063110/-/DCSupplemental.
References
- 1.Bridges CB. Non-disjunction as proof of the chromosome theory of heredity. Genetics. 1916;1(1):1–52. doi: 10.1093/genetics/1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schütt C, Nöthiger R. Structure, function and evolution of sex-determining systems in Dipteran insects. Development. 2000;127(4):667–677. doi: 10.1242/dev.127.4.667. [DOI] [PubMed] [Google Scholar]
- 3.Casper A, Van Doren M. The control of sexual identity in the Drosophila germline. Development. 2006;133(15):2783–2791. doi: 10.1242/dev.02415. [DOI] [PubMed] [Google Scholar]
- 4.Camara N, Whitworth C, Van Doren M. The creation of sexual dimorphism in the Drosophila soma. Curr Top Dev Biol. 2008;83:65–107. doi: 10.1016/S0070-2153(08)00403-1. [DOI] [PubMed] [Google Scholar]
- 5.Villella A, Hall JC. Neurogenetics of courtship and mating in Drosophila. Adv Genet. 2008;62:67–184. doi: 10.1016/S0065-2660(08)00603-2. [DOI] [PubMed] [Google Scholar]
- 6.Gelbart ME, Kuroda MI. Drosophila dosage compensation: A complex voyage to the X chromosome. Development. 2009;136(9):1399–1410. doi: 10.1242/dev.029645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siwicki KK, Kravitz EA. Fruitless, doublesex and the genetics of social behavior in Drosophila melanogaster. Curr Opin Neurobiol. 2009;19(2):200–206. doi: 10.1016/j.conb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salz HK, Erickson JW. Sex determination in Drosophila: The view from the top. Fly (Austin) 2010;4(1):60–70. doi: 10.4161/fly.4.1.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad T, Akhtar A. Dosage compensation in Drosophila melanogaster: Epigenetic fine-tuning of chromosome-wide transcription. Nat Rev Genet. 2011;13(2):123–134. doi: 10.1038/nrg3124. [DOI] [PubMed] [Google Scholar]
- 10.Gempe T, Beye M. Function and evolution of sex determination mechanisms, genes and pathways in insects. Bioessays. 2011;33(1):52–60. doi: 10.1002/bies.201000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhulst EC, van de Zande L, Beukeboom LW. Insect sex determination: It all evolves around transformer. Curr Opin Genet Dev. 2010;20(4):376–383. doi: 10.1016/j.gde.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Cline TW, et al. Evolution of the Drosophila feminizing switch gene Sex-lethal. Genetics. 2010;186(4):1321–1336. doi: 10.1534/genetics.110.121202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siera SG, Cline TW. Sexual back talk with evolutionary implications: Stimulation of the Drosophila sex-determination gene Sex-lethal by its target transformer. Genetics. 2008;180(4):1963–1981. doi: 10.1534/genetics.108.093898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKeown M, Belote JM, Boggs RT. Ectopic expression of the female transformer gene product leads to female differentiation of chromosomally male Drosophila. Cell. 1988;53(6):887–895. doi: 10.1016/s0092-8674(88)90369-8. [DOI] [PubMed] [Google Scholar]
- 15.Waterbury JA, Horabin JI, Bopp D, Schedl P. Sex determination in the Drosophila germline is dictated by the sexual identity of the surrounding soma. Genetics. 2000;155(4):1741–1756. doi: 10.1093/genetics/155.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans DS, Cline TW. Drosophila melanogaster male somatic cells feminized solely by TraF can collaborate with female germ cells to make functional eggs. Genetics. 2007;175(2):631–642. doi: 10.1534/genetics.106.066332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun S, Cline TW. Effects of Wolbachia infection and ovarian tumor mutations on Sex-lethal germline functioning in Drosophila. Genetics. 2009;181(4):1291–1301. doi: 10.1534/genetics.108.099374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cline TW. Autoregulatory functioning of a Drosophila gene product that establishes and maintains the sexually determined state. Genetics. 1984;107(2):231–277. doi: 10.1093/genetics/107.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dines JL (2001) New aspects of functional complexity for the master regulator of Drosophila melanogaster sex determination: Analysis of structures, expression patterns, and activities of Sex-lethal protein isoforms. PhD thesis (Univ of California, Berkeley) p 319.
- 20.Finley KD, Taylor BJ, Milstein M, McKeown M. dissatisfaction, a gene involved in sex-specific behavior and neural development of Drosophila melanogaster. Proc Natl Acad Sci USA. 1997;94(3):913–918. doi: 10.1073/pnas.94.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starr DJ, Cline TW. A host parasite interaction rescues Drosophila oogenesis defects. Nature. 2002;418(6893):76–79. doi: 10.1038/nature00843. [DOI] [PubMed] [Google Scholar]
- 22.Herskowitz I, Hagen D. The lysis-lysogeny decision of phage lambda: Explicit programming and responsiveness. Annu Rev Genet. 1980;14:399–445. doi: 10.1146/annurev.ge.14.120180.002151. [DOI] [PubMed] [Google Scholar]
- 23.Sosnowski BA, Belote JM, McKeown M. Sex-specific alternative splicing of RNA from the transformer gene results from sequence-dependent splice site blockage. Cell. 1989;58(3):449–459. doi: 10.1016/0092-8674(89)90426-1. [DOI] [PubMed] [Google Scholar]
- 24.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 25.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22(3):451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 26.Horabin JI. Splitting the Hedgehog signal: Sex and patterning in Drosophila. Development. 2005;132(21):4801–4810. doi: 10.1242/dev.02054. [DOI] [PubMed] [Google Scholar]
- 27.Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8(15):1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 28.Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121(5):795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14(2):341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 30.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302(5651):1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 31.Jenett A, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2(4):991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujii S, Amrein H. Genes expressed in the Drosophila head reveal a role for fat cells in sex-specific physiology. EMBO J. 2002;21(20):5353–5363. doi: 10.1093/emboj/cdf556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashiyama K, Hayashi Y, Kobayashi S. Drosophila Sex lethal gene initiates female development in germline progenitors. Science. 2011;333(6044):885–888. doi: 10.1126/science.1208146. [DOI] [PubMed] [Google Scholar]
- 34.Yang SY, Baxter EM, Van Doren M. Phf7 controls male sex determination in the Drosophila germline. Dev Cell. 2012;22(5):1041–1051. doi: 10.1016/j.devcel.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanowitz JL, Deshpande G, Calhoun G, Schedl PD. An N-terminal truncation uncouples the sex-transforming and dosage compensation functions of sex-lethal. Mol Cell Biol. 1999;19(4):3018–3028. doi: 10.1128/mcb.19.4.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grigliatti TA. In: Drosophila, a Practical Approach. Roberts DB, editor. New York: IRL; 1998. pp. 56–60. [Google Scholar]
- 37.Rørth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78(1-2):113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 38.Bell LR, Maine EM, Schedl P, Cline TW. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988;55(6):1037–1046. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.