Significance
Estrogens control the growth and development of the majority of breast cancers, the most prevalent malignancies of women in the developed world. Exemestane, a synthetic steroid commonly used clinically to prevent, delay progression of, and treat breast cancer, was designed to inhibit estrogen biosynthesis by aromatase. We have made the unexpected observation that exemestane also has widespread protective activities unrelated to blocking estrogens. Thus, exemestane is potently anti-inflammatory and antioxidant and can thereby protect against development of cancer and chronic degenerative diseases. Moreover, these activities are synergistic with sulforaphane and other cytoprotective phytochemicals. Use of such combinations could therefore provide a valuable strategy for reducing the risk of a variety of other malignancies and age-related chronic diseases.
Abstract
Exemestane (6-methyleneandrosta-1,4-diene-3,17-dione) is a synthetic steroidal inhibitor of the aromatase reaction that catalyzes the terminal and rate-limiting step of the biosynthesis of estrogens. It is active clinically in preventing, delaying progression of, and treating mammary cancers, many of which are estrogen receptor-positive. A striking feature of the structure of exemestane is an extended system of conjugated Michael reaction functions, which is also characteristic of inducers of a broad network of chemoprotective genes regulated by the Keap1 (Kelch-like ECA-associated protein)/Nrf2 (nuclear factor E2-related factor 2)/ARE (antioxidant response element) signaling system. These genes are largely involved in xenobiotic metabolism and antioxidative and anti-inflammatory protection, as well as the synthesis and reduction of glutathione. We show here that exemestane transcriptionally activates NAD(P)H:quinone oxidoreductase 1 (NQO1) and heme oxygenase 1 (HO-1), typical chemoprotective gene products, in a wide variety of mouse, rat, and human cells. It protects several cell lines against oxidative toxicity of tert-butyl hydroperoxide and 4-hydroxynonenal, against free radical damage arising from hypoxia–reoxygenation, and against UVA radiation damage. Exemestane also inhibits the inflammatory increases in inducible nitric oxide synthase (iNOS) in mouse macrophages exposed to LPS (lipopolysaccharide), thereby resembling the isothiocyanate sulforaphane derived from broccoli. Remarkably, combinations of exemestane and sulforaphane act highly synergistically, and this property is also displayed by several other phytochemicals. Thus, exemestane has a wide range of previously unrecognized protective activities, probably unrelated to aromatase inhibition. Its potential for reducing the risk, not only of breast cancer, but also of other chronic diseases that arise from inflammation, oxidative stress, and DNA-damaging electrophiles, requires exploration, particularly in view of the synergism with other phytochemicals.
Development of safe and effective agents for reducing the risk of cancer and other chronic diseases is a high priority of contemporary medicine. Implementation of strategies for chemoprevention/chemoprotection is beset with many problems (1, 2). In this paper, we describe the broad protective potential of a synthetic steroid, exemestane (Fig. 1A), that has already been safely administered to many thousands of human subjects as an estrogen biosynthesis inhibitor. We also find that exemestane acts synergistically in combination with a chemoprotective phytochemical, sulforaphane, derived from cruciferous vegetables, and already consumed widely as a regular component of the human diet.
Fig. 1.
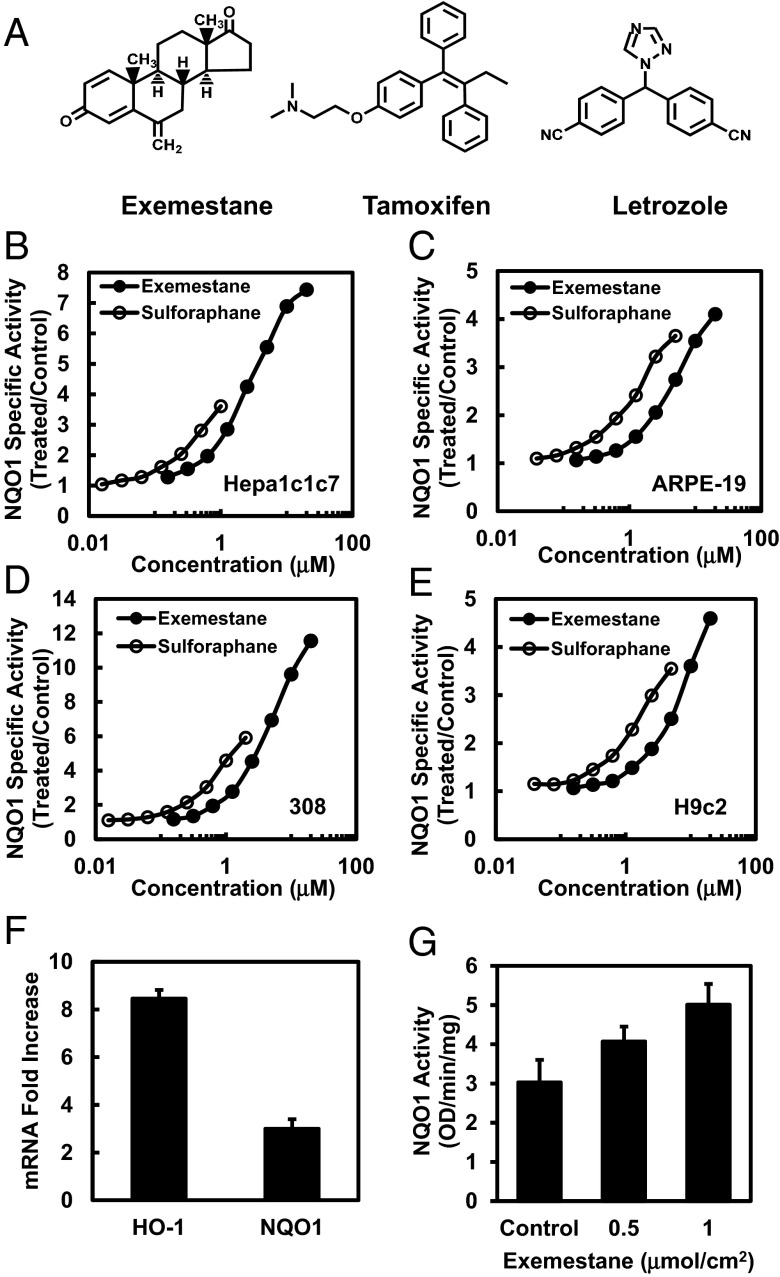
Exemestane induces cytoprotective enzymes in cultured cells and mouse skin. (A) Chemical structures of exemestane, tamoxifen, and letrozole. (B–E) Dose-dependent induction of NAD(P)H:quinone oxidoreductase 1 (NQO1) by exemestane and sulforaphane in different cell types: Hepa1c1c7, ARPE-19, 308 keratinocytes, and H9c2 cardiomyocytes. Cells were plated in 96-well plates, and 24 h later were exposed to serial dilutions of exemestane or sulforaphane for a further 48 h. NQO1 activity is expressed as mean ratios of treated over control specific activities by using eight replicate wells for each inducer concentration. SDs for all measurements were less than 10%. (F) Real-time PCR analysis of phase 2-related genes heme oxygenase-1 (HO-1) and NQO1 expression after 6 h of treatment with exemestane in H9c2 cells. β-Actin was used as an endogenous control for the target genes, and values are represented as the ratio of change in the mRNA levels of exemestane-treated versus vehicle-treated cells. Means ± SD are shown. (G) Induction of NQO1 in mouse skin by topical application of exemestane. The back of each SKH-1 hairless mouse (n = 5) was topically treated with two concentrations of exemestane [0.5 and 1.0 μmol/cm2 in 80% (vol/vol) acetone] and solvent only over about a 2.0-cm2 area for three doses at 24-h intervals. Mice were euthanized 24 h after the last dose, and dorsal skin was harvested. NQO1 specific activity was measured in supernatant fractions of homogenates of skin sections treated with exemestane or solvent (control). Means ± SD are shown.
In the economically developed world, mammary cancer is the most common malignancy in women. In the United States alone, 232,340 new diagnoses and 39,620 deaths of women from breast cancer are projected for 2013, and the global burden of new cases is enormous (3). The majority of breast malignancies are estrogen receptor-positive at the time of diagnosis, and their growth is under estrogen control. Thus, this process is an effective target for both prevention and treatment of breast cancer by two mechanisms: (i) selective modification of estrogen binding to their receptors, exemplified by such widely used agents as tamoxifen (Fig. 1A) and raloxifene (4), and (ii) blocking of estrogen biosynthesis by inhibition of its final and rate-limiting step, the aromatase reaction, by steroidal compounds such as exemestane and nonsteroidal letrozole (Fig. 1A) and anastrozole (5, 6). Both these approaches have been highly successful, and multiple clinical studies attest to their benefits (7). A recent study of 4,560 women at moderately increased risk for developing breast cancer showed that exemestane reduced the risk of breast cancer by 65% relative to placebo (8). Clinical comparisons of exemestane and other estrogen-function modifiers suggest that exemestane may have special, and even superior, protective properties over other agents such as tamoxifen (7, 9, 10). Combinations of exemestane with tamoxifen or with cyclooxygenase inhibitors have been administered to women with breast cancer. Suggestions of additive or even synergistic action, without increased adverse effects, have been reported (5, 7, 11, 12).
The chemical structure of exemestane (6-methyleneandrosta-1,4-diene-3,17-dione), designed as an irreversible, mechanism-based aromatase inhibitor, includes a system of highly electrophilic conjugated Michael acceptor groups (13). Interestingly, this structural feature was recognized by us long ago as a fundamental feature of many agents that induce the cytoprotective phase 2 response of aerobic cells via the Kelch-like ECA-associated protein (Keap1)/nuclear factor E2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway, which controls the gene expression of a large and diverse family of proteins that protect cells against electrophiles and oxidants, and enhance cell survival (14–18). This realization suggested to us that exemestane might react efficiently with the thiol groups of the reactive cysteine residues of Keap1 and thereby regulate Nrf2-dependent cytoprotective genes (14, 16–19). To our knowledge, these potentially much broader pharmacological activities of exemestane were not previously recognized.
We show here that the aromatase inhibitor exemestane exhibits a wide range of cytoprotective effects—that appear to be unrelated to aromatase inhibition. Thus, it potently activates the Keap1/Nrf2/ARE pathway, thereby stimulating expression of genes that regulate an extensive network of inducible cytoprotective phase 2 proteins that protect cells against reactive oxygen species (ROS), inflammation, and DNA-damaging electrophiles (19). In this respect, exemestane therefore resembles sulforaphane, the potent chemoprotective isothiocyanate isolated from broccoli and other cruciferous plants that activates the Nrf2-dependent phase 2 response (19, 20).
Results
Exemestane Induces Cytoprotective Enzymes in Cultured Cells and Mouse Skin.
We first confirmed the prediction, based purely on structural examination, that exemestane should induce the activity of NAD(P)H:quinone oxidoreductase 1 (NQO1), a prototypic phase 2 enzyme. In murine hepatoma Hepa1c1c7 cells, human retinal pigment epithelial ARPE-19 cells, murine 308 keratinocytes, and rat H9c2 myocardiocytes, inducer potencies of exemestane were comparable with, but modestly lower than, those of sulforaphane. The concentrations of exemestane required to double NQO1-specific activities (CD values) were 0.65 µM in Hepa1c1c7 and 308 cells, 2.2 µM in ARPE-19, and 2.9 µM in H9c2 cells whereas the corresponding CD values for sulforaphane were 0.2, 0.2, 0.7, and 0.9 µM, respectively. An impressive property of exemestane was its low toxicity. In all cell lines, exemestane showed no cytotoxicity up to 20 µM, the highest concentration tested, and increases in specific activities of NQO1 up to 4- to 12-fold were observed (Fig. 1 B–E). Exemestane therefore shows a very high Chemoprotective Index as defined by Pezzuto et al. (21). To evaluate the effect of the combination of exemestane and sulforaphane on induction of NQO1, we treated Hepa1c1c7 cells with mixtures of the two agents at constant ratios. Within attainable dose ranges for both agents, exemestane and sulforaphane showed no synergism on NQO1 induction at both 2:1 and 5:1 molar ratios. The combined effects of these agents are mostly additive, which suggests that exemestane induces NQO1 activity through the same Keap1/Nrf2/ARE mechanism as sulforaphane.
To determine whether the phase 2 enzyme inducer activity of exemestane was related to aromatase inhibition, we selected letrozole, a nonsteroidal and structurally unrelated aromatase inhibitor. In Hepa1c1c7 cells and at concentrations up to 20 μM, letrozole showed no inducer activity for NQO1. In the same system, tamoxifen, a selective estrogen receptor modifier and not an aromatase inhibitor, did not induce NQO1 activity but showed about 90% cytotoxicity at 20 μM.
We then examined whether treatment with exemestane changed expression of cytoprotective phase 2-related genes in H9c2 cardiomyocytes. Real-time PCR analysis of 10 μM exemestane-treated cells showed a significant more than eightfold increase in the mRNA levels of heme oxygenase 1 (HO-1), and a threefold increase in NQO1 expression over control cells (Fig. 1F). These results establish that exemestane treatment transcriptionally up-regulates the protective phase 2 response that contributes to its antioxidant and anti-inflammatory activities.
We next evaluated whether exemestane induced the cytoprotective enzyme NQO1 in the skin of female SKH-1 hairless mice. These mice are immunocompetent but hairless because of a defect in the keratin biosynthesis cycle. Three topical applications of exemestane (0.5 or 1.0 μmol/cm2 per application) at 24-h intervals to the dorsal skin of SKH-1 mice (n = 5) significantly elevated NQO1 activity (1.3- and 1.7-fold, respectively) in skin homogenates (Fig. 1G).
Exemestane Protects Human Retinal Pigment Epithelial Cells Against Oxidative Damage.
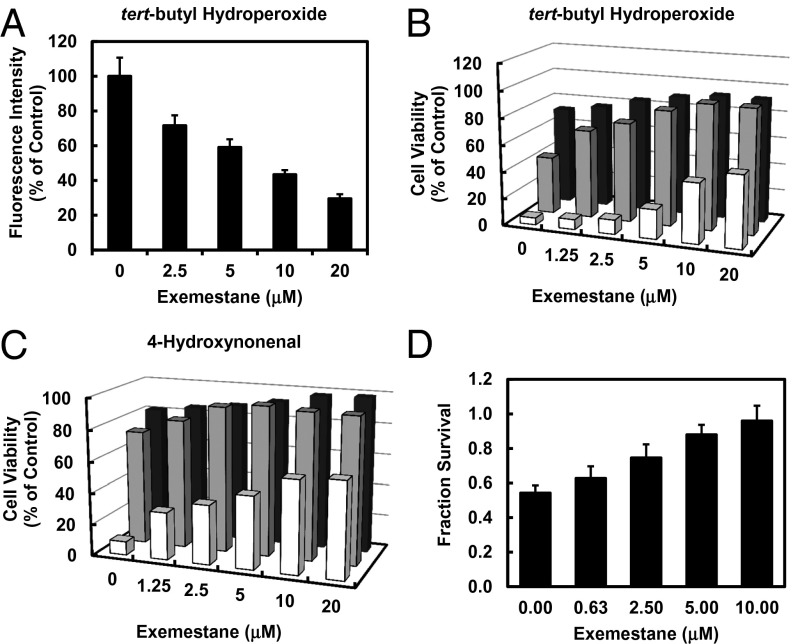
Much evidence indicates that induction of the phase 2 response plays a key role in cellular protection largely by detoxifying oxidants and electrophiles, and by blocking the effects of UV radiation. Age-related macular degeneration is the leading cause of blindness among the elderly in developed countries. ROS are probably involved in retinal pigment epithelium (RPE) cell dysfunction and contribute to disease development. Therefore, strategies for protecting RPE cells against oxidative damage may be particularly important in retarding development of macular degeneration. It has been reported that ROS generated by exogenous oxidants can damage cultured ARPE-19 cells (22, 23). We therefore tested whether exemestane could reduce ROS production in ARPE-19 by using the fluorescence-generating probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA). Treatment with exemestane dose-dependently suppressed ROS production stimulated by tert-butyl hydroperoxide (Fig. 2A). Treatment of ARPE-19 cells with a range of concentrations of exemestane for 24 h also protected against the cytotoxicity of oxidants (tert-butyl hydroperoxide and 4-hydroxynonenal) (Fig. 2 B and C). The degree of protection depended on both the concentrations of oxidants and exemestane.
Fig. 2.
Exemestane protects cells against oxidative stress. (A) ROS suppression by exemestane in human retinal pigment epithelial ARPE-19 cells. After 24 h of incubation with exemestane, the cells were exposed to 20 μM DCFDA for 30 min and then challenged with 500 μM tert-butyl hydroperoxide for 30 min. Fluorescence intensity was measured. Means ± SD are shown. Protection of ARPE-19 cells against toxicity of (B) tert-butyl hydroperoxide (0.2, 0.4, 0.8 mM for 4 h) and (C) 4-hydroxynonenal (10, 20, 40 μM for 4 h) as a function of 24-h prior exposure to 0–20 μM exemestane. Bar graphs show that cell viability is a function of both the concentrations of the oxidants and of exemestane. SDs for all measurements were less than 10%. Front, center, and rear series of bars refer to the highest, middle, and lowest concentrations of the oxidants, respectively. (D) Exemestane protects against hypoxia/reoxygenation-induced cardiomyocyte injury. Rat myocardial H9c2 cells were treated with exemestane for 24 h and then subjected to hypoxia for 1 h (2 mM Na2S2O4), and reoxygenation for 24 h. Cell viability, assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, was expressed as fractional survival compared with cells not exposed to hypoxia/reoxygenation. Means ± SD are shown.
Protection Against Hypoxia–Reoxygenation Injury.
Cellular injury resulting from ischemia and reperfusion (IR), and thereby generation of reactive oxygen intermediates, is a major contributor to morbidity and mortality of many medical procedures, including organ transplantation and cardiovascular surgery. A hypoxia/reoxygenation model was used to mimic IR injury in H9c2 rat cardiomyocytes in vitro and to evaluate protection by exemestane. Treatment of H9c2 cells with a range of concentrations of exemestane (0-10 µM) for 24 h dose-dependently protected cells against hypoxia/reoxygenation injury and almost totally restored cell viability at 10 µM concentration (Fig. 2D).
Protection Against UV Radiation.
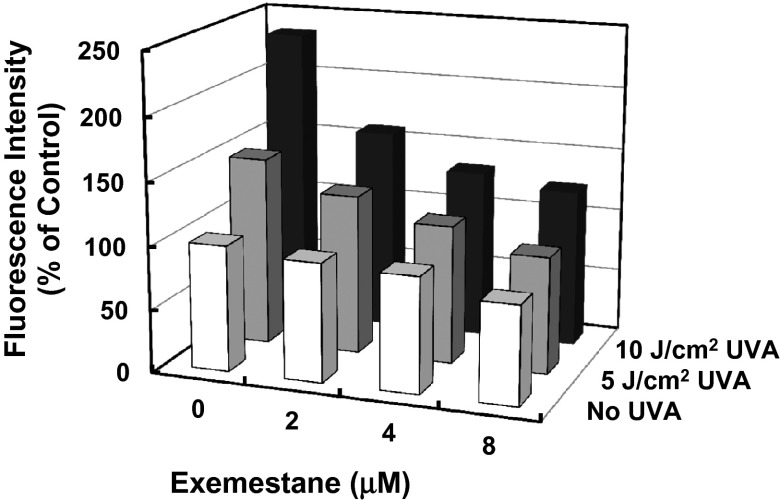
Solar UV radiation is a persistent damaging process. UVA radiation (320–400 nm) comprises more than 95% of solar radiation that reaches the surface of the earth and has deleterious effects on the skin. In response to UVA exposure, ROS are generated, which may directly result in damage to cellular proteins, lipids, and polysaccharides, and may also indirectly cause structural damage of DNA (24). The rate of skin aging and prevalence of skin cancer have been increasing dramatically (25), and inhibition of UVA-induced oxidative stress may therefore prevent such damage. To evaluate the potential UVA-protective effects of exemestane, mouse 308 keratinocytes were treated with exemestane 24 h before UVA irradiation, and ROS levels were measured by using DCFDA. Fig. 3 shows that exemestane treatment dose-dependently reduced ROS levels in UVA-irradiated mouse 308 keratinocytes.
Fig. 3.
Exemestane inhibits UVA-stimulated ROS production in murine 308 keratinocytes. Cells plated in 24-well plates were treated with exemestane for 24 h. After incubation with 20 μM DCFDA for 30 min, cells were exposed to 5 or 10 J/cm2 UVA, and fluorescence intensity was measured as indicator of ROS production in the cells. Cells that received no treatment were controls. SDs for all measurements were less than 10%.
Exemestane Inducer Activity Depends on the Keap1/Nrf2 Pathway.
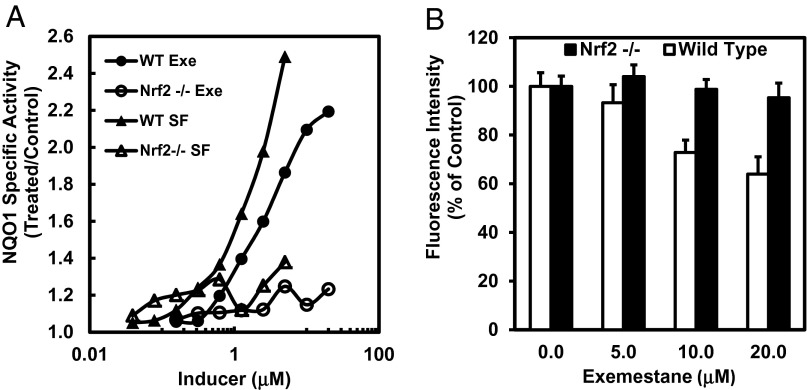
To determine whether exemestane induces NQO1 via the Nrf2 transcription factor, we examined NQO1 induction by exemestane in mouse embryonic fibroblasts (MEFs) derived from wild-type and Nrf2-knockout mice (18). Like sulforaphane, exemestane dose-dependently induced NQO1 activity in wild-type MEFs. In sharp contrast, in Nrf2-knockout (nrf2−/−) MEFs, NQO1 activity was not affected by either sulforaphane or exemestane (Fig. 4A). We also examined the protection by exemestane against oxidative stress and its dependence on nrf2 gene function by measuring tert-butyl hydroperoxide-stimulated ROS generation in MEF cells. The levels of ROS observed in wild-type MEFs were clearly suppressed by exemestane whereas exemestane showed no protection in nrf2−/− MEFs (Fig. 4B). The results establish that exemestane also protects against oxidative stress through the Nrf2 pathway.
Fig. 4.
Nrf2 is essential for exemestane to induce phase 2 response and suppress ROS production. (A) Dose-dependent induction of NQO1 by exemestane or sulforaphane in wild-type and Nrf2−/− MEF cells. Cells plated in 96-well plates were exposed to serial dilutions of exemestane or sulforaphane for 48 h. NQO1 activity was determined in cell lysates. SDs for all determinations were less than 10%. (B) Exemestane suppresses ROS production in wild-type MEF, but not in Nrf2−/− MEF cells. After 24 h of incubation with exemestane, wild-type and Nrf2−/− MEF cells were exposed to 20 μM DCFDA for 30 min and then challenged with 500 μM tert-butyl hydroperoxide for 30 min, and fluorescence intensity was measured. Means ± SD are shown.
Exemestane Inhibits LPS-Activated iNOS in Macrophages.
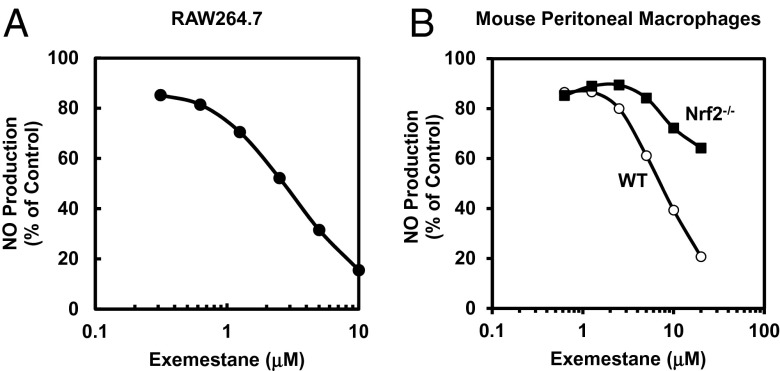
Many different chemical classes of inducers of the phase 2 response also display powerful anti-inflammatory activity (26, 27). Inducible nitric oxide synthase (iNOS) is an inflammatory biomarker, and induction of iNOS can contribute to cell injury in various diseases. Expression of iNOS can be up-regulated by inflammatory mediators such as the bacterial endotoxin LPS, through the I kappa B kinase (IKK)/NF-κB pathway. To analyze the anti-inflammatory potential of exemestane, we assessed the ability of exemestane to suppress the LPS-dependent transcriptional activation of iNOS by measuring NO production by the Griess reaction in RAW264.7 macrophages. Exemestane inhibited NO generation dose-dependently with an IC50 value of 2.5 µM (Fig. 5A), moderately less potent than sulforaphane in this assay (IC50 = 0.5 µM). For comparison, both letrozole and tamoxifen (up to 20 µM concentration) did not inhibit LPS-stimulated NO production in RAW264.7 cells. In this system, tamoxifen was 50% cytotoxic at 20 μM whereas exemestane and letrozole showed no toxicity at the same concentration. Thus, the anti-inflammatory effects of exemestane are not related to modifications of estrogen function.
Fig. 5.
Effects of exemestane on LPS-activated iNOS activity (NO production) in (A) RAW264.7 cells, and (B) WT and Nrf2−/− mouse peritoneal macrophages. Cells in 96-well plates were exposed to serial dilutions of exemestane in the presence of 10 ng/mL LPS for 48 h (RAW264.7) or 100 ng/mL LPS for 24 h (peritoneal macrophages). Then, NO production was measured as nitrite accumulation in the medium by the Griess reaction. Control cells were treated with LPS only. SDs for all determinations were less than 10%.
Because the Keap1/Nrf2/ARE pathway is essential for phase 2 gene inductive and antioxidative activities of exemestane, we further examined whether this pathway is also required for its anti-inflammatory activity. We compared inhibition by exemestane of LPS-dependent up-regulation of iNOS in peritoneal macrophages from wild-type and nrf2−/− mice. Exemestane was a much less effective inhibitor of NO production in Nrf2−/− macrophages than it was in wild-type macrophages (Fig. 5B). Our finding suggests that the inhibition of the up-regulation of iNOS by exemestane is partially, and probably largely, dependent on Nrf2.
Exemestane and Sulforaphane Synergistically Inhibit LPS-Activated iNOS in Mouse Peritoneal Macrophages.
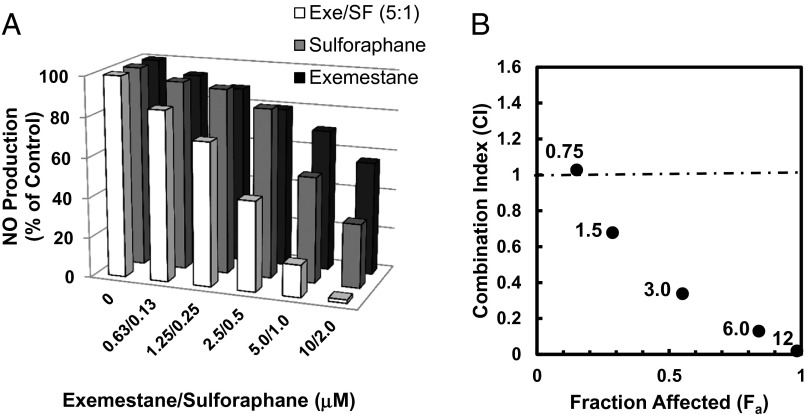
Both exemestane and sulforaphane showed dose-dependent inhibitory effects on LPS-stimulated NO production in mouse peritoneal macrophages, and the inhibition by exemestane is more highly Nrf2-dependent than the inhibition by sulforaphane (Fig. 5B) (27). These results suggest that exemestane and sulforaphane could inhibit LPS-activated iNOS in peritoneal macrophages by different molecular pathways and that combination of the two agents might result in enhanced inhibitory effects. Thus, we compared the inhibitory potencies of exemestane, sulforaphane, and their combinations (with exemestane and sulforaphane at 5:1 molar ratio) on NO production in LPS-stimulated mouse peritoneal macrophages. As shown in Fig. 6A, combinations of one-half doses of exemestane and of sulforaphane resulted in considerably more potent inhibition of NO production than did individual treatments with exemestane or sulforaphane at full doses. The mode of interaction between exemestane and sulforaphane in inhibiting iNOS was further analyzed by the CompuSyn program, which applies median effect equation methods (28–30). The combined drug effect is expressed as CI (combination index) versus Fa (fraction affected), with CI < 1 indicating synergism, CI = 1 indicating an additive effect, and CI > 1 indicating antagonism. Our experiments established that exemestane and sulforaphane combinations were potently synergistic (Fig. 6B). Thus, 50% inhibition of LPS-stimulated nitric oxide generation in mouse macrophages required 1.5 µM sulforaphane or 24.2 µM exemestane. A mixture of exemestane and sulforaphane (5:1) produced the same inhibitory effect at 0.29 µM sulforaphane together with 1.45 µM exemestane, a fivefold and more than 15-fold reduction in dosages, respectively. This constitutes powerful synergism (30). If such powerful synergism between exemestane and sulforaphane does indeed occur in vivo, combinations of these agents could potentially provide a valuable and well-tolerated strategy for prevention, avoiding recurrence, and even treatment of malignancies, and of other chronic diseases. Because suppression of inflammation has been identified as a consistent feature of many inducers of the phase 2 response (26, 27), combinations of exemestane with other phytochemical agents that have similar properties were examined. We compared the inhibitory potencies of exemestane in combination with three structurally unrelated phytochemicals—shikonin, zerumbone, and resveratrol—on NO production in LPS-stimulated RAW264.7 cells. At 90% inhibition of LPS-stimulated NO production, CI values for exemestane/shikonin (10:1), exemestane/zerumbone (2:1), or exemestane/resveratrol (1:1) were 0.55, 0.77, and 0.64, respectively, which are comparable with the CI value of exemestane/sulforaphane (5:1) 0.69 in RAW264.7 cells. These results indicate that synergism exists between exemestane and a broad range of phase 2 enzyme inducers.
Fig. 6.
Synergistic effect of exemestane and sulforaphane on LPS-activated iNOS activity in mouse peritoneal macrophages. (A) Peritoneal macrophages isolated from C57BL6 mice were plated in 96-well plates. Cells were exposed to exemestane, sulforaphane, or their combinations at a 5:1 molar ratio in the presence of 100 ng/mL LPS for 24 h. NO production was measured as nitrite accumulation in the medium. Control cells were treated with LPS only. Each bar represents the mean of eight replicates. SDs for all measurements were less than 10%. (B) Combination index (CI) values were plotted against the effects (fraction affected) mediated by five different drug combinations (0.75, 1.5, 3.0, 6.0, and12.0 μM total concentration shown next to each datum point), respectively. CI values from this experiment were obtained by using CompuSyn software (29).
Exemestane Enhances the Inhibition of Sulforaphane on NF-κB Activation in U937 Cells.
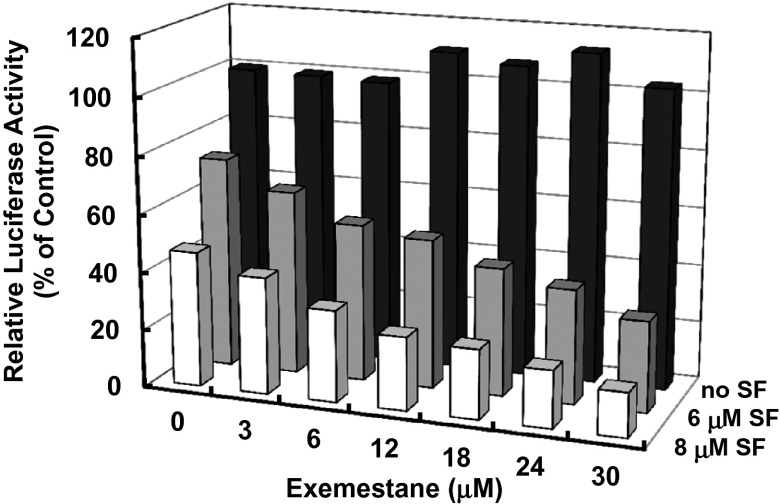
NF-κB is a transcription factor that plays a key role in inflammation (31). Activation of NF-κB signaling results in expression of proinflammatory cytokines as well as iNOS and COX-2 (32). To gain a better understanding of the mechanism of the profound synergism between exemestane and sulforaphane in suppressing LPS-stimulated iNOS up-regulation, we studied a human monocyte cell line (U937) stably transfected with a luciferase reporter containing three NF-κB binding sites (33). Fig. 7 shows that exemestane had no effect on LPS-activated NF-κB at up to 30 µM concentration whereas sulforaphane inhibited LPS-activated NF-κB–driven luciferase activity. Nonetheless, exemestane dramatically enhanced the inhibitory effect of sulforaphane on the activation of NF-κB. For comparison, letrozole alone showed no effect on LPS-activated NF-κB by itself and did not enhance the inhibitory effect of sulforaphane on NF-κB activation, which suggests that the above activity of exemestane is not aromatase pathway-dependent. Real-time PCR analysis of 10 ng/mL LPS-stimulated RAW264.7 cells showed a more than 90-fold increase in the mRNA levels of COX-2. Exemestane at 20 μM strongly enhanced the inhibitory effect of 4 μM sulforaphane on LPS-activated COX-2 expression from 18% to 41% whereas exemestane itself showed no inhibitory effect.
Fig. 7.
Effects of exemestane, sulforaphane, and their combination on LPS-induced NF-κB reporter gene activity. U937 3×κB-LUC cells were incubated with exemestane, sulforaphane, or their combination for 30 min. LPS (10 ng/mL) was added, and the NF-κB–driven luciferase activity was measured after 6 h of LPS exposure. Luciferase activity was normalized to LPS control (100%). Each bar represents the mean of triplicate experiments. SDs for all determinations were less than 10%.
Discussion
The steroid analog exemestane inhibits aromatase, the final and rate-limiting step of estrogen biosynthesis, restricts the growth of estrogen receptor-positive mammary cells, and reduces the risk of the recurrence of mammary cancer in patients. Exemestane is highly effective in inhibiting estrogen biosynthesis, as is well illustrated in peripheral fat, which is the principal site of estrogen biosynthesis in postmenopausal women (34). We have shown in this paper that, unexpectedly, exemestane exerts a wide range of other biological effects, seemingly mechanistically unrelated to aromatase inhibition, among which the up-regulation of the Keap1-Nrf2-ARE cytoprotective signaling system is probably the most prominent. Thus, in several cell lines [murine hepatoma Hepa1c1c7, murine 308 keratinocytes, adult human retinal pigment epithelial cells (ARPE-19), and rat myocardiocytes (H9c2)], NQO1 and HO-1 are up-regulated by exemestane. In this respect, exemestane action resembles that of sulforaphane. The up-regulation of NQO1 by exemestane is particularly significant because this enzyme plays an important role in protecting against estrogen-dependent carcinogenesis. It does so by promoting metabolic inactivation of reactive and carcinogenic estrogen intermediates and suppressing metabolic semiquinone cycling (35, 36). Combinations of exemestane with sulforaphane display largely additive effects in these systems.
Exemestane also resembles sulforaphane in that it protects retinal pigment epithelial cells, myocardiocytes, and keratinocytes against chemically generated oxidative stress resulting from exposure of these cells to tert-butyl hydroperoxide and 4-hydroxynonenal, against free radical damage arising from hypoxia–reoxygenation, and against UV radiation (UVA) damage. Limited experiments suggest that combinations of exemestane and sulforaphane also show additive effects in at least some of these systems. It is therefore not surprising that up-regulation of Nrf2-dependent cytoprotective genes results in closely parallel increases in phase 2 enzymes (e.g., NQO1) and enhanced protection against oxidative stress.
Exemestane also displays anti-inflammatory properties. It was therefore unexpected that the anti-inflammatory activities of exemestane, which resemble those of sulforaphane in many respects, are modulated in a highly synergistic manner by mixtures of exemestane and sulforaphane. We have very little insight into the mechanisms responsible for the surprisingly high synergism between exemestane and sulforaphane in suppression of LPS-stimulated iNOS up-regulation, as well as the enhancement by exemestane of the inhibitory effect of sulforaphane on NF-κB activation. Consideration should be given to the fact that the Keap1/Nrf2 system responds to a wide range of chemical compounds using different sensors (37, 38). In addition, some phase 2 enzyme inducers have been shown to suppress the NF-κB pathway by direct binding to critical cysteine residues in IKK activation loop and NF-κB DNA-binding loop (27). Furthermore, there is cross-talk between the Keap1/Nrf2 and IKK/NF-κB pathways (39). One or several of these mechanisms could be responsible for the synergism between exemestane and sulforaphane.
These deductions with respect to the unrecognized broad pharmacological effects of exemestane recall an earlier series of parallel conclusions with respect to a series of synthetic triterpenoid analogs related to oleanolic acid. Using potency of anti-inflammatory activity based on suppression of inducible nitric oxide synthase (iNOS) in mouse macrophages as the endpoint, we, in collaboration with colleagues at Dartmouth University, found that the anti-inflammatory potency of these triterpenoids depended on the critical positioning of Michael reaction acceptor groups in the triterpenoid structure (17, 26). Moreover, these structural features also controlled inhibition of proliferation, promotion of differentiation, and induction of apoptosis in cells. Because these structural features were also important for induction of the phase 2 response (controlled by the Keap1/Nrf2/ARE signaling pathway), we showed that the anti-inflammatory and phase 2 inducer potencies of 18 triterpenoids were closely linearly related over a range of six orders of potency magnitude, and both responses were abrogated in MEF lacking either Nrf2 or Keap1 (26). Several of these triterpenoids have subsequently been developed for clinical use in a variety of medical conditions (40).
In conclusion, in addition to its potent mechanism-dependent inhibition of estrogen biosynthesis, exemestane has chemoprotective properties that have hitherto not been explicitly recognized. Exemestane should therefore be considered for use also in chemoprotection against mammary tumors that are not estrogen receptor-positive, and against a wide variety of nonmammary tumors (and possibly other chronic diseases) that are not estrogen-dependent but have oxidative stress, inflammation, and electrophile-damaging etiologies. The additional finding that exemestane shows powerful synergism with another widely consumed Nrf2-activator, sulforaphane, and a number of other phytochemicals, increases the attractiveness of this strategy. Our findings favor the view that use of exemestane with its broad range of actions, and its potential synergism with sulforaphane and other phytochemicals, could be a valuable chemoprotective strategy for reducing the risk of many malignancies and possibly other chronic diseases.
Materials and Methods
The sources of all materials and cultured cells are described in SI Materials and Methods. The methods regarding biochemical analysis of NQO1 acitvity, ROS, cytotoxicity, iNOS induction, and luciferase activity are also described and referenced in SI Materials and Methods. The probes and procedures for real-time PCR to determine mRNA levels are detailed in SI Materials and Methods.
Supplementary Material
Acknowledgments
The human monocyte U937-3xκB-LUC cells were a generous gift from Prof. Rune Blomhoff (University of Oslo). These studies were generously supported by the Lewis B. and Dorothy Cullman Foundation. We are also grateful for an equipment grant from Murakami Noen.
Footnotes
Conflict of interest statement: The authors have filed patent applications.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318247110/-/DCSupplemental.
References
- 1.Fahey JW, Talalay P, Kensler TW. Notes from the field: “Green” chemoprevention as frugal medicine. Cancer Prev Res (Phila) 2012;5(2):179–188. doi: 10.1158/1940-6207.CAPR-11-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kensler TW, et al. Keap1-nrf2 signaling: A target for cancer prevention by sulforaphane. Top Curr Chem. 2013;329:163–177. doi: 10.1007/128_2012_339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Dunn BK, Cazzaniga M, DeCensi A. Exemestane: One part of the chemopreventive spectrum for ER-positive breast cancer. Breast. 2013;22(3):225–237. doi: 10.1016/j.breast.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Love RR, Hutson PR, Havighurst TC, Cleary JF. Endocrine effects of tamoxifen plus exemestane in postmenopausal women with breast cancer. Clin Cancer Res. 2005;11(4):1500–1503. doi: 10.1158/1078-0432.CCR-04-1610. [DOI] [PubMed] [Google Scholar]
- 6.Hong Y, et al. Molecular basis for the aromatization reaction and exemestane-mediated irreversible inhibition of human aromatase. Mol Endocrinol. 2007;21(2):401–414. doi: 10.1210/me.2006-0281. [DOI] [PubMed] [Google Scholar]
- 7.Deeks ED, Scott LJ. Exemestane: A review of its use in postmenopausal women with breast cancer. Drugs. 2009;69(7):889–918. doi: 10.2165/00003495-200969070-00007. [DOI] [PubMed] [Google Scholar]
- 8.Goss PE, et al. NCIC CTG MAP.3 Study Investigators Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 9.Davidson NE, Kensler TW. “MAPping” the course of chemoprevention in breast cancer. N Engl J Med. 2011;364(25):2463–2464. doi: 10.1056/NEJMe1106052. [DOI] [PubMed] [Google Scholar]
- 10.Visvanathan K, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942–2962. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 11.Hutson PR, Love RR, Havighurst TC, Rogers E, Cleary JF. Effect of exemestane on tamoxifen pharmacokinetics in postmenopausal women treated for breast cancer. Clin Cancer Res. 2005;11(24 Pt 1):8722–8727. doi: 10.1158/1078-0432.CCR-05-0915. [DOI] [PubMed] [Google Scholar]
- 12.Falandry C, Canney PA, Freyer G, Dirix LY. Role of combination therapy with aromatase and cyclooxygenase-2 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2009;20(4):615–620. doi: 10.1093/annonc/mdn693. [DOI] [PubMed] [Google Scholar]
- 13.Giudici D, et al. 6-Methylenandrosta-1,4-diene-3,17-dione (FCE 24304): A new irreversible aromatase inhibitor. J Steroid Biochem. 1988;30(1-6):391–394. doi: 10.1016/0022-4731(88)90129-x. [DOI] [PubMed] [Google Scholar]
- 14.Talalay P, De Long MJ, Prochaska HJ. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci USA. 1988;85(21):8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12(1-4):5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 16.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci USA. 2001;98(6):3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinkova-Kostova AT, et al. An exceptionally potent inducer of cytoprotective enzymes: Elucidation of the structural features that determine inducer potency and reactivity with Keap1. J Biol Chem. 2010;285(44):33747–33755. doi: 10.1074/jbc.M110.163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakabayashi N, et al. Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA. 2004;101(7):2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85(4):241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Talalay P, Cho C-G, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89(6):2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pezzuto JM, et al. Characterization of natural product chemopreventive agents. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Cancer Chemoprevention 2: Strategies for Cancer Chemoprevention. Totowa, NJ: Humana; 2005. pp. 3–37. [Google Scholar]
- 22.Gao X, Dinkova-Kostova AT, Talalay P. Powerful and prolonged protection of human retinal pigment epithelial cells, keratinocytes, and mouse leukemia cells against oxidative damage: The indirect antioxidant effects of sulforaphane. Proc Natl Acad Sci USA. 2001;98(26):15221–15226. doi: 10.1073/pnas.261572998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci USA. 2004;101(28):10446–10451. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svobodova A, Walterova D, Vostalova J. Ultraviolet light induced alteration to the skin. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150(1):25–38. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- 25.Pinnell SR. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J Am Acad Dermatol. 2003;48(1):1–19, quiz 20–22. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
- 26.Dinkova-Kostova AT, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA. 2005;102(12):4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Dinkova-Kostova AT, Talalay P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc Natl Acad Sci USA. 2008;105(41):15926–15931. doi: 10.1073/pnas.0808346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 29.Chou T-C, Martin N. CompuSyn for drug combinations: A Computer Software for Quantitation of Synergism and Antagonism, and the Determination of IC50, ED50 and LD50 Values. Paramus, NJ: ComboSyn; 2005. [PC software and user’s guide] [Google Scholar]
- 30.Chou T-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 31.Tak PP, Firestein GS. NF-kappaB: A key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: New opportunities for drug discovery. Nat Rev Drug Discov. 2004;3(5):401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 33.Carlsen H, Moskaug JØ, Fromm SH, Blomhoff R. In vivo imaging of NF-κB activity. J Immunol. 2002;168(3):1441–1446. doi: 10.4049/jimmunol.168.3.1441. [DOI] [PubMed] [Google Scholar]
- 34.Adams LS, Chen S. Phytochemicals for breast cancer prevention by targeting aromatase. Front Biosci (Landmark Ed) 2009;14:3846–3863. doi: 10.2741/3493. [DOI] [PubMed] [Google Scholar]
- 35.Dinkova-Kostova AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys. 2010;501(1):116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao Y, Brodie AM, Davidson NE, Kensler TW, Zhou Q. Inhibition of estrogen signaling activates the NRF2 pathway in breast cancer. Breast Cancer Res Treat. 2010;124(2):585–591. doi: 10.1007/s10549-010-1023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi M, et al. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29(2):493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci USA. 2010;107(44):18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who’s listening? Antioxid Redox Signal. 2010;13(11):1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liby KT, Sporn MB. Synthetic oleanane triterpenoids: Multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev. 2012;64(4):972–1003. doi: 10.1124/pr.111.004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.