Significance
Because lymphatic vessels (LVs) play critical roles not only in physiological processes such as maintenance of fluid homeostasis but also in pathological conditions including cancer metastasis, identification of factors that control LV formation is crucial. Signals mediated by activin receptor-like kinase 1 (ALK-1), a receptor for bone morphogenetic protein 9 (BMP-9), have been implicated in the formation of blood vessels because of its linkage to a human vascular disease. However, their roles in LV formation largely remain to be elucidated. Here, we show that BMP-9/ALK-1 signals inhibit LV formation in physiological and pathological conditions. Furthermore, we elucidated its molecular mechanisms in detail, using both in vivo and in vitro systems. These findings will help develop therapeutic strategies for LV-related diseases such as lymphedema and cancer.
Keywords: lymphangiogenesis, angiogenesis, blood vascular endothelial cells
Abstract
Lymphatic vessels (LVs) play critical roles in the maintenance of fluid homeostasis and in pathological conditions, including cancer metastasis. Although mutations in ALK1, a member of the transforming growth factor (TGF)-β/bone morphogenetic protein (BMP) receptor family, have been linked to hereditary hemorrhagic telangiectasia, a human vascular disease, the roles of activin receptor-like kinase 1 (ALK-1) signals in LV formation largely remain to be elucidated. We show that ALK-1 signals inhibit LV formation, and LVs were enlarged in multiple organs in Alk1-depleted mice. These inhibitory effects of ALK-1 signaling were mediated by BMP-9, which decreased the number of cultured lymphatic endothelial cells. Bmp9-deficient mouse embryos consistently exhibited enlarged dermal LVs. BMP-9 also inhibited LV formation during inflammation and tumorigenesis. BMP-9 downregulated the expression of the transcription factor prospero-related homeobox 1, which is necessary to maintain lymphatic endothelial cell identity. Furthermore, silencing prospero-related homeobox 1 expression inhibited lymphatic endothelial cell proliferation. Our findings reveal a unique molecular basis for the physiological and pathological roles of BMP-9/ALK-1 signals in LV formation.
Fluid homeostasis in vertebrates is maintained by two tubular networks, blood vessels (BVs) and lymphatic vessels (LVs), both of which are formed by endothelial cells and surrounding mural cells (1). The major role of LVs is to drain interstitial fluid that leaks from blood capillaries and return it to the BVs.
An insufficiency or obstruction in the lymphatic system results in lymphedema, which is characterized by disabling swelling in the affected tissues. LVs also provide a major pathway for tumor metastasis in many types of cancer, and regional lymph node metastasis has been correlated with cancer progression. Understanding the molecular mechanisms that govern lymphangiogenesis is crucial because of the importance of LVs in both normal and pathological conditions (1).
During embryogenesis, a subset of blood vascular endothelial cells (BECs) in cardinal veins begins to express the homeobox transcription factor Prox1. These prospero-related homeobox 1 (Prox1)-expressing cells differentiate into lymphatic endothelial cells (LECs) (2), which sprout from veins and migrate toward vascular endothelial growth factor (VEGF)-C-expressing mesenchymal cells (3). In Prox1-null mice, the sprouting of lymphatic endothelial progenitors from the veins appears unaffected at embryonic day (E)10.5; however, their migration is arrested at around E11.5–12.0, leading to a complete absence of lymphatic vasculature. This finding suggests that Prox1 is necessary to specify LEC phenotypes in a subset of venous endothelial cells (4). Furthermore, as a homeobox transcription factor, Prox1 is known to upregulate the expression of LEC markers and downregulate BEC markers in mature endothelial cells (5, 6). These findings suggest that Prox1 regulates the differentiation program of embryonic BECs to LECs by functioning as a binary transcriptional switch, turning the BEC program off and the LEC program on. Several lines of evidence have suggested that Prox1 is necessary not only for the differentiation of LECs but also for the maintenance of LEC identity (5–7). When the Prox1 gene is ablated postnatally, the expression of LEC markers decreases, resulting in the dedifferentiative reprogramming of LECs to BECs.
Postnatal lymphangiogenesis is regulated by the differentiation of CD11b+ macrophages into LECs (8) and proliferation of existing LECs (9). LEC proliferation is regulated by various growth factors and cytokines, such as VEGF-C/D, VEGF-A, fibroblast growth factor 2, hepatocyte growth factor, insulin-like growth factor 1, and angiopoietin 1 (reviewed in ref. 10).
Although many factors have been identified as prolymphangiogenic factors, there have been few reports on endogenous antilymphangiogenic factors. In addition to interferon-γ (11), we previously reported that TGF-β is a negative regulator of lymphangiogenesis (12). Although both these inhibitors decrease Prox1 expression, the molecular mechanisms and physiological relevance of their actions remain to be understood.
The TGF-β superfamily consists of more than 30 structurally related members, including TGF-βs, activin, and bone morphogenetic proteins (BMPs; reviewed in ref. 13). The BMP family consists of four subfamilies, including BMP-9, one of the TGF-β superfamily ligands, which has been implicated in angiogenesis (reviewed in ref. 14). Activin receptor-like kinase 1 (ALK-1) is a specific type I receptor for BMP-9. ALK1, its coreceptor endoglin (ENG), and the downstream signal-transmitting molecule SMAD4 have been identified as causal genes for the genetic vascular disorder known as hereditary hemorrhagic telangiectasia (HHT) (15–17). Alk1-deficient mice exhibit abnormal vascular phenotypes reminiscent of those of HHT patients (18–20).
Some groups have reported that BMP-9/ALK-1 signals negatively regulate angiogenesis in retina and in some in vitro experiments (21–23). However, Cunha et al. reported that treatment with ALK-1-Fc inhibited tumor angiogenesis (24). We also showed that BMP-9 induced the proliferation and migration of endothelial cells (25). Therefore, BMP-9 has a dual effect on angiogenesis that is dependent on context, the mechanisms of which still need to be elucidated.
Compared with their role in angiogenesis, the role of BMP-9/ALK-1 signals during lymphangiogenesis has not yet been clarified. Although Niessen et al. reported that ALK-1-Fc treatment perturbs the postnatal lymphangiogenesis in retina, tail, and ear skin (26), the precise mechanisms underlying this inhibition remain to be elucidated. In this study, we demonstrate that BMP-9/ALK-1 signals negatively regulate the formation of LVs in both physiological and pathological conditions by inhibiting the proliferation of LECs. We observed that this inhibition is caused by BMP-9-mediated decreases in Prox1 expression, resulting in the suppressed expression of cyclin family members. Furthermore, BMP-9 induces the dedifferentiation of LECs to BECs through a possible reduction in Prox1 expression, which is necessary to maintain LEC identity.
Results
Lymphatic Vessels Were Enlarged in Multiple Organs in Alk1-depleted Mice.
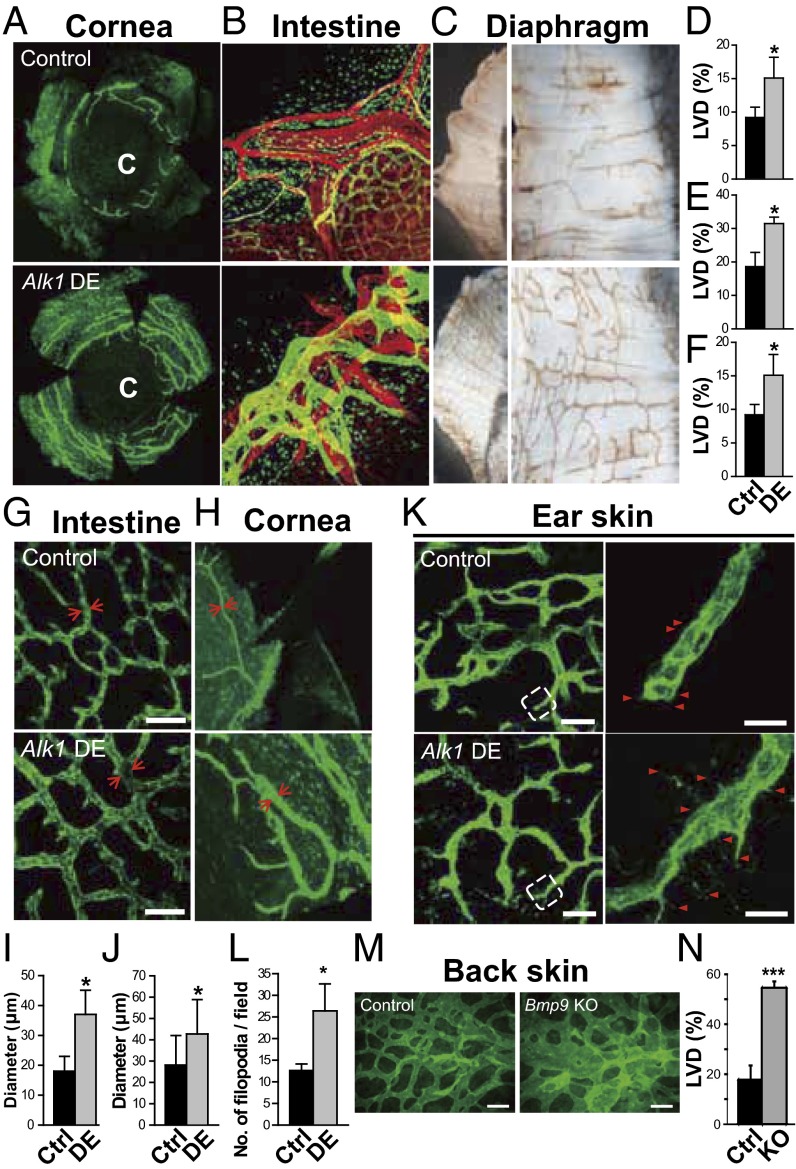
Although Alk1-inducible knockout (KO) mice reportedly exhibit arteriovenous malformations in the lungs, gastrointestinal tract, uterus, and wounded skin (20), the effects of Alk1 deletion on the lymphatic vasculature have not been investigated. To study the physiological roles of ALK-1-mediated signals in the formation of LVs, we first investigated the phenotypes of the LVs of multiple organs in Alk1-depleted mice [ROSA26-CreERT2 (tamoxifen-inducible expression system of Cre recombinase in the ROSA26 locus); ALK1floxed/floxed] (Fig. 1). The densities of LVs in the corneal limbus (Fig. 1 A and D), intestine (Fig. 1 B and E), and diaphragm (Fig. 1 C and F) of Alk1-depleted mice were higher than those of wild-type mice. The diameters of LVs were also significantly increased in the intestine (Fig. 1 G and I) and corneal limbus (Fig. 1 H and J) of Alk1-depleted mice compared with wild-type mice, suggesting enhanced proliferation of the LECs. Furthermore, although the structure of peripheral LVs in the ear skin of control mice was stable after birth at postnatal day (P)8, the number of filopodia in the LVs of Alk1-depleted mice increased with the appearance of typical tip cell-like structures, suggesting that these LVs were actively growing because of the loss of ALK-1 signals (Fig. 1 K and L). These results suggest that the signals mediated by ALK-1 physiologically maintain the structure of LVs by inhibiting excessive LV formation. We note that enlarged LVs were not observed in the tail dermis of Alk1-depleted mice, and their honeycomb structure was severely disorganized (SI Appendix, Fig. S1), which is consistent with a previous report (26). These results also imply that the roles of ALK-1 signals are context- and tissue-dependent.
Fig. 1.
Physiological roles of BMP-9/ALK-1 in the formation of LVs. (A–L) Phenotypes of the LVs from multiple organs in Alk1-depleted mice (DE) and control mice (Ctrl). C, center of the cornea. (A–F) LYVE-1+ lymphatic (green) and platelet endothelial cell adhesion molecule-1+ blood (red) vascular structures and graphs of the quantified density of LVs (LVD) in the cornea (A and D), intestine (B and E), and diaphragm (C and F) are shown. (G–J) LYVE-1+ lymphatic vascular structures (green) and graphs of the measured diameters of LVs in the intestine (G and I) and cornea (H and J). Arrows indicate representative lymphatics with measured diameters. Scale bars, 200 μm. (K) Structures of LYVE-1+ LVs in the postnatal ear skin of Alk1-depleted mice and control littermates in higher-magnification insets from left photos (dotted white line). (Left) Scale bars, 200 μm. Arrowheads indicate filopodia in the sprouting cells. (Right) Scale bars, 20 μm. (L) The number of filopodia in the LVs per field. (M and N) Phenotypes of dermal LVs in Bmp9 KO mice. (M) Morphology of LV formation in the back skin tissues of Bmp9 KO and control heterozygous mice at E15.5. Scale bars, 100 μm. (N) Quantification of the VEGFR3+ LV area in Bmp9 KO mice (n = 4) relative to heterozygous mice (n = 5).
Deletion of Bmp9 Expression Induced Dilation of the Lymphatic Vasculature in Mice.
Several lines of evidence have suggested that BMP-9 and BMP-10 are the physiological ligands for ALK-1 (27-29). To examine whether the loss of Bmp9 expression exhibits phenotypes similar to those observed in Alk1-depleted mice, we analyzed the structure of LVs in Bmp9 KO mice. Bmp9 KO mice were viable and fertile without gross abnormalities (29). We investigated the embryonic phenotypes in dermal lymphatics by performing whole-mount fluorescence immunostaining of embryonic back skin, using an antibody to VEGFR3. VEGFR3-positive LVs were actively formed at E15.5. The lymphatics of Bmp9 KO mice were larger than those of control (Bmp9 +/−) mice (Fig. 1 M and N). This enlargement of LVs was caused by increase in the number of LECs in Bmp9 KO LVs because the density of cell nuclei in Bmp9 KO LVs was not significantly different from that in control LVs (SI Appendix, Fig. S2). These results suggest that BMP-9 negatively regulates LV formation during embryogenesis.
BMP-9 Decreased the Number of Lymphatic Endothelial Cells.
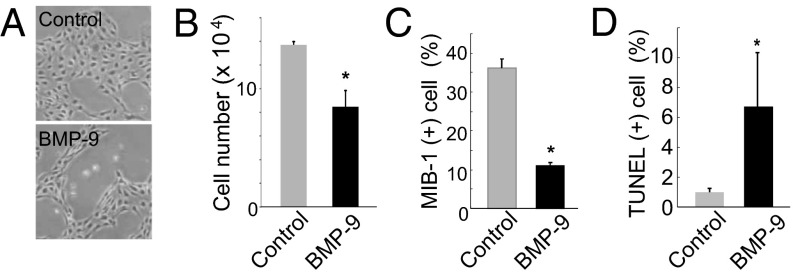
The formation of LVs in embryonic skin is mainly caused by the proliferation of existing dermal LECs. We used primarily cultured human dermal LECs (HDLECs) to examine whether the inhibitory effects of BMP-9 on embryonic dermal lymphangiogenesis were mediated by its direct effects on LECs. When HDLECs were cultured in the presence of 0.5% (vol/vol) serum and treated with 1 ng/mL BMP-9 for 48 h, the number of HDLECs was significantly lower than that of the control (Fig. 2 A and B). Next, we examined the effects of BMP-9 on the proliferation and apoptosis of HDLECs. When HDLECs were treated with BMP-9, the number of HDLECs that were positive for MIB-1, a mitotic index protein, decreased (SI Appendix, Fig. S3A; Fig. 2C), suggesting that BMP-9 reduces the size of a subpopulation of proliferating HDLECs. We also performed a TUNEL assay using HDLECs treated with BMP-9 and found that BMP-9 significantly increased the subpopulation of HDLECs that underwent apoptosis (SI Appendix, Fig. S3B; Fig. 2D). Next, we investigated whether BMP-9 also had negative effects on other types of LECs. The number of human lung lymphatic microvascular endothelial cells (HMVEC-LLy) was also decreased on stimulation by BMP-9 (SI Appendix, Fig. S4A). These results suggest that BMP-9 inhibits lymphangiogenesis by decreasing the number of multiple types of LECs.
Fig. 2.
Effects of BMP-9 on the number of lymphatic endothelial cells. (A) Morphological differences in HDLECs treated with BMP-9 for 24 h relative to control cells. (B) Number of HDLECs after 48 hours of treatment with BMP-9 compared with controls. (C and D) Quantification of MIB-1+ proliferating (C) and TUNEL+ apoptotic (D) HDLECs treated with BMP-9 vs. control.
BMP-9 Reduced the Number of HDLECs Through ALK-1.
Although Niessen et al. reported that the induction of SMAD6 expression by BMP-9 required ALK-1 in human microvascular dermal neonatal LECs, they did not describe whether BMP-9 changed the number of LECs (26). Therefore, we attempted to examine which type I receptor mediates the BMP-9 signals that reduce the number of HDLECs. BMP family members transduce their signals through receptor complexes that phosphorylate intracellular Smad proteins (14). We used semiquantitative RT-PCR analysis to study the expression profiles of TGF-β superfamily signaling components (SI Appendix, Fig. S5). To compare the expression of these signaling components in LECs and BECs, we used HDLECs and human dermal BECs (HDBECs) prepared from the same donor. HDLECs and HDBECs expressed transcripts for most components of the TGF-β superfamily signaling pathways, suggesting they are capable of transducing the signals mediated by TGF-βs, activins, and BMP members. To our interest, among the BMP-specific type I receptors (i.e., ALK-1, ALK-2, ALK-3, and ALK-6), only ALK-1 and ALK-2, both of which are reportedly activated by BMP-9 (22), were expressed in HDLECs and HDBECs. In accordance with these results, we observed that the addition of BMP-9 induced the phosphorylation of Smad1/5 at equivalent levels in HDLECs and HDBECs (SI Appendix, Fig. S4B). Phosphorylation of Smad1/5 by BMP-9 was also observed in HMVEC-LLy (SI Appendix, Fig. S4C), suggesting that BMP-9 activates BMP-specific intracellular Smad signals.
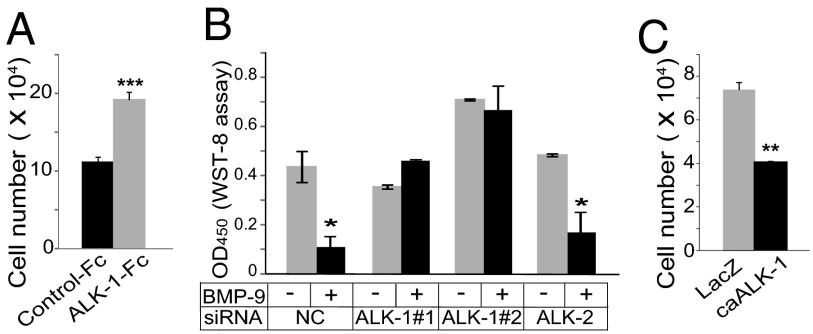
David et al. have shown that BMP-9 is present at a high level in serum, as the growth inhibitory effects of serum on BECs were canceled by an anti-BMP-9 neutralizing antibody (21). When an ALK-1-Fc decoy receptor was added to HDLEC culture in the presence of 5% (vol/vol) serum, the number of HDLECs grew significantly (Fig. 3A), suggesting that BMP-9 and/or BMP-10 in serum decreases the number of HDLECs.
Fig. 3.
Effects of ALK-1-mediated signals on the number of lymphatic endothelial cells. (A) Number of HDLECs after treatment with ALK-1-Fc for 48 h relative to control. (B) Number of HDLECs after treatment with siRNA for ALK-1 or ALK-2 for 48 h in the presence or absence of BMP-9 relative to control. NC, negative control. (C) Number of HDLECs infected with Ads encoding caALK-1 or LacZ for 48 h.
We next studied the physiological type I receptor through which BMP-9 signals elicit inhibitory effects on LEC proliferation. Previous reports have shown that BMP-9 binds both ALK-1 and ALK-2, both of which are expressed in HDLECs (SI Appendix, Fig. S5) (22). However, the receptor that is physiologically relevant to BMP-9-mediated signaling in HDLECs remains to be elucidated. When ALK-1 expression was knocked down by siRNAs in HDLECs, the BMP-9-induced expression of ID-1 was abrogated (SI Appendix, Fig. S6A). Furthermore, silencing ALK-1 expression also canceled the BMP-9-induced reduction of the number of HDLECs (Fig. 3B). In contrast, knocking down ALK-2 expression did not alter the expression of BMP-9 target genes or the number of HDLECs. These results suggest that ALK-1, but not ALK-2, is necessary for BMP-9-mediated signals in HDLECs. ALK-2 expression was also induced by BMP-9 via ALK-1 (SI Appendix, Fig. S6A).
Furthermore, when a constitutively active ALK-1 mutant (caALK-1) was adenovirally transduced into HDLECs (SI Appendix, Fig. S6B), the number of HDLECs fell in the absence of BMP-9 (Fig. 3C), suggesting that the activation of ALK-1 signals was sufficient to mimic the inhibitory effects of BMP-9 on the number of HDLECs. These results suggest that BMP-9 signals through ALK-1 to decrease the number of HDLECs.
BMP-9 Directly Downregulated PROX1 Expression via ALK-1.
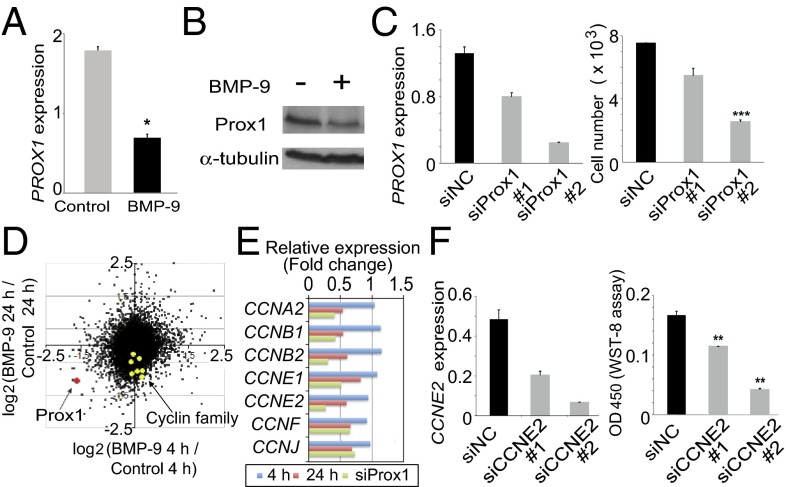
To screen for factors that are involved in the BMP-9-induced inhibition of HDLEC proliferation, we performed cDNA microarray analyses to investigate the genome-wide effects of BMP-9 on the HDLEC transcriptome profile (SI Appendix, Tables S1 and S2). Gene ontology analysis revealed that the top five gene ontology annotation clusters that corresponded to the early-response genes for BMP-9 treatment (4 h) included annotations associated with vascular development, transcriptional regulation, and BMP signaling (SI Appendix, Fig. S7A and Table S3). In contrast, the top five clusters that corresponded to the late-response genes for BMP-9 treatment (24 h) were associated with cell surface proteins, cytoskeletal regulation, cell cycle, and cell death (SI Appendix, Fig. S7B and Table S4). This result suggested that the BMP-9-induced modification of transcriptional programs that were involved in vascular development was in an early phase and subsequently caused phenotypic changes in HDLECs. Therefore, we hypothesized that BMP-9/ALK-1 signals directly modulate the expression of transcription factors that regulate the expression of cell-cycle-related factors. We identified PROX1 as one of the most downregulated genes of the early-response genes that reacted to BMP-9 treatment (SI Appendix, Fig. S7C and Table S1). We confirmed this finding at the RNA and protein levels, using quantitative RT-PCR analysis (Fig. 4A) and Western blotting (Fig. 4B), respectively.
Fig. 4.
Identification of Prox1 as a target of BMP-9/ALK-1 signaling and elucidation of its roles in regulating the number of HDLECs. (A and B) Prox1 mRNA (A) and protein (B) expression in HDLECs treated with BMP-9 for 4 h and 6 h, respectively. (C) PROX1 expression (Left) and HDLEC cell number (Right) after sham siRNA knockdown and Prox1 siRNA knockdown. (D) Scatter plot depicting the fold change of expression for every gene transcript in HDLECs treated with BMP-9 for 4 h and 24 h. The expression of PROX1 (red dot) and cyclin family members (yellow dots) are indicated. (E) Relative expression of several cyclin family members (yellow dots in D) among HDLECs treated with BMP-9 for 4 h and 24 h and treated with siRNA against Prox1. (F) Analysis of CYCLINE2 (CCNE2) expression (Left) and HDLEC cell number (Right) after sham siRNA knockdown (siNC) and CCNE2 siRNA knockdown (siCCNE2).
We next examined how BMP-9 directly downregulated PROX1 expression. Because the BMP-9-induced decrease in the expression of PROX1 and SMAD7, a direct target of BMP/Smad signals, was observed within 1 h of treatment (SI Appendix, Fig. S8A) and was not altered by the addition of cycloheximide (SI Appendix, Fig. S8B), PROX1 appeared to be directly regulated by BMP-9 without de novo protein synthesis. We also examined whether BMP-9 induced a decrease in PROX1 expression through ALK-1. When the expression of ALK-1, but not ALK-2, was knocked down by specific siRNAs, BMP-9 failed to reduce PROX1 expression (SI Appendix, Fig. S8C). Furthermore, the adenoviral expression of caALK-1 in HDLECs decreased PROX1 expression (SI Appendix, Fig. S8D). These results suggest that ALK-1 mediates the BMP-9-induced downregulation of PROX1 expression.
Silencing PROX1 Expression Reduced the Number of HDLECs.
To examine the causal relationship between BMP-9-induced decreases in cell number and reduced PROX1 expression, we knocked down PROX1 expression in HDLECs using siRNA and found that the cell number was decreased in the same manner as that observed with respect to BMP-9 (SI Appendix, Fig. S9). Furthermore, when two different Prox1-specific siRNAs that reduced PROX1 expression to different extents were used, the levels of PROX1 expression were correlated with the number of HDLECs (Fig. 4C). These results suggest that the effect of BMP-9 on the number of HDLECs results at least partly from the decreased expression of PROX1.
Decreased Expression of Cyclin Family Members by BMP-9 and Silencing of PROX1 Expression Were Involved in the BMP-9-induced Decrease in HDLEC Number.
Finding that treatment with BMP-9 and knockdown of PROX1 expression decreased the number of HDLECs prompted us to identify the factors whose expression is regulated by BMP-9 and Prox1. We performed cDNA microarray analysis using HDLECs transfected with control siRNA (siNC) or Prox1 siRNA (siProx1) and compared results using siProx1-treated or siNC-treated samples with results obtained from BMP-9-treated samples (SI Appendix, Tables S1, S2, and S5). We found that a number of genes were concomitantly decreased in both siProx1-treated samples and samples that had been treated with BMP-9 for 24 h. Consistent with the late effects of BMP-9 on cell-cycle-related genes in gene ontology analysis, we observed that some cyclin family genes were significantly suppressed by both the 24-h BMP-9 treatment and siProx1 treatment (Fig. 4 D and E).
Cyclin family members have been shown to regulate the cell cycle. In particular, the cyclin E2 gene (CYCLINE2, or CCNE2) has been reported as a target gene of Prox1 (5). To examine whether CCNE2 was a causative factor involved in the BMP-9-induced decrease in HDLEC number, we silenced CCNE2 expression in HDLECs, using siRNA. When CCNE2 expression decreased, the HDLEC number decreased in a dose-dependent manner (Fig. 4F). These results suggest that the reduction of Prox1 expression by BMP-9 leads to an alteration in cell-cycle-related gene expression, which in turn results in a decrease in the number of HDLECs.
BMP-9 Treatment and Prox1 Knockdown Each Induced the Reprogramming of LECs to BECs.
Several lines of evidence have suggested that endogenous Prox1 expression is necessary to maintain LEC identity (5–7). Johnson et al. reported that the loss of Prox1 expression not only reduced the expression of various LEC markers but also increased the expression of BEC markers (7). Furthermore, we reported that BMP-9 increases the expression of various BEC markers in various types of BECs (25). Taken together with the present finding that BMP-9 decreases Prox1 expression in LECs, we hypothesized that BMP-9 induces HDLECs to undergo phenotypic changes from the LEC phenotype to the BEC phenotype.
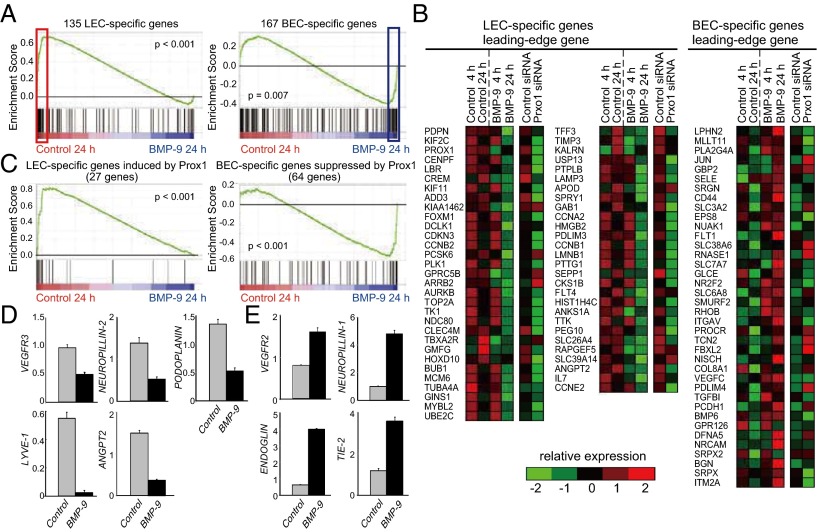
To test this possibility, we performed gene set enrichment analysis, using the list of BEC- and LEC-specific genes reported by Petrova et al. (5). This analysis demonstrated that the expression levels of most LEC-type genes were downregulated [false discovery rate (FDR) q < 0.001] and that many BEC-type genes were up-regulated as a result of BMP-9 treatment (FDR q = 0.002) (Fig. 5 A and B; SI Appendix, Table S6). Furthermore, BMP-9 more clearly suppressed and elevated the expression of Prox1-dependent LEC- and BEC-specific genes, respectively (Fig. 5 B and C), suggesting that the effects of BMP-9 on BEC- and LEC-specific genes may be largely attributable to the suppression of Prox1. Sixty-two of 167 LEC-type genes were downregulated, and 27 of 135 BEC-type genes were upregulated by the BMP-9 treatment, especially in 24-h observations (SI Appendix, Table S6). Representative genes that express well-known LEC markers (e.g., VEGFR3, ANGPT2, PODOPLANIN, NEUROPILIN-2, and LYVE-1) were downregulated (Fig. 5D), whereas those expressing well-known BEC markers (e.g., ENG, TIE-2, VEGFR2, and NEUROPILLIN-1) were upregulated (Fig. 5E). These results suggest that BMP-9 not only decreases the number of LECs but also induces the dedifferentiation of LECs to their ancestral BECs.
Fig. 5.
Effects of BMP-9 on the maintenance of lymphatic endothelial cell identity. (A) Gene set enrichment analysis of HDLECs treated with and without BMP-9 using the LEC-specific (Left) and BEC-specific (Right) gene sets. (B) A heat map representing the relative expression change in selected LEC-specific genes (Left and center) and selected BEC-specific genes (Right) in HDLECs treated with BMP-9 for 4 h and 24 h and treated with Prox1 siRNA. (C) Gene set enrichment analysis of HDLECs treated with and without BMP-9, using sets of LEC-specific (Left) and BEC-specific (Right) genes whose expression is regulated by Prox1. (D and E) The expression of representative LEC (D) and BEC (E) markers in HDLECs treated with BMP-9 for 24 h vs. control.
BMP-9 Inhibited Lymphangiogenesis in a Mouse Model of Chronic Aseptic Peritonitis.
Up to this point, we have shown that BMP-9/ALK-1 signals inhibit the proliferation of in vitro–cultured LECs and the in vivo formation of LVs under physiological conditions. Because lymphangiogenesis is observed and plays important roles in inflammation and cancer (1), we examined whether our findings were observed under such pathological conditions.
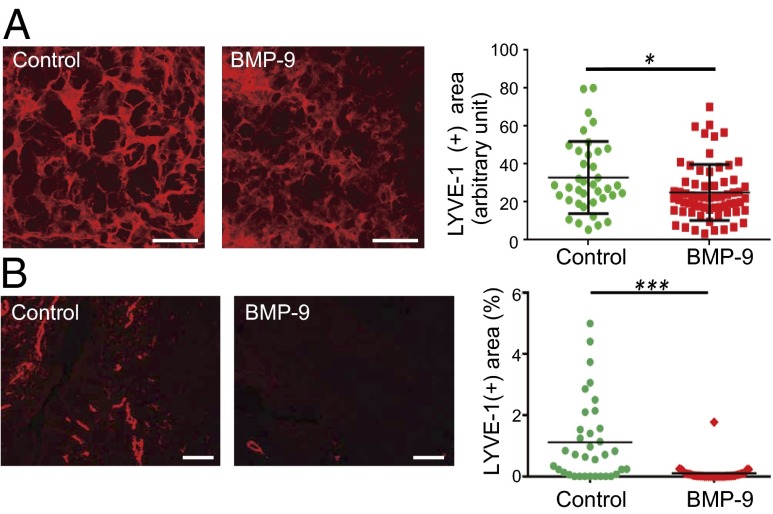
We used a mouse model of chronic aseptic peritonitis to examine whether BMP-9 inhibits inflammatory lymphangiogenesis (30). To examine the effects of BMP-9, adenoviruses (Ad) encoding lacZ (control) and BMP9 were intraperitoneally administered with thioglycollate medium (a proinflammatory agent) to immune-competent BALB/c mice. By day 16, inflammatory plaques consisting mainly of macrophages had formed on the peritoneal surface of the diaphragm. Diaphragms from killed mice were subjected to immunostaining for LYVE-1, a LEC marker. The area of LYVE-1-positive tissue was significantly diminished in the diaphragms of Ad-BMP9-injected mice compared with the control diaphragms of mice injected with Ad encoding lacZ (Fig. 6A). This result suggests that BMP-9 inhibits inflammatory lymphangiogenesis in vivo.
Fig. 6.
Roles of BMP-9 in inflammatory and tumor lymphangiogenesis. (A, left) Images of the thioglycollate-induced formation of LVs in the peritoneal surface of murine diaphragms that were adenovirally injected with lacZ (Control) or BMP9. Scale bars, 100 μm. (A, right) Quantification of the relative area of newly formed LVs in the diaphragm. (B, left) Images of the formation of LVs in tumors derived from allografted murine breast carcinomas expressing GFP (control) or BMP9. (B, right) Quantification of the relative area of LVs in these tumors. Scale bars, 100 μm.
BMP-9 Inhibited Tumor Lymphangiogenesis in Models of Mouse Breast Cancer Allografts.
We used a mouse breast cancer allograft model that employs 4T1 cells to study the roles of BMP-9 in tumor lymphangiogenesis. We established murine 4T1 breast carcinoma cells that stably expressed GFP (control) or BMP9 transgenes. We confirmed that expression of the BMP9 transgene did not affect the rate of 4T1 cell proliferation in vitro (SI Appendix, Fig. S10). Both of these cell lines were s.c. inoculated into immunocompromised BALB/c nude mice to obtain tumors. As we previously reported in a mouse xenograft model of human pancreatic BxPC3 cancer, expression of BMP-9 increased the density of blood vessels (SI Appendix, Fig. S11), suggesting that BMP-9 induced tumor angiogenesis in this breast cancer allograft model. In contrast, as shown in Fig. 6B, tumor LVs that stained positive for LYVE-1 were significantly decreased by treatment with BMP-9. These results suggest that BMP-9 inhibits lymphangiogenesis in tumors.
Discussion
In the present study, we have demonstrated that BMP-9/ALK-1 signaling inhibits lymphangiogenesis under both physiological and pathological conditions. We previously reported that BMP-9/ALK-1 signals induced the proliferation of BECs by inducing the expression of VEGFR2 and TIE-2, both of which induce BEC proliferation (25). It is of interest to note that this induction of VEGFR2 and TIE-2 by BMP-9 was observed in LECs (Fig. 5E). These results suggest that BMP-9/ALK-1 signals induce the expression of common target genes that activate the BEC program in both BECs and LECs. However, BMP-9 elicited opposite effects in LECs. We reasoned that these differential effects of BMP-9 on BECs and LECs were caused by the expression of Prox1 in LECs. BMP-9 downregulated Prox1 expression (Fig. 4; SI Appendix, Fig. S12), which led to the inhibition of LEC proliferation (Fig. 4; SI Appendix, Fig. S12) and the dedifferentiation of LECs to BECs (Fig. 5; SI Appendix, Fig. S12). In summary, the inhibitory effects of BMP-9/ALK-1 signaling to inactivate the LEC program won vs activation of the BEC program (SI Appendix, Fig. S12). In agreement with this model, the inhibitory effects of BMP-9 on LECs were partially canceled when HDLECs were treated with BMP-9 in combination with VEGF-A (a ligand of VEGFR2) (SI Appendix, Fig. S13).
Although we showed that BMP-9 decreases the number of LECs through the downregulation of Prox1 expression, the BMP-9-induced reduction in the expression of other proliferation-related factors, including C-MYC, and antiapoptotic factors, including BCLXL/BCL2L1, was independent of Prox1; silencing PROX1 expression did not alter their expression (SI Appendix, Tables S1 and S5). These results suggest that BMP-9 decreases the LEC population through Prox1-dependent and Prox1-independent mechanisms (SI Appendix, Fig. S12).
Niessen et al. studied the roles of ALK-1 signaling in the formation of LVs by systemic injection of neonatal mice with an ALK-1-Fc decoy receptor or anti-ALK-1 neutralizing antibody to inhibit ALK-1 signaling, which resulted in defective lymphatic development in multiple organs (26). Although they observed that the blockade of ALK-1 signals led to the disorganization of lymphatic structures, they did not describe whether LVs were enlarged. The variance from our finding that deletion of the ALK-1 gene results in the enlargement of LVs in multiple organs (Fig. 1) may have been caused by differences in the method used to inhibit ALK-1 signaling. Furthermore, this variance may also have been caused by the contribution of BMP-10, which is another physiological ligand of ALK-1 (29). Although Bmp10-deficient mice exhibit severe defects in cardiac tissues, no phenotypes in vascular systems have been described in Bmp10-deficient mice (27), suggesting that the physiological roles of BMP-10 may be limited to the cardiac development. When BMP-10 was added to the culture of HDLECs, their number was decreased with the decrease in the Prox1 expression (SI Appendix, Fig. S14), suggesting that BMP-10 is capable of negatively regulating the formation of LVs in a similar manner as BMP-9. Because the expression of BMP-10 is restricted to heart and developmentally regulated, the contribution of BMP-10 in combination with BMP-9 to embryonic and postnatal lymphangiogenesis needs to be studied, using Bmp10-deficient mice.
TGF-β activates the ALK-5 type I receptor, which induces the phosphorylation of Smad2/3 and their nuclear translocation with Smad4 and inhibits the proliferation of vascular endothelial cells in vitro (13). In lens epithelium, nuclear accumulation of Smad4 has been implicated in the regulation of Prox1 expression (31). In addition, we previously reported that TGF-β also inhibited the proliferation of HDLECs in vitro and lymphangiogenesis in vivo (12). Notably, TGF-β also downregulates PROX1 expression in HDLECs. It is of interest whether or not this decrease in PROX1 expression is involved in the TGF-β-induced inhibition of lymphangiogenesis. Furthermore, the molecular mechanisms of how two distinct signaling pathways that are mediated by Smad1/5/8 and Smad2/3 regulate the PROX1 expression need to be elucidated in the future.
BMP-9/ALK-1 signals have been implicated in various human diseases. Mutations in the ALK1 gene cause HHT2 (16). LV dysfunction has not been reported in HHT patients. Because HHT is an autosomal dominant disorder, the mutation of one copy of the ALK1 gene may not be enough to cause the lymphatic phenotypes observed in the present study. Furthermore, we observed that the formation of LVs decreased in BMP-9-expressing tumors, whereas the formation of BVs increased. The expression of BMP-9 in various types of tumors has been reported previously. In ovarian tumors, BMP-9 expression was shown to be elevated, which led to the promotion of tumor cell proliferation in an autocrine fashion (32). In contrast, BMP-9 expression was reportedly decreased or absent in prostate cancer (33). Because BMP-9 induces apoptosis in prostate carcinoma cells, the effects of BMP-9 appear to be context-dependent. It will be of great interest to study whether LV density differs among tumor specimens that express different levels of BMP-9. Recently, BMP-9/ALK-1 signals have been attracting attention as a target of cancer therapy, as the inhibition of ALK-1 signaling interferes with tumor angiogenesis, which may inhibit tumor growth (24, 34). Because the formation of LVs in tumors is associated with lymph node metastases in cancer, it is of interest to know whether blocking ALK-1 signals might influence tumor lymphangiogenesis.
Materials and Methods
HDLECs and HMVEC-LLy were purchased from Lonza and characterized (SI Appendix, Fig. S15). The 4T1 murine breast carcinoma cell line was obtained from the American Type Culture Collection. 293FT cells were obtained from Invitrogen. More information is provided in SI Appendix, Materials and Methods and SI Appendix, Table S7.
Supplementary Material
Acknowledgments
We thank Dr. Masanori Hirashima at Kobe University for fruitful discussion and technical advice; Mr. Yasuyuki Morishita, Ms. Keiko Yuki, Ms. Michiko Arai, and Mr. Taichi Itoh for their technical assistance; and members of the Department of Molecular Pathology for their useful discussions. This research was supported by KAKENHI (Grant-in-Aid for Scientific Research) on Innovative Areas, Integrative Research on Cancer Microenvironment Network (22112002) and Cellular and Molecular Basis for Neuro-vascular Wiring (23122504) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, Japan Society for the Promotion of Science Core-to-Core Program “Cooperative International Framework in TGF-β Family Signaling” and a Research Grant from the Takeda Science Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 18746.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310479110/-/DCSupplemental.
References
- 1.Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- 2.Oliver G, Srinivasan RS. Endothelial cell plasticity: How to become and remain a lymphatic endothelial cell. Development. 2010;137(3):363–372. doi: 10.1242/dev.035360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karkkainen MJ, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5(1):74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 4.Wigle JT, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21(7):1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrova TV, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21(17):4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishima K, et al. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol Biol Cell. 2007;18(4):1421–1429. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson NC, et al. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008;22(23):3282–3291. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maruyama K, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115(9):2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y, et al. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64(11):3737–3740. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 10.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140(4):460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 11.Kataru RP, et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011;34(1):96–107. doi: 10.1016/j.immuni.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Oka M, et al. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood. 2008;111(9):4571–4579. doi: 10.1182/blood-2007-10-120337. [DOI] [PubMed] [Google Scholar]
- 13. Derynck R, Miyazono K (2008) TGF-β and the TGF-β family, Cold Spring Harbor Monograph Series, eds Derynck R, Miyazono K (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY)
- 14.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147(1):35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 15.McAllister KA, et al. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8(4):345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 16.Johnson DW, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13(2):189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 17.Gallione CJ, et al. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4) Lancet. 2004;363(9412):852–859. doi: 10.1016/S0140-6736(04)15732-2. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan S, et al. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet. 2003;12(5):473–482. doi: 10.1093/hmg/ddg050. [DOI] [PubMed] [Google Scholar]
- 19.Park SO, et al. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood. 2008;111(2):633–642. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SO, et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest. 2009;119(11):3487–3496. doi: 10.1172/JCI39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David L, et al. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102(8):914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scharpfenecker M, et al. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120(Pt 6):964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 23.Larrivée B, et al. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell. 2012;22(3):489–500. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunha SI, et al. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp Med. 2010;207(1):85–100. doi: 10.1084/jem.20091309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki Y, et al. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci. 2010;123(Pt 10):1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 26.Niessen K, Zhang G, Ridgway JB, Chen H, Yan M. ALK1 signaling regulates early postnatal lymphatic vessel development. Blood. 2010;115(8):1654–1661. doi: 10.1182/blood-2009-07-235655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pashmforoush M, et al. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell. 2004;117(3):373–386. doi: 10.1016/s0092-8674(04)00405-2. [DOI] [PubMed] [Google Scholar]
- 28.Brown MA, et al. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J Biol Chem. 2005;280(26):25111–25118. doi: 10.1074/jbc.M503328200. [DOI] [PubMed] [Google Scholar]
- 29.Ricard N, et al. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119(25):6162–6171. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimatsu Y, et al. Ets family members induce lymphangiogenesis through physical and functional interaction with Prox1. J Cell Sci. 2011;124(Pt 16):2753–2762. doi: 10.1242/jcs.083998. [DOI] [PubMed] [Google Scholar]
- 31.Simirskii VN, Wang Y, Duncan MK. Conditional deletion of β1-integrin from the developing lens leads to loss of the lens epithelial phenotype. Dev Biol. 2007;306(2):658–668. doi: 10.1016/j.ydbio.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrera B, van Dinther M, Ten Dijke P, Inman GJ. Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res. 2009;69(24):9254–9262. doi: 10.1158/0008-5472.CAN-09-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye L, Kynaston H, Jiang WG. Bone morphogenetic protein-9 induces apoptosis in prostate cancer cells, the role of prostate apoptosis response-4. Mol Cancer Res. 2008;6(10):1594–1606. doi: 10.1158/1541-7786.MCR-08-0171. [DOI] [PubMed] [Google Scholar]
- 34.Cunha SI, Pietras K. ALK1 as an emerging target for antiangiogenic therapy of cancer. Blood. 2011;117(26):6999–7006. doi: 10.1182/blood-2011-01-330142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.