Abstract
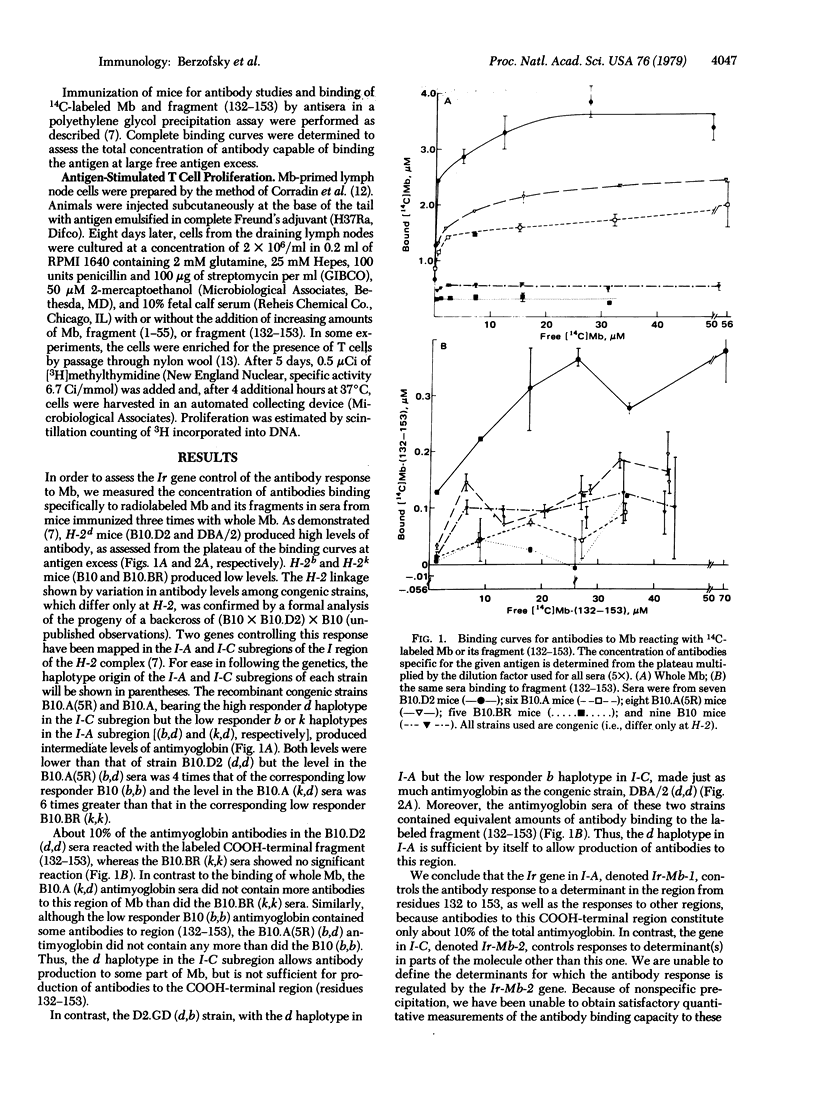
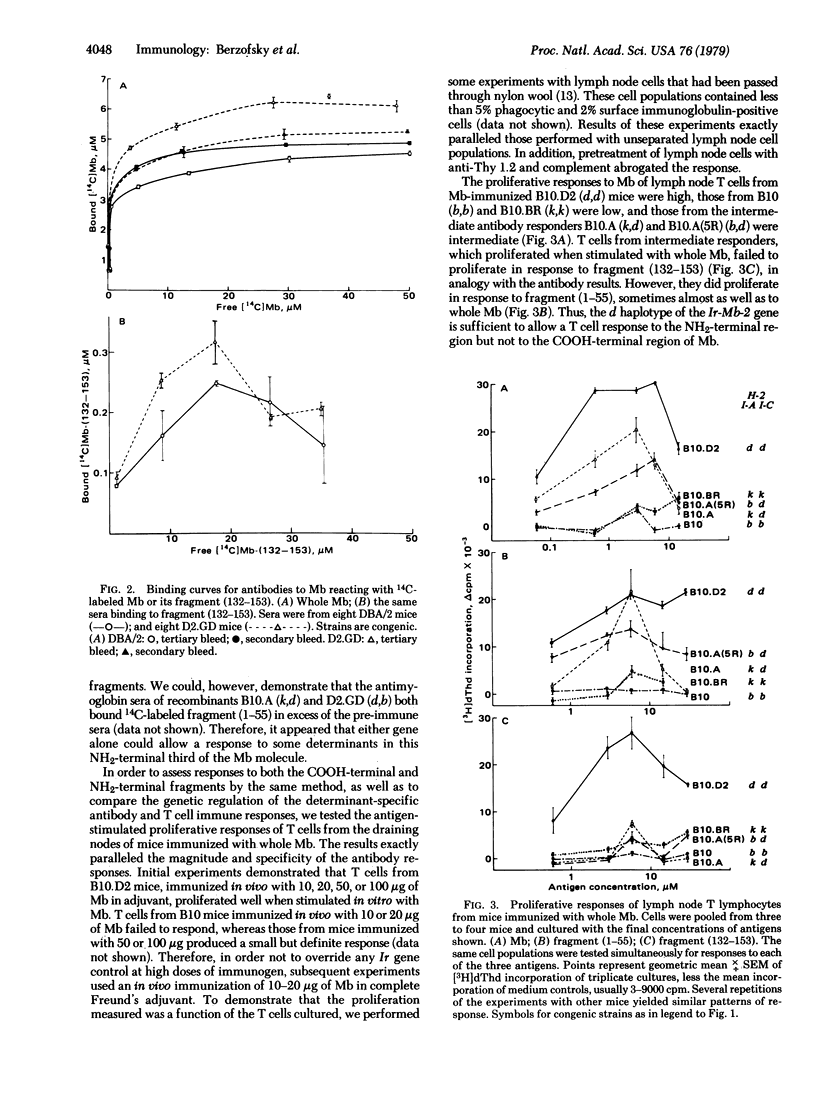
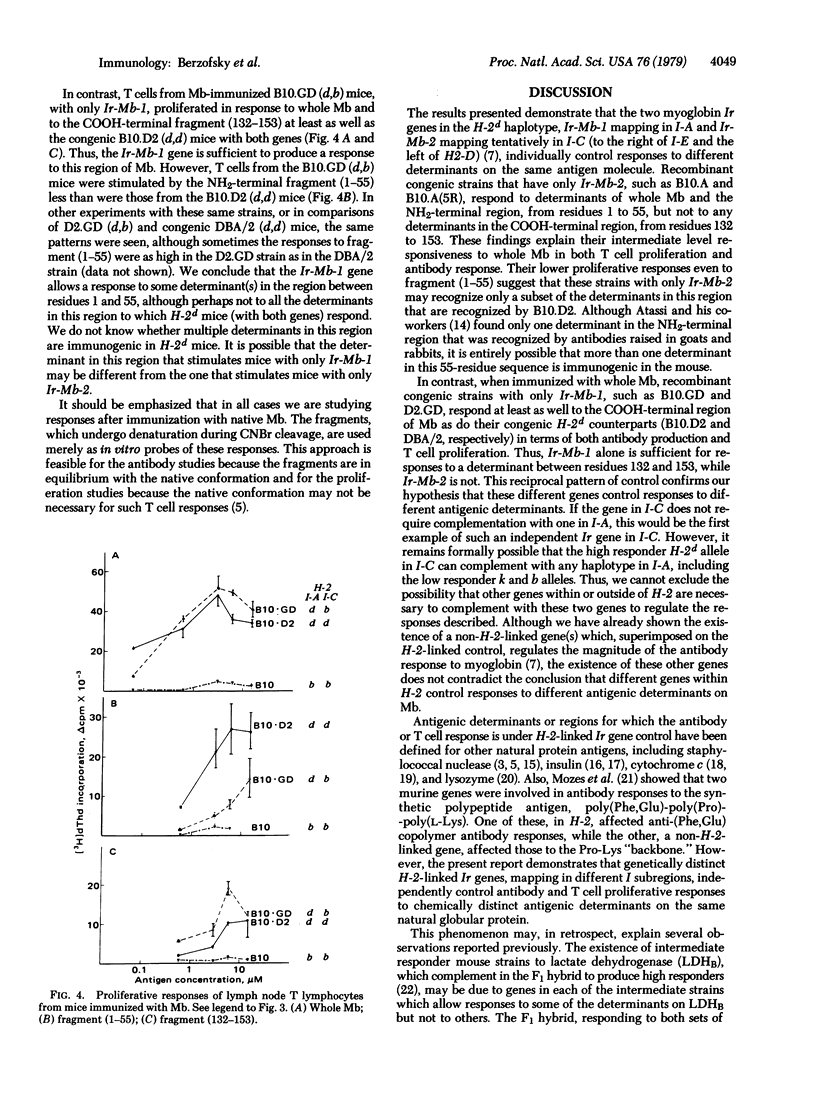
The murine antibody and T lymphocyte proliferative responses to sperm whale myoglobin (Mb) were found to be under control of two distinct H-2-linked immune response (Ir) genes (Ir-Mb-1, mapping in the I-A subregion, and Ir-Mb-2, mapping in I-C). H-2d mice (B10.D2 and DBA/2), with both genes, were high responders to Mb and its fragments for both antibody secretion and T cell proliferation, while H-2b (B10) and H-2k (B10.BR) mice were low responders. Strains with only Ir-Mb-2 [B10.A and B10.A(5R)], which were intermediate responders to Mb, made antibodies to and proliferated in response to the NH2-terminal fragment (1-55) but not the COOH-terminal fragment (132-153) when immunized with Mb. In contrast, mice carrying only the Ir-Mb-1 gene (D2.GD and B10.GD) made antibodies to and proliferated in response to both fragments. However, their proliferation to fragment (1-55) was often lower than that of their congenic high responders (DBA/2 and B10.D2, respectively), possibly because they respond to only some of the determinants on this NH2-terminal fragment. Thus, these data demonstrate that distinct Ir genes, mapping in separate I-subregions of H-2, control responses to different antigenic determinants on the same protein molecule. Moreover, the gene that controls the T lymphocyte responses to a given determinant also controls production of antibodies specific for that same determinant (or a closely associated one).
Keywords: major histocompatibility complex, immune response, genetics
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z. Antigenic structure of myoglobin: the complete immunochemical anatomy of a protein and conclusions relating to antigenic structures of proteins. Immunochemistry. 1975 May;12(5):423–438. doi: 10.1016/0019-2791(75)90010-5. [DOI] [PubMed] [Google Scholar]
- Barcinski M. A., Rosenthal A. S. Immune response gene control of determinant selection. I. Intramolecular mapping of the immunogenic sites on insulin recognized by guinea pig T and B cells. J Exp Med. 1977 Mar 1;145(3):726–742. doi: 10.1084/jem.145.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benacerraf B., Katz D. H. The histocompatibility-linked immune response genes. Adv Cancer Res. 1975;21:121–173. doi: 10.1016/s0065-230x(08)60972-0. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Genetic control of the antibody response to sperm whale myoglobin in mice. Adv Exp Med Biol. 1978;98:225–239. doi: 10.1007/978-1-4615-8858-0_12. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Genetic control of the immune response to mammalian myoglobins in mice I. More than one I-region gene in H-2 controls the antibody response. J Immunol. 1978 Feb;120(2):360–369. [PubMed] [Google Scholar]
- Berzofsky J. A., Pisetsky D. S., Schwartz R. H., Schechter A. N., Sachs D. H. Genetic control of the immune response to staphylococcal nuclease in mice. Adv Exp Med Biol. 1978;98:241–257. doi: 10.1007/978-1-4615-8858-0_13. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A., Schechter A. N., Shearer G. M., Sachs D. H. Genetic control of the immune response to staphylococcal nuclease. III. Time-course and correlation between the response to native nuclease and the response to its polypeptide fragments. J Exp Med. 1977 Jan 1;145(1):111–122. doi: 10.1084/jem.145.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzofsky J. A., Schechter A. N., Shearer G. M., Sachs D. H. Genetic control of the immune response to staphylococcal nuclease. IV. H-2-linked control of the relative proportions of antibodies produced to different determinants of native nuclease. J Exp Med. 1977 Jan 1;145(1):123–135. doi: 10.1084/jem.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Chiller J. M. Lymphocyte specificity to protein antigens. II. Fine specificity of T-cell activation with cytochrome c and derived peptides as antigenic probes. J Exp Med. 1979 Feb 1;149(2):436–447. doi: 10.1084/jem.149.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Complementation of H-2-linked Ir genes in the mouse. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3671–3675. doi: 10.1073/pnas.72.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELL P. G., BENACERRAF B. Studies on hypersensitivity. II. Delayed hypersensitivity to denatured proteins in guinea pigs. Immunology. 1959 Jan;2(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- Hill S. W., Sercarz E. E. Fine specificity of the H-2 linked immune response gene for the gallinaceous lysozymes. Eur J Immunol. 1975 May;5(5):317–324. doi: 10.1002/eji.1830050506. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Keck K. Ir-gene control of immunogenicity of insulin and A-chain loop as a carrier determinant. Nature. 1975 Mar 6;254(5495):78–79. doi: 10.1038/254078a0. [DOI] [PubMed] [Google Scholar]
- Marshall R. C., Jones W. C., Jr, Vigna R. A., Gurd F. R. Isolation of cyanogen bromide cleavage peptides from myoglobins. Z Naturforsch C. 1974 Jan-Feb;29(1):90–91. [PubMed] [Google Scholar]
- McDevitt H. O., Benacerraf B. Genetic control of specific immune responses. Adv Immunol. 1969;11:31–74. doi: 10.1016/s0065-2776(08)60477-0. [DOI] [PubMed] [Google Scholar]
- Melchers I., Rajewsky K. Specific control of responsiveness by two complementing Ir loci in the H-2 complex. Eur J Immunol. 1975 Nov;5(11):753–759. doi: 10.1002/eji.1830051105. [DOI] [PubMed] [Google Scholar]
- Mozes E., McDevitt H. O., Jaton J. C., Sela M. The genetic control of antibody specificity. J Exp Med. 1969 Dec 1;130(6):1263–1278. doi: 10.1084/jem.130.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S. Determinant selection and macrophage function in genetic control of the immune response. Immunol Rev. 1978;40:136–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A clonal deletion model for Ir gene control of the immune response. Scand J Immunol. 1978;7(1):3–10. doi: 10.1111/j.1365-3083.1978.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Berzofsky J. A., Horton C. L., Schechter A. N., Sachs D. H. Genetic control of the T lymphocyte proliferative response to staphylococcal nuclease: evidence for multiple MHC-linked Ir gene control. J Immunol. 1978 May;120(5):1741–1749. [PubMed] [Google Scholar]
- Schwartz R. H., Solinger A. M., Ultee M., Margoliash E. Genetic control of the T-lymphocyte proliferative response to cytochrome c. Adv Exp Med Biol. 1978;98:371–386. doi: 10.1007/978-1-4615-8858-0_20. [DOI] [PubMed] [Google Scholar]
- Yamashita U., Shevach E. M. The histocompatibility restrictions on macrophage T-helper cell interaction determine the histocompatibility restrictions on T-helper cell B-cell interaction. J Exp Med. 1978 Nov 1;148(5):1171–1185. doi: 10.1084/jem.148.5.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]



