Significance
The present study shows preferential activity of neurons in the medial and dorsolateral pulvinar to images of snakes. Pulvinar neurons responded faster and stronger to snake stimuli than to monkey faces, monkey hands, and geometric shapes, and were sensitive to unmodified and low-pass filtered images but not to high-pass filtered images. These results identify a neurobiological substrate for rapid detection of threatening visual stimuli in primates. Our findings are unique in providing neuroscientific evidence in support of the Snake Detection Theory, which posits that the threat of snakes strongly influenced the evolution of the primate brain. This finding may have great impact on our understanding of the evolution of primates.
Keywords: evolution, Snake Detection Theory, visual responses, low-pass filtered images
Abstract
Snakes and their relationships with humans and other primates have attracted broad attention from multiple fields of study, but not, surprisingly, from neuroscience, despite the involvement of the visual system and strong behavioral and physiological evidence that humans and other primates can detect snakes faster than innocuous objects. Here, we report the existence of neurons in the primate medial and dorsolateral pulvinar that respond selectively to visual images of snakes. Compared with three other categories of stimuli (monkey faces, monkey hands, and geometrical shapes), snakes elicited the strongest, fastest responses, and the responses were not reduced by low spatial filtering. These findings integrate neuroscience with evolutionary biology, anthropology, psychology, herpetology, and primatology by identifying a neurobiological basis for primates’ heightened visual sensitivity to snakes, and adding a crucial component to the growing evolutionary perspective that snakes have long shaped our primate lineage.
Snakes have long been of interest to us above and beyond the attention we give to other wild animals. The attributes of snakes and our relationships with them have been topics of discussion in fields as disparate as religion, philosophy, anthropology, psychology, primatology, and herpetology (1, 2). Ochre and eggshells dated to as early as 75,000 y ago and found with cross-hatched and ladder-shaped lines (3, 4) resemble the dorsal and ventral scale patterns of snakes. As the only natural objects with those characteristics, snakes may have been among the first models used in representational imagery created by modern humans. Our interest in snakes may have originated much further back in time; our primate lineage has had a long and complex evolutionary history with snakes as competitors, predators, and prey (1). The position of primates as prey of snakes has, in fact, been argued to have constituted strong selection favoring the evolution of the ability to detect snakes quickly as a means of avoiding them, beginning with the earliest primates (2, 5). Across primate species, ages, and (human) cultures, snakes are indeed detected visually more quickly than innocuous stimuli, even in cluttered scenes (6–11). Physiological responses reveal that humans are also able to detect snakes visually even before becoming consciously aware of them (12). Although the visual system must be involved in the preferential ability to detect snakes rapidly and preconsciously or automatically, the neurological basis for this ability has not yet been elucidated, perhaps because an evolutionary perspective is rarely incorporated in neuroscientific studies. Our study helps to fill this interdisciplinary gap by investigating the responses of neurons to snakes and other natural stimuli that may have acted as selective pressures on primates in the past.
Here, we identify a mechanism for the visual system’s involvement in rapid snake detection by measuring neuronal responses in the medial and dorsolateral pulvinar to images of snakes, faces of monkeys, hands of monkeys, and geometric shapes in a catarrhine primate, Macaca fuscata. The medial and the dorsal part of the traditionally delimited lateral pulvinar are distinctive in primates, with no homologous structures found in the visual systems of nonprimate mammals (13), and the medial pulvinar appears to be involved in visual attention and fast processing of threatening images (14). Based on this and other indirect evidence, the Snake Detection Theory (2) hypothesized that these primate-specific regions of the pulvinar evolved in part to assist primates in detecting and thus avoiding snakes. If true, then we would expect snake-sensitive neurons to be found in those regions. Here we present unique neuroscientific evidence in support of the snake detection theory (2).
Results
Preferential Responses to Snakes.
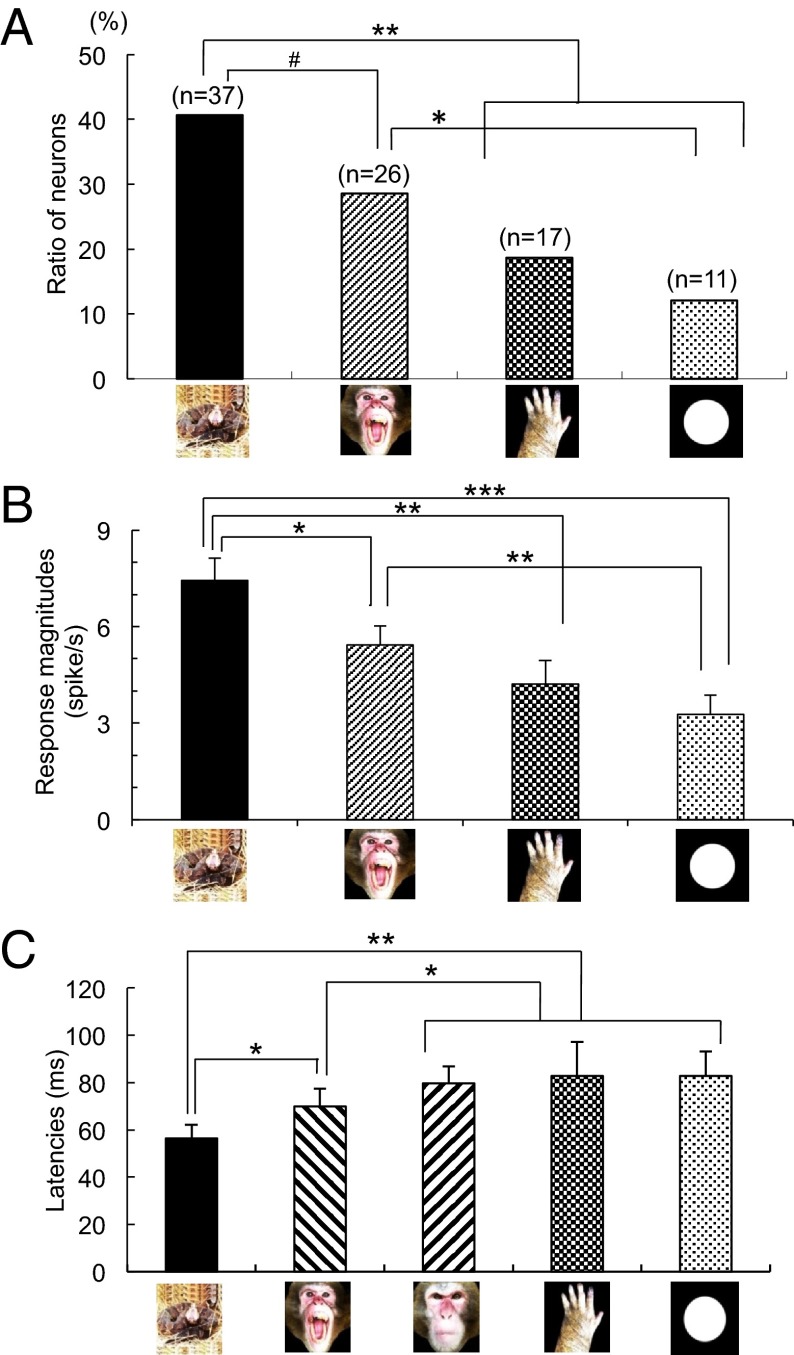
Of 745 pulvinar neurons recorded, 105 (14.1%) responded to at least one of the visual stimuli. Of these, 91 neurons were tested with all stimuli. These neurons responded differentially to the categories of visual stimuli. The pulvinar neurons were categorized by the stimulus that elicited the largest responses. “Snake-best” neurons were defined as those in which the mean response to all snake images was the largest among the four stimulus categories. “Face-best,” “hand-best,” and “simple geometrical shape-best” neurons were similarly defined for their respective images. Of the 91 neurons tested, snake-best neurons were most common (n = 37; 40.6%), followed by face-best neurons (n = 26; 28.6%), hand-best neurons (n = 17; 18.7%), and simple geometrical shape-best neurons (n =11; 12.1%) (Fig. 1A).
Fig. 1.
Neurophysiological characteristics of pulvinar neurons. (A) Ratio of the neurons that responded best to each stimulus category. **P < 0.01, *P < 0.05, #P < 0.10, significant difference (χ2 test). (B) Mean response magnitude to each stimulus category. ***P < 0.001, **P < 0.01, *P < 0.05, significant difference (Bonferroni test after one-way ANOVA). (C) Mean response latency to each stimulus category. **P < 0.01, *P < 0.05, significant difference (Bonferroni test after one-way ANOVA).
The proportion of snake-best neurons was significantly larger than those of hand- and simple geometrical figure-best neurons (χ2 tests, P < 0.01), and tended to be larger than that of face-best neurons (χ2 test, P < 0.10). The proportion of face-best neurons was significantly larger than that of simple geometrical shape-best neurons (χ2 test, P < 0.05).
There were also significant differences in mean response magnitudes to the four stimulus categories [repeated-measures one-way ANOVA; F(1, 90) = 101.096, P < 0.001] (Fig. 1B). Post hoc multiple comparisons indicated that the mean response was significantly greater to snakes than to other stimulus categories (Bonferroni test, P < 0.05), and that the mean response magnitude was significantly larger to faces than to simple geometrical shapes (Bonferroni test, P < 0.01). Differential responses of pulvinar neurons cannot be ascribed to luminance variations in this study because all stimuli used were controlled for equal luminance and size except the simple geometrical shapes (Materials and Methods). Furthermore, image scrambling decreased the selective responses to these stimuli (see below), suggesting that these responses were not attributed to local textures, but to the coherent images.
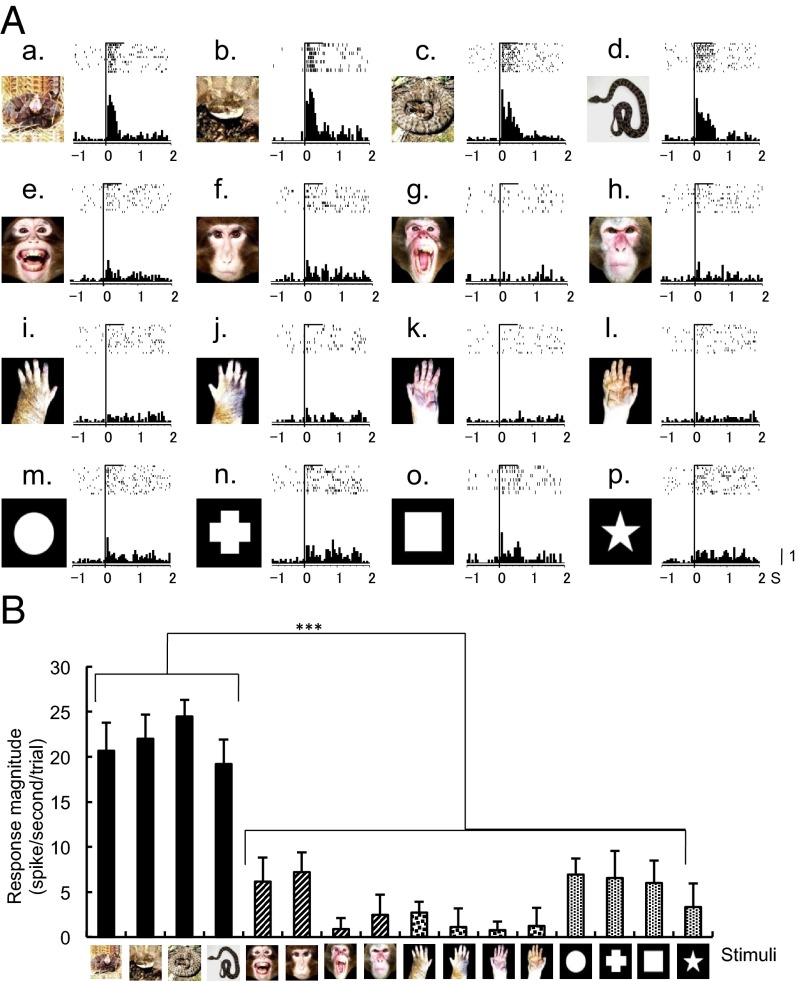
Fig. 2A shows an example of a neuron that responded selectively to snakes. This neuron responded strongly to all four snake images (Fig. 2A, a–d) and less to other stimuli (Fig. 2A, e–p). Fig. 2B shows response magnitudes of this neuron to all visual stimuli. There was a significant difference among the response magnitudes [one-way ANOVA; F(15, 177) = 13.81, P < 0.001]. Post hoc multiple comparisons indicated that the response magnitudes were significantly larger to the snakes than to the other stimuli for this neuron (Tukey test, P < 0.001). We further analyzed whether shapes of the four snake images (coiled or uncoiled) affected the responses using the 91 pulvinar neurons (Fig. S1). There was no significant difference in mean response magnitudes between the coiled and uncoiled snake images (paired t test, P > 0.05).
Fig. 2.
An example of a pulvinar neuron that responded most strongly to snakes. (A, a–p). Raster displays of neuronal activities and their summed histograms in response to each stimulus. (a–d) responses to snakes, (e–h) responses to monkey faces, (i–l) responses to monkey hands, and (m–p) responses to simple geometrical shapes. Horizontal bars above the raster displays indicate the stimulus presentation periods (500 ms). Vertical line in each of the raster displays and histograms indicates the stimulus onset. Calibration at the right bottom of the figure indicates the number of spikes per trial in each bin. Bin width = 50 ms. (B) Response magnitudes of the neuron shown in A to the 16 visual stimuli. The neuron responded most strongly to the snakes (***Tukey test after one-way ANOVA, P < 0.001).
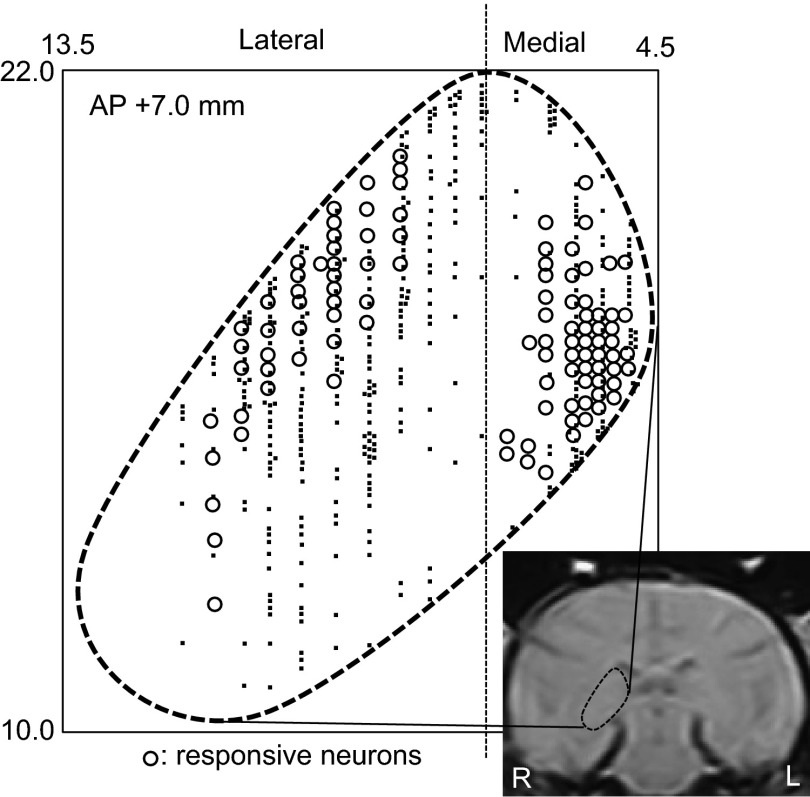
Most pulvinar neurons responding to the visual stimuli (Fig. 3, open circles) were located in the medial (medial pulvinar) and dorsolateral (lateral pulvinar) parts of the pulvinar. There was no significant difference in the ratio of the neurons responding to the visual stimuli between these two parts of the pulvinar (χ2 tests, P > 0.05).
Fig. 3.
Stereotaxic plots of the pulvinar neurons on the MRI photo of the monkey brain. The 745 pulvinar neurons were recorded from AP 8.0 to AP 5.0, but plotted on the plane at AP 7.0. The number in the left upper corner indicates the distance (in millimeters) anteriorly from the interaural line. The horizontal axis indicates the distance (in millimeters) from the midline; vertical axis indicates the distance (in millimeters) from the interaural line. Open circles, visually responsive neurons; dots, nonresponsive neurons.
Response Latencies of the Pulvinar Neurons.
Latencies of pulvinar neuronal responses ranged from 30 to 450 ms. The distribution of the latencies formed two peaks: a short latency group (30–120 ms) and a long latency group (170–450 ms). Mean latency of the short latency group was 60.6 ± 2.8 ms, whereas mean latency of the long latency group was 253.5 ± 26.7 ms. In the short latency group (Fig. 1C), the mean response latency to snakes was very short (55.4 ± 3.4 ms), and was significantly shorter than response latencies to angry faces, neutral faces, hands, and simple geometrical shapes (Bonferroni test after repeated measures one-way ANOVA, P < 0.05). There was also a significant difference between angry faces and the emotionally nonarousing neutral faces, hands, and simple geometrical shapes (Bonferroni test after repeated measures one-way ANOVA, P < 0.05).
Responses to the First Stimuli.
In this study, visual stimuli in the same categories were presented in the same blocks. Therefore, habituation to the visual stimuli could potentially occur through repetition of the stimuli of the same categories. To avoid a potential confounding habituation effect, we also analyzed the responses to only the first visual stimulus of each block. Statistical comparison indicated that similar results were obtained (Fig. S2). There were significant differences in mean response magnitudes to the four stimulus categories [repeated-measures one-way ANOVA; F(1, 90) = 31.725, P < 0.001] (Fig. S2A). Post hoc multiple comparisons indicated that the mean response was significantly greater to snakes than to other stimulus categories (Bonferroni test, P < 0.05). Furthermore, there were significant differences in mean response latencies to the four stimulus categories [repeated measures one-way ANOVA; F(1, 78) = 178.1, P < 0.001] (Fig. S2B). Post hoc multiple comparisons indicated that the mean response to snakes was significantly shorter than to other stimulus categories (Bonferroni test, P < 0.05).
Effects of Image Transformation.
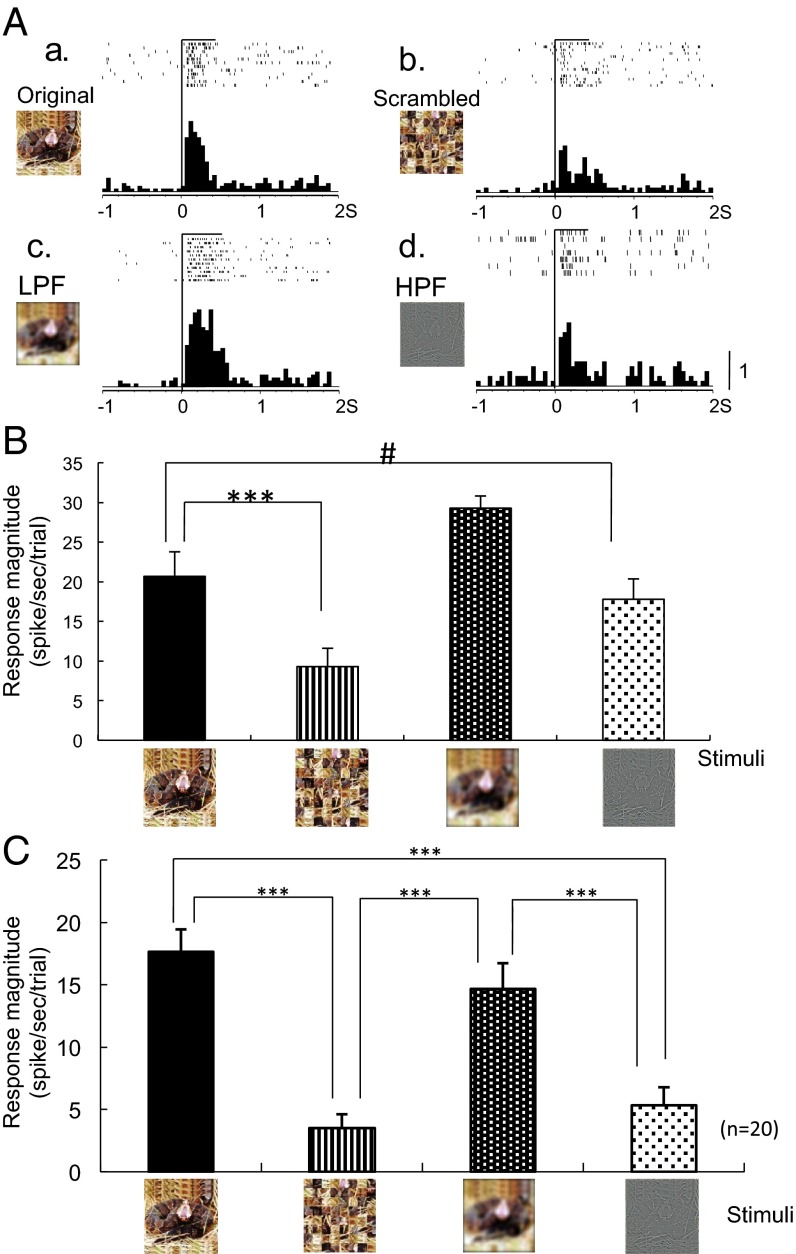
Fig. 4A shows an example of a neuron responding to scrambled and filtered images. This neuron responded strongly to the original snake image (Fig. 4A, a). Although low-pass filtering (LPF) did not affect the neuronal firing to the snake image (Fig. 4A, c), scrambling and high-pass filtering (HPF) decreased it (Fig. 4A, b and d). There was a significant difference among the response magnitudes to these stimuli [one-way ANOVA; F(3, 42) = 11.729, P < 0.001] (Fig. 4B). Post hoc multiple comparisons indicated that scrambling significantly decreased (Tukey test, P < 0.001) and HPF tended to decrease the response magnitudes to the snake image (Tukey test, P < 0.10).
Fig. 4.
Effects of scrambling and filtering of the images. (A) An example of neuronal responses to the original (a), scrambled (b), and filtered [c (LPF), d (HPF)] images (same neuron shown in Fig. 1). (B) Response magnitudes to the stimuli shown in A. Scrambling significantly decreased (***Tukey test after one-way ANOVA, P < 0.001) and HPF tended to decrease the responses to the original image (Tukey test after one-way ANOVA, P < 0. 10). (C) Mean response magnitudes to the scrambled and filtered images (n = 20). Scrambling and HPF significantly decreased the responses to the original image (***Tukey test after one-way ANOVA, P < 0.001).
A total of 20 neurons were tested with scrambled and filtered snake images in the same way; Fig. 4C displays averaged response magnitudes to these stimuli. Statistical analysis showed a significant difference among the response magnitudes [one-way ANOVA; F(3, 80) = 17.334, P < 0.001]. Post hoc multiple comparisons indicated that both scrambling and HPF significantly decreased the firing rate to the snake image (Tukey test, P < 0.001 for both comparisons).
Population Coding of Snakes by the Pulvinar Neurons.
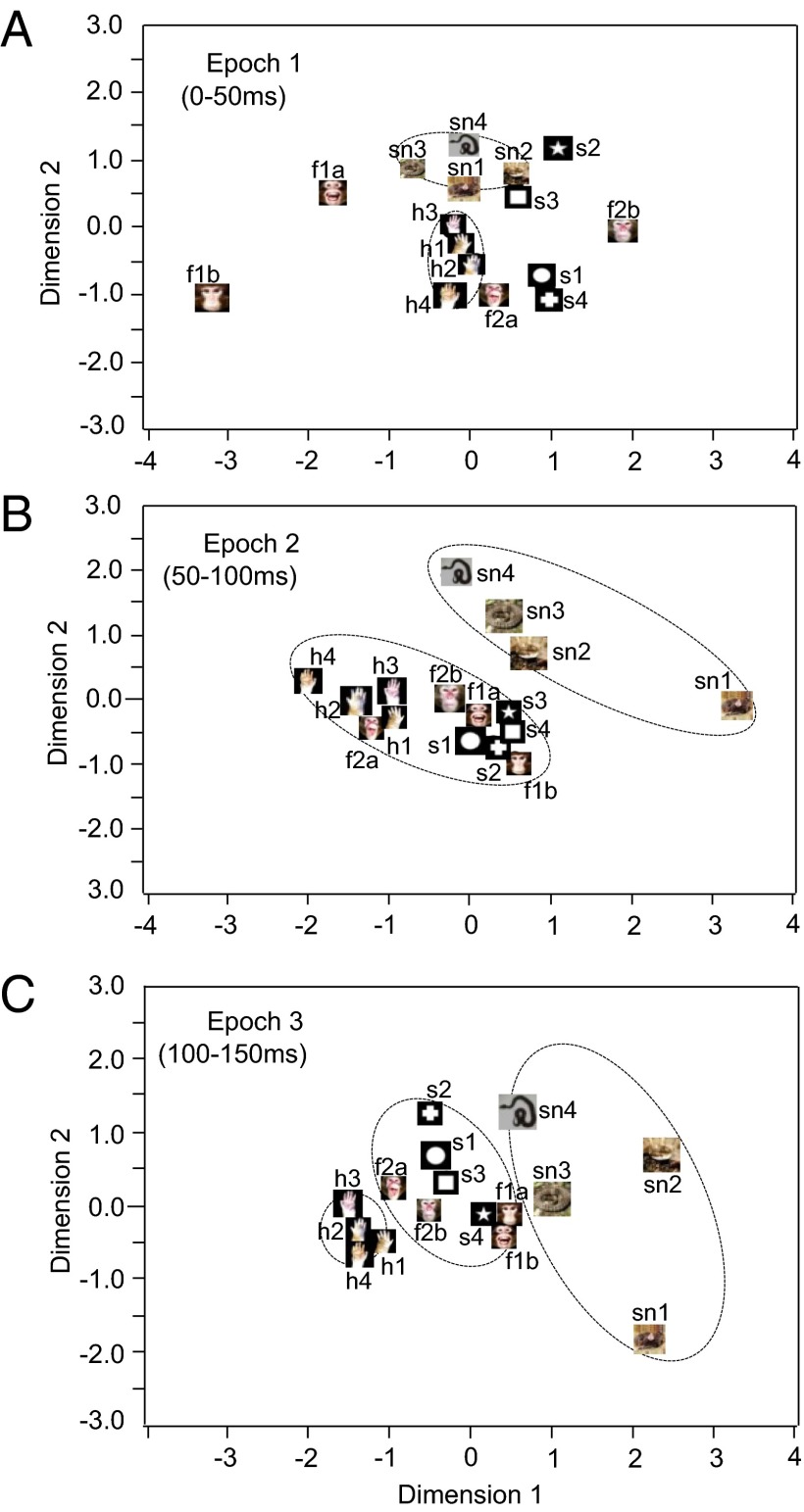
The datasets of response magnitudes of the 91 visually responsive pulvinar neurons in epochs 1 (0–50 ms), 2 (50–100 ms),and 3 (100–150 ms) after stimulus onset were subjected to multidimensional scaling (MDS) analysis (Fig. 5). After measurement of R2 and stress value for up to four dimensions, 2D spaces showed the best results. In the 2D spaces, R2 values of epoch 1, 2, and 3 were 0.843, 0.938, and 0.871, respectively. In epoch 1 (Fig. 5A), two groups were recognized: a cluster containing the snakes and the other containing hands. Discriminant analyses indicated significant separation between snake and hand pictures and between snake and all nonsnake stimuli (P < 0.05) (Table S1). There was also significant separation between hand pictures and simple geometrical shapes (P < 0.05). In epoch 2 clustering becomes clearer (Fig. 5B). Discriminant analyses indicate significant separations of snakes vs. faces, snakes vs. hands, snakes vs. simple geometrical shapes, and snakes vs. all nonsnake stimuli (P < 0.01). Separations of hands vs. faces, and hands vs. simple geometrical shapes were also significant (P < 0.05) (Table S1). The results in epoch 3 (Fig. 5C) were similar to those in epoch 2; hands were more clearly separated from the other stimuli (P < 0.01) (Table S1).
Fig. 5.
Distributions of the 16 visual stimuli in a 2D space resulting from MDS using responses of the 91 neurons to these stimuli in epoch 1 (A), epoch 2 (B), and epoch 3 (C). In epochs 1 and 2 (A and B), the snakes were separated from the remaining stimuli. In epoch 3 (C), three groups were separated: snakes, hands, and a cluster containing the faces and simple geometrical shapes.
Discussion
Primates have substantially modified and expanded the vertebrate visual system, and they rely heavily on vision as their primary sensory interface with the environment. Among other changes, the medial and dorsolateral pulvinar exist only in primates (13). Major modification of the visual system, including the addition of complex and energetically costly neural components, demands an adaptive explanation.
We show that neurons located especially in the medial and dorsolateral pulvinar respond selectively to snakes and in ways that facilitate their rapid visual detection: (i) the ratio of neurons that responded best to snakes was larger than those of neurons that responded best to other categories; (ii) mean response magnitudes were larger to snakes than to other stimuli; and (iii) snakes elicited neuronal responses with the shortest latencies. These responses were dependent on low-frequency images; HPF of the visual stimuli decreased neuronal responses but LPF did not. Distinct spatial frequencies of visual stimuli convey different information; high spatial frequencies of images convey fine visual details, whereas low spatial frequencies encode coarse visual information. Our results provide clear evidence that snakes provide coarse visual information that is effective in eliciting strong and rapid responses from a subset of visually active pulvinar neurons.
The medial and lateral pulvinar have been suggested to assist in shifting attention to relevant visual stimuli (15–17). Biologically meaningful stimuli relevant to our primate ancestors must have included snakes (1, 2, 5). Even today, deadly interactions with snakes are best avoided with early detection and a shifting of attention to them. The medial pulvinar receives direct inputs from the retina (13, 18) and the deeper layers of the superior colliculus (15, 19). Although the superior colliculus is considered a visual structure, it is also involved in threat-relevant motor behavior. Stimulation of its deeper layers causes animals to turn, dart, or freeze (20). Infant monkeys with bilateral neurotoxic lesions of the superior colliculus continue to reach for food in the presence of a snake model, whereas sham-operated monkeys avoid the food (21). Evasive movements such as these are typical of animals that are surprised or threatened by others (22, 23). The pulvinar is also connected to the amygdala (24). Studies of humans have implicated a pathway involving the superior colliculus, pulvinar, and amygdala in fast, automatic visual detection of fear-related stimuli, including snakes, at low spatial frequency (25–31). Similarly, the short response latencies and dependence on low spatial frequency to snakes in neurons of the nonhuman primate medial and dorsolateral pulvinar that we found corroborate the view that the pulvinar plays a crucial role in quickly conveying essential information affecting survival via bottom-up fast visual information processing (14).
This is not to suggest the subcortical route is the only pathway to detecting and avoiding danger. We also found medial and dorsolateral pulvinar neurons with longer latencies, and we suggest that these may receive top-down inputs from cortical visual areas (32). We also note that both the medial and dorsolateral pulvinar have reciprocal connections with association cortices (33). Interactive activity via reciprocal connections between subcortical nuclei and cortical areas likely enhances stimulus recognition and attention (34, 35). Although threatening visual inputs to the pulvinar and conveyed to the amygdala may be quickly processed, the pulvinar also likely coordinates cortical evaluation of the biological significance of affective visual stimuli (36).
The neuronal response to angry faces, albeit weaker than to snakes, was also faster and stronger than to hands and simple geometrical shapes. As is the case with snakes, being able to quickly detect and evade an angry conspecific undoubtedly has substantial survival value (1, 37). However, because the degree of facial expression in primates varies by body size, phylogenetic history, and group size (as a proxy for sociality) (38, 39), detecting threat from facial expression alone may not be universal among primates. In contrast, since the origin of primates, snakes have been a universal threat; both primates and snakes that can kill them (i.e., constrictors and venomous snakes) have their greatest diversity in tropical ecosystems (1, 2, 40, 41). Our data provide unique neuronal evidence supporting the hypothesis that snakes provided a novel selective pressure that contributed to the evolution of the primate order by way of visual modification (2, 5). We urge neurophysiologists to engage in similar studies across a wide range of primate species and closely related mammals to further examine the phylogenetic fingerprint of fast snake detection.
Materials and Methods
Animals.
Two adult (one female and one male) macaque monkeys (M. fuscata), weighing 7.0–8.8 kg, were used in this experiment. The monkeys were born and kept in a walled-off enclosure at a national monkey farm in Amami Island in Japan for 2 y, and then kept inside at the University of Toyama, Japan for the experiment. We believe that they had no chance to encounter snakes before the experiment. Each monkey was individually housed with food available ad libitum. The monkeys were deprived of water in their home cage and received juice as a reward during training and recording sessions. Supplemental water and vegetables were given after each day’s session. To assess the monkeys’ health, their weight was routinely monitored. The monkeys were treated in strict compliance with the United States Public Health Service Policy on Human Care and Use of Laboratory Animals, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Guidelines for the Care and Use of Laboratory Animals of the University of Toyama. This study has been approved by the Committee for Animal Experiments and Ethics at the University of Toyama.
The subject monkey sat in a monkey chair 68 cm away from the center of a 19-inch computer display for behavioral tasks during the training and recording sessions in a shielded room. The CRT monitor was set so that its center was on the same horizontal plane as the monkey’s eyes. The monkey chair was equipped with a responding button, which was positioned so that the monkey could easily manipulate it. An infrared charge-coupled device camera for eye-movement monitoring was firmly attached to the chair by a steel rod. During training and recording sessions, the monkey’s eye position was monitored with 33-ms time resolution by an eye-monitoring system (42). The juice reward was accessible to the monkey through a small spout controlled by an electromagnetic valve. A visual stimulus generator (ViSaGe MKII Visual Stimulus Generator, Cambridge Research Systems) controlled the electromagnetic valve and the timing of visual stimuli onset.
Visual Stimuli.
Fig. S3A shows the stimulus set, consisting of consisting of photos of snakes, photos of monkey faces (angry and neutral faces), photos of monkey hands, and simple geometrical figures (circle, cross, square, and star) used in the present study. The species of snakes used in the study were a Cottonmouth (Agkistrodon piscivorus, sn1), a Tsushima Island pitviper (Gloydius tsushimaensis, sn2 and sn3), and a Japanese mamushi (Gloydius blomhoffii, sn4). We used color images in the present study because previous studies reported that color of the stimuli affected detection of snakes (43, 44). The stimuli were 256 digitized RGB color-scale images with their resolution of 270 × 270 pixels. Stimuli were presented on a black background of 0.7 cd/m2 with their centers at the center of the display. The luminance of each stimulus was determined by measuring luminance of the circular area (radius, 6.35 cm) including each stimulus inside the circle by means of a luminance meter (BM-7A; Topcon). The luminance of these color stimuli was almost identical (6.005–6.445 cd/m2) [luminous intensity (total luminance) ranged from 38.432 to 41.248 mcd]. Luminance of the white areas inside the simple geometric patterns was 36.5 cd/m2 (total luminance of the circle, cross, square, and star was 45.6, 38.72, 53.592, and 20.64 mcd, respectively). These stimuli were displayed on a CRT monitor with a resolution of 640 × 480 pixels, and the size of the stimulus area was 5–7 × 5–7°.
Transformation of Visual Stimuli.
To analyze what features of the stimuli the neurons responded to, the visual stimuli were transformed. In scrambling, original images were cut into 64 pieces (8 × 8 pieces), and the fragments were randomly reassembled. Fig. S3B, b showed a scrambled image of the original snake image (Fig. S3B, a). In spatial filtering, we chose LPF with six cycles per image and HPF with 20 cycles per image based on previous studies (31, 45). First, colors of each image were separated into three color channels (red, green, and blue) and converted to grayscale images so that both LPF and HPF could be rendered in grayscale. Then, these three channel images were converted into frequency domain by the Fourier transform. Next, these images in each channel were processed with Gaussian LPF and HPF. Finally, these images in three channels were merged (Fig. S4 for HPF). Fig. S3B, c and d show the images processed with LPF and HPF, respectively. These images were processed using MATLAB 7.0.
Behavioral Tasks.
The monkeys were trained to perform a sequential delayed nonmatching-to-sample task (DNMS) that required the discrimination of the visual stimuli (Fig. S3 A and B). As illustrated in Fig. S3C, the task was initiated by a buzzer tone. Next, a fixation cross appeared in the center of the display. When the monkeys fixated on the cross for 1.5 s within a 0.5–1.0° window, a sample stimulus was presented for 500 ms (sample phase). The control phase was defined as the 100-ms period before the sample phase. Then, after an interval of 1.5 s, the same fixation cross and stimulus appeared again for 500 ms between one and four times (selected randomly for each trial). Finally, a new stimulus was presented (target phase). When the target appeared, the monkey was required to press a button within 2 s to receive a juice reward (0.8 mL). When the monkey failed to respond correctly during the target phase or to press the button before the target phase, the trials were aborted and a 620-Hz buzzer tone was presented. The intertrial intervals lasted 15–25 s (Fig. S3C). Visual stimuli were presented in separate blocks; stimulus pairs in the DNMS task within the same block consisted of the same category of stimuli (snakes, faces, hands, and simple geometric patterns). The presentation sequence of each category of the stimuli and presentation sequence of each stimulus within the same category were pseudorandomly determined so that presentation number of times of each stimulus was equal. After completion of behavioral training, a head-restraining device was attached to the skull under anesthesia (46, 47). Upon recovery from the surgery, the monkeys were retrained in the DNMS task while the head was painlessly fixed to the stereotaxic apparatus with the head-restraining device.
Stereotaxic Localization of the Pulvinar for Recording.
Before recording from the pulvinar in each hemisphere, a tungsten marker (diameter: 500 µm) was inserted near the target area under anesthesia, and 3D MRI scans of the monkey head were performed. The 3D pictures of the monkey brain with the marker were reconstructed by computer rendering. Three-dimensional stereotaxic coordinates of the target area were determined in reference to the marker in the 3D reconstructed brain (48). The locations of pulvinar neurons were based on the zero coordinates defined in the stereotaxic atlas of the brain of M. fuscata individuals (49).
Recording and Analysis of Pulvinar Neurons.
Neuronal activity was recorded from each hemisphere in both subjects by stereotaxically inserting a glass-insulated tungsten microelectrode into the pulvinar. Spike sorting was performed with the offline sorter program for cluster analysis (Off-line sorter, Plexon). Each cluster was checked manually to ensure that the cluster boundaries were well separated and that the waveform shapes were consistent with the action potentials. Finally, superimposed waveforms of the isolated units were drawn to check the consistency of the waveforms. Furthermore, all pulvinar neurons were analyzed by autocorrelograms. The autocorrelograms indicated that the refractory periods of the all pulvinar neurons were greater than 2 ms throughout the recording sessions, which indicates that the isolated spikes were recorded from single neurons.
We analyzed single neuronal activity during the following two periods: 100 ms before (pre) and 500 ms after (post) the onset of stimulus presentation in the sample phase. The baseline firing-rate was defined as the mean firing rate during the 100-ms preperiod. Significant excitatory or inhibitory responses to each stimulus were defined by a Wilcoxon signed-rank test (P < 0.05 for statistical significance) of the neuronal activity between the 100-ms pre- and the 500-ms postperiods. Furthermore, to investigate the temporal changes in the neuronal responses, the 500-ms postperiod was divided into ten 50-ms epochs. The mean neuronal firing rate was calculated for each of these epochs. The response magnitude was defined as follows: the mean firing rate in each epoch minus the mean firing rate during the 100-ms preperiod.
For each neuron, the response magnitudes during the visual stimulation period (for the whole 500-ms period and for each epoch) for all visual stimuli were analyzed by one-way ANOVA (P < 0.05). Response magnitudes between the stimuli were compared by Tukey post hoc tests (P < 0.05).
In addition, we analyzed the response latency to each visual stimulus. For each neuron, one perievent histogram was constructed with the entire set of data for all trials and all stimuli. Neuronal response latency was defined as the interval from the onset of stimulus presentation to the time at which the neuronal firing rate exceeded the mean ± 2 SD of the baseline firing-rate. All data were expressed as mean ± SEM.
MDS Analysis.
MDS is a method that is used to simplify the analysis of relationships that exist within a complex array of data. MDS constructs a geometric representation of the data to show the degree of the relationship between stimuli that are represented by the data matrix [see Young (50) for more details]. In the present study, the 16 visual stimuli were used to elicit neural activity in pulvinar neurons.
Data matrices of neural activity in a 91 × 16 array derived from the 91 visually responsive neurons were generated. Euclidean distances as dissimilarity between all possible pairs of two visual stimuli were calculated by using the visual responses of the 91 pulvinar neurons. Then, the MDS program (PROXSCAL procedure, SPSS statistical package, v16) positioned the visual stimuli in the 2D space with the distances between the stimuli representing the original relationships (i.e., Euclidean distances in the present study) (51, 52). Finally, the clusters of the visual stimuli were evaluated by discriminant analysis.
Supplementary Material
Acknowledgments
The authors thank Drs. Ralph Adolphs, Stephane Molotchnikoff, and two anonymous reviewers for valuable comments on earlier versions of this manuscript. This research was supported in part by Asian Core Program, Japan Society for the Promotion of Science; Ministry of Education, Culture, Sports, Science and Technology (MEXT), a Grant-in-Aid for Scientific Research (B) (25290005); and the National Bio-Resource Project “Japanese Monkeys” of the MEXT, Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312648110/-/DCSupplemental.
References
- 1.Headland TN, Greene HW. Hunter-gatherers and other primates as prey, predators, and competitors of snakes. Proc Natl Acad Sci USA. 2011;108(52):E1470–E1474. doi: 10.1073/pnas.1115116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isbell LA. The Fruit, the Tree, and the Serpent: Why We See so Well. Cambridge, MA: Harvard Univ Press; 2009. [Google Scholar]
- 3.Henshilwood CS, et al. Emergence of modern human behavior: Middle Stone Age engravings from South Africa. Science. 2002;295(5558):1278–1280. doi: 10.1126/science.1067575. [DOI] [PubMed] [Google Scholar]
- 4.Texier P-J, et al. From the cover: A Howiesons Poort tradition of engraving ostrich eggshell containers dated to 60,000 years ago at Diepkloof Rock Shelter, South Africa. Proc Natl Acad Sci USA. 2010;107(14):6180–6185. doi: 10.1073/pnas.0913047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isbell LA. Snakes as agents of evolutionary change in primate brains. J Hum Evol. 2006;51(1):1–35. doi: 10.1016/j.jhevol.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. J Exp Psychol Gen. 2001;130(3):466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- 7.LoBue V, DeLoache JS. Superior detection of threat-relevant stimuli in infancy. Dev Sci. 2010;13(1):221–228. doi: 10.1111/j.1467-7687.2009.00872.x. [DOI] [PubMed] [Google Scholar]
- 8.Shibasaki M, Kawai N. Rapid detection of snakes by Japanese monkeys (Macaca fuscata): An evolutionarily predisposed visual system. J Comp Psychol. 2009;123(2):131–135. doi: 10.1037/a0015095. [DOI] [PubMed] [Google Scholar]
- 9.Masataka N, Hayakawa S, Kawai N. Human young children as well as adults demonstrate ‘superior’ rapid snake detection when typical striking posture is displayed by the snake. PLoS ONE. 2010;5(11):e15122. doi: 10.1371/journal.pone.0015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soares SC. The lurking snake in the grass: interference of snake stimuli in visually taxing conditions. Evol Psychol. 2012;10(2):187–197. [PubMed] [Google Scholar]
- 11.Penkunas MJ, Coss RG. A comparison of rural and urban Indian children’s visual detection of threatening and nonthreatening animals. Dev Sci. 2013;16(3):463–475. doi: 10.1111/desc.12043. [DOI] [PubMed] [Google Scholar]
- 12.Öhman A, Soares JJF. On the automatic nature of phobic fear: Conditioned electrodermal responses to masked fear-relevant stimuli. J Abnorm Psychol. 1993;102(1):121–132. doi: 10.1037//0021-843x.102.1.121. [DOI] [PubMed] [Google Scholar]
- 13.Preuss TM. In: Primate Origins and Adaptations. Ravosa MJ, Dagosto M, editors. New York: Kluwer Academic/Plenum; 2007. pp. 625–675. [Google Scholar]
- 14.Ward R, Danziger S, Bamford S. Response to visual threat following damage to the pulvinar. Curr Biol. 2005;15(6):571–573. doi: 10.1016/j.cub.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 15.Stepniewska I. In: The Primate Visual System. Kaas JH, Collins CE, editors. New York: CRC; 2004. pp. 53–80. [Google Scholar]
- 16.Benevento LA, Miller J. Visual responses of single neurons in the caudal lateral pulvinar of the macaque monkey. J Neurosci. 1981;1(11):1268–1278. doi: 10.1523/JNEUROSCI.01-11-01268.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benevento LA, Port JD. Single neurons with both form/color differential responses and saccade-related responses in the nonretinotopic pulvinar of the behaving macaque monkey. Vis Neurosci. 1995;12(3):523–544. doi: 10.1017/s0952523800008439. [DOI] [PubMed] [Google Scholar]
- 18.Itaya SK, Van Hoesen GW. Retinal projections to the inferior and medial pulvinar nuclei in the Old-World monkey. Brain Res. 1983;269(2):223–230. doi: 10.1016/0006-8993(83)90131-2. [DOI] [PubMed] [Google Scholar]
- 19.Benevento LA, Fallon JH. The ascending projections of the superior colliculus in the rhesus monkey (Macaca mulatta) J Comp Neurol. 1975;160(3):339–361. doi: 10.1002/cne.901600306. [DOI] [PubMed] [Google Scholar]
- 20.Sewards TV, Sewards MA. Innate visual object recognition in vertebrates: Some proposed pathways and mechanisms. Comp Biochem Physiol A Mol Integr Physiol. 2002;132(4):861–891. doi: 10.1016/s1095-6433(02)00119-8. [DOI] [PubMed] [Google Scholar]
- 21.Maior RS, et al. Superior colliculus lesions impair threat responsiveness in infant capuchin monkeys. Neurosci Lett. 2011;504(3):257–260. doi: 10.1016/j.neulet.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 22.Ramakrishnan U, Coss RG, Schank J, Dharawat A, Kim S. Snake species discrimination by wild bonnet macaques (Macaca radiata) Ethology. 2005;111(4):337–356. [Google Scholar]
- 23.Carter AJ, Marshall HH, Heinsohn R, Cowlishaw G. How not to measure boldness: Novel object and antipredator responses are not the same in wild baboons. Anim Behav. 2012;84(3):603–609. [Google Scholar]
- 24.Jones EG, Burton H. A projection from the medial pulvinar to the amygdala in primates. Brain Res. 1976;104(1):142–147. doi: 10.1016/0006-8993(76)90654-5. [DOI] [PubMed] [Google Scholar]
- 25.Liddell BJ, et al. A direct brainstem-amygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage. 2005;24(1):235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Mulckhuyse M, Theeuwes J. Unconscious attentional orienting to exogenous cues: A review of the literature. Acta Psychol (Amst) 2010;134(3):299–309. doi: 10.1016/j.actpsy.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Morris JS, Öhman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA. 1999;96(4):1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maior RS, et al. A role for the superior colliculus in the modulation of threat responsiveness in primates: Toward the ontogenesis of the social brain. Rev Neurosci. 2012;23(5–6):697–706. doi: 10.1515/revneuro-2012-0055. [DOI] [PubMed] [Google Scholar]
- 29.Csatho A, Tey F, Davis G. Threat perception and targeting: The brainstem-amygdala-cortex alarm system in action? Cogn Neuropsychol. 2008;25(7-8):1039–1064. doi: 10.1080/02643290801996360. [DOI] [PubMed] [Google Scholar]
- 30.Tamietto M, de Gelder B. Neural bases of the non-conscious perception of emotional signals. Nat Rev Neurosci. 2010;11(10):697–709. doi: 10.1038/nrn2889. [DOI] [PubMed] [Google Scholar]
- 31.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci. 2003;6(6):624–631. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- 32.Olshausen BA, Anderson CH, Van Essen DC. A neurobiological model of visual attention and invariant pattern recognition based on dynamic routing of information. J Neurosci. 1993;13(11):4700–4719. doi: 10.1523/JNEUROSCI.13-11-04700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shipp S. The functional logic of cortico-pulvinar connections. Philos Trans R Soc Lond B Biol Sci. 2003;358(1438):1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grieve KL, Acuña C, Cudeiro J. The primate pulvinar nuclei: Vision and action. Trends Neurosci. 2000;23(1):35–39. doi: 10.1016/s0166-2236(99)01482-4. [DOI] [PubMed] [Google Scholar]
- 35.Pessoa L, Adolphs R. Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11(11):773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen MN, et al. Neuronal responses to face-like stimuli in the monkey pulvinar. Eur J Neurosci. 2013;37(1):35–51. doi: 10.1111/ejn.12020. [DOI] [PubMed] [Google Scholar]
- 37.Öhman A, Soares SC, Juth P, Lindström B, Esteves F. Evolutionary derived modulations of attention to two common fear stimuli: Serpents and hostile humans. J Cogn Psychol. 2012;24(1):17–32. [Google Scholar]
- 38.Dobson SD. Allometry of facial mobility in anthropoid primates: Implications for the evolution of facial expression. Am J Phys Anthropol. 2009a;138(1):70–81. doi: 10.1002/ajpa.20902. [DOI] [PubMed] [Google Scholar]
- 39.Dobson SD. Socioecological correlates of facial mobility in nonhuman anthropoids. Am J Phys Anthropol. 2009b;139(3):413–420. doi: 10.1002/ajpa.21007. [DOI] [PubMed] [Google Scholar]
- 40.Harcourt AH. Rarity in the tropics: Biogeography and macroecology of the primates. J Biogeogr. 2006;33(12):2077–2087. [Google Scholar]
- 41.Ricklefs RE, Losos JB, Townsend TM. Evolutionary diversification of clades of squamate reptiles. J Evol Biol. 2007;20(5):1751–1762. doi: 10.1111/j.1420-9101.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 42.Matsuda K. Measurement system of the eye positions by using oval fitting of a pupil. Neurosci Res Suppl. 1996;25:270. [Google Scholar]
- 43.LoBue V, DeLoache JS. Detecting the snake in the grass: Attention to fear-relevant stimuli by adults and young children. Psychol Sci. 2008;19(3):284–289. doi: 10.1111/j.1467-9280.2008.02081.x. [DOI] [PubMed] [Google Scholar]
- 44.Hayakawa S, Kawai N, Masataka N. The influence of color on snake detection in visual search in human children. Sci Rep. 2011;1:80. doi: 10.1038/srep00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rotshtein P, Vuilleumier P, Winston J, Driver J, Dolan R. Distinct and convergent visual processing of high and low spatial frequency information in faces. Cereb Cortex. 2007;17(11):2713–2724. doi: 10.1093/cercor/bhl180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishijo H, Ono T, Nishino H. Single neuron responses in amygdala of alert monkey during complex sensory stimulation with affective significance. J Neurosci. 1988;8(10):3570–3583. doi: 10.1523/JNEUROSCI.08-10-03570.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tazumi T, Hori E, Maior RS, Ono T, Nishijo H. Neural correlates to seen gaze-direction and head orientation in the macaque monkey amygdala. Neuroscience. 2010;169(1):287–301. doi: 10.1016/j.neuroscience.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 48.Asahi T, et al. A method for accurate determination of stereotaxic coordinates in single-unit recording studies in monkeys by high-resolution three-dimensional magnetic resonance imaging. Neurosci Res. 2003;47(2):255–260. doi: 10.1016/s0168-0102(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 49.Kusama T, Mabuchi M. Stereotaxic Atlas of the Brain of Macaca fuscata. Tokyo: Tokyo Univ Press; 1970. [Google Scholar]
- 50.Young FW. Multidimensional Scaling: History, Theory, and Applications. Hillsdale, NJ: Lawrence Erlbaum; 1987. [Google Scholar]
- 51.Kruskal JB. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika. 1964;29(1):1–27. [Google Scholar]
- 52.Shepard RN. The analysis of proximities: Multidimensional scaling with an unknown distance function. Psychometrika. 1962;27(2):125–140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.