Significance
Meningococcal serogroup X has recently emerged as a cause of meningitis outbreaks with epidemic potential in sub-Saharan Africa. Novel conjugation technologies, compatible with a reproducible production process, have been successfully used to develop immunogenic polysaccharide conjugate vaccine candidates that are likely to protect against meningococcal X disease. The timely development of an anti-meningococcal X conjugate vaccine appears a logical next step in the broadest control of meningococcal disease and requires commitment now.
Abstract
Neisseria meningitidis is a major cause of bacterial meningitis worldwide, especially in the African meningitis belt, and has a high associated mortality. The meningococcal serogroups A, W, and X have been responsible for epidemics and almost all cases of meningococcal meningitis in the meningitis belt over the past 12 y. Currently no vaccine is available against meningococcal X (MenX). Because the development of a new vaccine through to licensure takes many years, this leaves Africa vulnerable to new epidemics of MenX meningitis at a time when the epidemiology of meningococcal meningitis on the continent is changing rapidly, following the recent introduction of a glycoconjugate vaccine against serogroup A. Here, we report the development of candidate glycoconjugate vaccines against MenX and preclinical data from their use in animal studies. Following optimization of growth conditions of our seed MenX strain for polysaccharide (PS) production, a scalable purification process was developed yielding high amounts of pure MenX PS. Different glycoconjugates were synthesized by coupling MenX oligosaccharides of varying chain length to CRM197 as carrier protein. Analytical methods were developed for in-process control and determination of purity and consistency of the vaccines. All conjugates induced high anti-MenX PS IgG titers in mice. Antibodies were strongly bactericidal against African MenX isolates. These findings support the further development of glycoconjugate vaccines against MenX and their assessment in clinical trials to produce a vaccine against the one cause of epidemic meningococcal meningitis that currently cannot be prevented by available vaccines.
A major cause of bacterial meningitis worldwide, Neisseria meningitidis has significant associated mortality (1). Among the 13 distinct meningococcal serogroups, which are classified on the structure of their capsular polysaccharide (PS), serogroups A, B, C, Y, W, and X most commonly cause invasive disease, including meningitis and septicemia, in humans. The highest incidence of meningococcal meningitis occurs in the meningitis belt of sub-Saharan Africa, extending from Senegal to Ethiopia.
Since records began, meningococcal serogroup A (MenA) has been the dominant cause of epidemics of meningococcal meningitis in this region (2), but MenW (3) and MenX (4–6) have also been responsible for epidemics. From 2010 to 2012, MenX was responsible for annual meningitis outbreaks in Burkina Faso. In 2011, MenX accounted for 59% of confirmed cases of meningococcal meningitis in this country (7). Higher case fatality rates have been reported for meningitis caused by MenX compared with MenA (4, 6), and children aged 1–9 y constitute the most affected age group (4, 8).
In 2010, a MenA conjugate vaccine (MenAfriVac) was rolled out in a mass vaccination program in Burkina Faso, Mali, and Niger (9). Early reports indicate that this has been highly effective at reducing cases of MenA meningitis. Removal of serogroup A strains from circulating among the population may confer an advantage to MenX, previously less able to compete with the more virulent serogroup A (10, 11). Capsule replacement of carried meningococci did not occur following the implementation of serogroup C conjugate vaccines in the United Kingdom (12). However, the conditions in the meningitis belt are very different from those in industrialized nations and a recent study of carriage before and after the introduction of the MenA conjugate vaccine in Burkina Faso found significantly higher levels of MenX carriage following the introduction of the vaccine (13).
MenW PS vaccine is used for outbreak control of meningitis caused by MenW in the meningitis belt and a change in the epidemiology of meningitis due to MenW could necessitate its increased demand. Polyvalent vaccines, including MenW glycoconjugate, are currently produced and used in developed countries and could potentially be mobilized for use in Africa. In contrast, although the need for a vaccine against serogroup X Neisseria meningitidis has been recognized for many years (4, 5, 14, 15), none is currently available.
Given the success of other meningococcal glycoconjugate vaccines (16), the MenX PS antigen is a logical target for vaccine design. Plain PS could facilitate epidemic control, whereas conjugation to a carrier protein would provide enhanced immunogenicity, particularly from early infancy, by converting the PS into a T-cell–dependent antigen (17, 18). As recognized for other PS, conjugation to an appropriate carrier protein overcomes the limits of PS vaccines, such as poor efficacy in children less than 2 y, lack of immunological memory with poor booster responses, and relatively short duration of protection (19–21). Meningococcal conjugate vaccines are also able to overcome the immune hyporesponsiveness that is induced by PS vaccines (22, 23). Additionally, as documented for group C, meningococcal conjugate vaccines can reduce carriage of N. meningitidis in the nasopharynx, decreasing transmission (24), whereas PS vaccines have not been shown to provide substantial herd immunity (25). The impact of vaccination with the MenAfriVac conjugate vaccine in Burkina Faso on carriage and herd immunity has been recently reported (13).
The MenX PS was first characterized and defined as a distinct serogroup in the 1960s (26, 27) and was shown to be immunogenic in rabbits (28, 29). The structure of MenX PS consists of N-acetylglucosamine-4-phosphate residues held together by α-(1-4) phosphodiester bonds without O-acetyl groups (28, 30–33).
Here, we describe the process of synthesis of MenX glycoconjugate vaccines, together with data from preliminary immunological evaluation in mice. MenX oligosaccharides (OS) of different length and three different conjugation chemistries were compared, using CRM197, a nontoxic mutant of diphtheria toxin (34), as carrier protein. When tested in mice, all MenX–CRM197 conjugates resulted in high IgG antibody levels and serum bactericidal activity (SBA) titers, indicating the path ahead for the clinical development of a vaccine to prevent the remaining cause of epidemic meningococcal meningitis in Africa for which no vaccine is available.
Results
MenX PS Production.
To identify bacterial growth conditions for the optimal production and release of MenX PS into the supernatant, three different media were tested using the MenX strain 5967 (ST 750) (SI Materials and Methods). Modified Frantz medium resulted in the highest amount of PS expression (Table 1) and therefore was selected for higher scale (18 L) fermentation, which yielded 356 µg/mL MenX PS. Proton NMR spectroscopy (1H NMR) was used to monitor PS expression (SI Materials and Methods).
Table 1.
MenX 5967 (ST-750) strain growth in different media and relative PS production
| Growth medium | OD, 600 nm | Saccharide, µg/mL | µg Saccharide/OD |
| Modified Catlin v.6 | 2 | 22.55 | 13.3 |
| MCDM1 | 6 | 42.73 | 7.1 |
| Modified Frantz | 2.8 | 62.6 | 22.4 |
The process for purifying MenX PS was built on the knowledge of equivalent processes for the quadrivalent meningococcal A, C, W-135, and Y glycoconjugate vaccine (Menveo) and for Salmonella Vi (35, 36). Applying our high-performance anion-exchange chromatography with pulsed amperometric detection method (37), able to quantify very low MenX PS concentrations (≥0.5 μg/mL) and, unlike other methods (32, 38), applicable to all process intermediates including fermentation broth, we demonstrated that our purification process provides high yields at each step (six steps in total), with an overall recovery of 56% when scaled to 16 L of clarified fermentation broth.
All purified MenX PS preparations contained low levels of contaminants [protein and nucleic acid <1% [weight to weight (wt/wt)] of PS, and endotoxin <5 EU/µg of PS]. The structural integrity of MenX PS was confirmed by NMR analysis (31–33) (SI Materials and Methods). Applying high-performance liquid chromatography–size exclusion chromatography (HPLC-SEC), the PS eluted at a Kd of 0.34 from a TSK gel 6000PW connected in series with a TSK gel 5000PW column, without evidence of PS depolymerization or aggregation (SI Materials and Methods).
MenX–CRM197 Conjugates.
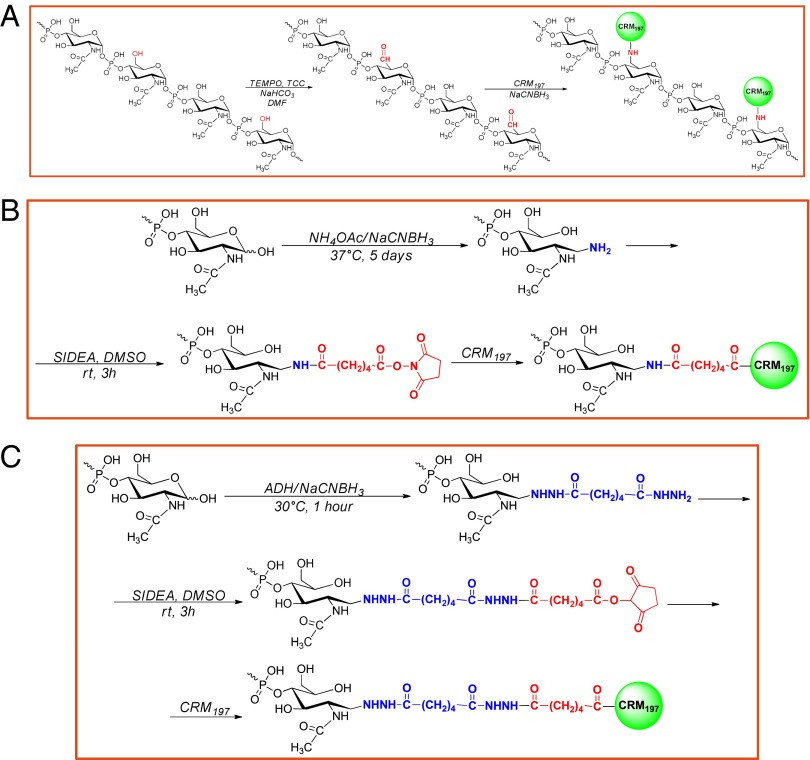
To identify a feasible and reproducible conjugation process, low-scale preparations were first performed using the native MenX PS. By applying a random activation of the phosphate groups along the saccharide chain with carbodiimide, complex products difficult to purify and characterize were obtained. MenX OS preparations with shorter chain lengths, obtained by mild acid hydrolysis (see SI Materials and Methods for details of preparation and characterization), were consequently used to prepare conjugates with three different coupling strategies (Fig. 1).
Fig. 1.
Reaction scheme for the synthesis of MenXOX–CRM197 conjugate (A), MenX–NH2–SIDEA–CRM197 conjugate (B), and MenX–ADH–SIDEA–CRM197 conjugate (C).
MenXOX–CRM197 (Fig. 1A) was prepared using MenX OS of average degree of polymerization (avDP) 80–100 (average Mr, 24–30 kDa). The primary hydroxyl groups of N-acetyl glucosamine phosphate repeating unit were randomly oxidized using 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), to introduce aldehyde groups which were then directly coupled to Lys ε-amine groups of CRM197 by reductive amination. 1H NMR was used to confirm the presence and estimate the concentration of functional aldehyde groups generated at position C6 by oxidation (SI Materials and Methods). Although proton NMR signals were expected both for the aldehyde and the hydrate aldehyde forms, only the signal at 5.32 ppm representing the hydrate aldehyde form was detected, possibly because the conditions used to perform the experiment shifted the equilibrium toward this species. The saccharide oxidation was estimated by integration of the hydrate aldehyde peak (H6ha) in comparison with H1 peak (proton at position C1): one repeating unit every 15–25 units was found to be oxidized. As confirmed by HPLC-SEC analysis, no degradation or aggregation of MenX OS occurred due to oxidation.
The other two coupling strategies were based on MenX OS of avDP 15–20 (average Mr, 4.5–6 kDa). In one case, the Menveo conjugation chemistry (35), consisting of reductive amination at the end of the sugar chain with ammonium acetate (NH4OAc), followed by reaction with adipic acid bis(N-hydroxysuccinimmide) (SIDEA) and conjugation to CRM197, was applied (Fig. 1B). A similar chemistry was evaluated where the reductive amination was conducted using adipic acid dihydrazide (ADH) followed also in this case by derivatization with SIDEA, thus resulting in a longer linker (Fig. 1C) (39). The use of more reactive ADH molecule resulted in shorter reaction time of the first step (from 5 d to 1 h) and higher derivatization yield: 40% of MenX chains derivatized with NH4OAc, compared with 81% using ADH, as quantified by 2,4,6-trinitrobenzenesulfonic acid (TNBS) colorimetric method. In both cases, >80% of saccharide chains were derivatized with SIDEA in the following reaction step. For all these process intermediates, no changes of Mr distribution occurred, as verified by HPLC-SEC analysis.
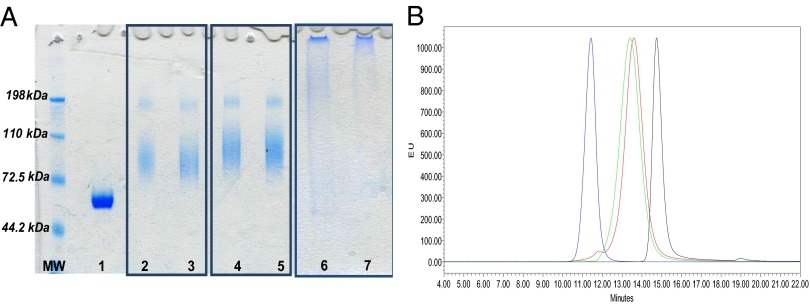
After verifying conjugate formation by SDS/PAGE and HPLC-SEC (Fig. 2), the three conjugation reaction mixtures were purified by preparative size exclusion column (Sephacryl S300 column). The fractions not overlapping with those corresponding to free CRM197 and free MenX OS were collected. Table 2 summarizes the characterization data of the purified conjugates tested in mice. The conjugates obtained by end-groups activation showed comparable OS to protein ratios, higher than that obtained for the random conjugate. All of the conjugates were of higher Mr than free CRM197 and free OS, as shown by the corresponding Kd values.
Fig. 2.
(A) SDS/PAGE analysis of CRM197 (lane 1); MenX–NH2–SIDEA–CRM197, MenX–ADH–SIDEA–CRM197, and MenXOX–CRM197 conjugation mixtures (2, 4, and 6, respectively); corresponding purified conjugates (3, 5, 7); 5 μg of protein loaded per each sample. (B) HPLC-SEC profiles (fluorescence emission) of purified MenXOX–CRM197 (blue), MenX–NH2–SIDEA–CRM197 (red), and MenX–ADH–SIDEA–CRM197 (green) in comparison with free CRM197 (black). Chromatograms were normalized with respect to y axis.
Table 2.
Characterization of the MenX–CRM197 conjugates tested in mice
| Conjugate | Conjugation chemistry | avDP MenX OS | MenX/CRM197, wt/wt | MenX/CRM197, mol/mol | Kd | Endotoxin, EU/µg |
| MenXOX–CRM197 | Random | 80 | 0.69 | 1.7 | 0.07 | 1.6 |
| MenX–ADH–SIDEA–CRM197 | Selective with longer spacer | 17 | 0.36 | 4.1 | 0.22 | 5.3 |
| MenX–NH2–SIDEA–CRM197 | Selective | 17 | 0.33 | 3.7 | 0.24 | 0.8 |
Kd values were of 0.33 for unconjugated CRM197 and of 0.24 and 0.40 for MenX OS of average degree of polymerization (avDP) 80 and 15, respectively.
Immunogenicity of Conjugates in Mice.
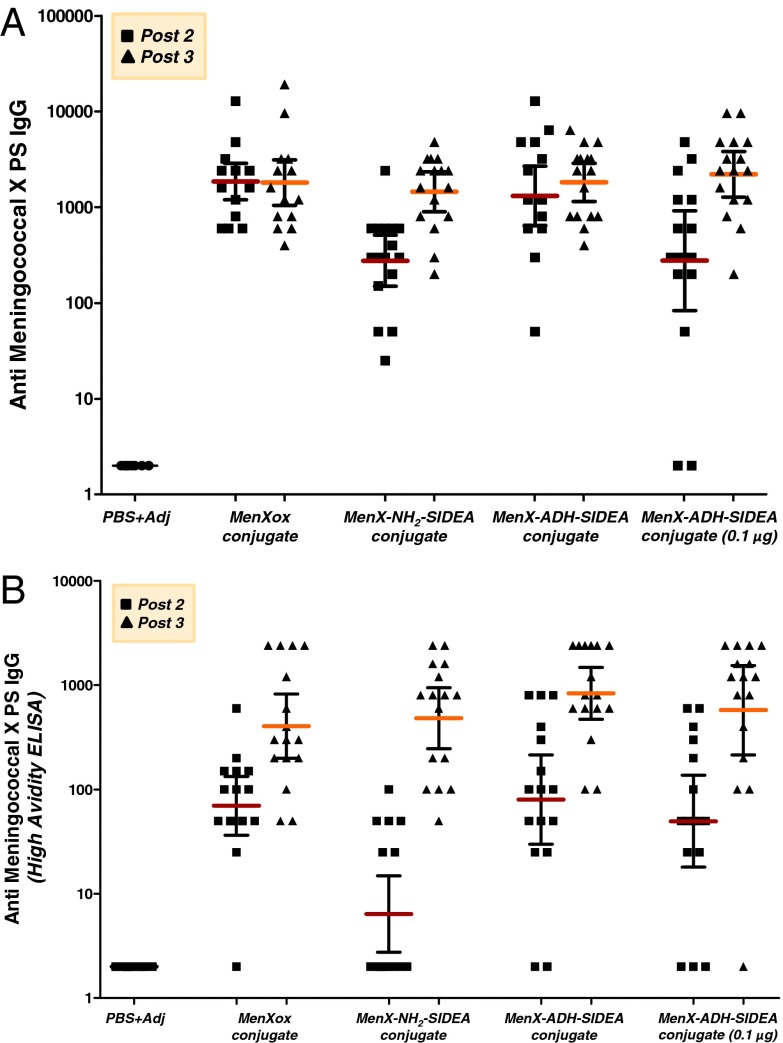
To assess the ability of conjugates to induce anti-MenX antibodies, female BALB/c (5–6 wk old) mice were immunized s.c. three times at 2-wk intervals with the vaccine candidates plus adjuvant. All MenX–CRM197 conjugates were immunogenic, eliciting significantly higher anti-MenX PS IgG ELISA units than the control group, which received PBS plus adjuvant (P value < 0.0001) (Fig. 3A). Mice immunized with unconjugated MenX PS or with the physical mixture MenX PS plus CRM197 did not show anti-IgG response to MenX PS. MenXOX–CRM197 and MenX–ADH–SIDEA–CRM197 conjugates, administered at a 1-μg saccharide dose, induced peak IgG antibody levels after two injections. In contrast, the MenX–NH2–SIDEA–CRM197 conjugate resulted in IgG antibody levels comparable to the other two conjugates only after the third dose, with a statistically significant booster effect compared with the level after the second dose (P value = 0.0002). By immunizing mice with a lower dose of conjugate (0.1 μg of saccharide of MenX–ADH–SIDEA–CRM197), lower IgG titers were obtained after the second dose, with a booster effect now observed after the third dose (P value = 0.002), that was capable of yielding comparable ELISA titers to the values obtained when immunizing with 1 μg of conjugate. Anti-MenX PS IgM ELISA units were low for all of the conjugates, as expected for conjugate vaccines and likely due to effective isotype switching from IgM to IgG (40, 41).
Fig. 3.
(A) Antibody response to meningococcal group X PS induced in female BALB/c mice (5–6 wk old) by MenX–CRM197 conjugate vaccines at 1-μg dose. MenX–ADH–SIDEA–CRM197 was also tested at 0.1-μg dose. Anti-MenX IgG ELISA units measured by standard ELISA in sera collected 2 wk after the second and third immunizations. Individual animals are represented by each point; the horizontal bars indicate geometric mean and 95% confidence intervals. (B) Anti-MenX IgG ELISA units measured by high-avidity ELISA using 0.1 M ammonium thiocyanate as chaotropic agent.
SBA assays were performed with pooled sera collected 2 wk after the second and the third immunization against the same strain used for MenX PS production, and indicated that antibodies induced by immunization with the MenX–CRM197 conjugates were functional (Table 3). Fourteen days after the second dose, the mean bactericidal titer was higher with MenX–ADH–SIDEA–CRM197 and MenXOX–CRM197 than MenX–NH2–SIDEA–CRM197, corresponding to IgG titers. Fourteen days after the third dose, the SBA titers were boosted for all conjugates, unlike IgG titers for MenXOX–CRM197 and MenX–ADH–SIDEA–CRM197. A possible explanation for this lack of correlation between ELISA and SBA titers is that ELISA detects both high- and low-avidity antibodies (42), whereas higher-avidity antibodies elicit more complement-mediated SBA (43, 44). To test this hypothesis, anti-MenX antibodies titers were measured by a modified ELISA, which uses a chaotropic salt to select higher avidity IgG antibodies. The results more closely matched the SBA data (Fig. 3B and Table 3). Anti-MenX PS IgG ELISA units, measured using this high-avidity ELISA, were low for all conjugates both after the second and third dose compared with units by standard ELISA. Now a statistically significant booster effect could be detected after the third dose for all conjugates (P from 0.0006 to <0.0001).
Table 3.
Serum bactericidal activity of pooled mouse sera against MenX strains and anti-MenX IgG GMT ELISA units 95% confidence interval 14 d after the second (post 2) and third (post 3) injection of MenX–CRM197 conjugate vaccines and control
| Group | SBA titers |
Standard ELISA |
High-avidity ELISA |
|||||
| Pooled sera post 2 | Pooled sera post 3 |
Sera post 2 | Sera post 3 | Sera post 2 | Sera post 3 | |||
| 5967 (ST 750) | 5967 (ST 750) | BF7/07 | Kenya 1/06 | |||||
| PBS + adjuvant | <16 | <4 | <4 | <4 | 2 | 2 | 2 | 2 |
| MenXOX–CRM197 | 1,024 | 4,096 | 4,096 | 2,048 | 1,857 | 1,810 | 69.8 | 404.6 |
| MenX–NH2–SIDEA–CRM197 | 128 | 4,096 | 4,096 | 2,048 | 276 | 1,447 | 6.4 | 482.7 |
| MenX–ADH–SIDEA–CRM197 | 1,024 | 4,096 | 4,096 | 2,048 | 1,313 | 1,816 | 80.1 | 836.1 |
| MenX–ADH–SIDEA–CRM197 (0.1 μg) | 512 | 4,096 | 2,048 | 2,048 | 277.5 | 2,208 | 49.6 | 576.8 |
Female BALB/c (5–6 wk old) mice were immunized three times at 2-wk intervals with MenX–CRM197 conjugate vaccines at 1-μg dose. MenX–ADH–SIDEA–CRM197 was also tested at 0.1-μg dose. All vaccines were tested with aluminum phosphate as adjuvant. Bactericidal titers are defined as the reciprocal serum dilution that gives a 50% decrease of colony-forming units after 60-min incubation in the reaction mixture, compared with the mean number of colony-forming units in the control reactions at time 0.
Pooled sera collected 2 wk after the third immunization were evaluated for functional activity against two additional MenX strains, BF7/07 and Kenya 1/06, respectively isolated in Burkina Faso (2007) and Kenya (2006), and provided by the World Health Organization Collaborating Centre for Reference and Research on Meningococci (Oslo, Norway). High SBA titers were found against each strain for all conjugates (Table 3).
Discussion
Despite the widespread implementation of a MenA–tetanus toxoid conjugate vaccine in the African meningitis belt and the availability of PS and conjugate MenW vaccines, this area is vulnerable to increased incidence of meningitis caused by MenX for which no vaccine is available (45). Because the development of a new vaccine through to licensure takes many years, this leaves Africa vulnerable to new epidemics of MenX meningitis at a time when the epidemiology of meningococcal meningitis on the continent is changing rapidly (13). Glycoconjugates are among the safest and most efficacious vaccines developed during the last 30 y (46). Following the success of serogroup C meningococcal conjugate vaccines (16), which widespread vaccination confirmed to be immunogenic, inducing immunological memory, reducing colonization, and providing herd immunity, tetravalent conjugate vaccines covering serogroup A, C, W135, and Y (47–49) have been developed to broaden protection against meningococcus. The timely development of an anti-MenX conjugate vaccine appears a logical next step in the broadest control of meningococcal disease and requires commitment now.
Using the process developed in this study, MenX PS was purified at high yields with good final product quality. The process is scalable and suitable for good manufacturing practice production. Approximately 200 mg of purified MenX PS was obtained from 1 L of fermentation broth, a productivity much higher than previously reported with MenX isolates (9.6–20 mg/L) (31, 50, 51). Studies on MenX PS and derived OS indicated that they are stable in aqueous solution (31, 33), providing the opportunity to develop a fully liquid vaccine formulation with clear advantages in terms of cost and practical utilization compared with freeze-dried formulations that require reconstitution with water.
Conjugation of native MenX PS, necessarily through the use of random chemistries, yielded heterogeneous products that were difficult to purify and characterize. As reported for other conjugate vaccines, cross-linked chemistries can be affected by low batch-to-batch consistency (52, 53). The use of shorter saccharides has allowed the production of more defined and less cross-linked conjugates, with advantages in relation to reproducibility and vaccine characterization. Parameters, including conjugation chemistry (random against selective carbohydrate modification), linker used for coupling and saccharide chain length, can have an impact on the immunogenicity of the corresponding conjugate vaccines (53–56). The effect of varying such parameters is antigen dependent and was investigated in the current study to optimize the design of a vaccine against MenX. Three different conjugate vaccines were synthesized using MenX OS of avDP 15–20 or 80–100 and CRM197 (34) as carrier protein. The chemistry developed for Menveo was adapted to the shorter OS (35). However, working with MenX PS, the first step of reductive amination (Fig. 1B) resulted in low activation yields. The use of ADH as a more reactive linker (Fig. 1C) (39) increased the yield of this first step and reduced the reaction time. Working with longer OS, a completely different chemistry was required (Fig. 1A). Random oxidation at position C6 of N-acetyl glucosamine phosphate repeating unit resulted in multiple activation sites along the saccharide chain instead of just at the terminal reducing end. This process was characterized by one step less compared with the other two methods. We estimated that a derivatization density of one repeating unit every 15–25 unit and the use of higher avDP OS should preserve epitopes of the native saccharide for interaction with the B cells.
All conjugates gave high anti-MenX IgG titers in mice (Fig. 3A) and induced functional antibodies when tested against African meningococcal X isolates (Table 3). High-avidity IgG antibody titers closely matched SBA titers, which is a good surrogate for meningococcal vaccine efficacy (42, 57, 58) (Table 3). Comparing MenXOX–CRM197 and MenX–ADH–SIDEA–CRM197, saccharide chain length and conjugation chemistry do not seem to affect immunogenicity significantly. However, MenX–ADH–SIDEA–CRM197, which contains a longer spacer compared with MenX–NH2–SIDEA–CRM197, resulted in higher antibody and SBA titers after two doses, indicating an effect from the length or the nature of the spacer on the antibody response.
MenX has recently emerged as a cause of meningitis outbreaks with epidemic potential in sub-Saharan Africa, where MenA and MenW have previously been the dominant causes of meningococcal disease (2, 45). Although it is uncertain how MenX will contribute to the problem of meningitis in Africa in the future, preparedness would be prudent in the face of changing epidemiology surrounding the introduction of the serogroup A conjugate vaccine. A trivalent conjugate vaccine covering MenX, MenA, and MenW would be particularly useful for prevention of meningococcal meningitis in the African meningitis belt. Such a vaccine could potentially have protected against over 90% of invasive meningococcal meningitis cases occurring over the past 10 y in this area. A major challenge to the development of a vaccine against MenX is the lack of commercial incentive, because cases of MenX meningitis are rare outside of Africa. Therefore, to progress new vaccine initiatives requires a public health demand from the population, health care professionals and ministries of health in the affected countries, together with foresight and support from global health policy makers and major funders of global health research. This successfully occurred with the formation of the Meningitis Vaccine Initiative, which was responsible for the development of the MenA conjugate vaccine.
This study has demonstrated that conjugation technologies, compatible with a reproducible production process, can be successfully used to develop immunogenic PS conjugate vaccine candidates that are likely to protect against MenX disease. This forms a basis for further vaccine development to identify the best candidate and formulations to proceed to clinical trials and indicates the next steps needed to develop a vaccine to protect against this important cause of epidemic meningitis in Africa.
Materials and Methods
SI Materials and Methods feature additional information to the section provided here.
MenX PS Production.
Neisseria meningitidis X 5967 strain (ST 750) was obtained from the Swiss Tropical Public Health Institute. It was originally isolated during a diagnostic procedure from the cerebrospinal fluid of a meningitis patient in Germany.
MenX PS was purified adapting methods previously developed for the purification of meningococcal and Vi PS (35, 36). A standard purification is described in SI Materials and Methods together with analytical methods to assess its purity and full characterization.
MenX–CRM197 Conjugates.
Random oxidation along the MenX chain and conjugation with CRM197 (Fig. 1A).
MenX OS (avDP 80–100), obtained by mild acid hydrolysis as described in SI Materials and Methods, was loaded in water onto a Sepharose SP column (∼ 4–5 mg of OS loaded per 1 mL of resin at 75 cm/h) preequilibrated with tetrabutyl ammonium bromide (TBABr) to introduce tetrabutyl ammonium as MenX counterion, allowing the subsequent dissolution of the saccharide chains in dimethylformamide (DMF). To MenX OS at 2.5 mg/mL in DMF, TEMPO (0.06 eq respect to MenX repeating units), NaHCO3 (9 eq respect to MenX repeating units), and trichloroisocyanuric acid (TCC) (2 eq respect to MenX repeating units) were added, and the suspension was incubated at 0 °C overnight with stirring. The reaction mixture was centrifuged (8,000 × g at 20 °C for 15 min), and the supernatant, containing the oxidized MenX, purified by precipitation, adding 1 M NaCl at a final concentration of 200 mM and acetone to 90% (vol/vol) in the final solution. The pellet was dissolved in water and desalted against water on a SEC Sephadex G-15 column (∼0.3 mg of OS loaded per 1 mL of resin at 30 cm/h).
For protein conjugation, CRM197 and NaH2PO4 buffer, pH 7.2, were added to oxidized MenX with a resulting protein concentration of 10 mg/mL, final buffer capacity of 10 mM, and a wt/wt ratio of saccharide to CRM197 of 4:1. NaBH3CN was added 1:1 in weight to the OS. The reaction was slowly stirred at 37 °C for 72 h. The conjugate was designated as MenXOX–CRM197.
Derivatization of the reducing end with NH4OAc or ADH by reductive amination, followed by reaction of with SIDEA and conjugation with CRM197 (Fig. 1 B and C).
The procedure developed for meningococcal serogroup A, C, W135, and Y OS was adapted to MenX OS (avDP 15–20) (35), as detailed in SI Materials and Methods.
Purification and characterization of MenX–CRM197 conjugates.
After verifying conjugate formation by SDS/PAGE and HPLC-SEC analysis, all of the conjugates were purified by size exclusion chromatography on a 1.6 × 90-cm SEC Sephacryl S-300 HR column eluted at 0.5 mL/min in 50 mM NaH2PO4, 150 mM NaCl, pH 7.2. Purified material was 0.2-μm filtered and stored at −20 °C. Methods used for MenX–CRM197 conjugates characterization are detailed in SI Materials and Methods.
Immunization Protocol and Serum Analysis.
All animal protocols were approved by the local animal ethical committee and by the Italian Ministry of Health in accordance with Italian law. Groups of 16 female BALB/c mice (5–6 wk old) were immunized on days 1, 14, and 28 with 0.1 or 1 µg of MenX conjugated saccharide antigen formulated in PBS buffer, pH 7.2, with aluminum phosphate as adjuvant (0.12 mg of Al3+-based content). All immunizations were performed by administering 200 μL of vaccine dilution via the s.c. route. Adjuvant alone was used for the negative control group. Sera were collected at days 0 (preimmune serum), 27 (post 2 serum), and 42 (post 3 serum). Specific anti-PS antibody titers and functional antibodies were estimated 2 wk after the second and the third immunization by IgG ELISA and SBA assay.
Supplementary Material
Acknowledgments
We thank Federica Sponga and Concetta M. Cicala of Novartis Vaccines and Oliver Koeberling of the Novartis Vaccines Institute for Global Health for support with meningococcal X strain selection and for optimization of bacterial growth conditions. We thank Prof. D. A. Caugant (Norwegian Institute of Public Health) for the serogroup X isolates BF7/07 and Kenya 1/06.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314476110/-/DCSupplemental.
References
- 1.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–B63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 2.Marc LaForce F, Ravenscroft N, Djingarey M, Viviani S. Epidemic meningitis due to Group A Neisseria meningitidis in the African meningitis belt: A persistent problem with an imminent solution. Vaccine. 2009;27(Suppl 2):B13–B19. doi: 10.1016/j.vaccine.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 3.Decosas J, Koama JB. Chronicle of an outbreak foretold: Meningococcal meningitis W135 in Burkina Faso. Lancet Infect Dis. 2002;2(12):763–765. doi: 10.1016/s1473-3099(02)00455-3. [DOI] [PubMed] [Google Scholar]
- 4.Boisier P, et al. Meningococcal meningitis: Unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis. 2007;44(5):657–663. doi: 10.1086/511646. [DOI] [PubMed] [Google Scholar]
- 5.Delrieu I, et al. Emergence of epidemic Neisseria meningitidis serogroup X meningitis in Togo and Burkina Faso. PLoS One. 2011;6(5):e19513. doi: 10.1371/journal.pone.0019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutonga DM, et al. Epidemiology and risk factors for serogroup X meningococcal meningitis during an outbreak in western Kenya, 2005–2006. Am J Trop Med Hyg. 2009;80(4):619–624. [PubMed] [Google Scholar]
- 7. Available at http://www.meningvax.org/files/BulletinMeningite2011_S44_47.pdf.
- 8.Djibo S, et al. Outbreaks of serogroup X meningococcal meningitis in Niger 1995–2000. Trop Med Int Health. 2003;8(12):1118–1123. doi: 10.1046/j.1360-2276.2003.01126.x. [DOI] [PubMed] [Google Scholar]
- 9.Frasch CE, Preziosi M-P, LaForce FM. Development of a group A meningococcal conjugate vaccine, MenAfriVac(TM) Hum Vaccin Immunother. 2012;8(6):715–724. doi: 10.4161/hv.19619. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood B. The changing face of meningococcal disease in West Africa. Epidemiol Infect. 2007;135(5):703–705. doi: 10.1017/S0950268807008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leimkugel J, et al. Clonal waves of Neisseria colonisation and disease in the African meningitis belt: Eight- year longitudinal study in northern Ghana. PLoS Med. 2007;4(3):e101. doi: 10.1371/journal.pmed.0040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maiden MC, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197(5):737–743. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristiansen PA, et al. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis. 2013;56(3):354–363. doi: 10.1093/cid/cis892. [DOI] [PubMed] [Google Scholar]
- 14.Gagneux S, et al. Clonal groupings in serogroup X Neisseria meningitidis. Emerg Infect Dis. 2002;8(5):462–466. doi: 10.3201/eid0805.010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagneux SP, et al. Prospective study of a serogroup X Neisseria meningitidis outbreak in northern Ghana. J Infect Dis. 2002;185(5):618–626. doi: 10.1086/339010. [DOI] [PubMed] [Google Scholar]
- 16.Gasparini R, Panatto D. Meningococcal glycoconjugate vaccines. Hum Vaccin. 2011;7(2):170–182. doi: 10.4161/hv.7.2.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An Acad Bras Cienc. 2005;77(2):293–324. doi: 10.1590/s0001-37652005000200009. [DOI] [PubMed] [Google Scholar]
- 18.Costantino P, Rappuoli R, Berti F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin Drug Discov. 2011;6(10):1045–1066. doi: 10.1517/17460441.2011.609554. [DOI] [PubMed] [Google Scholar]
- 19.Kelly DF, et al. CRM197-conjugated serogroup C meningococcal capsular polysaccharide, but not the native polysaccharide, induces persistent antigen-specific memory B cells. Blood. 2006;108(8):2642–2647. doi: 10.1182/blood-2006-01-009282. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald NE, et al. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: A randomized controlled trial. JAMA. 1998;280(19):1685–1689. doi: 10.1001/jama.280.19.1685. [DOI] [PubMed] [Google Scholar]
- 21.Bröker M, Fantoni S. Meningococcal disease: A review on available vaccines and vaccines in development. Minerva Med. 2007;98(5):575–589. [PubMed] [Google Scholar]
- 22.Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines. 2011;10(3):307–322. doi: 10.1586/erv.11.8. [DOI] [PubMed] [Google Scholar]
- 23.Richmond P, et al. Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. J Infect Dis. 2000;181(2):761–764. doi: 10.1086/315284. [DOI] [PubMed] [Google Scholar]
- 24.Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: Database analysis. BMJ. 2003;326(7385):365–366. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granoff DM, Feavers IM, Borrow R. In: Vaccines. 4th Ed. Plotkin SA, Orenstein WA, editors. Philadelphia: Elsevier; 2004. pp. 959–987. [Google Scholar]
- 26.Evans JR, Artenstein MS, Hunter DH. Prevalence of meningococcal serogroups and description of three new groups. Am J Epidemiol. 1968;87(3):643–646. doi: 10.1093/oxfordjournals.aje.a120854. [DOI] [PubMed] [Google Scholar]
- 27.Bories S, Slaterus KW, Faucon R, Audiffren P, Vandederdove M. Peut-on individualiser deux nouveaux groupes serologiques de Neisseria meningitidis? Med Trop. 1966;26:603–616. [Google Scholar]
- 28.Bundle DR, Jennings HJ, Kenny CP. An improved procedure for the isolation of meningococcal, polysaccharide antigens, and the structural determination of the antigen from serogroup X. Carbohydr Res. 1973;26(1):268–270. doi: 10.1016/s0008-6215(00)85053-3. [DOI] [PubMed] [Google Scholar]
- 29.Plescia OJ, Braun W, Palczuk NC. Production of antibodies to denatured deoxyribonucleic acid (DNA) Proc Natl Acad Sci USA. 1964;52(2):279–285. doi: 10.1073/pnas.52.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bundle DR, Smith IC, Jennings HJ. Determination of the structure and conformation of bacterial polysaccharides by carbon 13 nuclear magnetic resonance. Studies on the group-specific antigens of Neisseria meningitidis serogroups A and X. J Biol Chem. 1974;249(7):2275–2281. [PubMed] [Google Scholar]
- 31.Xie O, et al. Characterization of size, structure and purity of serogroup X Neisseria meningitidis polysaccharide, and development of an assay for quantification of human antibodies. Vaccine. 2012;30(40):5812–5823. doi: 10.1016/j.vaccine.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 32.Garrido R, et al. Quantitative proton nuclear magnetic resonance evaluation and total assignment of the capsular polysaccharide Neisseria meningitidis serogroup X. J Pharm Biomed Anal. 2012;70:295–300. doi: 10.1016/j.jpba.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Berti F, et al. Relative stability of meningococcal serogroup A and X polysaccharides. Vaccine. 2012;30(45):6409–6415. doi: 10.1016/j.vaccine.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 34.Bröker M, Costantino P, DeTora L, McIntosh ED, Rappuoli R. Biochemical and biological characteristics of cross-reacting material 197 CRM197, a non-toxic mutant of diphtheria toxin: Use as a conjugation protein in vaccines and other potential clinical applications. Biologicals. 2011;39(4):195–204. doi: 10.1016/j.biologicals.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Costantino P, et al. Development and phase 1 clinical testing of a conjugate vaccine against meningococcus A and C. Vaccine. 1992;10(10):691–698. doi: 10.1016/0264-410x(92)90091-w. [DOI] [PubMed] [Google Scholar]
- 36.Micoli F, et al. Production of a conjugate vaccine for Salmonella enterica serovar Typhi from Citrobacter Vi. Vaccine. 2012;30(5):853–861. doi: 10.1016/j.vaccine.2011.11.108. [DOI] [PubMed] [Google Scholar]
- 37.Micoli F, et al. Meningococcal X polysaccharide quantification by high-performance anion-exchange chromatography using synthetic N-acetylglucosamine-4-phosphate as standard. Anal Biochem. 2013;442(2):259–261. doi: 10.1016/j.ab.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Chen PS, Toribara TY, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–1758. [Google Scholar]
- 39.Micoli F, et al. O:2-CRM(197) conjugates against Salmonella Paratyphi A. PLoS One. 2012;7(11):e47039. doi: 10.1371/journal.pone.0047039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beuvery EC, van Rossum F, Nagel J. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect Immun. 1982;37(1):15–22. doi: 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guttormsen HK, et al. Rational chemical design of the carbohydrate in a glycoconjugate vaccine enhances IgM-to-IgG switching. Proc Natl Acad Sci USA. 2008;105(15):5903–5908. doi: 10.1073/pnas.0710799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granoff DM, et al. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin Diagn Lab Immunol. 1998;5(4):479–485. doi: 10.1128/cdli.5.4.479-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amir J, Liang X, Granoff DM. Variability in the functional activity of vaccine-induced antibody to Haemophilus influenzae type b. Pediatr Res. 1990;27(4 Pt 1):358–364. doi: 10.1203/00006450-199004000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Schlesinger Y, Granoff DM. The Vaccine Study Group Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA. 1992;267(11):1489–1494. [PubMed] [Google Scholar]
- 45.Xie O, Pollard AJ, Mueller JE, Norheim G. Emergence of serogroup X meningococcal disease in Africa: Need for a vaccine. Vaccine. 2013;31(27):2852–2861. doi: 10.1016/j.vaccine.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 46.Pace D. Glycoconjugate vaccines. Expert Opin Biol Ther. 2013;13(1):11–33. doi: 10.1517/14712598.2012.725718. [DOI] [PubMed] [Google Scholar]
- 47. [No authors listed] (2010) A new conjugate meningococcal vaccine (Menveo). Med Lett Drugs Ther 52(1343):59-60. [PubMed]
- 48. [No authors listed] (2005) Menactra: A meningococcal conjugate vaccine. Med Lett Drugs Ther 47(1206):29-31. [PubMed]
- 49.Papaevangelou V, Spyridis N. MenACWY-TT vaccine for active immunization against invasive meningococcal disease. Expert Rev Vaccines. 2012;11(5):523–537. doi: 10.1586/erv.12.32. [DOI] [PubMed] [Google Scholar]
- 50.Bundle DR, Jennings HJ, Kenny CP. Studies on the group-specific polysaccharide of Neisseria meningitidis serogroup X and an improved procedure for its isolation. J Biol Chem. 1974;249(15):4797–4801. [PubMed] [Google Scholar]
- 51.Robinson JA, Apicella MA. Isolation and characterization of Neisseria meningitidis groups A, C, X, and Y polysaccharide antigens. Infect Immun. 1970;1(1):8–14. doi: 10.1128/iai.1.1.8-14.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seppälä I, Mäkelä O. Antigenicity of dextran-protein conjugates in mice. Effect of molecular weight of the carbohydrate and comparison of two modes of coupling. J Immunol. 1989;143(4):1259–1264. [PubMed] [Google Scholar]
- 53.Beuvery EC, vd Kaaden A, Kanhai V, Leussink AB. Physicochemical and immunological characterization of meningococcal group A polysaccharide-tetanus toxoid conjugates prepared by two methods. Vaccine. 1983;1(1):31–36. doi: 10.1016/0264-410x(83)90010-5. [DOI] [PubMed] [Google Scholar]
- 54.Carmenate T, et al. Effect of conjugation methodology on the immunogenicity and protective efficacy of meningococcal group C polysaccharide-P64k protein conjugates. FEMS Immunol Med Microbiol. 2004;40(3):193–199. doi: 10.1016/S0928-8244(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 55.Richmond P, et al. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J Infect Dis. 2001;183(1):160–163. doi: 10.1086/317646. [DOI] [PubMed] [Google Scholar]
- 56.Halperin SA, et al. Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2–10 years of age. Vaccine. 2010;28(50):7865–7872. doi: 10.1016/j.vaccine.2010.09.092. [DOI] [PubMed] [Google Scholar]
- 57.Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine. 2009;27(Suppl 2):B112–B116. doi: 10.1016/j.vaccine.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 58.Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol. 2003;10(5):780–786. doi: 10.1128/CDLI.10.5.780-786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.