Significance
Comparing human blood cell types, nuclear diversity is visually striking but unexplained: quasi-spherical nuclei in stem/progenitor cells and T cells contrast with multilobed nuclei in neutrophils, giant nuclei in megakaryocytes, and anuclear erythrocytes. We hypothesized broad roles for the major nuclear structure proteins—lamins—and developed mass spectrometry-calibrated intracellular flow cytometry to quantify lamin-A:B ratios. This ratio controls both nuclear viscoelasticity and cell trafficking across microporous barriers. High A:B rigidifies erythroblast nuclei to favor marrow retention and also enhances erythropoiesis. Intermediate A:B enhances thrombopoiesis and opposes cell division to favor marrow anchorage of megakaryocytes. Human stem/progenitor cells have moderate lamin levels and reside in marrow whereas white cells are favored by low lamins and predominantly circulate.
Keywords: rheology, biophysics, hematopoiesis, nucleus, mechanobiology
Abstract
Hematopoietic stem and progenitor cells, as well as nucleated erythroblasts and megakaryocytes, reside preferentially in adult marrow microenvironments whereas other blood cells readily cross the endothelial barrier into the circulation. Because the nucleus is the largest organelle in blood cells, we hypothesized that (i) cell sorting across microporous barriers is regulated by nuclear deformability as controlled by lamin-A and -B, and (ii) lamin levels directly modulate hematopoietic programs. Mass spectrometry-calibrated intracellular flow cytometry indeed reveals a lamin expression map that partitions human blood lineages between marrow and circulating compartments (P = 0.00006). B-type lamins are highly variable and predominate only in CD34+ cells, but migration through micropores and nuclear flexibility in micropipette aspiration both appear limited by lamin-A:B stoichiometry across hematopoietic lineages. Differentiation is also modulated by overexpression or knockdown of lamins as well as retinoic acid addition, which regulates lamin-A transcription. In particular, erythroid differentiation is promoted by high lamin-A and low lamin-B1 expression whereas megakaryocytes of high ploidy are inhibited by lamin suppression. Lamins thus contribute to both trafficking and differentiation.
Hematopoietic cells that enter the circulation are seen to squeeze through small pores in the basement membrane and endothelium that partition bone marrow and blood (1). Retention within the marrow niche as well as trafficking into the circulation might therefore be regulated by cell deformability and the structural molecules responsible for it. Indeed, human polymorphonuclear neutrophils (PMNs) were shown decades ago to become more deformable upon differentiation in the marrow (2), with mature PMNs more capable of entering and exiting small capillaries (3). Leukemic cells are more rigid than normal, potentially explaining the interrupted blood flow and marrow hypercellularity in disease (4). Normal hematopoiesis has a well-characterized hierarchy, but it is unclear whether deformability factors into the program (3). Importantly, because of the high nucleus-to-cytoplasm ratio of hematopoietic cells, key processes such as sorting between marrow and blood could be based in part on nuclear deformability (Fig. 1A).
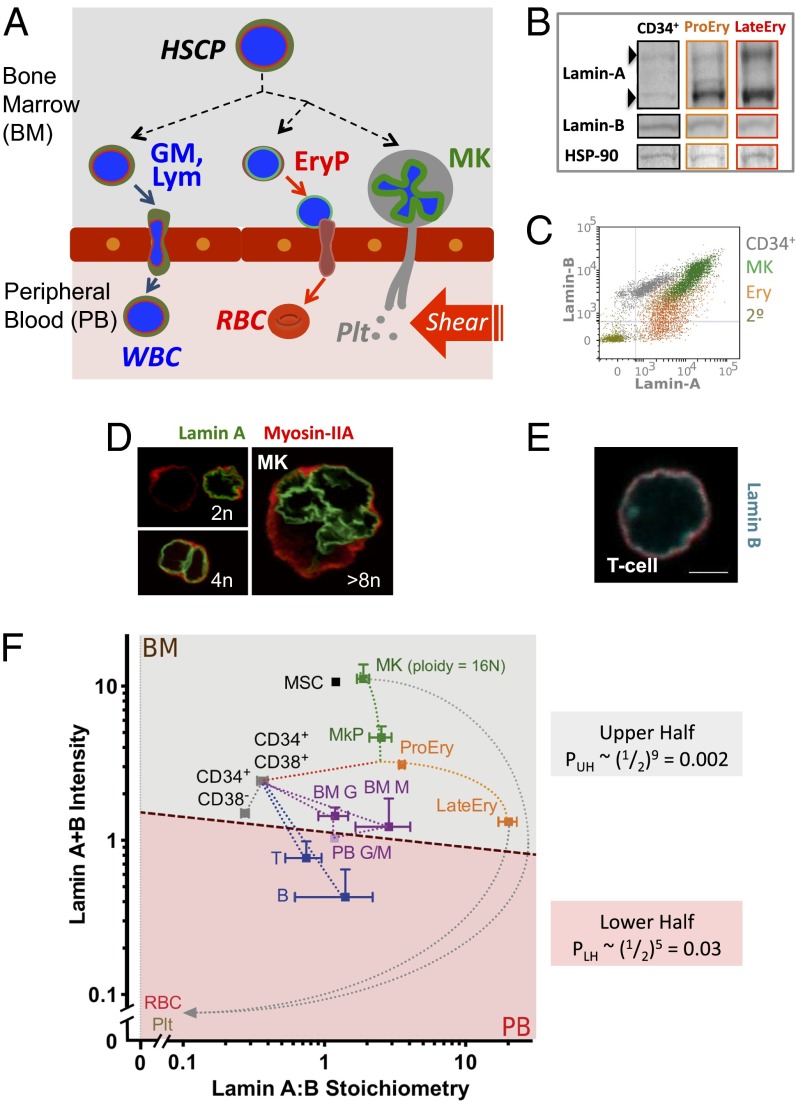
Fig. 1.
Lamin map of human hematopoiesis. (A) Hematopoietic cells are mostly in marrow or blood, and only a fraction of cells transmigrate through the endothelium with or without their nucleus. EryP, erythroid progenitors; GM, granulocytes and monocytes; HSCP, hematopoietic stem cell and progenitors; Lym, lymphocytes; MK, megakaryocytes; RBC, red blood cells; WBC, white blood cells. (B) Validation of flow cytometry by Western blot with cell numbers adjusted to give similar intensities of B-type lamins. Lamin-A,C splice-forms and B-type lamins are shown for fresh CD34+, early and late erythroid cells; densitometry shows rough agreement with MS-IF results. (C) Representative intracellular flow cytometry scatterplots show lamin-A (Alexa-488) and B-type lamin (Alexa-647) expression for indicated cells. (D) Confocal images of MKs immunostained for lamin-A (green) and myosin-II (red). (E) Confocal image of T cell immunostained for B-type lamins (cyan) and myosin-II (red) (Scale bar: 5 μm.) (F) Lamin-A relative to B-type lamins, transformed to a measurable A:B ratio versus calibrated sum intensity A+B (a.u.); on log scales, these are the respective difference and sum of chemical potentials for A and B (Fig. S2A). Mean fluorescent intensity of lamin for each subpopulation from flow cytometry was calibrated to an absolute ratio from MS analyses of a standard A549 cell line (lamin-A:B = 2.3) (Fig. S1). The dashed line schematically illustrates the semipermeable barrier between bone marrow (BM) and peripheral blood (PB), and the net probability of partitioning based on lamin expression can be estimated from the upper half and lower half probabilities: PTOT = PUH PLH = 0.00006. Measurements are mean ± SEM of n ≥ 3, with error bars omitted if <5% of mean. BM G, BM granulocytes (CD33mid); BM M, BM monocytes (CD33hi); CD34+CD38−, early progenitors; CD34+CD38+, common progenitors; LateEry, late erythroblasts (CD44−GPA+); MK, polyploid MKs (average 16N); MKP, MK progenitors (CD34−CD41+); MSC, mesenchymal stromal cells; PB G/M, PB granulocytes/monocytes; Plt, platelets; ProEry, proerythroblasts (CD44+GPA−); RBC, red blood cells; T, B, lymphoids. Representative MSC results from one donor are shown because the variation in A:B ratios between donors and cultured cells was minimal.
Lamins are intermediate filament proteins that assemble into “lamina” networks at the interface between chromatin and the inner nuclear membrane (5), conferring stiffness to the nucleus (6). In addition, the lamina is often proximal to heterochromatin, and, at least with embryonic stem cells, some genes alter their interactions with the lamina during cell-fate determination (7). In nearly all mammalian cells, A-type lamins (splice-forms A and C from LMNA) and B-type lamins (from LMNB1 and LMNB2) are detectable. In blood progenitors versus various mature cells, past studies appear at odds, even for the same cell type in reporting either decreased levels of both lamins (8–10) or else increased levels (11). One dramatic mutation in human LMNA leads to the accelerated aging syndrome Progeria (5), in which protein accumulates at the nuclear envelope and stiffens it (12), affecting many tissues and increasing platelet numbers by twofold or more (13). Mice with a large deletion in LMNA survive 6 wk postnatal (14), with defective lymphocytes (15), whereas mice deficient in the lamina-associated polypeptide 2α show hyperproliferation of erythroid progenitors and impaired differentiation (16). Relatively few mutations in B-type lamins have been reported (5), but defective lamin-B receptor in Pelger–Huet anomaly is characterized by hyposegmentation of neutrophils (17), defective chemotaxis, abnormal granulocytic differentiation, and also elevated lamin-A (18). Direct roles for lamins in normal human hematopoiesis, trafficking, and rheology otherwise remain unclear.
The synthesis and degradation of lamins is understudied in hematopoiesis. However, it is known that the lamin-A promoter has a retinoic acid (RA)-responsive element (19), and RA therapy for acute promyelocytic leukemia stimulates granulocyte differentiation (20) and decreases lamin-A expression, consistent with the early report of increased deformability of normal mature PMN (2). T cells also up-regulate lamin-A upon stimulation with phytohemagglutinin (21) although a functional effect is unknown. B-type lamins undergo proteolytic cleavage during early erythroid differentiation from burst forming unit-erythroid (BFU-E) and colony forming unit-erythroid (CFU-E) to proerythroblast (ProEry) stage via caspase-3 activation (22), and, in later stages, a distinct decrease in B-type lamins parallels the decrease in nuclear volume (23). The generality of such processes and their impact on nuclear flexibility are examined here.
High nuclear flexibility or compliance, with suitably low lamin levels, might facilitate migration of nucleated cells through constraining pores. On the other hand, the Erythroid (Ery) lineage undergoes nuclear condensation, which might stiffen the nucleus and limit trafficking but permit enucleated reticulocytes to egress more readily through small pores. Megakaryocytes (MK) undergo polyploidization, and the mature nucleus could be too large to pass through pores: such “nuclear anchorage” would permit MKs to extend motile membrane projections into blood so that shear fragmentation could produce platelets—as visualized recently (24). To investigate functional roles of lamins in differentiation-modulated trafficking, we began by determining the levels and stoichiometry of lamins in major lineages, uncovering a systematic expression map.
Results
Lamin-Based Lineage Map Sorts Marrow and Peripheral Blood.
Expression of both lamin-A and B-type lamins throughout hematopoiesis was assessed with antibodies in Western blotting, flow cytometry, and confocal microscopy (Fig. 1 B–E). To quantify lamin isoforms in terms of absolute stoichiometry within a given hematopoietic cell type (including rare cells), we applied a single-cell method of mass spectrometry-calibrated intracellular flow cytometry (MS-IF) (Fig. S1). In the flow cytometer, antibodies against surface markers first identify a cell type in a mixed population of cells whereas intracellular immunostaining for lamins provides relative intensity measures of lamins. Relative intensities are then calibrated by similar measurements on a standard cell line with lamin stoichiometry that has been quantified by mass spectrometry (Materials and Methods). Unlike solid tissues where B-type lamins are often “constitutive” (5), B-type lamins vary here by ∼30-fold in diploid cells whereas lamin-A varies much less (Table S1). MS-IF reveals the lowest lamin-A:B ratio ∼ 1:4 in CD34+ cells (mostly progenitors) whereas Erythroblasts (Ery) exhibit the highest A:B ratio [∼100-fold higher for LateEry: glycophorin A (GPA)+, CD44lo/−]. At the same time, the total intensity (A+B) is nearly constant as summarized in a “map” of sum-versus-ratio for lamins in hematopoietic cells (Fig. 1F). A modest two- to threefold decrease in A+B from ProEry (ploidy of 2n, and some premitotic 4n) to LateEry (2n) is consistent with loss of B-type lamins and a smaller nuclear area (23). Although this observation reveals an isoform switch from B to A before Ery enucleation, it establishes together with the low A:B ratio in CD34+ cells a very broad A:B range.
MK lineages show an intermediate A:B ratio, but, during maturation, they up-regulate both types (A+B) by up to 10-fold (Fig. 1F and Table S1). Even for MK progenitors (MKP) of low ploidy (2n, 4n cells), lamins increase about fivefold relative to CD34+CD38−. Further A+B increases in late MK (∼16n) are likely due to lamins accumulating in high ploidy nuclei because lamins interact with chromatin (Fig. 1D) (7). The extremes in lamin expression (A:B for Ery, and A+B for MK) seem consistent with the hypothesis, as examined by biophysical methods below, that nuclear rigidity and/or size limit nuclear entry into small pores and marrow sinusoids. As a consequence, human RBCs are enucleated, and the resultant lamin-less reticulocytes traverse the endothelium. Motile membrane projections likewise extend from MKs into blood, allowing platelets to be generated by blood-shear fragmentation.
Nucleated lineages that traffic into blood have lower lamin levels than Ery and MK. Lymphoid cells in blood show only ∼sixfold higher A:B ratio compared with CD34+ cells but nearly 10-fold lower A+B, with similar trends for granulocyte/monocyte (G/M) lineages (Fig. 1 E and F and Table S1). On the other hand, marrow-resident mesenchymal stromal cells (MSCs) are not found in circulation, and their total lamin levels are similar to late MK despite the low ploidy of MSCs.
In the lineage map of lamins (Fig. 1F), a simple line is drawn that separates all marrow-resident cells from all peripheral blood cells. The upper half of this map has the nine measured cell types that are predominantly in marrow, and the lower half has the five cell types that are predominantly in circulation (Tables S2–S4). The probability of such a simple partitioning with all laminhi in the marrow “Upper Half” (UH: PUH = 0.002) and all laminlo in the blood “Lower Half” (LH: PLH = 0.03) is estimated to be PTOT = 0.00006. The endothelium is, after all, a barrier that physically partitions two cellularized compartments, and what sets the probability of crossing that barrier is the ability of a given cell to move and deform through the small pores of the boundary (Fig. S2).
Lamin-A Limits 3D Migration of Nucleated Hematopoietic Cells.
Transendothelial migration involves large deformation as nucleated cells pass through much smaller pores (3), and we hypothesized that transwell filters with different size micropores could be used to show that lamin-A knockdown will enhance constrained migration (Fig. 2A). Aspiration of a cell into a micropipette illustrates the requisite nuclear deformation (e.g., G/M cells, Fig. 2B). Our previous aspiration experiments on a lung epithelial cell line showed that disruption of the cytoskeleton with or without lamin knockdown can reveal the contribution of lamins to nuclear properties (6). This previous study prompted a similar approach here, but with care to use low stresses commensurate with those generated by cells during migration (25). With G/M cells, lamin-A knockdown by ∼50% (Fig. S3A) indeed softens the nucleus ∼twofold (Fig. 2B). We hypothesized therefore that lamins in intact cells should also regulate migration through transwell filters. Using a broad range of filters, we tested whether migration would be impeded when cells squeeze through micropores similar to the size of blood capillaries (∼3 μm) whereas migration through large pores would be unaffected.
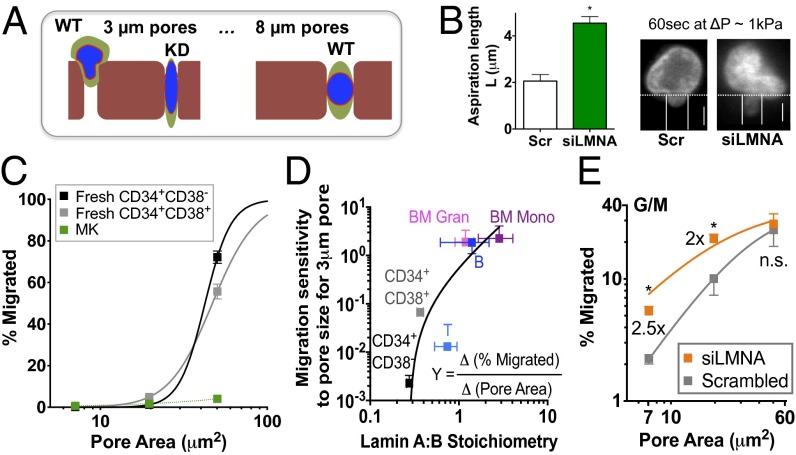
Fig. 2.
Lamin-A:B regulates transmigration of nucleated blood cells. (A) Schematic illustration of hypothesis that cell migration and nuclear deformation through sufficiently small micropores will be facilitated by lamin knockdown (KD). (B) Lamin-A confers nuclear stiffness, based on aspiration of G/M cells with or without knockdown with lamin-A siRNA (siLMNA). (Left) Quantitation of aspirated nuclear length (n = 3 donors, *P < 0.05). (Right) Representative image is for ΔP < 1 kPa at 60 s. (Scale bars: 5 μm.) (C) Migration of fresh CD34+ and MK cells. Cells were incubated on top of transwell with different pore sizes (3, 5, or 8 μm) with an SDF-1 (100 ng/mL) gradient for 4 h before analysis by flow cytometry to evaluate % migrated cells. Data were fit to y = a/[1 + b(x-xc)-m], where y = %migrated, x = pore area (μm2), a = 100 (% maximal migration when pore size is infinite), b relates to the half-maximum migration area (Fig. S3C), xc = critical pore area, m = Hill coefficient. Values for b, xc, and m for each cell type are as follows: CD34+CD38− (0.024, 1.44, and 5.4, respectively), CD34+CD38+ (0.022, 1.44, and 3.2, respectively), MK (0.0006, 0, and 0.9, respectively). (D) Lamin ratios predict migration sensitivity to pore size at small pores: the first derivative of y(x) was estimated for 3-μm pores and then fit to a power law yielding Y(at 3 μm) = 0.90 (A:B – 0.28)1.55 (R2 > 0.95). (E) Lamin-A limits 3D migration. Before transwell migration, cells were transfected with siLMNA for 3 d to give ∼50% knockdown (Fig. S3A). Results from granulocyte/macrophage cells derived from CD34+ culture are shown. Data fit per C. All results are mean ± SEM of n ≥ 3. *P < 0.05 in paired t test.
Migration of different hematopoietic lineages across the different filters was driven (in 4 h) by a standard chemotactic gradient of stromal derived factor-1 (SDF-1). For 3-μm pores, the percentage of cells that migrated is lowest for both CD34+ cells and MKs, but, for 8-μm pores, the majority of CD34+ cells cross the filter whereas the percentage of migrated MKs was relatively unchanged. Such transwell results seem qualitatively consistent with finite numbers of circulating CD34+ cells compared with no measurable MKs (Tables S2–S4). Given that the percentage of migration within a set assay time relates to migration velocity and also because pore area relates inversely to the requisite stress for migration through the constriction, the migration data are fit to a standard hyperbolic “Hill” equation for “velocity versus force” motility. The pore area for half-maximal migration of each lineage (A1/2) could thus be estimated. Although the general trend is that CD34+ and T-cells have a low A1/2, and the “extreme” cell types in the lineage map, such as high ploidy MKs, have a high A1/2 (Fig. 2C and Fig. S3B), this parameter correlates weakly with lamin A+B or A:B stoichiometry (Fig. S3C). Instead, the change in percent migration with change in pore size (Materials and Methods) reveals the migration sensitivity to pores, and the lineage-dependent migration sensitivity for small pores (3 μm) correlates well with lamin-A:B stoichiometry (Fig. 2D) (and not A+B, Fig. S3D). In other words, cells with lower A:B are less sensitive to pore size; conversely, cells with higher A:B are more sensitive to pore-size differences.
To determine whether lamin-A directly influences cell migration through a constraining micropore, siRNA knockdown was used. The percentage of CD34+-derived granulocyte/macrophage cells migrating through 3-μm or 5-μm pores increased by about twofold after knockdown (Fig. 2E), which approximates the measured softening in Fig. 2A. Importantly, no significant change was observed with 8-μm pores, which indicates that lamin-A limits migration of cells only through small pores. Knockdown of lamin-A did not change the expression levels of other proteins typically involved in migration through pores, such as myosin-II (26). These results indicate a major role for lamin-A in regulating pore trafficking.
Lamin Isoform Stoichiometry Predicts Nuclear Rheology.
Of the many studies of hematopoietic cell deformability (e.g., ref. 2), nuclear deformability has not yet been directly characterized and compared across lineages. We had shown that fresh human CD34+ cells are highly deformable compared with solid-tissue cell types (6), perhaps indicative of the stem cell/progenitor’s ability to traffic between marrow and circulation. The progressive increase in aspirated length of a nucleus at a constant pressure (Fig. 3A) gives the nuclear compliance and often fits a power law J(t) = a·tb that is typical for polymer rheology (6). The initial compliance is given by a, whereas b = 1 indicates a simple fluid that flows and does not recover after release of stress, and b = 0 indicates a simple elastic solid that will fully recover its shape.
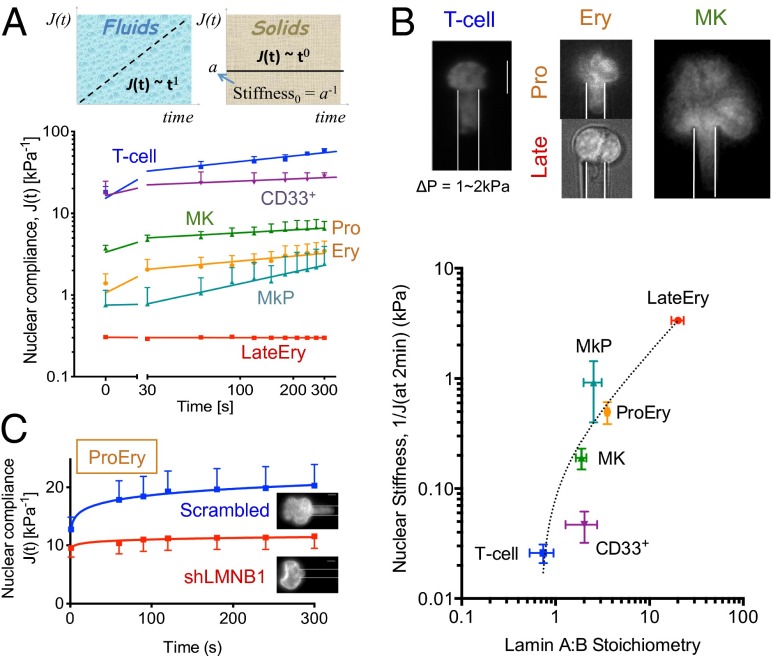
Fig. 3.
Lamin ratios predict nuclear stiffness in hematopoietic lineages. (A) Nuclear compliance change versus time at constant pressure ΔP = 0.3–6 kPa. (Upper) A power law fit, J(t) (kPa−1) = a·tb (t = sec) for each blood cell type, where (b = 1 for fluids), (b = 0 for solids). (Lower) Values for (a, b) in each cell type are as follows: (T cell: 15.2, 0.2), (CD33+: 16.4, 0.1), (MkP ≥ 30s: 0.16, 0.5), (MK: 3.4, 0.1), (ProEry: 1.1, 0.2), (LateEry: 0.3, 0). (B) High lamin-A:B correlates with stiff nuclei. (Upper) Images at 2 min of nuclear aspiration at ΔP = 1–2 kPa. (Scale bar: 5 μm.) (Lower) Correlation between nuclear stiffness (at 2 min) and lamin-A:B fits J (at 2 min) = 0.23(A:B – 0.68)0.91 (R2 = 0.96). All results are mean ± SEM of n ≥ 5 for each cell type. (C) Lamin-B1 knockdown stiffens nuclei in proerythroblasts, with no change in viable cell numbers (n = 10, P < 0.05). Nuclear compliance change with ΔP = 1–2 kPa where values for (a, b) in each sample are as follows: (scrambled: 12.8, 0.1), (shLMNB1: 9.4, 0.03). (Scale bars: 5 μm.)
Lymphoid and myeloid nuclei show high initial compliance (a = 20 kPa−1) (Fig. 3A), as predicted from their low lamin levels (Fig. 1B), and are roughly similar in this respect to fresh CD34+ cells (6). T-cell nuclei are also more viscous (b = 0.22) than myeloid nuclei (b = 0.08) (Fig. 3A), consistent with a previous report that granulocytes do not permanently deform as they pass through marrow (3). In contrast, Ery progenitors generally show at least 10-fold lower initial nuclear compliance (Fig. 3A). Although ProEry nuclei exhibit viscoelastic behavior similar to that of T-cell nuclei, late Ery nuclei are completely stiff and solid, consistent with their condensation during terminal differentiation. MK nuclei also have lower initial compliance than myeloid or lymphoid nuclei. Compared with MK progenitor nuclei (with b = 0.47), mature MK nuclei have 20-fold higher compliance despite being more solid-like (b = 0.11) (Fig. 3A). The large size and complex shape of polyploid MK nuclei, rather than compliance itself, are therefore likely to limit MK traffickability. Consistent with this, lamin-A:B stoichiometry correlates strongly with local nuclear stiffness [reciprocal of compliance 1/J(t) in Fig. 3B] measured at different time points (Fig. S4A), which is not the case for A+B intensity (Fig. S4B).
Given that B-type lamins localize to the lamina during erythroid differentiation (23) and that nuclear stiffness increases with lamin A:B (Fig. 3B), we hypothesized that down-regulation of B-type lamin(s) would effectively stiffen the nucleus during maturation. Typically, one expects that stiffness correlates positively with protein abundance, and so the prediction here is highly unusual for biopolymer physics. ProEry indeed shows decreased expression of B-type lamins compared with fresh CD34+ cells (Fig. 1F and Table S1), and nuclear stiffness of ProEry is high compared with circulating nucleated cells (Fig. 3A). To directly address the hypothesis, we knocked down lamin-B1, which is expressed at much higher levels than lamin-B2 in hematopoietic cells based on mass spectrometry analyses (Tables S5 and S6). In erythroid precursors, partial knockdown of lamin-B1 by RNAi (∼50%) (Fig. S4C) increases nuclear stiffness (Fig. 3C plot shows decreased compliance). Nuclear stiffening after lamin-B1 knockdown was also measured for a U251 glioma cell line in which the lamin-B1 isoform is only slightly more abundant and where knockdown-induced differentiation is unlikely (Fig. S4D). Lamin-B1 down-regulation in erythroid lineages is thus sufficient to promote nuclear stiffening, which is likely to favor marrow retention of a cell (Fig. 1). A preliminary study using pharmacological perturbations of erythropoietic pathways suggests the association with nuclear rigidification (Fig. S5).
Lamins Modulate Differentiation: Erythroid Lineage.
Recent studies of a few primary cells have shown significant modulation of differentiation by lamins. In particular, lamin-A overexpression in marrow-derived MSCs increases osteogenesis (27) whereas knockdown promotes adipogenesis (28). However, lamin knockout mice develop all tissue types before succumbing at birth or shortly after (14, 29), and so most or all tissue lineages seem to develop to some extent, which implies lamins function as lineage maturation factors. Here, we manipulated lamin levels in those hematopoietic lineages with extreme expression, Ery and MK, to assess direct modulation of differentiation (Fig. 4A).
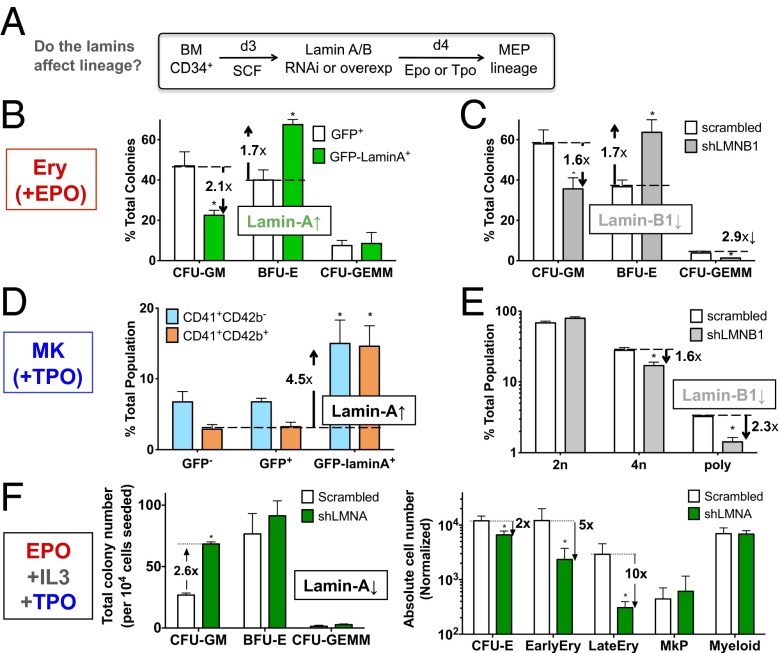
Fig. 4.
Lamins regulate erythroid and MK differentiation. (A) Scheme for in vitro differentiation. MEP, myeloid and erythroid progenitor; SCF, stem cell factor. Epo is erythropoietin and induces erythropoiesis; Tpo is thrombopoietin and induces MK-poiesis. (B and C) Colony-forming assays in methylcellulose medium show a shift to erythroid progenitors after (B) overexpression of GFP-lamin-A (∼40% transfection efficiency) or (C) knockdown of lamin-B1 (shLMNB1, ∼50% efficiency) in the presence of Epo for 3 d. *P < 0.05, GFP vs. GFP-lamin-A or scrambled vs. shLMNB1 (n = 3). (D and E) MK progenitor population enumerated by flow cytometry with CD41 and CD42b. (D) MK progenitors increase with lamin-A overexpression. CD41+CD42b−, early progenitor; CD41+CD42b+, late progenitor. *P < 0.05, GFP+ vs. GFP-lamin-A+ for each progenitor (n = 4). (E) Lamin-B1 knockdown decreases average MK ploidy. *P < 0.01, scrambled vs. shLMNB1 (n = 3). (F) Lamin-A is required for erythroid differentiation and restricts myeloid progenitor number. Cells were transduced with either scrambled or lamin-A shRNA (shLMNA) and cultured in the presence of Epo, IL-3 and Tpo. Functional progenitors (CFU-GM, BFU-E, CFU-GEMM) were quantified by colony-forming assay (Left) whereas differentiated subpopulations were quantified by flow cytometry as per ref. 39 (Right), normalized by 104 initial cell number. CFU-E, CD34−CD36+IL-3R−; EarlyEry, CD44+GPA+; LateEry, CD44−GPA+; MkP, CD41+; Myeloid, CD33+. *P < 0.05, scrambled vs. shLMNA.
Overexpression of lamin-A by ∼threefold in CD34+ cells (Fig. S6A) is close to the increased level measured during erythropoiesis (Fig. 1F) and resulted in a significant shift toward BFU-E from colony forming unit (CFU)-granulocyte and macrophage (GM) (Fig. 4B), consistent with the map (Fig. 1F). The multipotent CFU-granulocyte erythroid macrophage megakaryocyte (GEMM) was unaffected (Fig. 4B). B-type lamins decrease during erythroid development (22, 23) (Fig. 1F), and knockdown of lamin-B1 by RNAi in early hematopoietic progenitors indeed favors BFU-E (Fig. 4C). In addition, lamin-B1 RNAi decreases CFU-GEMM in contrast to lamin-A overexpression, revealing lamin-B1 as more influential to the maintenance of multipotent progenitors. The results thus show that lamins regulate lineage specification induced by soluble factors.
Lamins Modulate Differentiation: MK Lineage.
MKs normally show an increase in total lamin levels (Fig. 1F), but also an increase in A:B ratio. Overexpression of lamin-A indeed increases the number of both early and late MK progenitors cultured from BM CD34+ cells based on surface markers (Fig. 4D). Furthermore, although lamin-B1 knockdown suppresses polyploid and 4n populations (Fig. 4E), overexpression of lamin-A enhances polyploidy (Fig. S6B). Because Progeria patients reportedly have twofold more platelets in circulation (13), we also tested the impact of the progerin mutant (Δ50) on MK polyploidy. Overexpression of progerin, which tends to drive lamin accumulation at the envelope (12), does not greatly enhance the number of 8n MKs, but both wild-type and mutant overexpression increase high ploidy (≥16n) MKs (Fig. S6B). The finding here that overexpression of both wild-type and progerin lamin-A gave the same phenotype is consistent with similar findings of increased osteogenesis in MSCs (27), suggesting a common mechanism of increased lamina assembly.
Phosphorylation of lamin-A promotes disassembly and is high during cell division (30), and so we hypothesized that an S22A mutant that cannot be phosphorylated would limit nuclear division and again facilitate polyploidization. Overexpression of this mutant indeed shows a greater gain in 8n MKs than WT (Fig. S6B). Overall, the MK results suggest that, whereas lamina regulatory mechanisms might play a role during the formation of early polyploid MKs, the accumulation of lamin-A content per se appears sufficient to drive the formation of late polyploidy MKs through unknown mechanisms.
Lamin-A Modulates Lineage Decisions.
Lamin-A knockdown with shRNA (∼75% efficiency) (Fig. S6C) was performed to assess fate choices in the presence of a cytokine mixture that promotes multiple lineages (myeloid, erythroid, and MK). In such cultures, knockdown increases the total number of CFU-GM (Fig. 4F, Left) but decreases CFU-E and differentiated erythroid lineages without affecting BFU-E or the other differentiated lineages (Fig. 4F, Right). Together with the restriction of BFU-E by lamin-B1 (Fig. 4C), the results confirm predictions from the lamin map (Fig. 1F) that lamin-A and B1 play opposite roles in erythroid differentiation.
Lamin-A Is Modulated by Physiological Agonists in Differentiation.
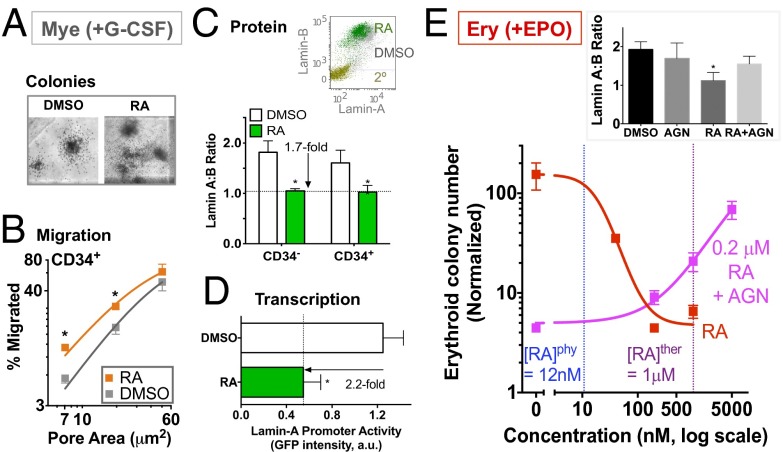
Retinoic acid (RA) in human serum normally is about 12 nM, and the lamin-A promoter is known to have multiple RA-responsive elements (19). RA is also known to promote terminal differentiation of granulocytes and self-renewal of hematopoietic stem cells while decreasing erythroid progenitor numbers (30, 31). Indeed, the RA receptor antagonist AGN194310 (AGN) increases granulocyte progenitors in mice by twofold, by blocking differentiation, with no significant effect on other lineages (31). Here, sustained RA treatment not only enhances both the number and size of myeloid colonies under G-CSF (Fig. 5A and Fig. S6 D and E), but also enhances migration of CD34+ progenitors through 3- to 5-μm pores (Fig. 5B). The ∼twofold effect is similar to the gain seen with direct lamin-A knockdown (Fig. 2E) and once again shows no significant impact on migration through the largest, nonconstraining pores (8 μm) (Fig. 5B). Indeed, RA represses lamin-A but not B-type lamin protein expression by ∼twofold for both CD34+ and CD34− cells (Fig. 5C). As predicted from the previous promoter analysis (19), the repression (∼twofold) occurs at the transcriptional level as indicated by lamin-A promoter activity measured here (Fig. 5D). The results are consistent with the role of lamin-A in suppressing the myeloid progenitor number (Fig. 4F, Left).
Fig. 5.
Lamin-A levels change with physiological agonists. (A) Retinoic Acid (RA) modulates lamin-A in myeloid (Mye) differentiation. CD34+-derived cells treated with either DMSO or RA (1 μM) for 3 d with G-CSF (10 ng/mL) before assays. CFU-GM colonies are more abundant and larger with RA (Fig. S6 D and E). (B) Effect of RA on transwell migration of CD34+ cells. *P < 0.05 (n = 3) for DMSO vs. RA in paired t tests (unless noted). (C) RA down-regulates lamin-A protein expression for both CD34− and CD34+ cells. (Inset) A representative flow cytometry plot (2°, secondary alone). *P < 0.01, DMSO vs. RA (n = 4). (D) RA represses lamin-A transcription. Cells were transiently transfected with a GFP reporter construct driven by a human lamin-A promoter, consisting of the 1,132-base pair upstream region and the first 385-base pair mRNA transcript region, followed by drug treatment. *P < 0.05 (n = 4). (E) RA modulates lamin-A in erythroid (Ery) differentiation. Absolute values of erythroid colony numbers were normalized to initial cell input and fit to dose–response curves. (IC50 or EC50, Hill coefficient) for: RA (20 nM), AGN in the presence of 0.2 μM RA (250 nM). R2 > 0.9. (n = 3). (Inset) Lamin A:B ratios with drug treatment (AGN: 5 μM, RA: 0.2 μM). *P < 0.05, one-way ANOVA (n = 3). All results are mean ± SEM.
The effects of RA on lamin-A levels seem to also apply to erythroid lineages, repressing lamin-A when CD34+ cells are treated with Epo supplemented medium (Fig. 5E, Inset). Consistent with the lamin map (Fig. 1F), RA suppresses erythroid colony formation in a dose-dependent manner (Fig. 5E): the ∼12 nM RA in human plasma is insufficient to suppress erythroid number, but a therapeutic dose for acute promyelocytic leukemia (∼1 μM peak in plasma) (20) inhibits erythroid differentiation ∼30-fold. AGN rescues both erythroid colony number and lamin A:B ratios in the presence of RA, but AGN has no effect on its own (Fig. 5E). Because pharmacological actions on nuclear receptors involve cell type-specific cofactors (32), AGN as a neutral antagonist in erythroid differentiation could reflect differences in cofactor expression. The results nonetheless indicate that RA regulates lamin-A, and the knockdown and overexpression studies indicate that lamin-A changes are sufficient to influence lineage.
Discussion
The epigenetic changes of lineage specification are likely affected by the nuclear lamina, which might selectively, if indirectly, interact with different chromatin regions during differentiation (7). Here, we show roles of the lamins in deformability-based sorting as well as lineage induction of blood cells. This characterization of lamin expression stoichiometry in freshly isolated hematopoietic cells from human marrow and blood demonstrates that absolute lamin A:B ratios correlate with nuclear stiffness (Fig. 3B). Although prior studies suggest that lamin-A is “below the detection limit” in terminally differentiated myeloid and lymphoid cells (8), another study suggested that it is restricted to the late stage of lymphoid development but not the early (11). Some studies suggest that B-type lamins are constitutive (9, 11) whereas other studies indicate that they are down-regulated in primary neutrophils (10). One study also acknowledges that, whereas blood granulocytes have much lower levels of both lamin-A and -B than the granulocytic HL-60 cell line, both lamins are detectable by confocal microscopy with distinct polymorphonuclear morphology (10). Our efforts to take a standardized approach with MS-IF show that A- and B-type lamins are generally present across different blood-cell types and at different levels. Although our data support some prior studies that total lamin expression is significantly decreased in both lymphoid and myeloid lineages upon differentiation from progenitors (CD34+CD38+), lamin A:B ratios are increased to ∼1 (Fig. 1F) and correlate with the known distribution of these lineages in marrow versus blood (Tables S2–S4).
Because most small openings between endothelial cells in marrow sinusoids are filled by marrow cells in situ (3), nuclear stiffness seems a reasonable limiting factor. Although it was previously appreciated that granulocytes are highly traffickable due to their unique polymorphonuclear morphology, our study reveals that T cells may be even more amenable to trafficking than granulocytes due to (i) lower lamin A:B ratio (Fig. 1F), (ii) lower marrow:blood cell ratio (Tables S2–S4), (iii) lower nuclear stiffness (Fig. 3B), and (iv) lower half-maximal pore area for migration (Fig. S3C). In contrast, although MK nuclei remain deformable (Fig. 3A), their size and complex morphology generally limit MK migration through small pores. Intact MKs could therefore be occasionally observed in circulation, but further chromatin condensation after exhaustive platelet generation would tend to inhibit traffickability (3). Flow-cytometry analyses of what crosses the micropore filters in our MK experiments indeed show that many more small-cell fragments (e.g., platelet-like particles) traffic across than cells (Fig. S7), consistent with the platelet-generating scheme in Fig. 1A.
Although lamin-A,C splice-form ratios require further study, a major finding here is that lamin-B1 regulates lineage specification as well as nuclear stiffening. Consistent with wide variation in lamin A:B ratios between neuronal cell types (33) and between solid tissue cell types in general (34), our measurements of absolute stoichiometry demonstrate widely differing ratios of A- and B-type lamins in hematopoiesis, which might reflect selective cleavage of B-type lamins during early erythropoiesis (22) and general accumulation of lamins during MK polyploidization (35). One previous study of mouse embryonic fibroblasts suggested that lamin-B1 does not contribute to nuclear mechanics, but such cells could be dominated by lamin-A (36). In contrast, during late erythroid differentiation here, reduction of lamin-B1 by knockdown as well as inhibition of HSP90 or histone acetylation stiffens the nucleus. Because avian erythrocytes undergo chromatin condensation without enucleation and no large change in lamin-B1 was observed in late murine erythropoiesis (37), detailed mechanisms of nucleus-based sorting and lamin regulation could be species specific.
Our results indicate that lamin-A:B stoichiometry defines nuclear stiffness, indicating separable roles as distinct polymers in defining viscoelasticity of nuclei. Given that decreasing lamin-A softens nuclei (6) (Fig. 2B) and that migration sensitivity to pore size increases as the relative amount of lamin-A increases (Fig. 2D), lamin-A dictates time-dependent deformability, which is indicative of a viscous fluid. On the other hand, lamin-B1 confers nuclear elasticity and resilience (Fig. 3C) so that its decrease during erythropoiesis produces a nucleus that is slow to recover and effectively stiffer. These results are consistent with previous measurements by fluorescence correlation spectroscopy (38) showing lamin-A is more mobile than B-type lamins. Our studies already implicate RA as a physiological factor in lamin-A expression (Fig. 5), but additional soluble or insoluble factors could likewise influence lamin levels, lineage, and cell trafficking.
Materials and Methods
Cell culture, micropipette analyses, pore migration assays, and other standard techniques are described in SI Materials and Methods. Commercially available primary human cells from marrow were obtained from either AllCells, Inc. (Emeryville, CA) or anonymous donors to the University of Pennsylvania Stem Cell Core with University of Pennsylvania Institutional Review Board approval (n > 10). Because lamins vary greatly across hematopoietic cells and because a standard method such as immunoblotting is a (nonlinear) bulk method requiring purification of cells that are sometimes rare in small samples of marrow or blood, immunoblots were used to confirm expression of lamins in a few cell-sample types. Quantitation of relative lamin levels by flow cytometry of a given lineage was done systematically using mean fluorescent intensity (MFI), and these measurements were then compared with lamin levels in a standard cell line (A549) for which the lamin-A:B ratio is calibrated by mass spectrometry (SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank Dr. Robert Goldman for lamin-B1 shRNA. This work was supported by National Institutes of Health Grants P01DK032094 and R01HL062352, and National Science Foundation and Human Frontier Science Program grants (to D.E.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304996110/-/DCSupplemental.
References
- 1.Sabin FR. Bone marrow. Physiol Rev. 1928;8:151–190. [Google Scholar]
- 2.Lichtman MA. Cellular deformability during maturation of the myeloblast: Possible role in marrow egress. N Engl J Med. 1970;283(18):943–948. doi: 10.1056/NEJM197010292831801. [DOI] [PubMed] [Google Scholar]
- 3.Lichtman MA, Packman CH, Constine LS. Molecular and Cellular Traffic Across the Marrow Sinuses. In: Tavassoli M, editor. Handbook of the Hemopoietic Microenvironment. Clifton, NJ: Humana Press; 1989. [Google Scholar]
- 4.Lam WA, Rosenbluth MJ, Fletcher DA. Chemotherapy exposure increases leukemia cell stiffness. Blood. 2007;109(8):3505–3508. doi: 10.1182/blood-2006-08-043570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD. Nuclear lamins. Cold Spring Harb Perspect Biol. 2010;2(11):a000547. doi: 10.1101/cshperspect.a000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci USA. 2007;104(40):15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peric-Hupkes D, et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 2010;38(4):603–613. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerner C, Sauermann G. Nuclear matrix proteins specific for subtypes of human hematopoietic cells. J Cell Biochem. 1999;72(4):470–482. [PubMed] [Google Scholar]
- 9.Röber RA, Sauter H, Weber K, Osborn M. Cells of the cellular immune and hemopoietic system of the mouse lack lamins A/C: Distinction versus other somatic cells. J Cell Sci. 1990;95(Pt 4):587–598. doi: 10.1242/jcs.95.4.587. [DOI] [PubMed] [Google Scholar]
- 10.Olins AL, et al. The human granulocyte nucleus: Unusual nuclear envelope and heterochromatin composition. Eur J Cell Biol. 2008;87(5):279–290. doi: 10.1016/j.ejcb.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilly MN, Kolb JP, Gosti F, Godeau F, Courvalin JC. Lamins A and C are not expressed at early stages of human lymphocyte differentiation. Exp Cell Res. 1990;189(1):145–147. doi: 10.1016/0014-4827(90)90267-e. [DOI] [PubMed] [Google Scholar]
- 12.Dahl KN, et al. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2006;103(27):10271–10276. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merideth MA, et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358(6):592–604. doi: 10.1056/NEJMoa0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahn D, et al. A truncated lamin A in the Lmna -/- mouse line: Implications for the understanding of laminopathies. Nucleus. 2012;3(5):463–474. doi: 10.4161/nucl.21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hale JS, Frock RL, Mamman SA, Fink PJ, Kennedy BK. Cell-extrinsic defective lymphocyte development in Lmna(-/-) mice. PLoS ONE. 2010;5(4):e10127. doi: 10.1371/journal.pone.0010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naetar N, et al. Loss of nucleoplasmic LAP2alpha-lamin A complexes causes erythroid and epidermal progenitor hyperproliferation. Nat Cell Biol. 2008;10(11):1341–1348. doi: 10.1038/ncb1793. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann K, et al. Mutations in the gene encoding the lamin B receptor produce an altered nuclear morphology in granulocytes (Pelger-Huët anomaly) Nat Genet. 2002;31(4):410–414. doi: 10.1038/ng925. [DOI] [PubMed] [Google Scholar]
- 18.Zwerger M, Herrmann H, Gaines P, Olins AL, Olins DE. Granulocytic nuclear differentiation of lamin B receptor-deficient mouse EPRO cells. Exp Hematol. 2008;36(8):977–987. doi: 10.1016/j.exphem.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okumura K, Nakamachi K, Hosoe Y, Nakajima N. Identification of a novel retinoic acid-responsive element within the lamin A/C promoter. Biochem Biophys Res Commun. 2000;269(1):197–202. doi: 10.1006/bbrc.2000.2242. [DOI] [PubMed] [Google Scholar]
- 20.Warrell RP, Jr, et al. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid) N Engl J Med. 1991;324(20):1385–1393. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- 21.Andrade R, Alonso R, Peña R, Arlucea J, Aréchaga J. Localization of importin alpha (Rch1) at the plasma membrane and subcellular redistribution during lymphocyte activation. Chromosoma. 2003;112(2):87–95. doi: 10.1007/s00412-003-0247-3. [DOI] [PubMed] [Google Scholar]
- 22.Zermati Y, et al. Caspase activation is required for terminal erythroid differentiation. J Exp Med. 2001;193(2):247–254. doi: 10.1084/jem.193.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krauss SW, et al. Nuclear substructure reorganization during late-stage erythropoiesis is selective and does not involve caspase cleavage of major nuclear substructural proteins. Blood. 2005;106(6):2200–2205. doi: 10.1182/blood-2005-04-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junt T, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 25.Oakes PW, et al. Neutrophil morphology and migration are affected by substrate elasticity. Blood. 2009;114(7):1387–1395. doi: 10.1182/blood-2008-11-191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beadle C, et al. The role of myosin II in glioma invasion of the brain. Mol Biol Cell. 2008;19(8):3357–3368. doi: 10.1091/mbc.E08-03-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol. 2008;10(4):452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naito M, Omoteyama K, Mikami Y, Takagi M, Takahashi T. Suppression of lamin A/C by short hairpin RNAs promotes adipocyte lineage commitment in mesenchymal progenitor cell line, ROB-C26. Histochem Cell Biol. 2012;137(2):235–247. doi: 10.1007/s00418-011-0890-3. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y, et al. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334(6063):1706–1710. doi: 10.1126/science.1211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labbaye C, et al. Retinoic acid downmodulates erythroid differentiation and GATA1 expression in purified adult-progenitor culture. Blood. 1994;83(3):651–656. [PubMed] [Google Scholar]
- 31.Purton LE, et al. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J Exp Med. 2006;203(5):1283–1293. doi: 10.1084/jem.20052105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3(11):950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 33.Jung HJ, Lee JM, Yang SH, Young SG, Fong LG. Nuclear lamins in the brain: New insights into function and regulation. Mol Neurobiol. 2013;47(1):290–301. doi: 10.1007/s12035-012-8350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swift J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149):1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitrat N, et al. Endomitosis of human megakaryocytes are due to abortive mitosis. Blood. 1998;91(10):3711–3723. [PubMed] [Google Scholar]
- 36.Lammerding J, et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem. 2006;281(35):25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 37.Popova EY, et al. Chromatin condensation in terminally differentiating mouse erythroblasts does not involve special architectural proteins but depends on histone deacetylation. Chromosome Res. 2009;17(1):47–64. doi: 10.1007/s10577-008-9005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimi T, et al. The A- and B-type nuclear lamin networks: Microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22(24):3409–3421. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu J, et al. Isolation and functional characterization of human erythroblasts at distinct stages: Implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013;121(16):3246–3253. doi: 10.1182/blood-2013-01-476390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.