Significance
Childhood maltreatment is a major risk factor for internalizing disorders including depression and anxiety, which cause significant disability. Altered connectivity of the brain’s fear circuitry represents an important candidate mechanism linking maltreatment and these disorders, but this relationship has not been directly explored. Using resting-state functional brain connectivity in adolescents, we show that maltreatment predicts lower prefrontal–hippocampal connectivity in females and males but lower prefrontal–amygdala connectivity only in females. Altered connectivity, in turn, mediated the development of internalizing symptoms. These results highlight the importance of fronto–hippocampal connectivity for both sexes in internalizing symptoms following maltreatment. The additional impact on fronto–amygdala connectivity in females may help explain their higher risk for anxiety and depression.
Keywords: child maltreatment, sex differences, ventromedial prefrontal cortex
Abstract
Maltreatment during childhood is a major risk factor for anxiety and depression, which are major public health problems. However, the underlying brain mechanism linking maltreatment and internalizing disorders remains poorly understood. Maltreatment may alter the activation of fear circuitry, but little is known about its impact on the connectivity of this circuitry in adolescence and whether such brain changes actually lead to internalizing symptoms. We examined the associations between experiences of maltreatment during childhood, resting-state functional brain connectivity (rs-FC) of the amygdala and hippocampus, and internalizing symptoms in 64 adolescents participating in a longitudinal community study. Childhood experiences of maltreatment were associated with lower hippocampus–subgenual cingulate rs-FC in both adolescent females and males and lower amygdala–subgenual cingulate rs-FC in females only. Furthermore, rs-FC mediated the association of maltreatment during childhood with adolescent internalizing symptoms. Thus, maltreatment in childhood, even at the lower severity levels found in a community sample, may alter the regulatory capacity of the brain’s fear circuit, leading to increased internalizing symptoms by late adolescence. These findings highlight the importance of fronto–hippocampal connectivity for both sexes in internalizing symptoms following maltreatment in childhood. Furthermore, the impact of maltreatment during childhood on both fronto–amygdala and –hippocampal connectivity in females may help explain their higher risk for internalizing disorders such as anxiety and depression.
In both clinical and community samples, maltreatment during childhood represents one of the strongest risk factors for developing depression and anxiety (1–3). Childhood maltreatment and other adversities account for up to a third of the risk for mood and anxiety disorders (4). Further, depression and anxiety disorders are major public health problems, affecting 15 and 32% of youth, respectively, by the age of 18 y (5). The burden of these disorders is significant, representing the second and fifth leading causes, respectively, of years lived with disability in the United States (6). Some evidence suggests that maltreatment may impart greater risk for the development of internalizing symptoms in females than in males (e.g., refs. 7–9). This differential risk could account, in part, for the higher incidence of internalizing problems in females than in males (10, 11). However, the neurobiological pathways from maltreatment during childhood to the expression of internalizing problems, including potential differences for females and males, remain poorly understood. Such information is crucial for improving the treatment of depression and anxiety disorders and for mitigating the effects of maltreatment during childhood.
Both maltreatment during childhood (12) and internalizing disorders (13, 14) have been associated with altered activity in specific brain circuits involved in the processing and regulation of threat and fear, including the amygdala, hippocampus, and prefrontal cortex (PFC). Maltreated children show increased amygdala and hippocampus activation in response to threatening faces (15–18), reduced hippocampus activation in a declarative memory task (19), and variable findings regarding PFC activation (12). These brain areas do not act in isolation but interact to regulate the fear response. The ventromedial (vm)PFC inhibits amygdala-based expression of fear responses and is required for fear extinction (14), whereas the hippocampus contextually limits fear responses via connections to both the amygdala and vmPFC (20). In rats, chronic stress impairs hippocampus–vmPFC long-term potentiation, which is required for the proper gating of conditioned fear (21, 22). Together, these findings suggest that prolonged exposure to stress may impair connectivity among the components of the fear circuitry, thereby impairing the regulation of emotion and amplifying fear responses.
The impact of maltreatment during childhood on the functional connectivity of this circuitry in humans has been explored only recently. Symptomatic adults reporting a history of maltreatment during childhood show altered local connectivity and hub-like properties of the amygdala and PFC (23), decreased functional connectivity strength of the PFC (24), and decreased functional connectivity of the amygdala with neighboring limbic areas (25). However, to our knowledge, no studies have investigated how maltreatment during childhood may affect brain functional connectivity in adolescence, and none have included connectivity of the hippocampus, a key node in the fear-regulatory network. Furthermore, no studies have investigated which, if any, of these differences in connectivity may mediate the link between maltreatment during childhood and the development of internalizing symptoms. Finally, no published work has investigated sex differences in the alterations in functional connectivity related to maltreatment during childhood that may lead to the increased rates of internalizing disorders seen in females.
We addressed these knowledge gaps by examining the associations between experiences of maltreatment in childhood, resting-state brain functional connectivity (rs-FC), and internalizing symptoms in a group of 64 late adolescents (30 female; age 18 y) who had been followed longitudinally from birth as part of the Wisconsin Study of Families and Work. Experiences of maltreatment were assessed via the Childhood Trauma Questionnaire (CTQ) (26) completed at age 18 y. As expected, adolescent reports of experiences of maltreatment in childhood were significantly and substantially associated with earlier maternal reports of family stress during late childhood and early adolescence (SI Methods and Table S1). We then examined the correlation of experiences of maltreatment in childhood with seed-based rs-FC of the hippocampus and amygdala. We predicted that maltreatment during childhood would be associated with decreased connectivity of the hippocampus and amygdala with the vmPFC. Next, we investigated whether altered rs-FC mediated the association of maltreatment during childhood and persistent adolescent internalizing symptoms (anxiety and depression symptoms averaged across four annual assessments at ages 15–18 y). Finally, we predicted that these associations would be stronger for females, given known sex differences in the development and stress sensitivity of the amygdala and hippocampus (11) and our prior study in this sample linking family stress during infancy and cortisol levels during childhood with adolescent amygdala–vmPFC rs-FC in girls but not in boys (27).
Results
All results of rs-FC analysis associated with experiences of maltreatment in childhood are displayed in Table 1. Here we feature rs-FC findings that also were predictive of adolescent internalizing symptoms. Additional connectivity findings that were not predictive of internalizing symptoms are detailed in SI Methods.
Table 1.
Summary of adolescent rs-FC estimates with amygdala and hippocampus seeds as predicted by experiences of maltreatment during childhood (CTQ total score)
| Seed | Identified cluster | Talairach coordinates | Peak t | Volume, μL |
| Left hippocampus | sgACC (BA 25) | 2, 22, −6 | −4.67 | 1,304 |
| Left amygdala | R. PCG (BA 4) | 22, −32, 28 | −4.53 | 2,912 |
| L. dlPFC (BA 10) | −36, 50, 20 | 4.80 | 1,176 | |
| Right amygdala | sgACC (BA 25) | 28, −24, 38 | −4.73 | 976 |
| R. PCG (BA 4) | 2, 18, −10 | −4.22 | 1,080 |
Results were significant at P < 0.05 with family-wise error correction at the whole-brain level. Note that the CTQ score also predicted lower connectivity between left amygdala and sgACC, but this prediction did not survive family-wise error correction.
Experiences of Maltreatment in Childhood and Adolescent Amygdala rs-FC.
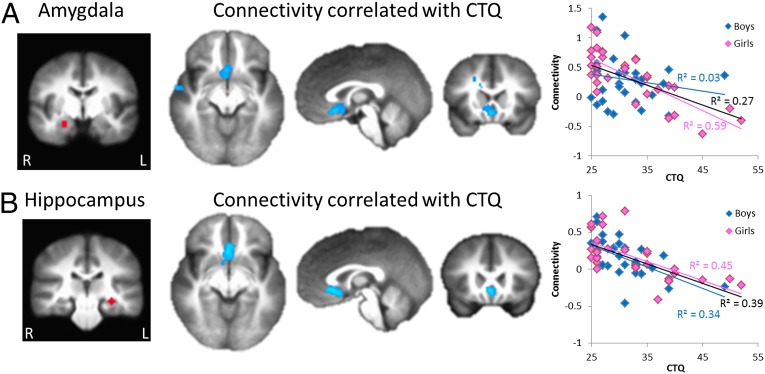
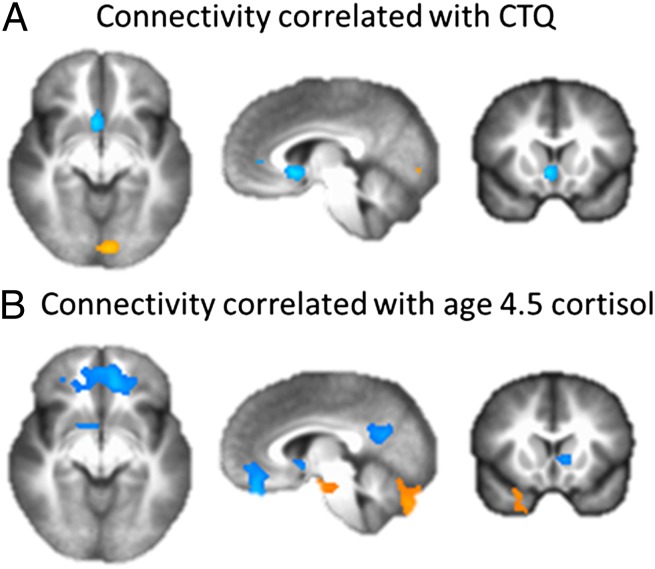
Across all participants, experiences of maltreatment in childhood predicted lower connectivity (R2 = 0.27) between the right amygdala and the vmPFC, specifically the subgenual anterior cingulate cortex (sgACC, Brodmann area 25) (Fig. 1A and Table 1). A similar association was found with the left amygdala but did not survive multiple comparison correction (cluster size = 62 voxels). The association of experiences of maltreatment in childhood with right amygdala–sgACC connectivity remained statistically significant when controlling for the afternoon cortisol level in childhood (age 4.5 y), which in our prior study (27) mediated the association between family stress during infancy and resting connectivity between the amygdala and a more anterior portion of the vmPFC (Fig. 2). Sex differences were examined by ANCOVA on the extracted rs-FC values. Results revealed a significant sex by CTQ score interaction (F1,60 = 4.20, P = 0.045). The association of CTQ score with amygdala–sgACC rs-FC was driven entirely by females (R2 = 0.59 and 0.03 for females and males, respectively), even though the CTQ scores of the two sexes were quite similar (t62 = 0.85, P = 0.40). These findings remained significant when controlling for adolescent current life stress (CTQ score main effect: F1,59 = 17.44, P < 0.001; sex–CTQ score interaction: F1,59 = 3.94, P = 0.05). The CTQ score also significantly predicted amygdala rs-FC to postcentral gyrus and dorsolateral PFC, but these connectivity results did not predict adolescent internalizing symptoms (see below and SI Methods).
Fig. 1.
Childhood experiences of maltreatment predict lower rs-FC of the amygdala and hippocampus (n = 64; 30 female). (A) CTQ scores negatively correlated with rs-FC between the right amygdala and sgACC across all participants. This association was specific to females (scatterplots, Right). (B) CTQ scores negatively correlated with rs-FC between the left hippocampus and sgACC in all participants. This association was present in both females and males (scatterplots, Right). Seed regions are displayed in the left panels. Connectivity results are displayed at P < 0.05, family-wise error corrected at the whole brain level.
Fig. 2.
In girls, experiences of maltreatment during childhood remain negatively correlated with amygdala–sgACC connectivity when cortisol values at age 4.5 y are included in the model (A). In addition, experiences of maltreatment and cortisol values at age 4.5 y are associated with amygdala connectivity to different regions of the vmPFC (B).
Experiences of Maltreatment in Childhood and Adolescent Hippocampus rs-FC.
Childhood experiences of maltreatment predicted lower connectivity between the left hippocampus and the sgACC across all participants (R2 = 0.39) (Fig. 1B and Table 1). Analysis of extracted rs-FC values in an ANCOVA revealed only a main effect of CTQ score (F1,60 = 36.69, P < 0.001), with similar associations in females and males (R2 = 0.45 and 0.34 respectively). This finding remained significant when controlling for adolescent current life stress (CTQ score main effect: F1,59 = 33.32, P < 0.001). No significant associations were found between CTQ score and right hippocampus connectivity.
Experiences of Maltreatment in Childhood, Adolescent rs-FC, and Internalizing Symptoms.
We next investigated the association of experiences of maltreatment during childhood with adolescent internalizing symptoms, considering potential sex differences and mediating effects of rs-FC. ANCOVA revealed that there was no statistically significant sex by CTQ score interaction (F1,60 = 1.94, P = 0.17); rather, both CTQ score (F1,60 = 11.35, P = 0.001) and sex (F1,60 = 4.24, P = 0.04) were significant predictors of internalizing symptoms. As expected, higher CTQ scores predicted more internalizing symptoms, and females had higher levels of internalizing symptoms than males (average 2.50 ± 0.55 and 1.99 ± 0.53, respectively). Of the rs-FC regions associated with CTQ score, bivariate correlations revealed that only amygdala– and hippocampus–sgACC rs-FC significantly predicted adolescent internalizing symptoms (SI Methods and Table S2). These results indicated that only amygdala– and hippocampus–sgACC rs-FC were potential mediators of the link between CTQ score and adolescent internalizing symptoms; thus these links became the focus of subsequent path-model analyses.
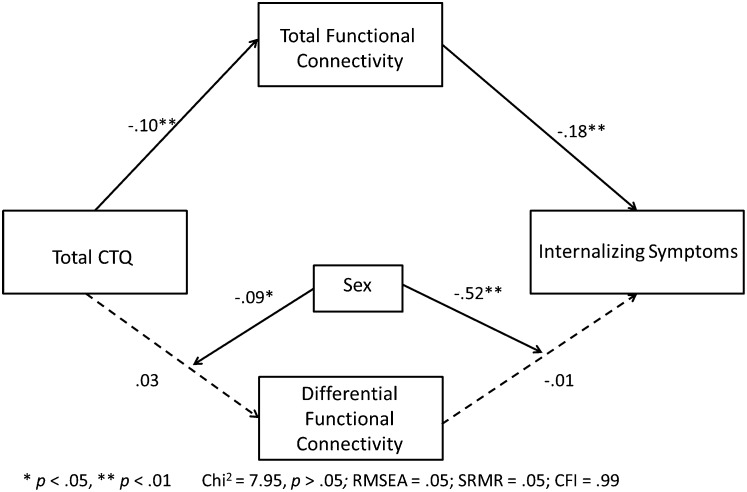
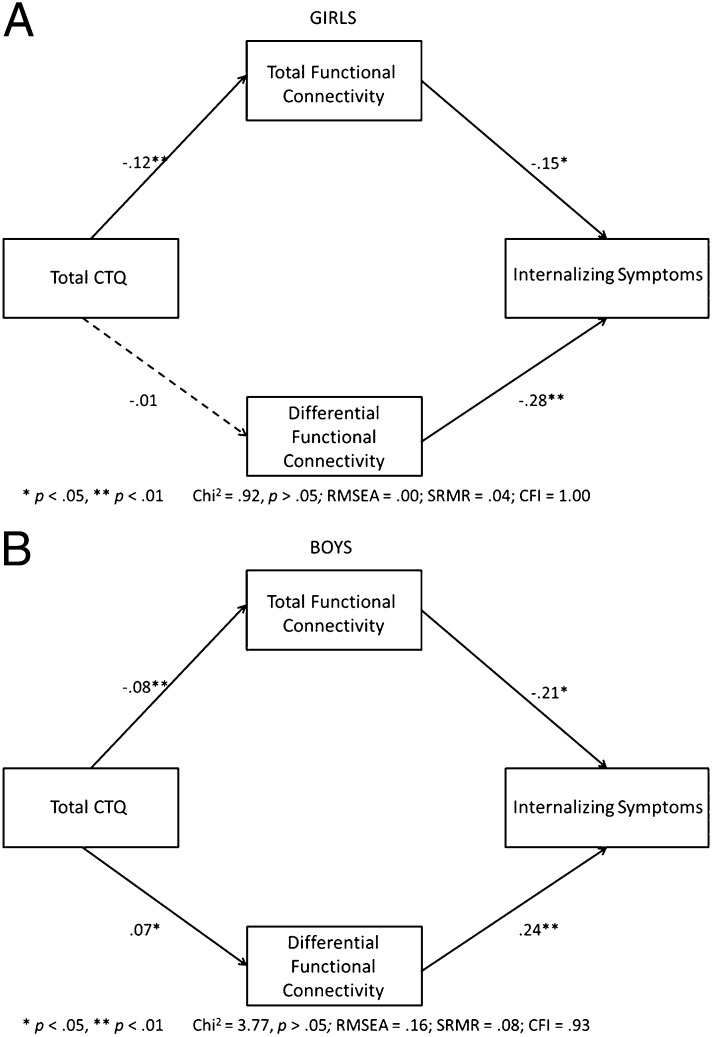
Using structural equation modeling (SEM), we then examined the mediating effects of amygdala– and hippocampus–sgACC rs-FC on the association between CTQ score and internalizing symptoms, as well as possible sex differences. Amygdala–sgACC connectivity and hippocampus–sgACC connectivity were highly correlated (r = 0.52, P < 0.001). Thus, to reduce problems of multicollinearity that occur when both connectivity measures are included in the same model, we constructed two independent (uncorrelated) measures that distinguish the total connectivity, or what the amygdala– and hippocampus–sgACC measures share in common (i.e., the sum of the two connectivity measures), from what differentiates them (i.e., amygdala–sgACC minus hippocampus–sgACC). For both females and males, maltreatment experiences predicted lower total rs-FC of the amygdala and hippocampus to sgACC, and the lower rs-FC in turn mediated the association of maltreatment experiences with internalizing symptoms (Fig. 3). However, the mediating effect of differential connectivity was moderated by sex. In males, maltreatment experiences predicted lower rs-FC primarily between the hippocampus and sgACC, predicting greater internalizing symptoms; in females, maltreatment experiences predicted lower rs-FC between both the hippocampus and the sgACC and the amygdala and the sgACC, again predicting greater internalizing symptoms (Figs. 3 and 4). Reversal of this model (CTQ score→internalizing→functional connectivity) yielded poor model fit and decreased fit statistics, suggesting that altered rs-FC mediates internalizing symptoms but not the reverse (Methods).
Fig. 3.
SEM examining the effect of rs-FC in mediating between childhood experiences of maltreatment (measured by CTQ scores) and internalizing symptoms at age 18 y, as moderated by sex. Connectivity variables included total connectivity of the amygdala and hippocampus to sgACC and differential connectivity (the difference between the amygdala– and hippocampus–sgACC scores) to examine overall and relative effects of each region. Models are shown by sex in Fig. 4.
Fig. 4.
SEM examining the effect of functional connectivity in mediating between childhood experiences of maltreatment (measured by CTQ scores) and adolescent internalizing symptoms in girls (A) and boys (B). Connectivity variables included both total connectivity of the amygdala and hippocampus to sgACC and differential connectivity (the difference between the amygdala– and hippocampus–sgACC scores) to examine overall and relative effects of each region. (A) In girls, lower total functional connectivity of the amygdala and hippocampus to sgACC mediated the association between CTQ scores and internalizing symptoms. Lower amygdala–sgACC connectivity (i.e., a lower differential score) contributed more substantially to the association between CTQ scores and internalizing symptoms. (B) In boys, findings were similar except that lower hippocampus–sgACC connectivity (i.e., a higher differential score) contributed more substantially to this effect. The full model with moderation by sex is shown in Fig. 3.
Discussion
Our study details findings suggesting a direct neural mechanism mediating the link between childhood experiences of maltreatment and the development of anxiety and depressive symptoms in adolescence. This neural mechanism involves altered connectivity within the brain’s fear-regulatory circuit including the hippocampus, amygdala, and sgACC. The sgACC, as part of the vmPFC, is a putative homolog of the rat infralimbic (IL) cortex, which mediates recall of fear extinction by suppressing amygdala-based fear responses through activation of inhibitory intercalated neurons in the amygdala (14). Consistent with rodent data, fear-extinction studies in humans demonstrate increased activation of the vmPFC during fear-extinction learning and recall (14, 28). In humans, the sgACC also is involved more broadly in the automatic (nonconscious) regulation of negative affect. sgACC activation has been observed with the induction of sadness and the recall of traumatic events, and persistent hyperactivation has been observed in clinical depression (29). Furthermore, exogenous glucocorticoids reduce sgACC activation and simultaneously increase arousal to sadness-invoking stimuli in healthy individuals (30). Consistent with these findings, both adolescents and adults with anxiety disorders show reduced sgACC activation when appraising their own fear (31). Within this framework, maltreatment-associated uncoupling of the amygdala and sgACC may result in impaired modulation of negatively valenced emotional responses, including a failure to extinguish fear responses in the absence of threat.
Maltreatment-associated uncoupling of the hippocampus and sgACC may lead to additional disruptions in the regulation of negative affect. In this case, impaired communication between the hippocampus and sgACC may reduce contextual gating of the expression of conditioned fear, leading to more generalized and persistent negative affect states. In rodents, both the IL cortex and hippocampus are required for the recall of fear-extinction memory (22, 32, 33), which involves enhanced synaptic plasticity between the hippocampus and medial PFC (mPFC) (21, 34). Furthermore, chronic stress in rodents blocks extinction-related enhancement of hippocampus–mPFC plasticity and the recall of fear extinction (21). Our findings are consistent with this effect and suggest that experiences of maltreatment during childhood may reduce the capacity of the hippocampus to engage PFC-mediated recall of fear extinction, thereby leading to greater internalizing symptoms.
The uncoupling of the hippocampus and sgACC associated with maltreatment in childhood also may have relevance for emotional disorders such as posttraumatic stress disorder (PTSD). Adult PTSD has been characterized by impaired fear extinction recall and lower activation of the hippocampus and vmPFC during extinction recall (20, 35). Interestingly, adult trauma (combat) exposure actually increases hippocampus–vmPFC connectivity, whereas failure to increase this connectivity was associated with PTSD symptoms (36). Together with our findings, this observation suggests that fronto–hippocampal connectivity may be uniquely vulnerable to trauma exposure during development. The failure of this circuit to modulate fear and anxiety responses contextually would be expected to lead to internalizing symptoms as observed in our sample. In addition, impairments in hippocampal connectivity induced by maltreatment in childhood may place the brain at further risk following subsequent adult trauma by reducing the capacity of the brain to engage this fear-gating circuit appropriately.
Of note, our study did not reveal any significant associations between maltreatment experiences in childhood and amygdala/hippocampus connectivity to the dorsal anterior cingulate cortex (dACC), even at an uncorrected P = 0.001. The dACC is a putative homolog of the rat prelimbic (PL) cortex which, in contrast to the IL cortex, facilitates the expression of conditioned fear via excitatory connections to the basolateral amygdala (33, 37). In human studies, the dACC shows greater activation during acquisition and expression of fear (14). dACC activation to threat is associated with both childhood and adult exposure to trauma (38), and increased dACC–amygdala connectivity has been observed following combat exposure in adults (39, 40). Our findings suggest that maltreatment experiences were associated more strongly with impaired vmPFC connectivity. However, it is possible that abnormalities in dACC connectivity would emerge with additional task provocation, as in the studies cited above, or with greater levels of maltreatment than were observed in this community sample.
Childhood experiences of maltreatment also were associated with altered connectivity between the amygdala and other brain regions, including lower connectivity to the postcentral gyrus and greater connectivity to dlPFC. However, none of these other areas mediated the development of internalizing symptoms. Thus, the functional significance of altered connectivity in these areas remains unclear at this time. Further studies would be warranted to explore their potential relevance to other psychiatric domains such as externalizing symptoms.
Our study also has revealed sex differences in the neural impact of exposure to maltreatment during childhood. Specifically, our results suggest that at the neural level females are more vulnerable to childhood experiences of maltreatment, because in females these experiences impact both the amygdala– and hippocampal–sgACC regulatory pathways. In contrast, in males maltreatment during childhood appeared to impact only the hippocampus–sgACC pathway. This “double hit” in females may explain, in part, their higher levels of internalizing symptoms in our sample and the broadly observed greater risk for anxiety and depression in females. The amygdala and hippocampus are known to exhibit sex differences in their developmental trajectories that could confer differing vulnerability to experiences of maltreatment. Initial studies using linear modeling suggested that, between the ages of 4–18 y, the amygdala has a more extended period and greater rate of growth in males, whereas the hippocampus has a more extended period and greater rate of growth in females (41, 42). More recent work using nonlinear modeling and a wider age range (1 mo to 25 y) revealed greater rates of growth for both amygdala and hippocampus in females during the first several years of life, with sex differences in the period of growth only in the amygdala (i.e., longer for males) (43). Periods of rapid brain maturation are particularly sensitive to the negative effects of early experiences (44) and may account for the greater neural impact of maltreatment in females by affecting functional connectivity of both the amygdala and hippocampus with the sgACC. In addition, prolonged amygdala growth in males may confer greater protection in terms of neuroplasticity following repeated stress, perhaps allowing sgACC–amygdala connectivity to remain more intact in males.
Sex differences in white matter development also may have particular relevance for our functional connectivity findings. Males have more rapid increases in white matter volume during development (45) and have greater structural integrity in white matter tracts connecting the vmPFC and amygdala/hippocampus (46, 47). These differences may confer greater protection in males following maltreatment experiences, although this hypothesis would require further study.
Aside from the period and rate of growth, the amygdala, hippocampus, and sgACC also may exhibit inherent sex differences in sensitivity to stress. Unfortunately there is very little work in humans (especially developmentally) directly examining this question. A meta-analysis of volumetric brain studies of PTSD revealed that exposure to trauma had a relatively greater effect on hippocampal volume in males than in females (48). Consistent with this finding, animal studies have shown that chronic stress impairs neurogenesis and causes dendritic retraction in the hippocampus, along with spatial memory deficits, to a much greater degree in adult male than in female rats (49). These sex differences in sensitivity to the effects of chronic stress may reflect, in part, the effects of estrogen, which exhibits a number of neuroprotective effects in the hippocampus (49, 50). However, in our sample, maltreatment during childhood showed similar associations with hippocampal rs-FC in both males and females. One possibility is that the greater vulnerability of the male hippocampus to stress may be counterbalanced by greater structural connectivity between the vmPFC and hippocampus in males, yielding similar effects for both sexes in terms of the functional connectivity in this pathway. Even less is known about sex differences in the impact of stress on the amygdala and PFC. Chronic stress increases dendritic arborization of the amygdala and decreases arborization of the PFC in male rats, but there are few such studies in female rats (49, 51). In humans, structural connectivity between the vmPFC and amygdala/hippocampus is reduced in children who have suffered early neglect/maltreatment (52, 53), but whether there are sex differences in the effects of stress on these white matter tracts remains unclear. Ultimately, more studies exploring developmental sex differences in the sensitivity of these brain regions and their connecting fibers to stress will be needed in humans as well as in animal models.
The results of this study suggest important brain pathways linking maltreatment in childhood to the development of anxiety and depression. Strengths of the study include the relatively large sample size, longitudinal design, and the inclusion of measures of adolescent brain connectivity. However, there are potential caveats regarding these findings. First, it is possible that altered brain connectivity represents a preexisting factor (trait) for vulnerability to experiences of maltreatment or for the development of internalizing symptoms. Although our path modeling suggests otherwise, it will be important in future studies to supplement this type of data with earlier brain measures, before maltreatment has occurred, to address this question. Second, connectivity measures based on the resting state may not be equivalent to those obtained during a task. On the other hand, resting-state analysis avoids task performance as a potential confound. Finally, although our data suggest strong developmental sensitivities to trauma exposure in the fear circuit, it will be important in future work to tease out more precisely which developmental periods are the most vulnerable in different brain areas and how these periods may differ by sex.
In conclusion, the current data suggest a direct neural mechanism, via altered connectivity of the brain’s fear circuitry, by which experiences of maltreatment during childhood lead to anxiety and depressive symptoms by late adolescence. These findings highlight the importance of sgACC–hippocampal connectivity for both sexes in internalizing symptoms following maltreatment in childhood. Furthermore, the impact of maltreatment in childhood on both sgACC–amygdala and –hippocampal connectivity in females may help explain their higher risk for internalizing disorders such as anxiety and depression. We observed these associations even with the relatively low levels of maltreatment experiences found in a community sample. These results suggest that maltreatment, even below the threshold of reportable childhood maltreatment, leads to significant changes in the brain’s emotion-regulating circuitry. These findings will help point the way to new and developmentally sensitive interventions following maltreatment with the goal of averting the development of internalizing disorders, which are major public health problems. Our findings also suggest that additional or more extensive interventions may be needed to help female victims recover from the many deleterious effects of maltreatment during childhood.
Methods
Participants.
Participants were 64 adolescents [30 female; age 18.79 ± 0.19 y (mean ± SD)] from the larger Wisconsin Study of Families and Work (originally the Wisconsin Maternity Leave and Health Project) (54). For further details see SI Methods.
Behavioral Measures.
Experiences of maltreatment during childhood were assessed by self-report at the age of 18 y using the CTQ (26). The total CTQ score (sum of physical abuse and neglect, emotional abuse and neglect, and sexual abuse) was used in the analyses. Variance in the CTQ score was driven most strongly by emotional abuse and neglect (Subscore means: emotional abuse 6.83 ± 2.45, emotional neglect 8.06 ± 2.53, physical abuse 5.33 ± 0.82, physical neglect 5.73 ± 1.23, sexual abuse 5.69 ± 2.58). Maternal reports of earlier childhood stress were based on averages for three developmental periods: infancy/preschool, late childhood (age 9–11 y), and early/midadolescence (age 13–15 y). Maternal stress measures included maternal depression, negative parenting, marital conflict/family anger, maternal role overload, and financial stress (54). Adolescent anxiety and depression symptoms were assessed four times annually from ages 15–18 y via self-report with the adolescent version of the MacArthur Health and Behavior Questionnaire (HBQ) (55), a well-validated measure of mental health, physical health, and social and academic functioning. Of interest for the current analysis were the HBQ subscales measuring symptoms of anxiety and depression, which were averaged across the 4 y to provide a measure of persistent internalizing symptoms. Finally, current adolescent life stress was indexed using a 61-item life-events inventory modeled on the Adolescent Perceived Events Scale (56) and the Life Experiences Survey (57). Events covered age-appropriate life domains (e.g., relationships, change in parental marital status or finances, serious illnesses and deaths). The current analyses include the summed impact of negative events in the past 6 mo.
Imaging Data Acquisition and Processing.
Structural and resting-state functional images were collected on a 3T MRI scanner (GE Discovery MR750) with an eight-channel RF head coil array. Data preprocessing was conducted with Analysis of Functional and Neural Images (AFNI) and FMRIB Software Library software. For further details, see SI Methods.
Functional Connectivity Analyses.
rs-FC estimates were computed using a seed region-based approach. Binary masks of the left and right amygdala and hippocampus were defined in AFNI by placing spheres with a 4-mm radius at the locations of the amygdala and hippocampus. Participant connectivity maps were entered into two-tailed regressions (AFNI’s 3dttest++) while covarying childhood experience of maltreatment (CTQ scores), childhood basal cortisol levels, and/or other behavioral variables of interest. Note that CTQ scores were not correlated with subject motion (Table S3). At an individual voxel P < 0.001, a minimum cluster size of 111 voxels is required to have a corrected P ≤ 0.05. For further details, see SI Methods.
Path Modeling.
Mplus software (version 5.2, Muthén & Muthén, Los Angeles) was used to construct a structural equation model testing the mediating effects of brain connectivity in the association between childhood experiences of maltreatment and persistent internalizing symptoms. To test for mediation, both direct and indirect effects were examined. To determine if pathways differed in males and females, main and appropriate interactive effects of sex were included on all indirect pathways (58).
Because the measures of hippocampus–sgACC and amygdala–sgACC connectivity were highly correlated, including both in the model introduced potential problems of multicollinearity. To address this problem, we chose to conduct a principal components analysis specifying two independent (uncorrelated) components. This approach allowed us to isolate what the two measures had in common (i.e., the first component reflected total, or shared, connectivity) from what was different about them (i.e., the second component reflected differential connectivity, with a positive score reflecting increased amygdala connectivity and a negative score reflecting increased hippocampal connectivity). We chose this approach because we are equally interested in both connectivity measures; other approaches, such as residualization, would require us to attribute the shared variance arbitrarily to only one of the measures (e.g., including in the model hippocampus–sgACC connectivity and amygdala–sgACC connectivity residualized for hippocampus–sgACC connectivity, or vice versa). Given that previous analyses did not identify significant associations with any control variables, no additional predictors were included in the model because of unnecessary reductions in power. This model demonstrated good fit [χ2 = 7.95, P > 0.05, root mean square error of approximation (RMSEA) = 0.05, standardized root mean square residual (SRMR) = 0.05, comparative fit index (CFI) = 0.99) and accounted for 49.7% of the variance in persistent internalizing symptoms. Furthermore, this model revealed that experiences of maltreatment during childhood led to lower total connectivity, which in turn led to internalizing symptoms (Fig. 3). The differential pathway revealed significant sex interactions, suggesting that for boys the total connectivity findings were driven by less connectivity in the hippocampus–sgACC pathway (Figs. 3 and 4). Formal testing of both mediating pathways suggests significant mediating effects of connectivity (total connectivity estimate = 0.02, P < 0 0.01; differential connectivity estimate = 0.04, P = 0.05). Finally, because the timing in the measurement of connectivity and internalizing symptoms overlapped, a second SEM was constructed with the positions of internalizing symptoms and connectivity reversed. The fit statistics of this model were all within the unacceptable range, suggesting that the observed data did not fit the proposed model (χ2 = 47.71, P < 0.05, RMSEA = 0.33, SRMR = 0.13, CFI = 0.37). For further details see SI Methods.
Supplementary Material
Acknowledgments
We thank J. Armstrong for general management of the Wisconsin Study of Families and Work; M. Anderle, R. Fisher, L. Angelos, A. Dyer, C. Hermes, A. Koppenhaver, and C. Boldt for assistance with data collection and recruitment; and J. Ollinger, G. Kirk, N. Vack, J. Koger, and I. Dolski for general, technical, and administrative assistance. This work was supported by National Institutes of Health Grants P50 MH084051, R01-MH044340, and P50-MH052354; the John D. and Catherine T. MacArthur Foundation Research Network on Psychopathology and Development; and the HealthEmotions Research Institute, Department of Psychiatry, University of Wisconsin School of Medicine and Public Health. Partial support for R.J.H. was provided by the American Academy of Child and Adolescent Psychiatry and the Brain and Behavior Research Foundation. Partial support for P.L.R. was provided by the Canadian Institutes of Health Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310766110/-/DCSupplemental.
References
- 1.Gilbert R, et al. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373(9657):68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- 2.Moffitt TE, et al. Generalized anxiety disorder and depression: Childhood risk factors in a birth cohort followed to age 32. Psychol Med. 2007;37(3):441–452. doi: 10.1017/S0033291706009640. [DOI] [PubMed] [Google Scholar]
- 3.Anda RF, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green JG, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merikangas KR, et al. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murray CJL et al. (2013) The State of US Health, 1990-2010: Burden of Diseases, Injuries, and Risk Factors. JAMA 310(6):591–608. [DOI] [PMC free article] [PubMed]
- 7.McGee RA, Wolfe DA, Wilson SK. Multiple maltreatment experiences and adolescent behavior problems: Adolescents’ perspectives. Dev Psychopathol. 1997;9(1):131–149. doi: 10.1017/s0954579497001107. [DOI] [PubMed] [Google Scholar]
- 8.Lansford JE, et al. A 12-year prospective study of the long-term effects of early child physical maltreatment on psychological, behavioral, and academic problems in adolescence. Arch Pediatr Adolesc Med. 2002;156(8):824–830. doi: 10.1001/archpedi.156.8.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacMillan HL, et al. Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry. 2001;158(11):1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 11.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 12.Hart H, Rubia K. Neuroimaging of child abuse: A critical review. Front Hum Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCrory EJ, et al. Heightened neural reactivity to threat in child victims of family violence. Curr Biol. 2011;21(23):R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Tottenham N, et al. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrett AS, et al. Brain activation to facial expressions in youth with PTSD symptoms. Depress Anxiety. 2012;29(5):449–459. doi: 10.1002/da.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maheu FS, et al. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrión VG, Haas BW, Garrett A, Song S, Reiss AL. Reduced hippocampal activity in youth with posttraumatic stress symptoms: An FMRI study. J Pediatr Psychol. 2010;35(5):559–569. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maren S, Phan KL, Liberzon I. The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14(6):417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia R, Spennato G, Nilsson-Todd L, Moreau J-L, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem. 2008;89(4):560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76(4):804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cisler JM, et al. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol Med. 2013;43(3):507–518. doi: 10.1017/S0033291712001390. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, et al. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22241. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Werff SJA, et al. Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol Med. 2013;43(9):1825–1836. doi: 10.1017/S0033291712002942. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein DP, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 27.Burghy CA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 29.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sudheimer KD, et al. Exogenous glucocorticoids decrease subgenual cingulate activity evoked by sadness. Neuropsychopharmacology. 2013;38(5):826–845. doi: 10.1038/npp.2012.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Britton JC, et al. Response to Learned Threat: An fMRI study in adolescent and adult anxiety. Am J Psychiatry. 2013;170(10):1195–1204. doi: 10.1176/appi.ajp.2013.12050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25(39):8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36(2):529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hugues S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn Mem. 2007;14(8):520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milad MR, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Admon R, et al. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proc Natl Acad Sci USA. 2009;106(33):14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13(6):728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herringa RJ, Phillips ML, Fournier JC, Kronhaus DM, Germain A. Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans. Psychol Med. 2013;43(7):1533–1542. doi: 10.1017/S0033291712002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Wingen GA, Geuze E, Vermetten E, Fernández G. Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry. 2011;16(6):664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Wingen GA, Geuze E, Vermetten E, Fernández G. The neural consequences of combat stress: Long-term follow-up. Mol Psychiatry. 2012;17(2):116–118. doi: 10.1038/mp.2011.110. [DOI] [PubMed] [Google Scholar]
- 41.Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(8):1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- 42.Giedd JN, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4-18. Cereb Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 43.Uematsu A, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS ONE. 2012;7(10):e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology (Berl) 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: Magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ. 2012;3(1):19. doi: 10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menzler K, et al. Men and women are different: Diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage. 2011;54(4):2557–2562. doi: 10.1016/j.neuroimage.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 47.Chou K-H, Cheng Y, Chen I-Y, Lin C-P, Chu W-C. Sex-linked white matter microstructure of the social and analytic brain. Neuroimage. 2011;54(1):725–733. doi: 10.1016/j.neuroimage.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 48.Karl A, et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30(7):1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 49.McEwen BS. Stress, sex, and neural adaptation to a changing environment: Mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: Implications for anxiety disorders. Biol Mood Anxiety Disord. 2012;2(1):3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol Neurobiol. 2009;40(2):166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- 52.Eluvathingal TJ, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics. 2006;117(6):2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- 53.Kumar A, et al. Microstructural abnormalities in language and limbic pathways in orphanage-reared children: A diffusion tensor imaging study. J Child Neurol. 2013 doi: 10.1177/0883073812474098. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biol Psychiatry. 2002;52(8):776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- 55.Essex MJ, et al. MacArthur Assessment Battery Working Group The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the Macarthur Health and Behavior Questionnaire. J Am Acad Child Adolesc Psychiatry. 2002;41(5):588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 56.Compas BE, Davis GE, Forsythe CJ, Wagner BM. Assessment of major and daily stressful events during adolescence: The Adolescent Perceived Events Scale. J Consult Clin Psychol. 1987;55(4):534–541. doi: 10.1037/0022-006X.55.4.534. [DOI] [PubMed] [Google Scholar]
- 57.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: Development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46(5):932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 58.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.