The lymphatic system regulates tissue fluid homeostasis and acts as a conduit for leukocytes. Under high hydrostatic pressure, the blood system delivers oxygen, nutrients, and hormones to distant cells, while acellular fluid is forced out of blood capillaries into interstitial space. Most of this fluid drains back into lymphatic capillaries that are under low pressure and leaky due to their unique endothelial lining with microvalves between endothelial cells (ECs). Lymphatic ECs (LECs) are distinguished from blood ECs (BECs) by expression of the transcription factor Prox1, the cell surface proteins LYVE-1 and podoplanin, and the growth factor receptors VEGFR3 and neuropilin 2, whereas BECs express PECAM-1, endoglin, VEGFR2, and neuropilin 1 (1, 2). The lymphatic system develops from an endothelial outgrowth of the anterior cardinal vein, with several proteins required to induce LEC differentiation, most notably transcription factors Sox18 and Prox1 (1, 3, 4). Others are required for maturation of the lymphatic system, including some that function in arterial EC specification (1, 2) (Fig. 1). Yoshimatsu et al. (5) highlight the role of a ligand–receptor system that represses LEC differentiation, proliferation, and lymphangiogenesis. The receptor ALK-1, previously implicated in arteriovenous specification (6), is activated by the TGF-β–related ligand bone morphogenetic protein-9 (BMP-9) and inhibits the switch from BEC to LEC differentiation by repressing the latter and promoting the former. Their study adds another dimension to the molecular control of EC specification.
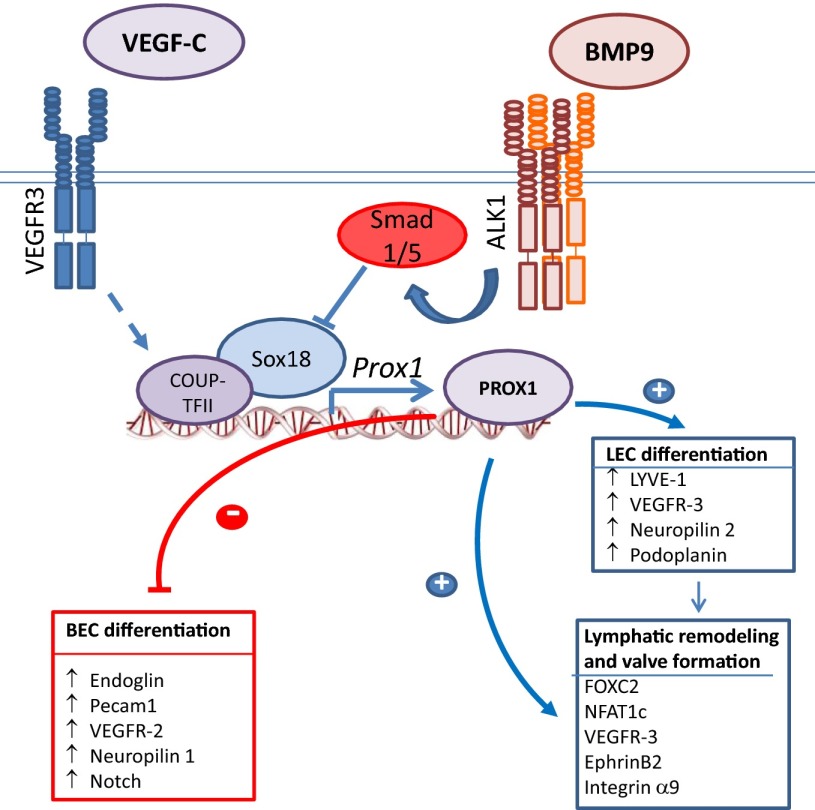
Fig. 1.
Model for the inhibitory effect of BMP-9–ALK-1 signaling on LEC differentiation, and enhanced BEC differentiation through suppression of Prox1. Prox1 either directly or indirectly activates expression of LEC markers, whilst suppressing expression of BEC markers.
Gene targeting and lineage tracing analyses in model organisms identified several molecular players that orchestrate budding of LECs from venous endothelium (1, 2). In early development, VEGFR3 and LYVE-1 are not only expressed in LECs but also in venous BECs, together with the transcription factor COUP-TFII. Sox18 is expressed with the appearance of the lymphatic rudiment and cooperates with COUP-TFII to activate Prox1 (Fig. 1). Prox1 and COUP-TFII then interact to instigate a LEC transcription program that enables continued expression of VEGFR3, Neuropilin 2, LYVE-1, and podoplanin (7). As development ensues, VEGFR3 expression is repressed in BECs but, due to persistent Prox1 expression, remains active in LECs. Prox1 is a master regulator of lymphatic development required to maintain LEC identity (1, 2, 7), because repression of Prox1 reprograms LECs into BECs (4).
A number of signaling pathways regulate LEC generation and lymphatic vessel development. The tyrosine kinase receptor VEGFR3 is activated by the ligands VEGF-C and VEGF-D, secreted from mesenchyme at sites of lymphangiogenesis, and is required for expansion of the LEC population and lymphangiogenesis (1, 2). EphrinB2 controls internalization of VEGFR3, and its loss also results in lymphatic defects (8). Angiopoietins, acting through the Tie-2 tyrosine kinase receptor, also control lymphangiogenesis. Whereas Tie-2 is expressed in both BECs and LECs, and angiopoietins control both hem- and lymphangiogenesis, only angiopoietin-2 expression is essential for development of the lymphatic system (1, 2). Notch signaling is also required for lymphatic vessel development, by regulating EphrinB2 expression and, consequently, VEGFR3 signaling (9). However, none of these signaling pathways directly controls Prox1 expression and LEC lineage commitment, although Erk MAPK signaling was shown to activate Sox18 and Prox1 expression and LEC specification (10).
Yoshimatsu et al. (5) define the role of the BMP/activin receptor-like kinase 1 (ALK-1) ligand–receptor pathway as an inhibitor of LEC differentiation and lymphangiogenesis. Unlike receptor tyrosine kinases that mediate VEGF or angiopoietin signaling, TGF-β family proteins act through complexes of two type II and two type I receptors that primarily phosphorylate Ser and Thr, enabling the type I receptors to activate Smads through C-terminal phosphorylation on Ser. Using mouse models, the authors show that in LECs, the type I receptor ALK-1 relays signals in response to BMP-9 and that BMP-9–ALK-1 signaling represses LEC differentiation and lymphangiogenesis in normal development and tumors. This repression is accompanied by increased BEC-marker expression, positioning BMP-9–ALK-1 signaling as a switch that regulates the reciprocal balance of BEC and LEC differentiation (5), resulting from repression of the Prox1 gene by ALK-1–activated Smads (4, 7).
These observations extend previous findings on the roles of ALK-1 and BMP-9 in hem- and lymphangiogenesis. ALK-1 was shown to antagonize the activities of the canonical TGF-β type I receptor, TβRI/ALK-5, in the control of endothelial function by TGF-β (11). With the identification of BMP-9 as a ligand for ALK-1 in ECs, BMP-9–ALK-1 signaling was shown to inhibit EC proliferation and angiogenesis (12), yet was also seen to promote EC proliferation and capillary sprouting (13). In lymphatic development, blockade of ALK-1 signaling in mice results in impaired lymphangiogenesis (14), whereas BMP-9 deficiency causes abnormal lymphatic development and impaired lymphatic draining (15).
The role of BMP-9–ALK-1 signaling as a differentiation switch in LECs is reminiscent of similar key roles of TGF-β family proteins in differentiation. TGF-β induces epithelial–mesenchymal transition (EMT), thus repressing the epithelial state and activating mesenchymal differentiation (16). TGF-β also represses osteoblastic and myogenic differentiation, whereas some BMPs activate osteoblast differentiation (17), and BMP7 induces brown adipocyte differentiation in mesenchymal progenitors (18). The underlying mechanisms reveal a potent ability of Smads to either activate or repress the expression and/or activities of master transcription factors that drive differentiation programs (19). In EMT induction, TGF-β–activated Smad3 induces expression of Snail transcription factors (20), whereas, in inhibiting osteoblast differentiation, Smad3 cooperates with Runx2 to repress Runx2 target genes (21), and, in myogenesis inhibition, Smad3 associates with myogenic bHLH transcription factors to prevent activation of their target genes (22). By analogy, we surmise that ALK-1–activated Smad1 or Smad5 repress Sox18-mediated transcription (Fig. 1), consistent with the cooperation of Smads with other Sox transcription factors such as Sox2 and Sox9 (19, 23). Thus, functional silencing of Sox18 by Smad1 or Smad5 may repress Prox1, preventing LEC differentiation and expanding the BEC population. When during embryogenesis ALK-1 expression first limits LEC expansion remains to be tested, because Alk1−/− mice die before lymphangiogenesis begins, and BMP10 can substitute for loss of BMP9 in BMP9−/− embryos (24). Nevertheless, Yoshimatsu et al. (5) observed defective lymphatic development in Bmp9−/− embryos five days after first budding of the lymphatic rudiment.
Mutations in genes controlling lymphatic development, including SOX18 and FOXC2, were found to be causative in human lymphedema syndromes (1). In contrast, hemizygous loss-of-function ALK1 mutations cause a vascular malformation syndrome named hereditary hemorrhagic telangiectasia (HHT), rather than affecting lymphatics. HHT patients present with recurrent nosebleeds and develop cutaneous and mucosal blood vessel dilatations. Some patients also develop life-threatening arteriovenous malformations (25). HHT can be caused by mutations in other ALK-1 pathway components, including the ALK-1 coreceptor endoglin that aids ligand binding, and Smad4, which partners with TGF-β– and BMP-activated Smads (25). Recently, causative BMP9 missense mutations have been implicated in an HHT-related syndrome (26). Thus, HHT-causing mutations appear to define a BMP-9–endoglin–ALK-1–Smad4 pathway. Despite the identification of causative genes, the basis for HHT pathology is still uncertain. Mouse models of HHT show an arterial to venous EC transition within vascular lesions, evident by decreased EphrinB2 and increased COUP-TFII expression (6). It will be of interest to study transitions from BEC- to LEC-like properties within human HHT lesions.
The control of LEC differentiation by BMP-9–ALK-1 signaling raises interesting questions. Why do ALK-1 and BMP-9 mutations primarily affect the vascular system in humans, whereas mutations in other key regulators of lymphangiogenesis cause lymphedema syndromes? The answer to this question lies in the fact that regulatory molecules are often repurposed for varied functions in different tissues and at different stages of development. For example, EphrinB2 not only acts in axon guidance but also regulates angiogenesis, lymphangiogenesis, and arterial EC fate (8, 27). Similarly, VEGFR3, an LEC marker, is essential for hemangiogenesis, where it can act with or independently of VEGF or VEGFR-2 to activate angiogenesis (9, 28). In this respect, it is intriguing that polymorphisms in PTPN14, a gene mutated in a lymphedema syndrome (29), show genetic association with the incidence of arteriovenous malformations in HHT patients (30), suggesting an interaction with endoglin/ALK-1 signaling in vascular development. PTPN14 interacts with VEGFR3 (29) and regulates EphrinB2 expression (30), suggesting further functional interaction between ALK-1 and VEGFR3. Direct functional cross-talk between ALK-1–activated Smads and VEGFR3 signaling, possibly complementing ALK-1–Notch signaling cross-talk as seen in hemangiogenesis (31), may additionally coordinate LEC and BEC proliferation and differentiation. Finally, a lingering question is what other ligands might control LEC differentiation and lymphangiogenesis through ALK-1. TGF-β can activate ALK-1 in ECs (11), and BMP-10 acts through ALK-1 in early vascular development (24). Defining the context-dependent roles of ALK-1 and its ligands in determining EC differentiation status during vascular and lymphatic development continues to be challenging but is of great biomedical interest.
Footnotes
The authors declare no conflict of interest.
See companion article on page 18940.
References
- 1.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17(11):1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 2.Koltowska K, Betterman KL, Harvey NL, Hogan BM. Getting out and about: The emergence and morphogenesis of the vertebrate lymphatic vasculature. Development. 2013;140(9):1857–1870. doi: 10.1242/dev.089565. [DOI] [PubMed] [Google Scholar]
- 3.François M, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456(7222):643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 4.Wigle JT, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21(7):1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshimatsu Y, et al. Bone morphogenetic protein-9 inhibits lymphatic vessel formation via activin receptor-like kinase 1 during development and cancer progression. Proc Natl Acad Sci USA. 2013;110:18940–18945. doi: 10.1073/pnas.1310479110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26(3):328–331. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, et al. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood. 2009;113(8):1856–1859. doi: 10.1182/blood-2008-03-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465(7297):483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 9.Niessen K, et al. The Notch1-Dll4 signaling pathway regulates mouse postnatal lymphatic development. Blood. 2011;118(7):1989–1997. doi: 10.1182/blood-2010-11-319129. [DOI] [PubMed] [Google Scholar]
- 10.Deng Y, Atri D, Eichmann A, Simons M. Endothelial ERK signaling controls lymphatic fate specification. J Clin Invest. 2013;123(3):1202–1215. doi: 10.1172/JCI63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goumans M-J, et al. Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J. 2002;21(7):1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109(5):1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, et al. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci. 2010;123(Pt 10):1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 14.Niessen K, Zhang G, Ridgway JB, Chen H, Yan M. ALK1 signaling regulates early postnatal lymphatic vessel development. Blood. 2010;115(8):1654–1661. doi: 10.1182/blood-2009-07-235655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levet S, et al. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood. 2013;122(4):598–607. doi: 10.1182/blood-2012-12-472142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25(1):76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- 17.Derynck R, et al. TGF-β family signaling in mesenchymal differentiation. In: Derynck R, Miyazono K, editors. The TGF-β Family. New York: Cold Spring Harbor Press; 2008. pp. 613–666. [Google Scholar]
- 18.Tseng YH, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454(7207):1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullen AC, et al. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell. 2011;147(3):565–576. doi: 10.1016/j.cell.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent T, et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-β mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11(8):943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang JS, Alliston T, Delston R, Derynck R. Repression of Runx2 function by TGF-β through recruitment of class II histone deacetylases by Smad3. EMBO J. 2005;24(14):2543–2555. doi: 10.1038/sj.emboj.7600729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D, Black BL, Derynck R. TGF-β inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001;15(22):2950–2966. doi: 10.1101/gad.925901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem. 2005;280(9):8343–8350. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, et al. Context-dependent signaling defines roles of BMP9 and BMP10 in embryonic and postnatal development. Proc Natl Acad Sci USA. 2013;110(29):11887–11892. doi: 10.1073/pnas.1306074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shovlin CL. Hereditary haemorrhagic telangiectasia: Pathophysiology, diagnosis and treatment. Blood Rev. 2010;24(6):203–219. doi: 10.1016/j.blre.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Wooderchak-Donahue WL, et al. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet. 2013;93(3):530–537. doi: 10.1016/j.ajhg.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104(5):576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 28.Benedito R, et al. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484(7392):110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- 29.Au AC, et al. Protein tyrosine phosphatase PTPN14 is a regulator of lymphatic function and choanal development in humans. Am J Hum Genet. 2010;87(3):436–444. doi: 10.1016/j.ajhg.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benzinou M, et al. Mouse and human strategies identify PTPN14 as a modifier of angiogenesis and hereditary haemorrhagic telangiectasia. Nat Commun. 2012;3:616. doi: 10.1038/ncomms1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larrivée B, et al. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell. 2012;22(3):489–500. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]