Significance
We examined the variability of neuron packing densities across cortical regions and areas in two baboons with spontaneous, untreated epilepsy and two baboons without epilepsy. The two baboons without epilepsy had the distribution of neocortical neurons expected for Old World monkeys and baboons, whereas the baboons with untreated epilepsy had reduced numbers of cortical neurons overall, with the greatest reductions in motor and frontal areas of the cortex, and with little or no reduction in the primary visual cortex. The results suggest that neuron loss may follow untreated seizure activity, and this loss is greatest in areas of the cortex related to motor functions.
Keywords: primates, plasticity, neuronal density
Abstract
Epilepsy is characterized by recurrent seizure activity that can induce pathological reorganization and alter normal function in neocortical networks. In the present study, we determined the numbers of cells and neurons across the complete extent of the cortex for two epileptic baboons with naturally occurring seizures and two baboons without epilepsy. Overall, the two epileptic baboons had a 37% average reduction in the number of cortical neurons compared with the two nonepileptic baboons. The loss of neurons was variable across cortical areas, with the most pronounced loss in the primary motor cortex, especially in lateral primary motor cortex, representing the hand and face. Less-pronounced reductions of neurons were found in other parts of the frontal cortex and in somatosensory cortex, but no reduction was apparent in the primary visual cortex and little in other visual areas. The results provide clear evidence that epilepsy in the baboon is associated with considerable reduction in the numbers of cortical neurons, especially in frontal areas of the cortex related to motor functions. Whether or not the reduction of neurons is a cause or an effect of seizures needs further investigation.
Epilepsy is associated with structural changes in the cerebral cortex (e.g., refs. 1–6), and partial epilepsies (i.e., seizures originating from a brain region) may lead to loss of neurons (7) and altered connectivity (8). The cerebral cortex is a heterogeneous structure comprised of multiple sensory and motor information-processing systems (e.g., refs. 9 and 10) that vary according to their processing demands, connectivity (e.g., refs. 11 and 12), and intrinsic numbers of cells and neurons (13–16). Chronic seizures have been associated with progressive changes in the region of the epileptic focus and in remote but functionally connected cortical or subcortical structures (3, 17). Because areas of the cortex are functionally and structurally different, they may also differ in susceptibility to pathological changes resulting from epilepsy.
The relationship between seizure activity and neuron damage can be difficult to study in humans. Seizure-induced neuronal damage can be convincingly demonstrated in animals using electrically or chemically induced status epilepticus (one continuous seizure episode longer than 5 min) to reveal morphometric (e.g., refs. 18 and 19) or histological changes (e.g., refs. 20 and 21). Subcortical brain regions are often studied for vulnerability to seizure-induced injury (21–27); however, a recent study by Karbowski et al. (28) observed reduction of neurons in cortical layers 5 and 6 in the frontal lobes of rats with seizures. Seizure-induced neuronal damage in the cortex has also been previously demonstrated in baboons with convulsive status epilepticus (29).
The goal of the present study was to determine if there is a specific pattern of cell or neuron reduction across the functionally divided areas of the neocortex in baboons with epilepsy. Selected strains of baboons have been studied as a natural primate model of generalized epilepsy (30–36) that is analogous to juvenile myoclonic epilepsy in humans. The baboons demonstrate generalized myoclonic and tonic-clonic seizures, and they have generalized interictal and ictal epileptic discharges on scalp EEG. Because of their phylogenetic proximity to humans, baboons and other Old World monkeys share many cortical areas and other features of cortical organization with humans (e.g., refs. 9 and 10). Cortical cell and neuron numbers were determined using the flow fractionator method (37, 38) in epileptic baboon tissue obtained from the Texas Biomedical Research Institute, where a number of individuals develop generalized epilepsy within a pedigreed baboon colony (31–36). Our results reveal a regionally specific neuron reduction in the cortex of baboons with naturally occurring, generalized seizures.
Results
We present cell and neuron numbers for small blocks of tissue across all cortical areas and regions in four baboons: two epileptic baboons and two neurologically normal controls. We focused only on neocortex cell and neuron numbers in the present report. One hemisphere from each case was used to determine cell and neuron numbers. The brain of case 09-27 was purchased from the University of Washington National Primate Research Center. Cases 11-31, 10-04, and 11-45 were provided by the Texas Biomedical Research Institute. All baboons were female, between 12 and 17 y of age, and with body weights between 17.8 and 23.4 kg. Table S1 summarizes specific details on the age, sex, size, and perfusion method for each case. Cases 09-27 and 11-31 were neurologically normal, and cases 10-04 and 11-45 were epileptic. Case 10-04 experienced multiple seizures at age 13 y, based upon witnessed seizure activity and craniofacial injury typically related to seizure activity. The first reported craniofacial injury occurred at age 10 y. Two witnessed seizures were reported in case 11-45, the first occurring at age 7 y after the delivery of an infant, and at age 16 y. Case 11-45 also underwent a scalp electroencephalogram at age 12 y, indicating generalized interictal epileptic discharges, but no evidence of photosensitivity. Both epileptic baboons were clinically otherwise unremarkable, and neither had experienced an episode of status epilepticus. There were no reports of motor impairment or any other type of behavioral impairment. Neither epileptic baboon was treated with antiepileptic medications.
Overall Reduction of Cell and Neuron Number in the Cortex with Epilepsy.
One cortical hemisphere from each of two normal baboons and two epileptic baboons was manually flattened. Flattened cortex varied in surface area between 186 and 242 cm2. Each flat cortex was viewed on a light box to identify borders of myelin-dense primary sensory areas [primary visual cortex (V1), primary sensory cortex (S1), and primary motor cortex (M1)] (15, 16, 39). Cortex was dissected into tissue pieces that were processed as 142 (09-27; average surface area = 1.307 cm2), 194 (11-31; average surface area = 1.201 cm2), 177 (10-04; average surface area = 1.389 cm2), and 428 (11-45; average surface area = 0.4478 cm2) samples. Tissue pieces for case 11-45 were dissected with a smaller area in an effort to further localize specific regions or areas of cell or neuron loss. For each dissected tissue sample, we determined the total number of cells and percentage of neurons per piece. Cell and neuron densities for each tissue piece were calculated by dividing the numbers of cells and neurons by square centimeter of cortical surface area. Table S2 summarizes the data for each case.
The numbers of cells and neurons across the entire cortical sheet were remarkably consistent between the two neurologically normal baboon cases. For case 09-27, there were 4.67 billion cells in the cortex, of which 2.36 billion (51%) were neurons. Similarly, for case 11-31, there were 4.29 billion cells in the cortex, of which 2.27 billion (52%) were neurons.
The overall numbers of neurons across the entire cortical sheet were markedly lower in epileptic baboons (Fig. 1B′) relative to normal baboons (Fig. 1A′) (U = 1826.0; P = 0.0001), and the overall number of cells per cortical hemisphere was also lower (Fig. 1 A and B) (U = 1436.0; P = 0.0001). For epileptic baboon 10-04, there were 4.00 billion cells in the cortex, of which 1.61 billion (39%) were neurons; and for epileptic baboon 11-45, there were 4.24 billion cells across the cortex, of which 1.79 billion (41%) were neurons. Epileptic baboons had a lower range of cells overall (4.00–4.24 billion cells) relative to control baboons (4.29–4.67 billion cells). In contrast, the reduced neuron number was evident, with a range of 2.26–2.39 billion cortical neurons in neurologically normal baboons, and 1.61–1.79 billion cortical neurons in epileptic baboons. Thus, epileptic baboon 10-04 had ∼48% fewer neurons across the extent of the cortex, and epileptic baboon 11-45 has ∼26% fewer cortical neurons (mean reduction of ∼37%).
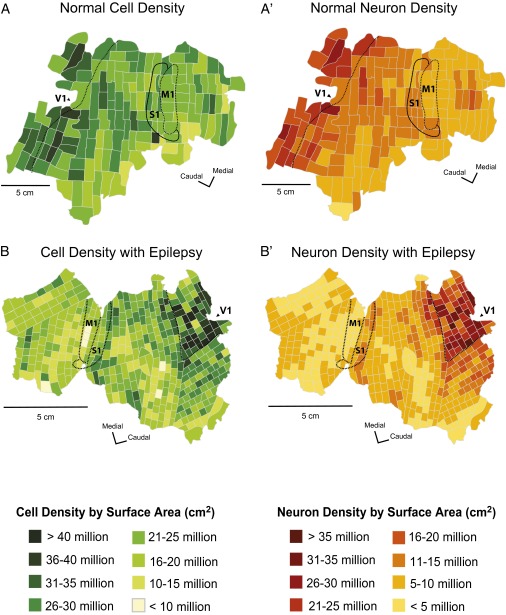
Fig. 1.
Cell and neuron density maps from a normal baboon (case 09-27) and an epileptic baboon (case 11-45). The normal cell (A) and neuron (A′) distribution in baboons shows a general caudal-to-rostral decrease in cells and neurons across the cortical sheet, with the highest cell and neuron densities located within primary sensory areas, which is consistent with findings from other primates. Epileptic baboons have consistently lower cell (B) and neuron (B′) densities relative to control. Neuron reduction appears to be regional specific with the most neuron loss observed in cortex rostral to the central sulcus, including M1.
Cell and Neuron Reduction Associated with Epilepsy Is Specific to Cortical Area.
We previously showed that the neuron density and the ratio of neurons to nonneuronal cells (mainly glia cells) vary to a great extent across cortical areas in a nonuniform pattern (15) (Fig. 2). In all cases, there was a caudal-to-rostral decrease in the numbers of cells and neurons across the cortical sheet, with primary sensory areas having the highest neuron densities, which is consistent with previous reports of normal primates (Figs. 1 A and A′, and 2 A and B) (15, 16). In normal baboons, V1 was ∼25% more cell dense than S1 (U = 114.0, P = 0.006) and M1 (U = 262.0, P = 0.108), and almost twice as neuron dense as S1 (U = 68.0, P = 0.0001) and M1 (U = 56.0, P = 0.0001), which is evident in Fig. 2. M1 and S1 were similarly cell dense (U = 59.0, P = 0.152), whereas M1 was 19% less neuron dense than S1 (U = 54.0, P = 0.093). Epileptic baboons also demonstrated a caudal-to-rostral decrease in cell and neuron densities across the cortical sheet (Figs. 1 B and B′, and 2 C and D) despite the overall reduction in the number of neurons. In epileptic baboons, V1 was on average, ∼43% more cell dense than both S1 (U = 115.0, P = 0.0001) and M1 (U = 207.0, P = 0.0001). V1 was on average ∼3.75-times more neuron dense than S1 (U = 14.0, P = 0.0001), and ∼5.63-times more neuron dense than M1 (U = 0.0, P = 0.0001). Cell densities in S1 and M1 were similar (U = 160.0, P = 0.813). However, M1 was roughly half as neuron dense as S1 (U = 99.0, P = 0.042).
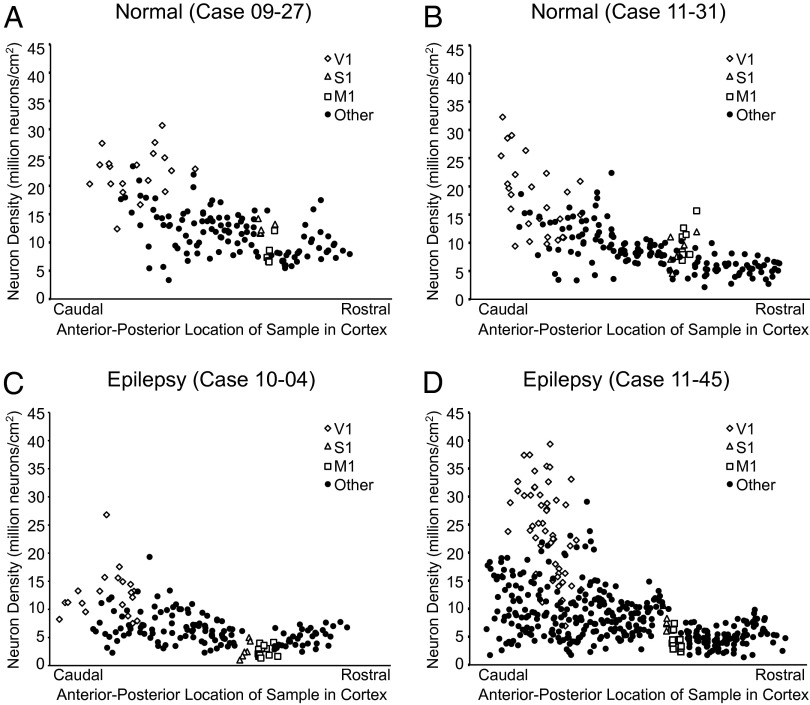
Fig. 2.
Neuron density versus the anterior-posterior dimension was plotted for each case. All flattened hemispheres were dissected into tissue pieces, and each piece was assigned an anterior-posterior coordinate by generating centroid measures. Normal neuron distribution (A and B) is shown to follow the caudal-to-rostral decrease in cortical neuron density that is typical of primates. Epileptic baboons show a reduction of neurons within this distribution, particularly in cortex rostral to the central sulcus (C and D).
We found that cell (U = 1457.0, P = 0.304) and neuron (U = 1483.0, P = 0.377) densities in V1 were similar in normal and epileptic baboons. The average cell density in V1 in normal baboons was 27.2 million cells/cm2 vs. 26.9 million cells/cm2 in epileptic baboons; the average neuron densities were 19.9 million neurons/cm2 and 18.5 million neurons/cm2, respectively (Fig. 2). Reductions in cells and neurons were evident outside of V1, particularly in the frontal lobe. In the primary somatosensory area S1, the average cell density was 23% lower in epileptic baboons relative to normal baboons but was not statistically significant (U = 38.0, P = 0.091). There was a 51% drop in S1 neuron density in epileptic baboons (5.32 million neurons/cm2 vs. 11.0 million neurons/cm2) that was statistically significant (U = 13.0, P = 0.001). The primary area most affected by cell and neuron reduction was M1. There was a 35% loss in cell density in M1 of epileptic baboons (15.0 million cells/cm2) relative to normal baboons (23.2 million cells/cm2) (U = 38.0, P = 0.0001). There was a drastic reduction in neuron density within M1, with a 65% reduction in neuron density in epileptic baboons (3.21 million neurons/cm2) compared with normal baboons (8.91 million neurons/cm2) (U = 0.0, P = 0.0001).
Neuron Reductions Within M1 Are Not Uniform.
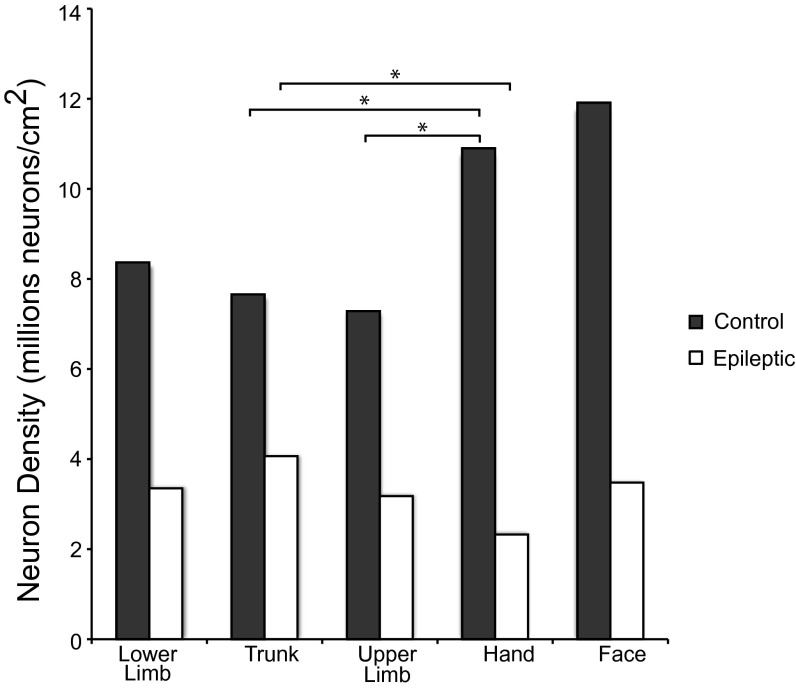
We found that the most substantial neuron reduction within M1 in epileptic baboons was localized to the most lateral aspect of the primary motor cortex, which generally corresponds to the locations of hand- and face-movement representations. In each baboon case, we estimated the boundaries of M1 and the subregions of M1 for movement representations of body parts based on visible M1 boundaries, sulcal landmarks, and reference to previously published depictions of M1 subdivisions (16), and dissected these regions accordingly. Cell densities across movement representations within M1 were found to be consistent within individuals. The data in Fig. 3 shows the distribution of neuron densities within the estimated representations. In normal baboons, the hand representation was found to be more neuron dense than the upper limb (U = 0.000, P = 0.050) and trunk (U = 0.000, P = 0.050) movement representations located in the more medial aspect of M1. Neuron density of the hand was not found to be different from the lower limb (U = 1.000, P = 0.077) or face representations. Conversely, in epileptic baboons, the hand representation was less neuron dense than the trunk representation (U = 2.000, P = 0.012). Averaged for both normal baboons, the face representation was ∼35% higher in neuron density relative to the upper limb, trunk, and lower limb representations. The hand representation of normal baboons was 24% more neuron dense than the upper limb, trunk, and lower limb regions. Conversely, averaged neuron densities for both epileptic baboons showed that the face representation was 13% lower than neuron densities of the upper limb, trunk, and lower limb regions. The hand representation of epileptic baboons was 36% lower compared with the upper limb, trunk, and lower limb regions. These data suggest that lateral M1, which is typically the most neuron-dense region of M1 (16), is the region that is most affected by neuron loss in epileptic baboons.
Fig. 3.
Histograms of neuron density in M1. Movement representation boundaries within M1 were estimated and dissected as described by Young et al. (16), and neuron densities by surface area were plotted for each case. There is neuron reduction within M1 of epileptic baboons relative to normal baboons. Lateral M1, which contains the face and hand representations, was the most neuron dense M1 region in normal baboons. In epileptic baboons, there is a substantial reduction of neurons in M1 in both cases, with the hand movement representation being the least neuron dense region of motor cortex.
Cell and Neuron Densities Within Representations of V1 and S1.
We found that foveal representation areas were consistently higher in cell and neuron densities than peripheral representation areas within V1, but that there was no overall difference related to epileptic or nonepileptic conditions. The average cell density in central vision areas in normal and epileptic baboons was ∼32 million cells/cm2. The average cell density for peripheral regions was 26.0 million cells/cm2 in epileptic baboons, and 22.2 million cells/cm2 in neurologically normal baboons. Foveal regions contained 22.9 million neurons/cm2 (normal) and 21.8 million neurons/cm2 (epileptic), and peripheral regions contained 17.9 million neurons/cm2 (normal) and 15.5 million neurons/cm2 (epileptic). No consistent internal variation according to somatotopic location was found within S1 in any case examined in this study.
Discussion
Using the flow fractionator method of counting cells and neurons (37, 38), we were able to demonstrate general and area-selective reductions in cortical neuron numbers and packing densities in the brains of epileptic compared with normal baboons. Although the numbers of cells and neurons were relatively equivalent in the primary visual cortex, they were dramatically reduced in the primary somatosensory and primary motor cortices. Differences in neuronal reduction were noted between functional regions of the motor cortex, with lateral M1 (subsuming face and hand functions) were more affected than medial M1 (proximal upper extremity, trunk, and lower extremities). This pattern of neuron reduction may have important implications in the etiology and pathophysiology of this natural model of genetic epilepsy.
Although epilepsy is associated with neuron loss and associated atrophy of cortical and subcortical structures, these effects have been described only following the prolonged and continuous seizure activity that characterizes status epilepticus (29). Neither of the two epileptic baboons in this study suffered from status epilepticus, and both appeared to have sporadic seizures. The baboon with the greatest neuronal reduction did experience more frequent seizures before euthanasia, but had no evidence of acute brain injury or infection to account for the seizure increase. Clinical reports (e.g., refs. 40 and 41) and behavioral studies (e.g., refs. 2 and 42) have reported impaired movement dexterity with epilepsy. There were no reports of motor impairment in either epileptic baboon, but it must be noted that motor dexterity was not explicitly studied. Behavioral studies are needed to carefully evaluate motor performance.
The reduction in neurons may account for the decrease in sulcal areas, particularly of the depths of cortical sulci in the epileptic baboons, most prominently revealed for the central, intraparietal, and cingulate sulci in MRI images (33). Although there was no decrease in cortical thickness, the reduction of cortex in cortical fissures suggests an overall reduction in cortical volume, consistent with the present evidence of neuron reduction in epileptic baboons. Decreased neuronal counts could be associated with decreased axonal and dendritic connections, particularly in U-fiber pathways that span the sulci. It is possible that neuron hypertrophy contributes to retention of some cortical thickness as microscopic examination of neocortex of temporal lobe epilepsy patients reported a 28% increase in neuron size with 3D unbiased stereology (43). Dendritic hypertrophy may also contribute to the retention of cortical thickness; however, it is dependent on cortical region and layer examined and the severity and the number of seizures (1, 43). These potential mechanisms can be further evaluated histologically and by diffusion weighted imaging.
Localized reduction in neuron numbers likely reflects regional differences in cortical microcircuitry. Although reduced numbers of neurons were found in M1 and S1, the number of neurons in V1 remained relatively constant. Laminar differences between V1 and M1 are readily apparent. In V1, a thick layer 4 contains densely packed, small neurons, and fewer pyramidal neurons. In contrast, M1 is defined by large pyramidal neurons in layers 2/3 and layer 5 (e.g., refs. 44–47), and a nearly absent layer 4. There is regional variation in the structural complexity of cortical pyramidal neurons that have implications for plasticity. V1 pyramidal neurons have the simplest dendritic arbors and fewest synaptic spines (48), whereas those in the frontal cortex have the most complex dendritic arbors (49), in support of more complex patterns of connectivity necessary for plasticity (50). With repeated seizures, there are decreased hyperpolarization-activated currents of layer 5 pyramidal neurons (51), a persistent expansion of movement representations areas (52), a decrease in movement threshold (53) in layer 5 of the motor cortex, and an increase in the polysynaptic component of the callosal-neocortical evoked potential (52), reflecting a source in superficial layer 5 and a sink in deep layer 5 (54). There are also a greater number of efficacious excitatory synapses (2) and alterations in hypertrophy of basilar dendrites (1), both in layer 5, which probably support the generation and maintenance of epileptic networks. The basal dendritic territories of pyramidal neurons of layer 5 in the somatosensory cortex also contain the largest number of parvalbumin-immunoreactive cell bodies (55), an inhibitory population of interneurons important for seizure propagation control (56), which is reduced in the cortex of epileptic patients (e.g., ref. 57) and the motor cortex of rats with seizures (58). In epileptic baboons, M1 and S1 maximally express interictal epileptic discharges, both spontaneous and elicited by intermittent light stimulation (e.g., ref. 36). Myoclonic seizures, which are the most common seizure types in the baboon, mainly affect the face and upper extremities (59), the somatopic regions most affected by neuron reduction. Motor cortex hyperexcitability in juvenile myoclonic epilepsy has been linked to impaired functioning of intracortical inhibition in transcranial magnetic stimulation studies (60). Further study of the intact cortical architecture of M1 is needed to determine what specific cell types are affected and their laminar distribution.
Materials and Methods
All brains were flushed with 0.9% phosphate buffer saline (PBS) and shipped overnight in the same solution. Upon arrival, one hemisphere from each was manually flattened. Readily identifiable cortical areas were dissected from the cortical sheet with a scalpel, including the primary visual cortex (V1), primary sensory cortex (S1), and primary motor cortex (M1). The locations of dissection cuts were drawn onto a high-resolution photograph of the cortex. The surface area was measured for each piece using Image J software (National Institutes of Health). This software was also used to assign an anterior-posterior coordinate by generating a centroid measure for the location of each tissue piece. Each piece was processed for cell and neuron density using the flow fractionator method for cell and neuron counting (37, 38). The overall number of cells and neurons were compared for epileptic and neurologically normal conditions using a Mann–Whitney test. Comparisons were also made between V1, S1, and M1 between the two conditions using the Mann–Whitney test. Statistical significance was set at P < 0.05, and was analyzed using SPSS.
Additional methodological details are given in SI Materials and Methods (including Figs. S1 and S2).
Supplementary Material
Acknowledgments
We thank Laura Trice and Feyi Aworunse for laboratory assistance, and Dr. Jamie Reed for statistical assistance. Flow cytometry experiments were conducted in the Vanderbilt Medical Center Flow Cytometry Shared Resource supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). This work was supported by a grant from the G. Harold and Leila Y. Mathers Foundation (to J.H.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318894110/-/DCSupplemental.
References
- 1.Teskey GC, Monfils MH, Silasi G, Kolb B. Neocortical kindling is associated with opposing alterations in dendritic morphology in neocortical layer V and striatum from neocortical layer III. Synapse. 2006;59(1):1–9. doi: 10.1002/syn.20215. [DOI] [PubMed] [Google Scholar]
- 2.Henry LC, Goertzen CD, Lee A, Teskey GC. Repeated seizures lead to altered skilled behaviour and are associated with more highly efficacious excitatory synapses. Eur J Neurosci. 2008;27(8):2165–2176. doi: 10.1111/j.1460-9568.2008.06153.x. [DOI] [PubMed] [Google Scholar]
- 3.Ciumas C, Savic I. Structural changes in patients with primary generalized tonic and clonic seizures. Neurology. 2006;67(4):683–686. doi: 10.1212/01.wnl.0000230171.23913.cf. [DOI] [PubMed] [Google Scholar]
- 4.Ronan L, et al. Widespread cortical morphologic changes in juvenile myoclonic epilepsy: Evidence from structural MRI. Epilepsia. 2012;53(4):651–658. doi: 10.1111/j.1528-1167.2012.03413.x. [DOI] [PubMed] [Google Scholar]
- 5.Vollmar C, et al. Altered microstructural connectivity in juvenile myoclonic epilepsy: The missing link. Neurology. 2012;78(20):1555–1559. doi: 10.1212/WNL.0b013e3182563b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Govindan RM, Asano E, Juhasz C, Jeong JW, Chugani HT. Surface-based laminar analysis of diffusion abnormalities in cortical and white matter layers in neocortical epilepsy. Epilepsia. 2013;54(4):667–677. doi: 10.1111/epi.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garbelli R, et al. Architectural (Type IA) focal cortical dysplasia and parvalbumin immunostaining in temporal lobe epilepsy. Epilepsia. 2006;47(6):1074–1078. doi: 10.1111/j.1528-1167.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- 8.Vulliemoz S, et al. Connectivity of the supplementary motor area in juvenile myoclonic epilepsy and frontal lobe epilepsy. Epilepsia. 2011;52(3):507–514. doi: 10.1111/j.1528-1167.2010.02770.x. [DOI] [PubMed] [Google Scholar]
- 9.Van Essen DC, Glasser MF, Dierker DL, Harwell J. Cortical parcellations of the macaque monkey analyzed on surface-based atlases. Cereb Cortex. 2012;22(10):2227–2240. doi: 10.1093/cercor/bhr290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb Cortex. 2012;22(10):2241–2262. doi: 10.1093/cercor/bhr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaas JH, Garraghty PE. Hierarchical, parallel, and serial arrangements of sensory cortical areas: Connection patterns and functional aspects. Curr Opin Neurobiol. 1991;1(2):248–251. doi: 10.1016/0959-4388(91)90085-l. [DOI] [PubMed] [Google Scholar]
- 12.Scannell JW, Blakemore C, Young MP. Analysis of connectivity in the cat cerebral cortex. J Neurosci. 1995;15(2):1463–1483. doi: 10.1523/JNEUROSCI.15-02-01463.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skoglund TS, Pascher R, Berthold CH. Heterogeneity in the columnar number of neurons in different neocortical areas in the rat. Neurosci Lett. 1996;208(2):97–100. doi: 10.1016/0304-3940(96)12569-6. [DOI] [PubMed] [Google Scholar]
- 14.Beaulieu C, Colonnier M. Number of neurons in individual laminae of areas 3B, 4 gamma, and 6a alpha of the cat cerebral cortex: A comparison with major visual areas. J Comp Neurol. 1989;279(2):228–234. doi: 10.1002/cne.902790206. [DOI] [PubMed] [Google Scholar]
- 15.Collins CE, Airey DC, Young NA, Leitch DB, Kaas JH. Neuron densities vary across and within cortical areas in primates. Proc Natl Acad Sci USA. 2010;107(36):15927–15932. doi: 10.1073/pnas.1010356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young NA, Collins CE, Kaas JH. Cell and neuron densities in the primary motor cortex of primates. Front Neural Circuits. 2013;7:30. doi: 10.3389/fncir.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller SS, et al. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: Effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry. 2002;73(6):648–655. doi: 10.1136/jnnp.73.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitkänen A, et al. Progression of neuronal damage after status epilepticus and during spontaneous seizures in a rat model of temporal lobe epilepsy. Prog Brain Res. 2002;135:67–83. doi: 10.1016/S0079-6123(02)35008-8. [DOI] [PubMed] [Google Scholar]
- 19.Kubová H, Mareš P. Are morphologic and functional consequences of status epilepticus in infant rats progressive? Neuroscience. 2013;235:232–249. doi: 10.1016/j.neuroscience.2012.12.055. [DOI] [PubMed] [Google Scholar]
- 20.Castro OW, et al. Comparative neuroanatomical and temporal characterization of FluoroJade-positive neurodegeneration after status epilepticus induced by systemic and intrahippocampal pilocarpine in Wistar rats. Brain Res. 2011;1374:43–55. doi: 10.1016/j.brainres.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Gavrilovici C, Pollock E, Everest M, Poulter MO. The loss of interneuron functional diversity in the piriform cortex after induction of experimental epilepsy. Neurobiol Dis. 2012;48(3):317–328. doi: 10.1016/j.nbd.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Pitkänen A, Tuunanen J, Kälviäinen R, Partanen K, Salmenperä T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32(1–2):233–253. doi: 10.1016/s0920-1211(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 23.Kälviäinen R, et al. Recurrent seizures may cause hippocampal damage in temporal lobe epilepsy. Neurology. 1998;50(5):1377–1382. doi: 10.1212/wnl.50.5.1377. [DOI] [PubMed] [Google Scholar]
- 24.Kotloski R, Lynch M, Lauersdorf S, Sutula T. Repeated brief seizures induce progressive hippocampal neuron loss and memory deficits. Prog Brain Res. 2002;135:95–110. doi: 10.1016/S0079-6123(02)35010-6. [DOI] [PubMed] [Google Scholar]
- 25.Kälviäinen R, Salmenperä T. Do recurrent seizures cause neuronal damage? A series of studies with MRI volumetry in adults with partial epilepsy. Prog Brain Res. 2002;135:279–295. doi: 10.1016/S0079-6123(02)35026-X. [DOI] [PubMed] [Google Scholar]
- 26.Peredery O, Persinger MA, Parker G, Mastrosov L. Temporal changes in neuronal dropout following inductions of lithium/pilocarpine seizures in the rat. Brain Res. 2000;881(1):9–17. doi: 10.1016/s0006-8993(00)02730-x. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, et al. Preferential neuron loss in the rat piriform cortex following pilocarpine-induced status epilepticus. Epilepsy Res. 2007;74(1):1–18. doi: 10.1016/j.eplepsyres.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Karbowski LM, Parker GH, Persinger MA. Post-seizure drug treatment in young rats determines clear incremental losses of frontal cortical and hippocampal neurons: the resultant damage is similar to very old brains. Epilepsy Behav. 2013;27(1):18–21. doi: 10.1016/j.yebeh.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Meldrum BS, Pappy JJ, Toure MF, Brierley JB. Four models for studying cerebral lesions secondary to epileptic seizures. In: Meldrum BS, Marsden CD, editors. Advances in Neurology. Vol 10. New York: Raven Press; 1975. pp. 147–161. [PubMed] [Google Scholar]
- 30.Killam EK. Photomyoclonic seizures in the baboon, Papio papio. Fed Proc. 1979;38(10):2429–2433. [PubMed] [Google Scholar]
- 31.Szabó CA, et al. Scalp EEG for the diagnosis of epilepsy and photosensitivity in the baboon. Am J Primatol. 2004;62(2):95–106. doi: 10.1002/ajp.20018. [DOI] [PubMed] [Google Scholar]
- 32.Szabó CA, et al. Clinical and EEG phenotypes of epilepsy in the baboon (Papio hamadryas spp.) Epilepsy Res. 2005;65(1-2):71–80. doi: 10.1016/j.eplepsyres.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Szabó CA, et al. Cortical sulcal areas in baboons (Papio hamadryas spp.) with generalized interictal epileptic discharges on scalp EEG. Epilepsy Res. 2011;93(2-3):91–95. doi: 10.1016/j.eplepsyres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabó CA, Salinas FS, Narayana S. Functional PET evaluation of the photosensitive baboon. Open Neuroimaging J. 2011;5:206–215. doi: 10.2174/1874440001105010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabó CÁ, et al. Baboon model of generalized epilepsy: Continuous intracranial video-EEG monitoring with subdural electrodes. Epilepsy Res. 2012;101(1-2):46–55. doi: 10.1016/j.eplepsyres.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szabó CÁ, Knape KD, Leland MM, Williams JT. Electroclinical phenotypes in a pedigreed baboon colony. Epilepsy Res. 2013;105(1–2):77–85. doi: 10.1016/j.eplepsyres.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins CE, Young NA, Flaherty DK, Airey DC, Kaas JH. A rapid and reliable method of counting neurons and other cells in brain tissue: A comparison of flow cytometry and manual counting methods. Front Neuroanat. 2010;4:5. doi: 10.3389/neuro.05.005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young NA, et al. Use of flow cytometry for high-throughput cell population estimates in brain tissue. Front Neuroanat. 2012;6:27. doi: 10.3389/fnana.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campi KL, Collins CE, Todd WD, Kaas J, Krubitzer L. Comparison of area 17 cellular composition in laboratory and wild-caught rats including diurnal and nocturnal species. Brain Behav Evol. 2011;77(2):116–130. doi: 10.1159/000324862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helmstaedter C, Kemper B, Elger CE. Neuropsychological aspects of frontal lobe epilepsy. Neuropsychologia. 1996;34(5):399–406. doi: 10.1016/0028-3932(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez MT, et al. Deficits in executive functions and motor coordination in children with frontal lobe epilepsy. Neuropsychologia. 2002;40(4):384–400. doi: 10.1016/s0028-3932(01)00130-0. [DOI] [PubMed] [Google Scholar]
- 42.Flynn C, Young NA, Teskey GC. Seizures, but not lowered seizure thresholds, results in larger neocortical motor maps and concomitant disruptions in skilled motor behaviour. Behav Brain Res. 2010;214(1):60–65. doi: 10.1016/j.bbr.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Bothwell S, et al. Neuronal hypertrophy in the neocortex of patients with temporal lobe epilepsy. J Neurosci. 2001;21(13):4789–4800. doi: 10.1523/JNEUROSCI.21-13-04789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. J Comp Neurol. 1993;330(2):238–271. doi: 10.1002/cne.903300207. [DOI] [PubMed] [Google Scholar]
- 45.Preuss TM, Stepniewska I, Jain N, Kaas JH. Multiple divisions of macaque precentral motor cortex identified with neurofilament antibody SMI-32. Brain Res. 1997;767(1):148–153. doi: 10.1016/s0006-8993(97)00704-x. [DOI] [PubMed] [Google Scholar]
- 46.Preuss TM, Stepniewska I, Kaas JH. Movement representation in the dorsal and ventral premotor areas of owl monkeys: A microstimulation study. J Comp Neurol. 1996;371(4):649–676. doi: 10.1002/(SICI)1096-9861(19960805)371:4<649::AID-CNE12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 47.Elston GN, Rockland KS. The pyramidal cell of the sensorimotor cortex of the macaque monkey: Phenotypic variation. Cereb Cortex. 2002;12(10):1071–1078. doi: 10.1093/cercor/12.10.1071. [DOI] [PubMed] [Google Scholar]
- 48.Elston GN, Tweedale R, Rosa MGP. Cortical integration in the visual system of the macaque monkey: Large-scale morphological differences in the pyramidal neurons in the occipital, parietal and temporal lobes. Proc Biol Sci. 1999;266(1426):1367–1374. doi: 10.1098/rspb.1999.0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elston GN, Benavides-Piccione R, DeFelipe J. The pyramidal cell in cognition: A comparative study in human and monkey. J Neurosci. 2001;21(17):RC163. doi: 10.1523/JNEUROSCI.21-17-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stepanyants A, Hof PR, Chklovskii DB. Geometry and structural plasticity of synaptic connectivity. Neuron. 2002;34(2):275–288. doi: 10.1016/s0896-6273(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 51.Albertson AJ, Yang J, Hablitz JJ. Decreased hyperpolarization-activated currents in layer 5 pyramidal neurons enhances excitability in focal cortical dysplasia. J Neurophysiol. 2011;106(5):2189–2200. doi: 10.1152/jn.00164.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teskey GC, Monfils MH, VandenBerg PM, Kleim JA. Motor map expansion following repeated cortical and limbic seizures is related to synaptic potentiation. Cereb Cortex. 2002;12(1):98–105. doi: 10.1093/cercor/12.1.98. [DOI] [PubMed] [Google Scholar]
- 53.Young NA, Vuong J, Flynn C, Teskey GC. Optimal parameters for microstimulation derived forelimb movement thresholds and motor maps in rats and mice. J Neurosci Methods. 2011;196(1):60–69. doi: 10.1016/j.jneumeth.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 54.Chapman CA, et al. Changes in field potentials and membrane currents in rat sensorimotor cortex following repeated tetanization of the corpus callosum in vivo. Cereb Cortex. 1998;8(8):730–742. doi: 10.1093/cercor/8.8.730. [DOI] [PubMed] [Google Scholar]
- 55.Elston GN, DeFelipe J, Arellano JI, Gonzilez-Albo MC, Rosa MG. Variation in the spatial relationship between parvalbumin immunoreactive interneurones and pyramidal neurones in rat somatosensory cortex. Neuroreport. 1999;10(14):2975–2979. doi: 10.1097/00001756-199909290-00019. [DOI] [PubMed] [Google Scholar]
- 56.Cammarota M, Losi G, Chiavegato A, Zonta M, Carmignoto G. Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. J Physiol. 2013;591(Pt 4):807–822. doi: 10.1113/jphysiol.2012.238154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi M, Kumada S, Shioda K, Fukatsu R. Neuropathological analysis of the brainstem and cerebral cortex lesions on epileptogenesis in hereditary dentatorubral-pallidoluysian atrophy. Brain Dev. 2007;29(8):473–481. doi: 10.1016/j.braindev.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Silva AV, Sanabria ERG, Cavalheiro EA, Spreafico R. Alterations of the neocortical GABAergic system in the pilocarpine model of temporal lobe epilepsy: Neuronal damage and immunocytochemical changes in chronic epileptic rats. Brain Res Bull. 2002;58(4):417–421. doi: 10.1016/s0361-9230(02)00811-0. [DOI] [PubMed] [Google Scholar]
- 59.Caviness JN. Pathophysiology and treatment of myoclonus. Neurol Clin. 2009;27(3):757–777, vii. doi: 10.1016/j.ncl.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Manganotti P, Bongiovanni LG, Zanette G, Fiaschi A. Early and late intracortical inhibition in juvenile myoclonic epilepsy. Epilepsia. 2000;41(9):1129–1138. doi: 10.1111/j.1528-1157.2000.tb00318.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.