Significance
In higher plants, the destiny of apical meristems (stem cells) is specific organogenesis, which determines the pattern of plant growth, and therefore morphotype and fertility. We found that bacterial infection can derail the meristems from their genetically preprogrammed destiny, altering plant morphogenesis. We identified four abnormal growth patterns, symptoms, in tomato infected with a cell wall-less bacterium, and found that each symptom corresponds to a distinct phase in meristem fate derailment. We unveiled molecular events that underlie the pathogen-induced meristem fate derailment and proposed a model to explain the phenomenon. Through probing the pathologically altered destiny of stem cells, our findings contribute to understanding plant growth and development under both normal and pathological conditions.
Keywords: organogenesis, cauliflower-like inflorescence, sympodial growth, flower malformation, shoot proliferation
Abstract
In the life cycle of higher plants, it is the fate of meristem cells that determines the pattern of growth and development, and therefore plant morphotype and fertility. Floral transition, the turning point from vegetative growth to reproductive development, is achieved via genetically programmed sequential changes in meristem fate from vegetative to inflorescence, and to floral, leading to flower formation and eventual seed production. The transition is rarely reversible once initiated. In this communication, we report that a bacterial infection can derail the genetically programmed fate of meristem cells, thereby drastically altering the growth pattern of the host plant. We identified four characteristic symptoms in tomato plants infected with a cell wall-less bacterium, phytoplasma. The symptoms are a manifestation of the pathogen-induced alterations of growth pattern, whereas each symptom corresponds to a distinct phase in the derailment of shoot apical meristem fate. The phases include premature floral meristem termination, suppressed floral meristem initiation, delayed conversion of vegetative meristem to inflorescence meristem, and repetitive initiation and outgrowth of lateral vegetative meristems. We further found that the pathogen-induced alterations of growth pattern were correlated with transcriptional reprogramming of key meristem switching genes. Our findings open an avenue toward understanding pathological alterations in patterns of plant growth and development, thus aiding identification of molecular targets for disease control and symptom alleviation. The findings also provide insights for understanding stem cell pluripotency and raise a tantalizing possibility for using phytoplasma as a tool to dissect the course of normal plant development and to modify plant morphogenesis by manipulating meristem fate.
In higher plants, growth and development are achieved through genetically programmed, concerted processes that are governed by intrinsic signaling pathways that respond to environmental cues. Among the processes, floral transition is the pivotal event that triggers the switch from vegetative growth to reproductive development. Floral transition involves at least three crucial steps: (i) conversion of the vegetative shoot apical meristem to a meristem (IM), (ii) production of floral meristems (FMs) from the periphery of the IM, and (iii) activation of ABCDE floral organ identity genes that lead to flower morphogenesis (1). Under normal physiological conditions, the process is essentially irreversible once the programmed meristem switching cascade is triggered (2). Although it is well known that abiotic factors such as temperature and day length can modify meristem fate (3, 4), and thereby morphogenesis, the derailment of normal morphogenesis by biotic factors and its underlying molecular mechanisms have been largely overlooked.
Phytoplasmas are minute, cell wall-less bacteria (5) believed to have evolved from an Acholeplasma-like ancestor via genomic reduction and fusion (6). Characterized by possessing small, AT-rich genomes packed with phage remnant-derived sequence viable mosaics (6–8), extant phytoplasmas live a transkingdom parasitic life, infecting plant and insect hosts. In infected plants, phytoplasmas occupy sieve elements of phloem tissues and cause numerous diseases in diverse host species (9). Despite recent identifications of potential virulence factors (10–12), phytoplasmas remain one of the least characterized plant pathogens in terms of their mechanisms of pathogenesis. Plants with phytoplasmal diseases often exhibit symptoms of shoot proliferation, witches’-broom (WB) growth, and flower malformation (13). Studies have revealed that, in deformed flowers of phytoplasma-affected plants, expressions of FM and floral organ identity genes become altered (14–17) (Table 1). Although such findings provided some insights into molecular events involved in homeotic transformations of floral organs, the underlying response mechanisms that led to phytoplasma-induced multiple morphological abnormalities have remained unexplored.
Table 1.
Transcriptional reprogramming of meristem switching and downstream floral organ identity genes in response to phytoplasma infection
| Genes involved in floral signaling pathway | Previous reports |
This study |
|||||||
| STOL-infected tomato (14) | OY-infected petunia (15) | Italian ICPh-infected Arabidopsis (17) | PLY- or PnWB- infected periwinkle (16) | PPT phytoplasma-infected tomato |
|||||
| BB stages 3 and 6 | CLI | DSGP | WB | ||||||
| Flower organ identity genes | Class A | PFG↓ | AP1→ | MC↓↓ | |||||
| Class B | LeDEF↓ | PhGLO1↓ | AP3↓ | LePI↓↓ | |||||
| PI↓ | TAP3↓↓ | ||||||||
| TM6↓↓ | |||||||||
| TPI↓↓ | |||||||||
| Class C | TAG1↑↓ | pMADS3↑ | AG↓ | TAG1↓↓ | |||||
| FBP6↑ | TAGL1↓↓ | ||||||||
| Class D | FBP7↓ | SLMBP3→↓↓* | |||||||
| TAGL11↑↑↓* | |||||||||
| Class E | FBP2↑ | SEP3↓ | SEP3↓ | LeMADS1↓↓ | |||||
| FBP5↑ | TM29↓↓ | ||||||||
| SLMBP21↓↓ | |||||||||
| TM5 (SEP3)↓↓ | |||||||||
| RIN↓→ | |||||||||
| FM identity genes | FA↑ | ALF↓ | FA↓→ | FA↓ | |||||
| WUS↓ | DOT↓ | AN↓↓ | AN↓ | ||||||
| TER↓ | LeWUS↓↓ | LeWUS→ | |||||||
| IM identity genes | SFT→→ | SFT↑ | SFT↓ | ||||||
| SP→ | |||||||||
| Vegetative-inflorescence meristem integrity gene | LeWUS↓ | ||||||||
Abbreviations: BB, big bud; CLI, cauliflower-like inflorescence; DSGP, disrupted sympodial growth pattern; ICPh, Italian clover phyllody; OY, onion yellows phytoplasma; PLY, periwinkle leaf yellowing phytoplasma; PnWB, peanut witches’-broom phytoplasma; PPT, potato purple top phytoplasma; STOL, stolbur phytoplasma; WB, witches’ broom. ↓, down-regulation; ↑, up-regulation; →, no apparent transcriptional change.
For RT-PCR assay of class D genes SLMBP3 and TAGL11, samples from stages 3, 6, and 8 were used. During the morphogenesis of tomato flowers, the initiation of ovule development from the placenta does not occur until stage 8 (34).
Columbia Basin potato purple top (PPT) phytoplasma is a ‘Candidatus Phytoplasma trifolii’-related strain responsible for PPT disease epidemics that have repeatedly occurred in the Pacific Northwest region of the United States during the past decade (18). The phytoplasma belongs to a lineage previously termed as beet leafhopper-transmitted virescence agent, which has a broad plant host range (19). In tomato plants (Solanum lycopersicum Mill.), the most recognizable symptom induced by the PPT phytoplasma infection is formation of “big buds” (BBs), an aberrant floral structure consisting of enlarged and fused sepals and aborted inner whorls (20). In the present study, we identified three previously undescribed symptoms: cauliflower-like inflorescence (CLI), disrupted sympodial growth pattern (DSGP), and WB growth. We found that the four symptoms were mutually distinct, each representing a unique stage of derailment of programmed meristem fate and a modified pattern of growth. The abnormal modifications in growth pattern were correlated with spatial and temporal alterations in transcriptional regulation of a suite of meristem switching and organ identity genes, redefining the fate and determinacy of the meristem cells. Based on these findings, we propose a model to explain the pathogen-induced alterations of growth pattern (PIAGP).
Results and Discussion
Identification of Symptoms Induced by PPT Phytoplasma Infection in Tomato.
We found that the complexity of the symptoms induced by the PPT phytoplasma infection in tomato extends far beyond BB formation: following the infection, as the diseased plant grew, at least four different symptoms appeared sequentially, and eventually occurred in a complex array. Close morphological and anatomical examinations of the symptoms and the course of their development revealed the relationship between a given symptom type and a specific alteration of the fate of the apical meristem. Perhaps nowhere is this phenomenon more striking than in the case of PPT phytoplasma infection in tomato.
BB.
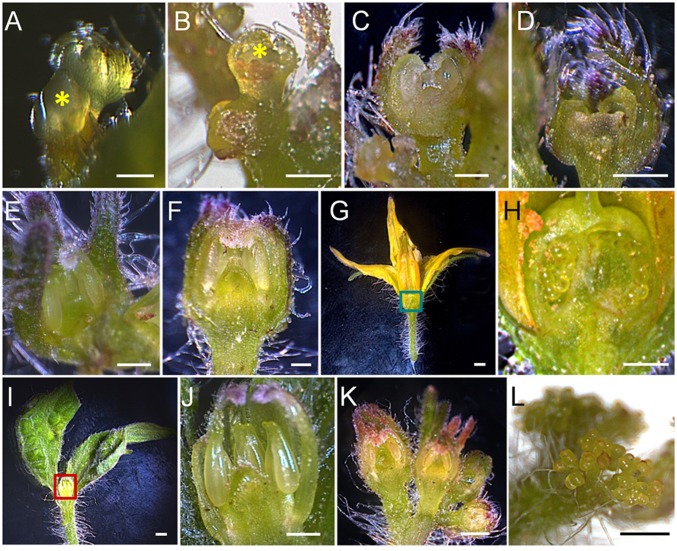
Beginning at ∼28 d postinoculation (dpi), the earliest inflorescence appeared and developed BBs in place of normal flowers (Fig. 1 A and B). Enlarged sepals fused along nearly their entire margins, forming a hollow bladder with a serrated opening at the distal end (Fig. 1B). In very few BB (less than 1%), the calyx opened, forming a fan- or trumpet-like structure (Fig. 1 C and D). All BBs, including fan- and trumpet-like BBs, had the same internal structure: petals and stamens were greenish, and stamens were loose and failed to form a cone (Fig. 2J); a carpel-like structure was formed, lacking the ovary, style, and stigma (Fig. 2 I and J). These buds never set fruit. During development of a given BB, initiation of sepal and petal primordia from FMs occurred at early stages (flower buds < 0.5 mm; Fig. 2B). However, when the buds reached stage 6 (sepal size, 1 mm), while sepals kept stretching out, petals bent downward toward inner whorls, and the emergence of stamens and carpels was delayed. The FM lost dome shape and became flat, a sign of premature termination (Fig. 2D). At stage 8 (sepal size, 2 mm), while the development of the three inner whorls became arrested completely (Fig. 2F), the sepals continued to grow in size, giving rise to the eventual BB structure (Fig. 2I).
Fig. 1.
Symptoms of potato purple top (PPT) phytoplasma infection in tomato and phenotypes of loss-of-function tomato lines. (A) A normal flower from a mock-inoculated healthy plant. (B) A typical big bud (BB) (marked with a red circle) structure formed by a PPT phytoplasma-infected plant. Occasionally, BB sepals open like a fan (C) or a trumpet (D). (E) An inflorescence from a mock-inoculated healthy plant. (F) Cauliflower-like inflorescence (CLI). (G) Highly branched inflorescence phenotype of anantha (an) tomato mutant. (H) Disrupted sympodial growth pattern (DSGP) (Right) vs. the normal three leaves–one inflorescence sympodial growth pattern (Left). In DSGP, more than nine, instead of three, leaves are present in a single sympodial unit. Individual leaves are numbered and inflorescence branches are marked by yellow dots. (I) An auxiliary shoot from a mock-inoculated healthy plant. (J) Witches’-broom (WB) growth induced by PPT phytoplasma infection. (K) WB growth shown in J but opened to facilitate viewing of auxiliary shoots (indicated by red arrowheads).
Fig. 2.
Microscopic observation of PPT phytoplasma infection-induced changes in meristem fate and flower morphology. (A and B) Stage 3 floral buds (0.5 mm, indicated by yellow asters) of mock-inoculated (A) and phytoplasma-infected (B) plants. (C and D) Stage 6 (1 mm) floral buds of mock-inoculated (C) and phytoplasma-infected (D) plants. (E and F) Stage 8 (2 mm) floral buds of mock-inoculated (E) and phytoplasma-infected (F) plants. (G) Longitudinal section of a mature flower from a mock-inoculated plant. (H) A close-up view of the green-boxed part of G, showing carpel with ovules. (I) Longitudinal section of a BB from a phytoplasma-infected plant. (J) A close-up view of the red-boxed area of I, showing aborted three inner whorl organs. (K) Normal floral transition leading to formation of typical tomato inflorescence and flowers. (L) A phytoplasma infection-induced CLI composed of dividing sympodial IMs. (Scale bar: 0.5 mm.)
CLI.
Beginning at appropriately 45 dpi, instead of producing BBs, new IMs began to branch into smaller and smaller segments, forming cauliflower-like structures (Fig. 1F). Unlike healthy tomato plants, in which the inflorescence is compound and consists of branches generated from the zigzag reiteration of sympodial inflorescence meristems (SIMs) with each SIM producing a new SIM before terminating in a FM (21), the cauliflower-like structures developed in PPT phytoplasma-affected plants were the result of repetitive initiation of SIM in place of FM (or homeotic transformation of FM to SIM) (Fig. 2L). Such a phenomenon indicated that the formation of FM had been suppressed at this disease stage. Occasionally, both BB and CLI symptoms appeared in the same inflorescence, indicating that the inflorescence was affected at the time when some FM had already initiated, whereas subsequent initiation of FM was suppressed.
DSGP.
In a normal, healthy tomato plant, at the 10- to 12-leaf stage, the primary shoot becomes terminated when the first inflorescence forms. Subsequently, a sympodial growth pattern is established, with each sympodial unit containing three leaves followed by an inflorescence (22). We observed that PPT phytoplasma infection did not affect the timing of initial floral transition and the consequent formation of the first inflorescence. However, starting from the fourth or fifth inflorescence, the normal sympodial growth pattern became disrupted. Instead of forming normal three leaves–one inflorescence sympodial units (Fig. 1H, Left), more leaves (up to 10) were produced in each unit, and the distal internodes were noticeably shortened (Fig. 1H, Right). Such sympodial growth pattern change indicated that the vegetative meristem (VM)-to-IM conversion had been delayed.
WB growth.
In healthy tomato plants, for each sympodial unit, lateral bud outgrowth only occurs at the axil of the leaf below the inflorescence. In PPT phytoplasma-infected plants, beginning at appropriately 45 dpi, lateral buds began to sprout from every leaf axil on upper shoots, a strong indication of apical dominance release. Following repeated lateral bud initiation and outgrowth, highly proliferated and compact branches had formed by around 60 dpi, resulting in the WB symptom (Fig. 1 J and K). Foliage in WB structures was smaller in size and was often chlorotic.
A Working Model for Understanding PIAGP.
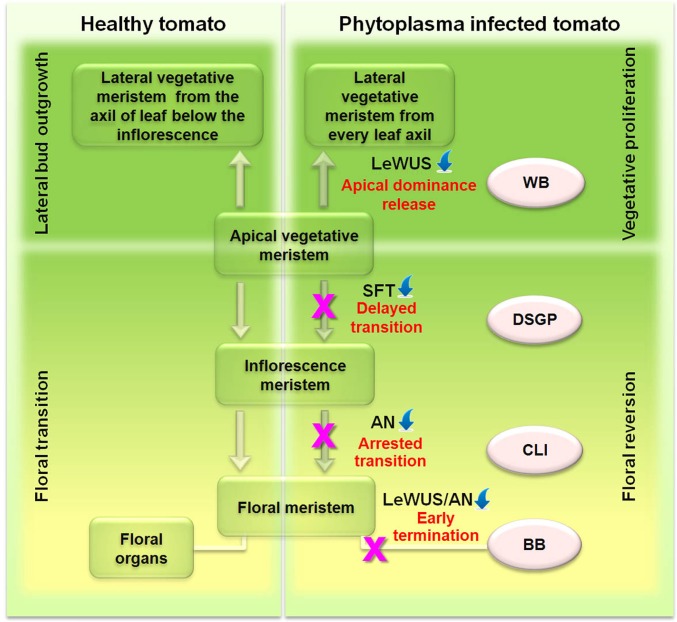
Based on the above identified symptoms, the timing of each symptom’s appearance, and the organ in which a given symptom appeared, we propose that each symptom induced by the phytoplasma infection, conceivably through the action of unknown effector molecule(s), represents a stage-specific alteration of shoot apical meristem fate. The fate of a given shoot apex and the final morphology (symptom) of the shoot, therefore, depends upon the developmental stage of the apex when it becomes affected by the phytoplasma. According to this hypothesis, if an apex originally destined to be a FM becomes affected by the phytoplasma after its initiation as an FM, the further development of the FM will be terminated prematurely, resulting in BB symptoms. If an apex becomes affected before FM initiation, the initiation of FM from IM will be arrested for that apex, resulting in CLI symptoms. If an apex is affected at or right before IM initiation, the conversion of VM to IM will be delayed, resulting in DSGP symptoms in the branch. If an apex becomes affected at earlier than any of the above-mentioned critical stages, apical dominance will be released and lateral VM will be initiated and outgrow repetitively, resulting in WB symptom. We next studied how these alterations of apical shoot meristem fate come about. Because PIAGP is due to changes in meristem fate, we hypothesized that the stem cells must be altered by reprogramming of the expressions of meristem switching genes and downstream organ identity genes. Our hypothesis is summarized in Fig. 3 as a working model for understanding PIAGP.
Fig. 3.
Proposed model of pathogen-induced alteration of meristem fates. In this model, PPT phytoplasma-induced floral reversion and vegetative proliferation takes place through transcriptional suppression of key meristem switching genes, altering meristem fate. Early termination of FM, arrested IM-to-FM transition, delayed transition from apical meristem to IM, and release of apical dominance. AN, anantha gene; BB, big bud; CLI, cauliflower-like inflorescence; DSGP, disrupted sympodial growth pattern; LeWUS, tomato Wuschel gene; SFT, single flower truss gene; WB, witches’ broom. The downward blue arrowheads indicate suppression of respective genes. The pink cross indicates point of meristem fate derailment.
Identification of Meristem Switching Genes Involved in PIAGP.
At the molecular level, normal floral transition is controlled by an array of meristem switching genes and downstream floral organ identity genes (1) (Fig. S1). In tomato, several single-gene mutants that impact normal floral transition and flower formation are available. To test our hypothesis ascribing a key role of altered expressions of host meristem switching and downstream organ identity genes in the development of the observed PPT phytoplasmal symptoms, we selected single-gene mutants and transgenic tomato lines that exhibit phenotypes similar to the disease symptoms, and performed comparative analyses on expression profiles of the selected genes in healthy and PPT phytoplasma-infected plants through quantitative reverse transcriptase–PCR (qRT-PCR).
Genes involved in WB growth.
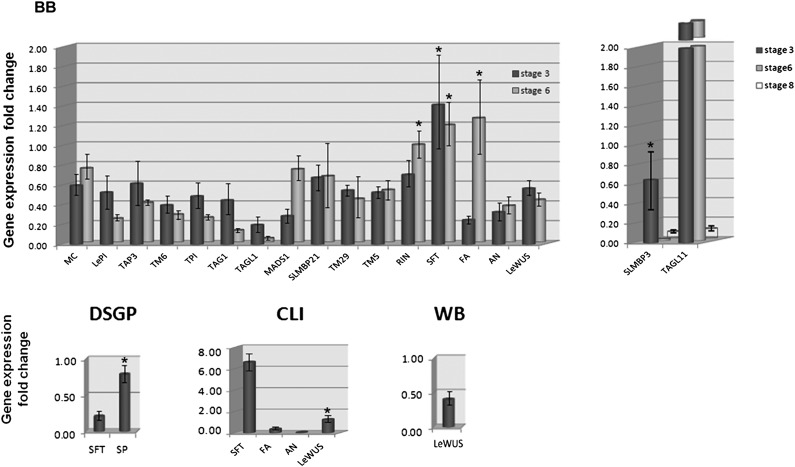
Although the phytoplasma-induced WB growth is characterized by highly proliferated shoots resulting from the apparent loss of apical dominance, this symptom does not phenocopy previously described meristem-maintenance tomato mutants such as gob, exp, spd, or mud (23). Rather, it resembles the phenotype of an Arabidopsis recessive mutant of the WUSCHEL (WUS) gene. The striking similarity between the PPT phytoplasma-induced WB symptom and the growth pattern of noninfected recessive wus mutant Arabidopsis was evident in the apparent repeated initiation and outgrowth of VM from the axils of previously produced leaves and generation of a “stop-and-go”-like pattern of auxiliary bud growth, giving rise to a bushy or wuschel (in German) appearance (24). Results from a qRT-PCR assay confirmed that the transcript levels of the tomato WUS ortholog, LeWUS, were decreased in the meristems of lateral shoot apices from phytoplasma-infected plants (Table 1 and Fig. 4); the low expression of LeWUS may result in a situation analogous to that in the Arabidopsis recessive wus mutant: a release of shoot apical dominance, giving rise to a stop-and-go growth pattern.
Fig. 4.
Expressions of meristem switching and floral organ identity genes in PPT phytoplasma-infected plants. Gene expression fold changes are relative to mock-inoculated controls to which a value of 1.00 was assigned. β-Actin gene was used as an internal reference. BB, big bud; CLI, cauliflower-like inflorescence; DSGP, disrupted sympodial growth pattern; WB, witches’-broom growth. The vertical bars indicate two times the value of the SD for the corresponding dataset (n = 9). Up- or down-regulations of all differentially expressed genes are statistically significant (P < 0.05). Genes that were not differentially expressed are marked with an asterisk. Refer to Table 1 for a summary of up- and down-regulation of the genes.
Genes involved in DSGP.
In healthy tomato, starting from 10- to 12-leaf stage, the plant exhibits a sympodial growth pattern, with each sympodial unit containing three leaves and one inflorescence. Two genes have been reported to influence sympodial growth; they are SELF PRUNING (SP) and SINGLE FLOWER TRUSS (SFT) (25–28). SP controls the number of leafs in sympodial units. Whereas the recessive loss-of-function mutant sp causes gradual reduction in leaf number with each successive sympodial unit, overexpression of SP increases the number of leaves in each sympodial unit. SFT, the tomato ortholog of Arabidopsis FLOWERING LOCUS T (FT), encodes a graft-transmissible florigen that regulates flowering time and sympodial development. The loss-of-function mutant sft produces either a solitary flower or inflorescences that frequently revert to leaves (25–28). Although the PPT phytoplasma-induced DSGP symptom mimicked the “leaf number increase” phenotype similar to the phenotypes of both the sft mutant and the overexpression of SP in transgenic tomato, the following two lines of evidence indicated that SFT was involved in the DSGP symptom development: (i) in PPT phytoplasma-affected VMs, the expression of the SFT gene was significantly down-regulated (Table 1, DSGP column, and Fig. 4, DSPG panel); and (ii) the normal “three leaves–one inflorescence” sympodial growth pattern was restored after the PPT phytoplasma was graft-inoculated onto a transgenic tomato line constitutively expressing the SFT gene (data not shown). However, there was no apparent change in SP gene transcript level in PPT phytoplasma-infected wild-type tomato, and when the PPT phytoplasma was inoculated to a sp mutant tomato plant, a new morphotype developed. Instead of displaying the phenotype characteristic of sp mutant (gradual reduction in leaf number with each successive sympodial unit, and eventual termination of the shoot by two consecutive inflorescences), the PPT phytoplasma-infected sp mutant tomato produced repeated sympodial units, each containing only a single leaf. This latter phenomenon suggests that SP plays a role in sympodial unit geometry that differs from that of SFT.
Genes involved in CLI formation.
The CLI symptom exhibited by PPT phytoplasma-infected tomato plants resembled the phenotype of a noninfected tomato having strong recessive mutant of ANANTHA (AN) (Fig. 1 F and G). AN, the tomato ortholog of Arabidopsis F-box protein UNUSUAL FLORAL ORGANS (UFO), regulates inflorescence branching and floral organ identity specification (29). In strong recessive an mutants, SIMs proliferate and fail to initiate FM, giving rise to CLI (22, 29). Indeed, our qRT-PCR results revealed that, in PPT phytoplasma-infected plants, the expression of AN gene was down-regulated more than 20-fold in the SIMs of PPT phytoplasma-induced CLI (Table 1 and Fig. 4), suggesting AN may play a major role in the formation of the CLI symptom. However, AN may not be the only factor involved, as our results showed that, in the SIMs of PPT phytoplasma-induced CLI, the expressions of FA and SFT were also altered (Fig. 4). A recent study revealed that, in tomato IMs, a cooperation of Jointless (J) and an unknown target of SFT represses FM identity gene FA, leading to compound inflorescence (30). It would be interesting to learn whether the up-regulation of SFT gene and the down-regulation of FA gene that we observed in the SIMs of PPT phytoplasma-induced CLI play a similar role in overall CLI symptom induction.
Genes involved in BB formation.
Formation of the four floral whorls (sepal, petal, stamen, and carpel), characteristically present in a normal tomato flower, is regulated by a series of MADS-box transcriptional factors encoded by five classes of floral organ identity genes (ABCDE model, Fig. S1). Functions of tomato floral homeotic genes are summarized in Table S1. In phytoplasma-induced BB, floral deformation occurred in all four whorls; no known tomato mutant has exhibited such phenotype, so available tomato floral mutants were not useful to study the genes involved in BB symptoms. Although no morphological alterations were detectable in the FMs destined to form BB until stage 6 of the floral development (Fig. 2 A and B), qRT-PCR results revealed that down-regulation of multiple floral organ identity genes occurred in earlier stages (Table 1 and Table S1, Fig. 4 and Fig. S2). Consistent with the roles of the respective floral organ identity genes in floral morphogenesis, the down-regulation of a class A gene (MC) and interacting class E genes (TM29 and LeMADS1) conceivably contributed to eventual sepal hypertrophy in BB. The suppression of class B (LePI, TAP3, TM6, TPI), class C (TAG1 and TAGL1), class D (SLMBP3, TAGL11), and class E (TM5) genes were likely responsible for the gross underdevelopment of the petals, stamens, and carpels. In addition to the floral organ identity genes, the expressions of key FM identity genes, AN and LeWUS, were also suppressed (Table 1 and Fig. 4 and Fig. S2). Because the FM identity genes control the downstream floral identity genes (1) (Fig. S2), down-regulation of AN and LeWUS may play a major role in terminating FMs prematurely, leading to eventual floral malformation as exhibited by the BB symptom.
Concluding Remarks.
In higher plants, apical meristems are major determinants of plant morphotype and fertility. The conversion of the stem cells from vegetative state to floral initial is a critical event that decides whether a flowering plant will produce the next generation of seeds. In nature, as well as in agriculture, this event assumes greater importance for survival of a plant species through a nongrowing season as well as for survival of other members of a biosphere. However, these important normal processes can be derailed. Whereas the destiny of a FM or IM is largely set due to already completed differentiation, our findings illustrate that a meristem can be diverted from its normal destiny. We have examined the fate of shoot apical meristems in the case of plant infection by a unique class of bacteria known as phytoplasma. Depending upon the developmental stage of a developing IM or FM when it became affected by the phytoplasma, the meristem produced distinct abnormal structures, DSGP, CLI, or BB. Our observations indicated that an IM or FM gradually becomes progressively more committed to irreversible destiny as a flower organ, and at the same time, the meristem becomes increasingly less susceptible to derailment from the normal process.
Our study also documents events in pathogen-induced release of shoot apical dominance. Normal apical dominance, exerted by a shoot apical meristem on proximal axillary buds, maintains a plant species’ characteristic growth habit. We found that disruption of the apical dominance by the phytoplasma infection resulted in repetitive initiation and outgrowth of axial shoots, WB growth, a characteristic of many phytoplasmal plant diseases that yields no FMs. Our model explains that the infection derails the meristem from its normal destiny by altering the expression of specific genes that are normally involved in production of a fertile flower. By proposing mechanisms whereby the pathogen induces abnormal plant morphogenesis, the model supports and reinforces the role in meristem development suggested by others for these genes, and it opens avenues for identifying molecular targets for mitigation of disease, while presenting insights for further understanding of stem cell pluripotency and regulation of plant morphogenesis.
Finally, one may ask whether the observed derailments of meristem fates have an effect upon the phytoplasma pathogen or its insect vector. Both the derailment of IM and FM from forming reproductive flowers, and the proliferation of axillary shoots lacking FMs, tend to produce increased, possibly prolonged, vegetative growth (Fig. 3). Thus, it might be speculated that the symptom syndrome, consisting of nonreproductive flower-like structures (BB and CLI), additional leaves in DSGP, and WB growths, and concomitant delay in plant senescence, may be advantageous for pathogen and vector through the presence of greater amounts of host tissue over a longer period.
Materials and Methods
Phytoplasma Strain and Plant Materials.
The Columbia Basin PPT phytoplasma, a Candidatus Phytoplasma trifolii-related strain and a member of the clover proliferation phytoplasma group, subgroup A, was originally identified in diseased potato plants growing in Washington State (31) and was maintained in tomato plants via serial shoot graft transmission. Seeds of wild-type tomato (Solanum lycopersicum Mill. cv. Moneymaker) were obtained commercially from Reimer Seeds. Seeds of tomato mutants falsiflora (fa, LA0854), single flower truss (sft, LA2460), macrocalyx (mc, LA0159), anantha (an, LA0536), and self pruning (sp, LA3133) were provided by the C. M. Rick Tomato Genetics Resource Center (Davis, CA). Seeds of the tomato transgenic line, 35S:SFT, were a gift from Dr. Eliezer Lifschitz of the Technion–Israel Institute of Technology (Haifa, Israel).
Graft Inoculation.
PPT phytoplasma infection in experimental plants was established by graft inoculation. Four-leaf–stage healthy (wild-type, mutant, or transgenic) tomato seedlings were used as recipient plants (rootstocks) and infected shoots exhibiting WB symptoms were used as phytoplasma inocula (scions). A freshly cut shoot to be used as inoculum was trimmed to a wedge at its lower end and the wedged region was inserted into a cleft cut in the main stem of the recipient plant. The graft union was held firm with a plastic clip. The grafted plants were placed on a mist bench for a week and then moved to a climate-controlled greenhouse (25 °C and 70–80% humidity) with a 16-h light/8-h dark photoperiod.
Symptom Recording and Stereomicroscopic Imaging.
Floral and foliage symptom development in infected plants was observed and recorded daily with respect to flowering timing, inflorescence formation, sympodial growth pattern, and flower and leaf morphology. Internal structures of floral organs, inflorescences, and shoot apex were dissected and examined under a SteREO Discovery V20 microscope (Zeiss). The images were captured by a digital camera (AxioCam; Zeiss) attached to the microscope.
qPCR Analysis of Gene Expression.
VM, IM, and FM tissues were dissected from vegetative shoot apices, inflorescences, and floral buds, respectively, of both PPT phytoplasma-infected and mock-inoculated control plants under a dissecting microscope. For each meristem type, 5-µg samples were pooled for RNA extraction using a modified TRIzol/RNeasy hybrid protocol (32). First-strand cDNA was synthesized from the above RNA preparations with oligo-dT primer using AffinityScript Multiple Temperature cDNA Synthesis kit (Stratagene). Gene expression profiles were analyzed by qPCR with gene-specific primers (Table S2). The amplification reactions were performed using Brilliant SYBR Green qPCR Master Mix on the Mx3000P Real-Time System (Stratagene). Relative quantitation of the target gene transcripts was carried out using the ∆∆Ct method (33). Gene expression fold changes were relative to healthy controls with β-actin gene as the internal reference. Experiments were repeated three times, and three independent samples were used for each repeat experiment (n = 9). Data were analyzed statistically with Student’s t test implemented in Microsoft Excel.
Supplementary Material
Acknowledgments
Tomato transgenic line 35S:SFT was provided by Dr. Eliezer Lifschitz of the Technion–Israel Institute of Technology. Tomato single-gene mutant lines fa, sft, mc, an, and sp were obtained from the C. M. Rick Tomato Genetics Resource Center.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318489110/-/DCSupplemental.
References
- 1.Kater MM, Dreni L, Colombo L. Functional conservation of MADS-box factors controlling floral organ identity in rice and Arabidopsis. J Exp Bot. 2006;57(13):3433–3444. doi: 10.1093/jxb/erl097. [DOI] [PubMed] [Google Scholar]
- 2.Levy YY, Dean C. The transition to flowering. Plant Cell. 1998;10(12):1973–1990. doi: 10.1105/tpc.10.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R, et al. Tomato flower abnormalities induced by low temperatures are associated with changes of expression of MADS-Box genes. Plant Physiol. 1998;117(1):91–100. doi: 10.1104/pp.117.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trevaskis B, et al. Short vegetative phase-like MADS-box genes inhibit floral meristem identity in barley. Plant Physiol. 2007;143(1):225–235. doi: 10.1104/pp.106.090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doi Y, Teranaka M, Yora K, Asuyama H. Mycoplasma or PLT-group-like organisms found in the phloem elements of plants infected with mulberry dwarf, potato witches' broom, aster yellows or paulownia witches' broom. Ann Phytopathol Soc Jpn. 1967;33(4):259–266. [Google Scholar]
- 6.Wei W, Davis RE, Jomantiene R, Zhao Y. Ancient, recurrent phage attacks and recombination shaped dynamic sequence-variable mosaics at the root of phytoplasma genome evolution. Proc Natl Acad Sci USA. 2008;105(33):11827–11832. doi: 10.1073/pnas.0805237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jomantiene R, Davis RE. Clusters of diverse genes existing as multiple, sequence-variable mosaics in a phytoplasma genome. FEMS Microbiol Lett. 2006;255(1):59–65. doi: 10.1111/j.1574-6968.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- 8.Jomantiene R, Zhao Y, Davis RE. Sequence-variable mosaics: Composites of recurrent transposition characterizing the genomes of phylogenetically diverse phytoplasmas. DNA Cell Biol. 2007;26(8):557–564. doi: 10.1089/dna.2007.0610. [DOI] [PubMed] [Google Scholar]
- 9.Weintraub PG, Beanland L. Insect vectors of phytoplasmas. Annu Rev Entomol. 2006;51:91–111. doi: 10.1146/annurev.ento.51.110104.151039. [DOI] [PubMed] [Google Scholar]
- 10.Hoshi A, et al. A unique virulence factor for proliferation and dwarfism in plants identified from a phytopathogenic bacterium. Proc Natl Acad Sci USA. 2009;106(15):6416–6421. doi: 10.1073/pnas.0813038106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugio A, Kingdom HN, MacLean AM, Grieve VM, Hogenhout SA. Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proc Natl Acad Sci USA. 2011;108(48):E1254–E1263. doi: 10.1073/pnas.1105664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacLean AM, et al. Phytoplasma effector SAP54 induces indeterminate leaf-like flower development in Arabidopsis plants. Plant Physiol. 2011;157(2):831–841. doi: 10.1104/pp.111.181586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee IM, Davis RE, Gundersen-Rindal DE. Phytoplasma: Phytopathogenic mollicutes. Annu Rev Microbiol. 2000;54(1):221–255. doi: 10.1146/annurev.micro.54.1.221. [DOI] [PubMed] [Google Scholar]
- 14.Pracros P, Renaudin J, Eveillard S, Mouras A, Hernould M. Tomato flower abnormalities induced by stolbur phytoplasma infection are associated with changes of expression of floral development genes. Mol Plant Microbe Interact. 2006;19(1):62–68. doi: 10.1094/MPMI-19-0062. [DOI] [PubMed] [Google Scholar]
- 15.Himeno M, et al. Unique morphological changes in plant pathogenic phytoplasma-infected petunia flowers are related to transcriptional regulation of floral homeotic genes in an organ-specific manner. Plant J. 2011;67(6):971–979. doi: 10.1111/j.1365-313X.2011.04650.x. [DOI] [PubMed] [Google Scholar]
- 16.Su YT, Chen JC, Lin CP. Phytoplasma-induced floral abnormalities in Catharanthus roseus are associated with phytoplasma accumulation and transcript repression of floral organ identity genes. Mol Plant Microbe Interact. 2011;24(12):1502–1512. doi: 10.1094/MPMI-06-11-0176. [DOI] [PubMed] [Google Scholar]
- 17.Cettul E, Firrao G. Development of phytoplasma-induced flower symptoms in Arabidopsis thaliana. Physiol Mol Plant Pathol. 2011;76(3-4):204–211. [Google Scholar]
- 18.Munyaneza JE, Crosslin JM, Upton JE, Buchman JL. Incidence of the beet leafhopper-transmitted virescence agent phytoplasma in local populations of the beet leafhopper, Circulifer tenellus, in Washington State. J Insect Sci. 2010;10(18):1–10. doi: 10.1673/031.010.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oldfield GN, Kaloostian GH, Pierce HD, Granett AL, Calavan EC. Beet leafhopper transmits virescence to periwinkle. Calif Agric. 1977;31(6):14–15. [Google Scholar]
- 20.Wu W, et al. Salicylic acid-mediated elicitation of tomato defence against infection by potato purple top phytoplasma. Ann Appl Biol. 2012;161(1):36–45. [Google Scholar]
- 21.Park SJ, Jiang K, Schatz MC, Lippman ZB. Rate of meristem maturation determines inflorescence architecture in tomato. Proc Natl Acad Sci USA. 2012;109(2):639–644. doi: 10.1073/pnas.1114963109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippman ZB, et al. The making of a compound inflorescence in tomato and related nightshades. PLoS Biol. 2008;6(11):e288. doi: 10.1371/journal.pbio.0060288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brand A, Shirding N, Shleizer S, Ori N. Meristem maintenance and compound-leaf patterning utilize common genetic mechanisms in tomato. Planta. 2007;226(4):941–951. doi: 10.1007/s00425-007-0540-0. [DOI] [PubMed] [Google Scholar]
- 24.Lenhard M, Jürgens G, Laux T. The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development. 2002;129(13):3195–3206. doi: 10.1242/dev.129.13.3195. [DOI] [PubMed] [Google Scholar]
- 25.Shalit A, et al. The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc Natl Acad Sci USA. 2009;106(20):8392–8397. doi: 10.1073/pnas.0810810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molinero-Rosales N, Latorre A, Jamilena M, Lozano R. SINGLE FLOWER TRUSS regulates the transition and maintenance of flowering in tomato. Planta. 2004;218(3):427–434. doi: 10.1007/s00425-003-1109-1. [DOI] [PubMed] [Google Scholar]
- 27.Pnueli L, et al. The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development. 1998;125(11):1979–1989. doi: 10.1242/dev.125.11.1979. [DOI] [PubMed] [Google Scholar]
- 28.Lifschitz E, et al. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci USA. 2006;103(16):6398–6403. doi: 10.1073/pnas.0601620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen KD, Sussex IM. Falsiflora and anantha control early stages of floral meristem development in tomato (Lycopersicon esculentum Mill) Planta. 1996;200(2):254–264. [Google Scholar]
- 30.Thouet J, Quinet M, Lutts S, Kinet JM, Périlleux C. Repression of floral meristem fate is crucial in shaping tomato inflorescence. PLoS ONE. 2012;7(2):e31096. doi: 10.1371/journal.pone.0031096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee IM, Bottner KD, Munyaneza JE, Secor GA, Gudmestad NC. Clover proliferation group (16SrVI), subgroup A (16SrVI-A) phytoplasma is a probable causal agent of potato purple top disease in Washington and Oregon. Plant Dis. 2004;88(4):429. doi: 10.1094/PDIS.2004.88.4.429B. [DOI] [PubMed] [Google Scholar]
- 32.Lai Z, Gross BL, Zou Y, Andrews J, Rieseberg LH. Microarray analysis reveals differential gene expression in hybrid sunflower species. Mol Ecol. 2006;15(5):1213–1227. doi: 10.1111/j.1365-294X.2006.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 34.Brukhin V, Hernould M, Gonzalez N, Chevalier C, Mouras A. Flower development schedule in tomato Lycopersicon esculentum cv. sweet cherry. Sex Plant Reprod. 2003;15(6):311–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.