Significance
Although most known Mendelian genes causing nonsymptomatic epilepsies encode ion channels, Mass1 (monogenic audiogenic seizure susceptible 1) is among the few known epilepsy genes that do not encode channels, suggesting that membrane hyperexcitability can arise indirectly. Here we found that the MASS1 protein is highly expressed in the colliculi of the adult brain, where MASS1 senses extracellular calcium and activates PKA and PKC signaling via Gαs and Gαq to regulate stability of MAG in myelin-forming cells. These data provide us with a unique working model where MASS1 regulates MAG in response to calcium in myelinated regions of critical sites for the initiation and propagation of audiogenic epilepsy.
Keywords: genetics, molecular basis
Abstract
VLGR1 (very large G protein-coupled receptor 1), also known as MASS1 (monogenic audiogenic seizure susceptible 1), is an orphan G protein-coupled receptor that contains a large extracellular N terminus with 35 calcium-binding domains. A truncating mutation in the Mass1 gene causes autosomal recessive, sound-induced seizures in the Frings mouse. However, the function of MASS1 and the mechanism underlying Frings mouse epilepsy are not known. Here, we found that MASS1 protein is enriched in the myelinated regions of the superior and inferior colliculi, critical areas for the initiation and propagation of audiogenic seizures. Using a panel of myelin antibodies, we discovered that myelin-associated glycoprotein (MAG) expression is dramatically decreased in Frings mice. MASS1 inhibits the ubiquitylation of MAG, thus enhancing the stability of this protein, and the calcium-binding domains of MASS1 are essential for this regulation. Furthermore, MASS1 interacts with Gαs/Gαq and activates PKA and PKC in response to extracellular calcium. Suppression of signaling by MASS1 RNAi or a specific inhibitor abrogates MAG up-regulation. We postulate that MASS1 senses extracellular calcium and activates cytosolic PKA/PKC pathways to regulate myelination by means of MAG protein stability in myelin-forming cells of the auditory pathway. Further work is required to determine whether MAG dysregulation is a cause or consequence of audiogenic epilepsy and whether there are other pathways regulated by MASS1.
Epilepsy is a complex brain disorder with recurrent and unprovoked seizures affecting ∼2.5% of the population (1). In a majority of epilepsy cases, the precise trigger of seizure induction is not understood but is often associated with various precipitants such as stress, fatigue, menstruation, alcohol ingestion, and dehydration. However, in the reflex epilepsies, the trigger can be remarkably specific. Flickering light, reading, startle, touch, or specific types of music are each known to precipitate seizures in different patients (2).
The Frings mouse has monogenic audiogenic seizures, for which the causative gene has already been identified, thereby making it an outstanding animal model for more detailed study of reflex epilepsy pathogenesis (3). The Frings mutation arose spontaneously on the Swiss albino genetic background. In response to a high-intensity sound (110-dB, 11-kHz tone stimulus of 20 s duration), mutant mice exhibit wild running, loss of righting reflex, tonic flexion, and tonic extension seizures (3). A 1-bp deletion leads to premature truncation of the very large G protein-coupled receptor 1 (VLGR1)/monogenic audiogenic seizure susceptible 1 (MASS1) (hereafter referred to as “MASS1”) protein and causes the audiogenic seizures in Frings mice (4). There are two Mass1-specific knockout mice, which either deleted exons 2–4 (5) or after exon 82 covering the transmembrane and carboxyl terminus of Mass1 (6). Both of them showed the same audiogenic seizures in response to high-intensity sound like the Frings mice. MASS1 is expressed in brain, kidney, and cochlea, although finer detail regarding cell type-specific expression is not available. It is an ∼700-kDa orphan G protein coupled receptor (GPCR) having several known domains. They include 35 CalX-β domains, a Pentraxin domain, and an epilepsy-associated repeat (EAR) (4, 7). In particular, CalX-β is known to be a calcium-binding regulatory region found in sodium–calcium exchangers (8), and the presence of 35 of these domains in the extracellular N terminus of MASS1 suggests that calcium may be an important regulator of MASS1. An additional clue regarding the function of MASS1 comes from recent evidence that MASS1 is required for normal maturation of auditory hair bundles (9). Stereocilia of cochlear hair cells are coupled to one another by a number of different link types (tip links, horizontal top connectors, shaft connectors, and ankle links) (10). Examination of a targeted knockout of Mass1 showed that ankle links failed to form in the cochlea, and the hair bundles became disorganized after birth. However, the underlying seizure mechanism in MASS1-deficient mice is still enigmatic.
Here we show that MASS1 regulates myelin-associated glycoprotein (MAG) expression via Gαs/Gαq and PKA/PKC in response to calcium in myelinated regions of superior and inferior colliculi (SC and IC, respectively), sites known to be critical for the initiation and propagation of audiogenic seizures (11). This evidence that mutation of a GPCR causes a mammalian epilepsy, possibly via dysregulation of a myelin protein, may provide insight into novel intracellular signaling pathways in epileptogenesis.
Results
MASS1 Is Expressed in Oligodendrocytes.
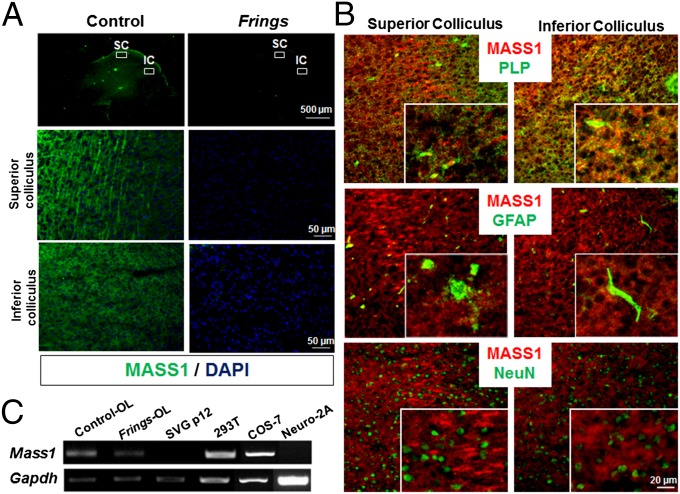
As part of our initial investigation of MASS1 function, we conducted immunohistochemical analyses of brain sections from control and Frings mice using a polyclonal MASS1 antibody (12), which was generated against the cytoplasmic tail (amino acids 6198–6307). Interestingly, the MASS1 protein was highly enriched in the SC and IC (Fig. 1A). The IC (and to a lesser extent, the SC) are nuclei in the auditory pathway and have important roles in audiogenic seizure initiation/propagation (11). No immunoreactivity of MASS1 was seen in Frings mouse brain. Next, the sections were colabeled with antibodies against MASS1 and either proteolipid protein (PLP) [oligodendrocytes (OLs)], NeuN (neurons), or GFAP (astrocytes). MASS1 signal overlapped with that of PLP but not other markers, suggesting that MASS1 is enriched in OLs (Fig. 1B). Expression of MASS1 in OLs was further confirmed by RT-PCR in total RNA extracts of purified primary OLs from both Frings and control mice (Fig. 1C). Mass1 transcript was expressed in both OLs and kidney cell lines but not in astrocytic or neuronal cell types. Expression of Mass1 transcript in the kidney has previously been reported (4), although its function in kidney is unknown. To confirm MASS1 expression in myelin-forming OL cells, myelin fractions were purified from both Frings and control mice (Fig. S1), and myelin protein complex was precipitated using anti-MBP (myelin basic protein) antibody. MASS1 was present in the myelin fraction associated with MBP. Taken together, they suggest that MASS1 is expressed in myelin-forming cells. However, we could not exclude the possibility of MASS1 expression in other types of brain cells in a spatiotemporal manner.
Fig. 1.
MASS1 is enriched in SC and IC. (A) SC and IC of control mice showed strong MASS1 immunostaining (green), whereas no signal was seen in Frings mice. (B) Double immunostaining for MASS1 (green) and markers (red) for OLs (PLP), astrocytes (GFAP), or neurons (NeuN) showed that MASS1 is enriched in myelin-forming OLs. (C) PCR of cDNAs from different cell types reveals that MASS1 is expressed in OLs and kidney cell lines (COS-7 and HEK293T) but not in SVGp12 (astrocytes) or Neuro-2A (neurons) cells.
Reduced MAG Expression in Frings Mice.
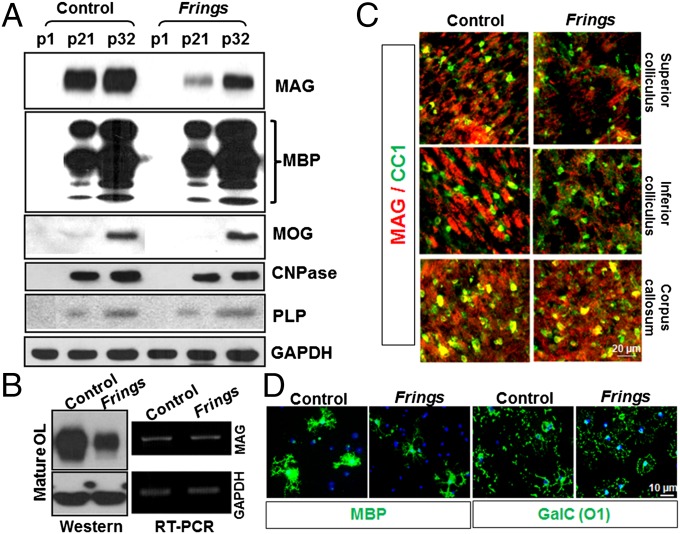
OLs are myelin-forming cells in the central nervous system (CNS), and their growth and differentiation are regulated by many factors: metabolites such as glucose and lactate (13), microRNAs (14, 15), and proteins (16). Therefore, to test the effects of MASS1 on physiology of OLs, we quantified major myelin proteins in both Frings and control brain homogenates. At all ages examined, MAG expression is dramatically decreased in the Frings mouse, whereas MBP, myelin oligodendrocyte glycoprotein (MOG), 2′,3′-Cyclic-nucleotide 3′-phosphodiesterase (CNPase), and PLP are not significantly changed (Fig. 2A). To further confirm reduced MAG expression, we prepared primary OLs by differentiating them from purified oligodendrocyte progenitor cells (OPCs). In cultured Frings OLs, MAG protein expression is significantly reduced compared with that of control. However, semiquantitative RT-PCR revealed that Mag mRNA levels were not different in mutant vs. control mice, implying that MASS1 regulates MAG posttranscriptionally (Fig. 2B). Immunohistochemical staining confirmed that MAG expression is significantly reduced in the SC and IC of the Frings mice but not in the corpus callosum (Fig. 2C), indicating that MASS1 in the colliculi might regulate MAG expression but not in corpus callosum where MASS1 is not enriched. Thus, there are likely other pathways affecting MAG expression. An OL marker (CC1) was included and showed no obvious differences in overall OL populations. Furthermore, there were no discernible differences between Frings and controls in the differentiation status of purified OLs as assessed by markers of mature OLs—MBP and galactosylceramide (GalC; O1) (Fig. 2D).
Fig. 2.
MAG protein levels are dramatically reduced in the Frings mouse. (A) Western blots of whole-brain extracts from control and Frings mice at different developmental stages show that MAG levels are reduced in the mutant Frings mice, but other myelin proteins, such as MBP, MOG, CNPase, and PLP, are not affected by the absence of MASS1 at different times (p1, p21, and p32). p, postnatal day. (B) OPCs were purified from both control and Frings mice and then differentiated into mature OLs for 5 d. MAG protein levels were dramatically decreased in mature OLs of Frings mice, whereas mRNA levels were not different. (C) Immunohistochemical analysis revealed reduced MAG expression in the SC and IC in Frings mice compared with control, but not in the corpus callosum. A glial marker (CC1) was used to define the glial cell population. (D) Mature OLs from Frings and control mice were stained for mature OL markers (MBP and GalC) and showed no obvious differences.
MASS1 Regulates MAG Stability.
Expressing exogenous MASS1 is difficult given the size of the cDNA (∼20 kb). Therefore, we generated a partial MASS1 construct (miniMass1) containing the recognized functional domains of MASS1 (Fig. S2A). Mini-MASS1 includes the signal peptide, four copies of the calcium-binding domain (CalX-β), the EAR and Pentraxin domains, GPCR-proteolytic site, seven transmembrane segments, and the intracellular carboxyl terminal region. Additionally, we constructed a miniMass1 lacking CalX-β domains (miniMass1ΔCalX-β) to test calcium-specific effects, and a Mass1 C terminus harboring only the intracellular carboxyl terminus of MASS1 (Fig. S2A). GST-tagged clones for Mag, Mass1 C terminus, and the Mass1 third cytoplasmic loop were also generated for overexpression (Fig. S2B).
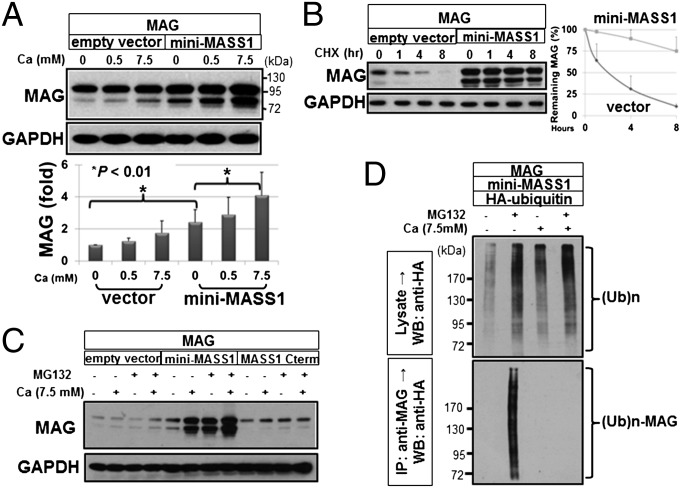
Expression of MAG protein in COS-7 cells was dramatically increased in a calcium-dependent manner when cotransfected with miniMass1 vs. empty vector (Fig. 3A). We tested whether the increased MAG resulted from altered degradation by treating transfected cells with cycloheximide before collecting cells at different time points and showed that MAG degradation is slower in cells cotransfected with miniMass1 vs. control, indicating that MASS1 decreases MAG degradation in vitro (Fig. 3B). Treatment with a proteasomal inhibitor (MG132) in cells cotransfected with miniMass1 showed significantly higher MAG levels than in nontreated cells (Fig. 3C). In all cases (Fig. 3 A–C) the cells were maintained in calcium-free DMEM with 0.1% serum before calcium treatment. It is possible that higher expression of MAG, even with 0 mM Ca2+, resulted from a trace amount of calcium in the serum (0.1%) that is necessary to maintain cell viability. In addition, MG132 treatment did not affect the MAG levels in samples coexpressed with MASS1 C terminus or empty vector, suggesting that MASS1 slows proteasomal degradation of MAG and that the extracellular region of MASS1 is critical for this effect. Next, we examined ubiquitylation of MAG when coexpressed with mini-MASS1 and HA-ubiquitin. Immunoprecipitation of MAG from extracts and Western blot analysis against ubiquitin showed increased ubiquitylated MAG in the absence of calcium, suggesting that mini-MASS1 blocks MAG ubiquitylation in a calcium-dependent manner (Fig. 3D). Expression of mini-MASS1 lacking CalX-β repeats (Fig. S2A) did not result in increased expression of MAG, implying that the calcium-binding repeats of MASS1 are necessary for regulation of MAG levels (Fig. 4A). Interestingly, MAG is expressed at lower levels in cells of mini-MASS1ΔCalX-β compared with empty vector control, suggesting that mini-MASS1ΔCalX-β might have a dominant-negative effect on MAG regulation.
Fig. 3.
MASS1 enhances MAG stability in a calcium-dependent manner. (A) MAG plasmid was cotransfected into COS-7 cells with empty vector or miniMass1. The cells were transferred to serum- and calcium-free media for 24 h and then treated with the indicated amounts of calcium for 1 h and analyzed by Western blot analyses for MAG and GAPDH. Calcium levels in the media correlated with MAG protein levels. MAG levels were increased in the presence of mini-MASS1 compared with control. Data are expressed as means of triplicate ± SD. (B) Cycloheximide (CHX) was used to monitor the degradation rate of MAG. Pixel intensity of protein bands was quantified using TINA software (Fuji) and plotted as normalized intensity units (Right). The degradation of MAG is much slower in the presence of mini-MASS1 compared with empty vector, suggesting that mini-MASS1 stabilizes MAG protein. Data are expressed as means of triplicate ± SD. (C) After transfection and 24 h starvation, cells were treated with 10 µM MG132 with the indicated calcium levels for 5 h. Mini-MASS1 greatly increases the effect of MG132 on MAG stabilization. (D) Cells were cotransfected with HA-tagged ubiquitin and the indicated plasmids, lysed, immunoprecipitated with MAG antibody, blotted, and probed with HA antibody. Higher calcium levels suppress the ubiquitin modification of MAG protein.
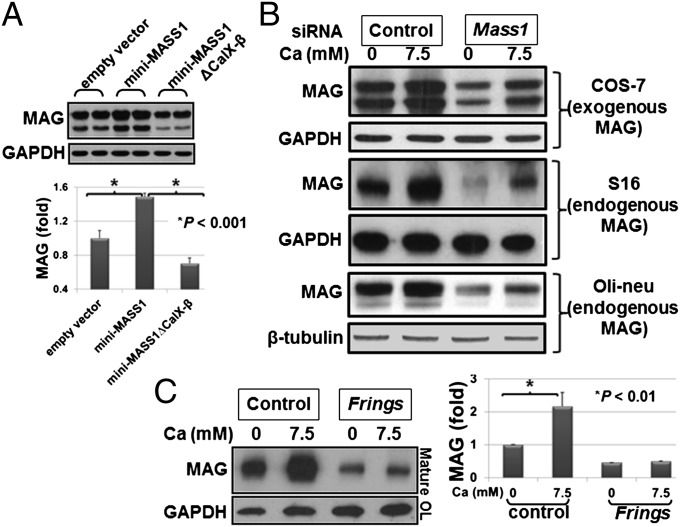
Fig. 4.
Either absence of MASS1 or calcium-binding domains of MASS1 abolishes the enhanced MAG expression. (A) mini-MASS1, with or without CalX-β repeats, and empty vector were coexpressed with MAG in HEK293T cells. Cells expressing mini-MASS1 show increased MAG expression, and removal of the CalX-β domains abrogates this effect. Quantification of MAG proteins is shown below. Data are expressed as means of triplicate ± SD. (B) Introduction of siRNA targeting MASS1 results in the suppression of both exogenous (COS-7) and endogenous (S16 Schwann cell and oli-neu oligodendrocytes) MAG expression, indicating that the suppression of endogenous MASS1 destabilizes MAG protein. (C) OPC cells were purified from both Frings and control mice and differentiated into mature OL cells for 5 d, serum starved without calcium for 5 h, and then treated with the indicated amount of calcium for 1 h. Lysates were immunostained with MAG and GAPDH antibodies. A dramatic increase in MAG levels was noted in the control OLs compared with the Frings OLs. Data are expressed as means of triplicate ± SD.
To further assess whether repression of endogenous MASS1 reduces MAG levels, we used Mass1-specific siRNA duplexes to suppress MASS1 expression and efficiently reduced Mass1 expression in Mass1-positive cells (oli-neu) (Fig. S3). Transfection of MASS1 siRNAs in COS-7 cells cotransfected with MAG and in S16 (Schwann cell line) and oli-neu (OL cell line) cells expressing endogenous MAG led to dramatically reduced MAG levels (Fig. 4B). To confirm the MAG regulation by MASS1 in OLs, we exposed primary differentiated OLs from control and Frings (Mass1-null) mice to different concentrations of calcium. MAG levels were significantly higher in control OLs vs. Frings OLs (Fig. 4C). Together, these data suggest that MASS1 inhibits the ubiquitylation and hence the degradation of MAG in a calcium-dependent manner and that this effect requires the extracellular region of MASS1.
Intracellular MASS1 Domains Interact with Gαs and Gαq.
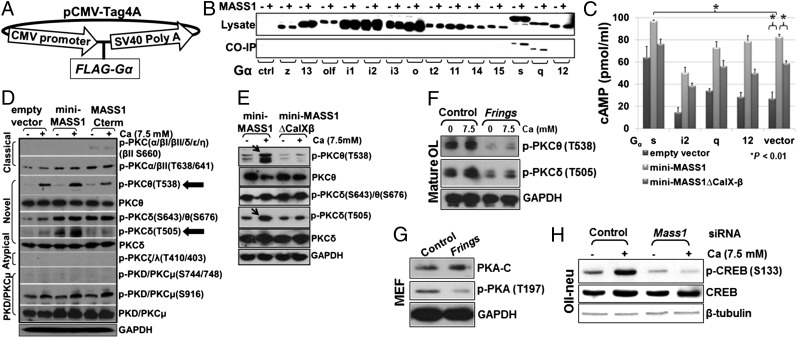
Because MASS1 is a GPCR, we set out to characterize specific intracellular signals that it regulates. We tested for interactions to identify the G proteins through which MASS1 signals. Because subunits are critical for specificity of intracellular signaling (17), we cloned α subunits that we were able to amplify from mouse brain cDNAs (Fig. 5A) and tested each for interaction with the intracellular regions of MASS1 using a GST pulldown assay. Because G proteins interact with the third loop and/or C-terminal region of MASS1, we cotransfected Gα protein clones with plasmids expressing the third loop and C-terminal region of MASS1. Interestingly, both Gαs and Gαq isotypes bound to MASS1 (Fig. 5B). Gαs activates adenylyl cyclase and up-regulates cAMP, whereas Gαq protein activates the PKC pathway. Therefore, we postulated that MASS1 regulates PKA and PKC signals via Gαs and Gαq, respectively.
Fig. 5.
MASS1 activates PKA and PKCδ/θ in response to extracellular calcium via Gαs/Gαq. (A) For the expression of 14 Gα proteins, each full coding sequence was subcloned into FLAG-fusion vector (pCMV-Tag 4A). (B) FLAG-tagged Gα protein-encoding genes were cotransfected into HEK293T cells with clones encoding the third cytoplasmic loop and carboxyl terminal region of MASS1 each fused to GST. The extracts were precipitated with GST-Sepharose beads and analyzed by FLAG antibody. Both Gαs and Gαq were coimmunoprecipitated. (C) Four representative Gα proteins (Gαs, Gαi2, Gαq, and Gα12) and empty control vector were cotransfected into COS-7 with miniMass1 or miniMass1ΔCalX-β. ELISA revealed that calcium-activated mini-MASS1 increases intracellular cAMP via Gαs much more than controls (empty vector and miniMass1ΔCalX-β). Data are expressed as means of triplicate ± SD. (D) Activation of PKCδ/θ by MASS1. Cells were transfected with empty vector, mini-MASS1, and MASS1 C terminus, without serum or calcium, and then treated with 7.5 mM calcium for 1 h. Extracts were analyzed by Western blotting with various phospho-PKC antibodies. Novel PKC isozymes (PKCδ/θ) were up-regulated only in the presence of both mini-MASS1 and calcium. (E) Comparing mini-MASS1 to mini-MASS1ΔCalX-β confirmed that specific activation of PKCδ/θ requires the calcium-binding CalX-β repeats. (F) Mature OL lysates were used to test whether phospho-PKCδ/θ levels are affected by the absence of MASS1. Activation of both PKC δ and θ is dramatically reduced in the Frings OLs. (G) The phosphorylation in the catalytic subunits of PKA (T197) was dramatically reduced, which is an indicator of PKA activation, in primary cultured MEFs from Frings and control. (H) The phosphorylation of CREB (S133), a substrate of PKA, was increased by the addition of calcium (control siRNA), whereas this propensity was absent in oli-neu cells treated with Mass1-siRNA.
Calcium-Dependent Regulation of PKA and PKCδ/θ by MASS1.
Because PKA activity is dependent on cAMP levels in the cell, we transfected miniMass1 either containing or lacking CalX-β repeats and empty vector into COS-7 cells with four representative Gα proteins (Gαs, Gαi2, Gαq, and Gα12) and empty plasmid. After serum starvation in calcium-depleted media, 7.5 mM calcium was added to the media for 1 h, and intracellular cAMP was measured by ELISA. Higher cAMP levels were seen when mini-MASS1 was expressed compared with mini-MASS1ΔCalX-β or empty vector regardless of Gα proteins, suggesting that mini-MASS1 up-regulates intracellular cAMP levels via endogenous Gα proteins. Coexpression of Gαs protein with mini-MASS1 resulted in the highest cAMP concentrations compared with Gαi2, Gαq, Gα12, or vector control (Fig. 5C and Fig. S4), indicating that calcium-dependent MASS1 signaling regulates cAMP via Gαs.
To investigate MASS1 regulation of PKC via Gαq, phospho-specific PKC antibodies were used to detect activation of specific PKC isozymes. Calcium was used to activate MASS1 in HEK293T cells transfected with exogenous miniMass1 or Mass1 C terminus. Because the MASS1 C terminus has neither extracellular nor transmembrane domains (Fig. S2A), it cannot receive extracellular signals directly and was therefore used as a negative control for the effect of intracellular calcium. Cells were treated with calcium for 1 h after serum starvation, and the phosphorylation status of PKC isozymes was monitored by Western blot analyses using phospho-specific antibodies. Interestingly, PKCδ and PKCθ were specifically up-regulated only in the presence of both mini-MASS1 and calcium (Fig. 5D). Three phosphorylation sites are sufficient for fully activated PKC isozymes (18). We found that a threonine in the activation site (T505 or T538), which plays a critical role in overall catalytic activity and regulates other phosphorylations of hydrophobic/turn motif regions in PKCδ/θ (18), is hyper-phosphorylated relative to empty vector and MASS1 C terminus controls. Because MASS1 is expressed endogenously in HEK293T cells (Fig. 1C), empty vector and MASS1 C terminus controls also increased the phosphorylation of the activation site (T505 or T538) in PKCδ/θ, although to a lesser extent than the sample transfected with mini-MASS1. We further asked whether calcium-binding domains mediate PKC activation. After transfecting miniMass1 and miniMass1ΔCalX-β into HEK293T cells, we checked the phosphorylation status of PKCδ/θ using the same conditions (above). As seen in Fig. 5E, activating phosphorylations of PKCδ/θ were much less robust in the presence of mini-MASS1ΔCalX-β. Taken together, these data show that MASS1 regulates PKC signaling in a calcium-sensitive manner.
Absence of MASS1 Prevents MAG Regulation.
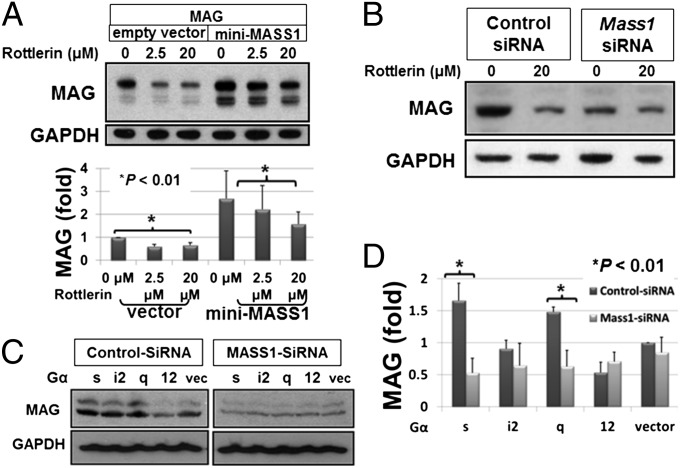
PKC was previously shown to play a role in regulating myelin proteins levels (19). To further reveal the mechanism and assess MASS1 regulation of PKC in vivo, we used primary OL cultures and found that phosphorylated PKCδ/θ levels were dramatically decreased in Frings OLs vs. control (Fig. 5F). For PKA activation, the catalytic subunits of PKA must be phosphorylated, which occurs on threonine 197 (T197) and helps orientate catalytic residues in the active site of PKA (20). The phosphorylation of PKA at T197 was dramatically reduced in primary cultured mouse embryonic fibroblast cells (MEFs) of the Frings compared with that of control mice (Fig. 5G). Furthermore, phosphorylation at serine 133 of cAMP responsive element binding protein (CREB) that is a substrate of PKA (21) was increased by the addition of calcium (control siRNA), whereas this propensity was absent in oli-neu cells treated with Mass1-siRNA (Fig. 5H). These results imply that endogenous MASS1 may regulate MAG stability via PKC and PKA in myelin-forming cells. To confirm the specificity of PKC signaling on MAG regulation, we treated cell cultures with a specific PKCδ/θ inhibitor, Rottlerin (22), at different doses in the presence or absence of mini-MASS1. Rottlerin, a compound from Mallotus philippinensis, is known to inhibit protein kinases with high specificity for PKCδ/θ (IC50 range of 3–6 μM), PKCα/β/γ (IC50 range of 30–42 μM), and PKCε/ζ/η (IC50 range of 80–100 μM) (22). Whereas MAG levels were significantly higher in the presence of overexpressed MASS1 relative to control (empty vector; only endogenous MASS1 present), MAG levels decreased in a dose-dependent manner by Rottlerin under both conditions (Fig. 6A). Knockdown of Mass1 expression by siRNA dramatically reduced the effect of Rottlerin (Fig. 6B). These results suggest that MASS1-mediated PKA and PKCδ/θ act together as key regulators of MAG expression.
Fig. 6.
MASS1 up-regulates MAG via PKA and PKCδ/θ. (A) MAG was expressed in COS-7 cells with mini-MASS1 and then treated with Rottlerin, a specific PKCδ/θ inhibitor, for 16 h. Dose-dependent reductions in MAG expression were seen with Rottlerin treatment, indicating that PKC activation is necessary for the regulation of MAG. Data are expressed as means of triplicate ± SD. (B) Knockdown of Mass1 expression by siRNA dramatically reduced the effect of Rottlerin on MAG regulation. (C) Loss of MASS1 by siRNA abolishes Gαs/Gαq-mediated up-regulation of MAG expression. (D) Densitometric quantification of MAG proteins was analyzed by TINA software (Fuji) and indicated Gαs/Gαq-specific MAG up-regulation by MASS1. Data are expressed as means of triplicate ± SD.
We next asked whether there is a direct correlation between Gαs/Gαq and MASS1-mediated MAG regulation. Overexpression of G proteins with control siRNA in COS-7 cells resulted in higher MAG levels for both Gαs and Gαq but not for Gαi2 and Gα12. However, knockdown of MASS1 by siRNA blocked up-regulation of MAG by Gαs and Gαq (Fig. 6 C and D). Thus, we concluded that MASS1 up-regulates MAG expression via Gαs/Gαq.
Discussion
As we predicted years ago, most known Mendelian genes causing nonsymptomatic epilepsies encode ion channels (23). MASS1 is among the few known epilepsy genes that do not encode channels, suggesting that membrane hyperexcitability can arise indirectly (24). The precise role of MASS1 is not known and, as an orphan GPCR, its large size has been an obstacle for such studies. Therefore, we used a minigene containing known or predicted functional domains of MASS1. With this construct we investigated intracellular signaling pathways associated with MASS1 function and found that MASS1 up-regulates PKA/PKC signaling in a calcium-dependent manner, thus inhibiting MAG ubiquitylation and degradation by the proteasome. To control for overexpression effects of exogenous mini-MASS1, we showed similar regulation of MAG by endogenous MASS1 in purified OLs.
Under physiological conditions, calcium concentrations are regulated by homeostatic mechanisms such as the calcium sensing receptor (25). The plasma membrane calcium-ATPase, the Na+/Ca2+ exchanger, and Ca2+ permeable ion channels also contribute to extracellular calcium homeostasis, largely as a function of driving forces defined by ionic gradients. Although these mechanisms function to maintain extracellular calcium, decreases in extracellular calcium can occur in the brain as a consequence of neuronal activity (particularly excessive activity such as seizures). It is interesting to speculate that MASS1 might function to monitor extracellular calcium levels and mediate intracellular signaling events that enable cellular responses to changes in the extracellular ionic environment in the CNS.
PKC is known to regulate steady-state levels of myelin proteins such as MBP, PLP, MAG, CNPase, O4, and GALC. PKC-mediated posttranscriptional suppression of myelin gene expression is most profound in differentiating OLs at or before the onset of myelin protein synthesis (19). This correlates well with our data showing that MASS1 regulates MAG protein stability. PKC signaling has a proliferative effect on immature OLs and increases process extension and myelin formation in mature OLs (26). PKC activation also results in increased phosphorylation of CNPase and MBP. cAMP and PKA signaling can induce MAG and PLP expression and trigger the final stages of OL maturation (27). We now show that MASS1 mediates MAG expression through PKA and cAMP, suggesting that in addition to PKC, a unique signaling pathway exists in OLs through which PKA regulates MAG.
MAG is a functional ligand for the neurite outgrowth inhibitory protein (NOGO) receptor (NgR) (28) and is involved in the process of myelination, cell adhesion, and signaling in myelin-forming cells (29). MAG-deficient mice show developmental abnormalities including multiple ensheathed axons, delayed myelination, and dying-back oligodendrogliopathy in older mice (30), but it is not known whether they have any seizure phenotype. MAG is localized in periaxonal membranes of myelin sheath, where it functions in the maintenance of periaxonal space and ganglioside-mediated axon–glial interaction (29). In this study there were no discenible changes in the gross structure of myelin, even though we need to further characterize subtle myelin changes. In another way, it might suggest it could affect ganglioside-mediated axon–glial interaction. The mice expressing only monosialoganglioside GM3, which were generated by double disruption of both GD3 synthase, a sialytransferase that synthesizes b-series gangliosides, and 1,4-N-acetylgalatosaminyltransferase (GalNAcT), were extremely susceptible to induction of lethal seizures by sound stimulus, indicating that gangliosides might play an essential role in certain audiogenic seizure pathways (31). Nerve cell surface gangliosides GD1a and GT1b are specific functional ligands for MAG-mediated inhibition of neurite outgrowth, which gangliosides are synthesized from simpler precursors by the addition of GalNAc to the growing carbohydrate chain via GalNAcT. Knockout mice with an inactivated GalNAcT gene do not express GD1a and GT1b. Instead, these mice express compensatory amounts of the simpler gangliosides GD3 (which is not a MAG ligand) and GM3 (which binds MAG, but with lower affinity than GD1a or GT1b) (32). Frings mice crossed with GalNAcT knockout will provide better evidence for the involvement of axon–glia interaction-mediated epileptic pathology.
Mass1 transcripts were reported to be highly expressed in restricted regions of the developing CNS (33). Because MASS1 is strongly expressed in the ventricular zone, a center of neural progenitor cells during embryonic neurogenesis, MASS1 may play a fundamental role in CNS development. In this study we have shown that the MASS1 protein is also expressed in the SC and IC of the adult brain. These regions are critical for initiation and propagation of audiogenic seizures. Immunohistochemistry for c-Fos in the Frings mouse showed audiogenic seizure-induced transcriptional activation in neurons of the brainstem seizure pathway, including the external and dorsal nuclei of IC (34). PKC can also transduce signals to the nucleus in OLs, activating transcription of genes such as c-fos (35). Given high MASS1 protein expression in SC and IC, we postulate that MASS1 regulates the myelination of the SC and IC, and this myelination may be modified by the absence of MASS1 and could be a contributor to the epilepsy in Frings mice. However, preliminary data on myelin structure of SC with fluoromyelin staining (Fig. S5) and electron microscopy (Fig. S6) did not reveal any gross myelin abnormalities such as altered g-ratio. Therefore, more detailed ultrastructural morphometry of Frings mice is an important next step for characterization of the axon and its surrounding myelin structure, along with the audiogenic brainstem using transmission electron microscopy. Besides MASS1, other well-known myelin-enriched proteins, such as myelin protein zero, GALC, and inverted formin-2 (INF2), are also expressed in the kidney with unknown functions, except that INF2 is enriched in endoplasmic reticulum of podocytes, where it might regulate membrane structure for filtration complex (36). We speculate they are necessary in both myelin-forming cells and kidney for unique membrane structures: myelin sheath of brain and podocyte-filtration barrier of kidney.
Given the restricted expression of MASS1 protein in the SC and IC, the null mutation of this gene causes alterations of MAG abundance in the entire brain (Fig. 2). There are two possibilities. First, it could present complex ganglioside-dependent regional oligodendroglial heterogeneity, because MAG expression is progressively and selectively regulated in the brains of mice lacking complex brain gangliosides in a ganglioside-dependent manner (37). Second, it could reflect the difference between calcium-activated MAG up-regulation and degradation rates. Under physiological conditions in brain, the dynamic changes of calcium in response to environment may result in MAG accumulation in the colliculi of normal control, owing to the difference of fast posttranscriptional up-regulation (Fig. 4C; 1 h for a 2.5-fold increase) vs. slower degradation rates (Fig. 3B; >8 h of half-life of MAG).
In summary, we have shown that MASS1 senses extracellular calcium and activates PKA and PKC signaling via Gαs and Gαq to regulate stability of MAG in myelin-forming cells. This study provides insight into a unique pathway of myelin regulation via a GPCR associated with audiogenic epilepsy. Hopefully, such insights may ultimately lead to identification of novel anticonvulsants.
Materials and Methods
Details for antibodies, chemicals, mice, immunochemistry, transmission electron microscopy, in vitro binding and ubiquitylation assay, animal cell culture, plasmids, and quantification of cAMP are provided in SI Materials and Methods. All animal experiments were performed under approval by the Animal Care and Use Committee at University of California, San Francisco. ImageJ (NIH) or TINA (Fuji) software were used for densitometric analyses.
Supplementary Material
Acknowledgments
We thank Dr. Uwe Wolfrum for providing the rabbit polyclonal MASS1 antibody; Dr. Ben Barres for help in implementing the OPC primary culture system; and Drs. Devon Ryan, Samuel Pleasure, Jonah Chan, Andrew Charles, and Henry Bourne for helpful discussions and critical review of the manuscript. This work was supported by National Institutes of Health Grants NS038616 and NS044379 (to L.J.P.), U54 RR19481, and a grant from Parents Against Childhood Epilepsy (PACE). L.J.P. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318501110/-/DCSupplemental.
References
- 1.Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349(13):1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 2.Burgess DL. Listen carefully: Positional cloning of an audiogenic seizure mutation may yield Frings benefits. Neuron. 2001;31(4):507–508. doi: 10.1016/s0896-6273(01)00400-7. [DOI] [PubMed] [Google Scholar]
- 3.Frings H, Frings M, Kivert A. Behavior patterns of the laboratory mouse under auditory stress. J Mammal. 1951;32(1):60–76. [Google Scholar]
- 4.Skradski SL, et al. A novel gene causing a mendelian audiogenic mouse epilepsy. Neuron. 2001;31(4):537–544. doi: 10.1016/s0896-6273(01)00397-x. [DOI] [PubMed] [Google Scholar]
- 5.Yagi H, et al. Vlgr1 knockout mice show audiogenic seizure susceptibility. J Neurochem. 2005;92(1):191–202. doi: 10.1111/j.1471-4159.2004.02875.x. [DOI] [PubMed] [Google Scholar]
- 6.McMillan DR, White PC. Loss of the transmembrane and cytoplasmic domains of the very large G-protein-coupled receptor-1 (VLGR1 or Mass1) causes audiogenic seizures in mice. Mol Cell Neurosci. 2004;26(2):322–329. doi: 10.1016/j.mcn.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7(4):339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 8.Nikkila H, et al. Sequence similarities between a novel putative G protein-coupled receptor and Na+/Ca2+ exchangers define a cation binding domain. Mol Endocrinol. 2000;14(9):1351–1364. doi: 10.1210/mend.14.9.0511. [DOI] [PubMed] [Google Scholar]
- 9.Michalski N, et al. Molecular characterization of the ankle-link complex in cochlear hair cells and its role in the hair bundle functioning. J Neurosci. 2007;27(24):6478–6488. doi: 10.1523/JNEUROSCI.0342-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodyear R, Richardson G. The ankle-link antigen: An epitope sensitive to calcium chelation associated with the hair-cell surface and the calycal processes of photoreceptors. J Neurosci. 1999;19(10):3761–3772. doi: 10.1523/JNEUROSCI.19-10-03761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Cairasco N. A critical review on the participation of inferior colliculus in acoustic-motor and acoustic-limbic networks involved in the expression of acute and kindled audiogenic seizures. Hear Res. 2002;168(1-2):208–222. doi: 10.1016/s0378-5955(02)00371-4. [DOI] [PubMed] [Google Scholar]
- 12.Maerker T, et al. A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Hum Mol Genet. 2008;17(1):71–86. doi: 10.1093/hmg/ddm285. [DOI] [PubMed] [Google Scholar]
- 13.Rinholm JE, et al. Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci. 2011;31(2):538–548. doi: 10.1523/JNEUROSCI.3516-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin D, Shin JY, McManus MT, Ptácek LJ, Fu YH. Dicer ablation in oligodendrocytes provokes neuronal impairment in mice. Ann Neurol. 2009;66(6):843–857. doi: 10.1002/ana.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin D, Howng SYB, Ptáček LJ, Fu Y-H. miR-32 and its target SLC45A3 regulate the lipid metabolism of oligodendrocytes and myelin. Neuroscience. 2012;213:29–37. doi: 10.1016/j.neuroscience.2012.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330(6005):779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- 17.Downes GB, Gautam N. The G protein subunit gene families. Genomics. 1999;62(3):544–552. doi: 10.1006/geno.1999.5992. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Graham C, Li A, Fisher RJ, Shaw S. Phosphorylation of the protein kinase C-theta activation loop and hydrophobic motif regulates its kinase activity, but only activation loop phosphorylation is critical to in vivo nuclear-factor-kappaB induction. Biochem J. 2002;361(Pt 2):255–265. doi: 10.1042/bj3610255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asotra K, Macklin WB. Protein kinase C activity modulates myelin gene expression in enriched oligodendrocytes. J Neurosci Res. 1993;34(5):571–588. doi: 10.1002/jnr.490340509. [DOI] [PubMed] [Google Scholar]
- 20.Moore MJ, Kanter JR, Jones KC, Taylor SS. Phosphorylation of the catalytic subunit of protein kinase A. Autophosphorylation versus phosphorylation by phosphoinositide-dependent kinase-1. J Biol Chem. 2002;277(49):47878–47884. doi: 10.1074/jbc.M204970200. [DOI] [PubMed] [Google Scholar]
- 21.Delghandi MP, Johannessen M, Moens U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell Signal. 2005;17(11):1343–1351. doi: 10.1016/j.cellsig.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Gschwendt M, et al. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199(1):93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 23.Ptácek LJ, et al. Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell. 1991;67(5):1021–1027. doi: 10.1016/0092-8674(91)90374-8. [DOI] [PubMed] [Google Scholar]
- 24.Robinson R, Gardiner M. Molecular basis of Mendelian idiopathic epilepsies. Ann Med. 2004;36(2):89–97. doi: 10.1080/07853890310019952. [DOI] [PubMed] [Google Scholar]
- 25.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4(7):530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 26.Stariha RL, Kim SU. Protein kinase C and mitogen-activated protein kinase signalling in oligodendrocytes. Microsc Res Tech. 2001;52(6):680–688. doi: 10.1002/jemt.1052. [DOI] [PubMed] [Google Scholar]
- 27.Ye P, et al. Cyclic AMP-induced upregulation of proteolipid protein and myelin associated glycoprotein gene expression in C6 cells. J Neurosci Res. 1992;31(3):578–583. doi: 10.1002/jnr.490310324. [DOI] [PubMed] [Google Scholar]
- 28.Liu BP, Fournier A, GrandPré T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297(5584):1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 29.Quarles RH. Myelin-associated glycoprotein (MAG): Past, present and beyond. J Neurochem. 2007;100(6):1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 30.Li C, et al. Myelination in the absence of myelin-associated glycoprotein. Nature. 1994;369(6483):747–750. doi: 10.1038/369747a0. [DOI] [PubMed] [Google Scholar]
- 31.Kawai H, et al. Mice expressing only monosialoganglioside GM3 exhibit lethal audiogenic seizures. J Biol Chem. 2001;276(10):6885–6888. doi: 10.1074/jbc.C000847200. [DOI] [PubMed] [Google Scholar]
- 32.Vyas AA, et al. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc Natl Acad Sci USA. 2002;99(12):8412–8417. doi: 10.1073/pnas.072211699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMillan DR, Kayes-Wandover KM, Richardson JA, White PC. Very large G protein-coupled receptor-1, the largest known cell surface protein, is highly expressed in the developing central nervous system. J Biol Chem. 2002;277(1):785–792. doi: 10.1074/jbc.M108929200. [DOI] [PubMed] [Google Scholar]
- 34.Klein BD, Fu Y-H, Ptacek LJ, White HS. c-Fos immunohistochemical mapping of the audiogenic seizure network and tonotopic neuronal hyperexcitability in the inferior colliculus of the Frings mouse. Epilepsy Res. 2004;62(1):13–25. doi: 10.1016/j.eplepsyres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Bhat NR, Hauser KF, Kindy MS. Cell proliferation and protooncogene induction in oligodendroglial progenitors. J Neurosci Res. 1992;32(3):340–349. doi: 10.1002/jnr.490320306. [DOI] [PubMed] [Google Scholar]
- 36.Boyer O, et al. INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N Engl J Med. 2011;365(25):2377–2388. doi: 10.1056/NEJMoa1109122. [DOI] [PubMed] [Google Scholar]
- 37.Sheikh KA, et al. Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc Natl Acad Sci USA. 1999;96(13):7532–7537. doi: 10.1073/pnas.96.13.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.