Significance
High-fat diet–induced obesity (DIO) increases the activity of endocannabinoids, the body’s own marijuana-like substances. Endocannabinoids activate CB1 receptors in the liver to cause insulin resistance and promote the synthesis of lipids by inducing stearoyl-CoA desaturase-1 (SCD1), the enzyme responsible for generating monounsaturated fatty acids (MUFAs). Here we show that endogenous, but not diet-derived, MUFAs act as potent inhibitors of fatty acid amide hydrolase, the enzyme that degrades the endocannabinoid anandamide, and are thus responsible for the increased hepatic levels of anandamide in mice with DIO. These findings reveal a positive feedback loop between the hepatic endocannabinoid/CB1 receptor system and SCD1 that contributes to increased lipogenesis and insulin resistance in DIO.
Abstract
High-fat diet (HFD)–induced obesity and insulin resistance are associated with increased activity of the endocannabinoid/CB1 receptor (CB1R) system that promotes the hepatic expression of lipogenic genes, including stearoyl-CoA desaturase-1 (SCD1). Mice deficient in CB1R or SCD1 remain lean and insulin-sensitive on an HFD, suggesting a functional link between the two systems. The HFD-induced increase in the hepatic levels of the endocannabinoid anandamide [i.e., arachidonoylethanolamide (AEA)] has been attributed to reduced activity of the AEA-degrading enzyme fatty acid amide hydrolase (FAAH). Here we show that HFD-induced increased hepatic AEA levels and decreased FAAH activity are absent in SCD1−/− mice, and the monounsaturated fatty acid (MUFA) products of SCD1, palmitoleic and oleic acid, inhibit FAAH activity in vitro at low micromolar concentrations. HFD markedly increases hepatic SCD1 activity in WT mice as well as in CB1R−/− mice with transgenic reexpression of CB1R in hepatocytes, but not in global CB1R−/− mice. Treatment of HFD-fed mice with the SCD1 inhibitor A939572 prevents the diet-induced reduction of hepatic FAAH activity, normalizes hepatic AEA levels, and improves insulin sensitivity. SCD1−/− mice on an HFD remain insulin-sensitive, but develop glucose intolerance and insulin resistance in response to chronic treatment with the FAAH inhibitor URB597. An HFD rich in MUFA or feeding mice pure oleic acid fail to inhibit hepatic FAAH activity. We conclude that MUFAs generated via SCD1 activity, but not diet-derived MUFAs, function as endogenous FAAH inhibitors mediating the HFD-induced increase in hepatic AEA, which then activates hepatic CB1R to induce insulin resistance.
The endocannabinoid system includes cannabinoid receptors, endogenous ligands that activate them—with arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG) being the two most widely investigated—and mechanisms for endocannabinoid biosynthesis and inactivation (1, 2). The latter occurs through cellular reuptake facilitated by a putative membrane transporter and is followed by enzymatic degradation by hydrolytic enzymes, including fatty acid amide hydrolase (FAAH) (3) and monoacylglycerol lipase (MAGL) (4, 5). FAAH was the first enzyme responsible for endocannabinoid hydrolysis to be purified and characterized. It exists as a dimer in its membrane-associated form, and possesses an unusual binding site and mechanism of catalytic action using a Ser-Ser-Lys triad (6, 7). Several endogenous compounds have been identified as good substrates for FAAH, including AEA and related fatty acid amides such as oleoylethanolamide (OEA) and palmitoylethanolamide, the tissue levels of which increase following genetic ablation or pharmacological inhibition of FAAH. Although FAAH can also degrade 2-AG in vitro (7), the enzyme that most specifically controls the levels of 2-AG is MAGL (4, 5), and 2-AG tissue levels remain unchanged in the absence of FAAH or FAAH activity in vivo (8).
Endocannabinoids produce a broad range of biological effects, some of which can be exploited for therapeutic purposes, as in the case of analgesic, anxiolytic, and antispasticity effects (9). Other effects, such as increased lipogenesis and decreased insulin and leptin signaling, contribute to the pathology of diet-induced obesity and its metabolic complications (10–12). Inhibition of FAAH results in localized elevations in the tissue levels of AEA, causing a spectrum of effects that do not include the psychotropic effects of global CB1 receptor (CB1R) activation caused by direct-acting agonists (13) and therefore having better therapeutic potential. Indeed, FAAH inhibitors have been developed for the treatment of anxiety and neuropathic and inflammatory pain (14). On the contrary, pharmacological inhibition or genetic deletion of FAAH has been shown to promote the development of obesity and insulin resistance (15, 16). Furthermore, the increase in AEA in the liver of diet-induced obese (DIO) mice, which contributes to insulin resistance, has been linked to reduced FAAH activity in the liver (11).
Stearoyl-CoA desaturase-1 (SCD1) catalyzes the rate-limiting reaction of the synthesis of monounsaturated fatty acids (MUFAs), mainly palmitoleate (C16:1n-7) and oleate (C18:1n-9), which are the major endogenous MUFAs derived from membrane phospholipids, triglycerides, wax esters, and cholesteryl esters. Elevated SCD1 activity has been implicated in a wide range of disorders, including obesity, diabetes, and atherosclerosis (17), whereas mice lacking SCD1 are lean and hypermetabolic (18). Leptin, the primary signal through which the hypothalamus senses nutritional state and modulates food intake and energy balance (19), represses the expression and enzymatic activity of hepatic SCD1 (18), and obesity is associated with resistance to leptin. SCD1 is an important metabolic control point in the development of obesity and insulin resistance, but the mechanism through which SCD1 or its MUFA products modulate metabolism is unknown. The parallel functions of SCD1 and AEA in promoting DIO and insulin resistance suggest that they share the same metabolic pathways. Based on the role of reduced FAAH activity in causing the increase in hepatic AEA in DIO mice (11), we hypothesized that MUFAs, generated in the body exclusively via SCD1, act as endogenous FAAH inhibitors. We tested this hypothesis by analyzing the metabolic profile of WT and SCD1−/− mice fed normal chow or a high-fat diet (HFD). Our findings indicate that palmitoleic (C16:1n-7) and oleic acids (C18:1n-9) act as endogenous inhibitors of FAAH.
Results
HFD Increases Hepatic AEA and OEA Levels and Decreases FAAH Activity in WT but Not in SCD1−/− Mice.
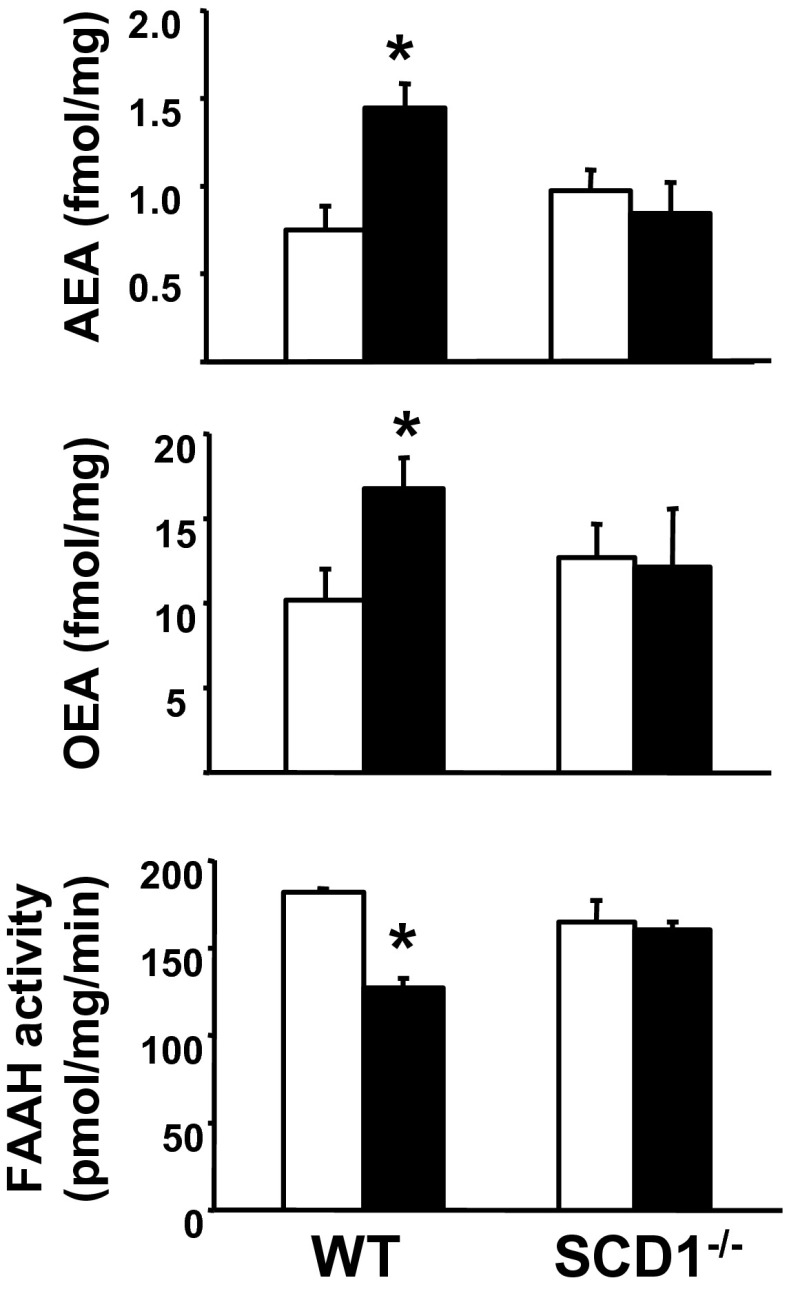
Male SCD1−/− mice on a C57BL/6J background and their WT littermates were maintained on standard diet (STD) or an HFD rich in saturated fatty acids for 12 wk. In WT littermates of SCD1−/− mice, HFD induced an increase in the hepatic levels of FAAH substrates AEA and OEA and a parallel reduction in hepatic FAAH activity (Fig. 1), whereas, in whole brain tissue, there were no HFD-induced changes in these parameters (Fig. S1). To test the relationship between FAAH activity and hepatic AEA levels, a group of WT mice were treated with various doses of the FAAH inhibitor URB597 or vehicle and killed 2 h later (when the effect of URB597 peaks; Fig. S2A) to measure hepatic FAAH activity and AEA levels (Fig. S2 B and C). Analysis of the inverse correlation between the two parameters indicated that an approximately twofold increase in AEA occurs at a ∼20% to 30% decrease in FAAH activity (Fig. S2D), similar to the effects of HFD. The HFD-induced reduction in FAAH activity was not accompanied by any change in hepatic FAAH mRNA levels or in the activity of N-acyltransferase (NAT), the rate-limiting step in AEA biosynthesis (Fig. S3), in agreement with previous observations (11). In contrast to WT mice, in SCD1−/− mice, the same diet failed to alter AEA and OEA content or FAAH activity in the liver, relative to tissue from STD-fed mice (Fig. 1). In agreement with an earlier report (17), SCD1−/− mice were resistant to the diet-induced metabolic changes.
Fig. 1.
SCD1−/− mice are resistant to DIO and its metabolic complications, including decreased FAAH activity and increased N-acylethanolamide levels in the liver. Columns and bars represent means ± SE, n = 4 to 10 mice per group. [*P < 0.05 between corresponding values in STD-fed (open columns) and HFD-fed mice (filled columns).]
HFD Up-Regulates SCD1 Activity in a CB1R-Dependent Manner.
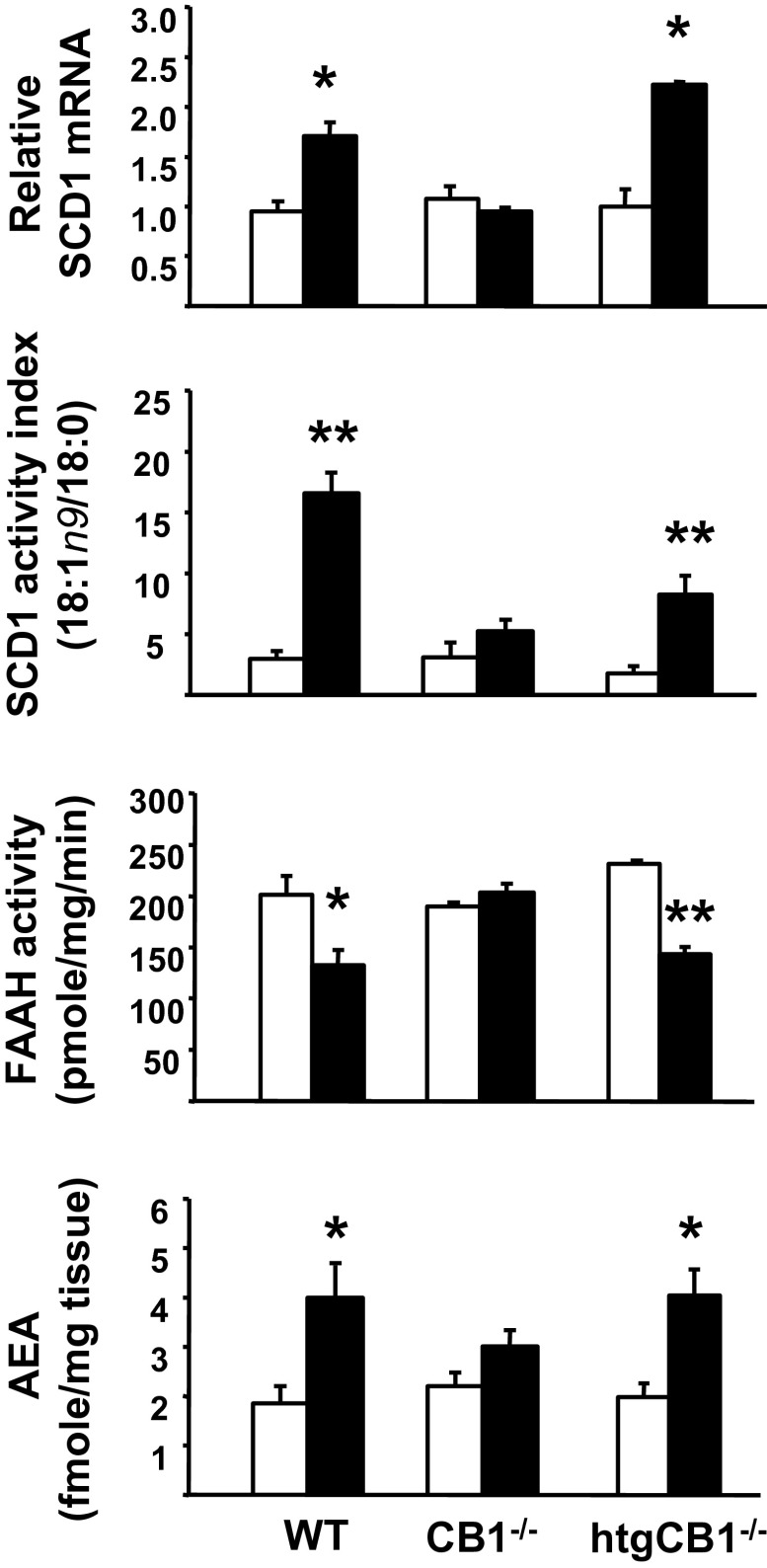
To examine the possible relationship between hepatic CB1R and SCD1 activity, we quantified SCD1 gene expression and enzyme activity in hepatocytes isolated from WT mice, CB1R−/− mice, and CB1R−/− mice with hepatocyte-specific transgenic reexpression of CB1R (htgCB1R−/− mice). Male mice from the three strains were maintained on STD or HFD for 14 wk. As illustrated in Fig. 2, HFD significantly increased hepatic SCD1 gene expression in WT and htgCB1R−/− mice, but not in CB1R−/− mice. There were corresponding changes in SCD1 enzyme activity index, estimated from the C18:1n-9 to C18:0 fatty acid ratio in the liver, which was increased by HFD by 5.6- or 4.4-fold in WT or htgCB1R−/− mice, respectively, but remained unchanged in the CB1R−/− group. The hepatic levels of the individual saturated and MUFAs are illustrated in Fig. S4. In parallel, HFD caused a reduction of FAAH activity and a corresponding increase in hepatic AEA levels in WT and htgCB1R−/− mice, again with no change in these parameters in the CB1R−/− mice (Fig. 2). These results suggest that MUFAs generated via SCD1 mediate the HFD-induced inhibition of FAAH activity and increase in hepatic AEA.
Fig. 2.
HFD increases SCD1 gene expression and activity in WT and htgCB1−/− mice, but not in CB1−/− mice. Mice were fed STD (open column) or an HFD (black column) for 12 wk, at which time they were killed, and snap-frozen liver tissue was used for RNA or lipid extraction and enzyme activity assays. (*P < 0.05 and **P < 0.001 vs. corresponding group fed STD; n = 6–8 per group).
Palmitoleic Acid (C16:1n-7) and Oleic Acid (C18:1n-9) Inhibit Human Recombinant FAAH Activity in Vitro.
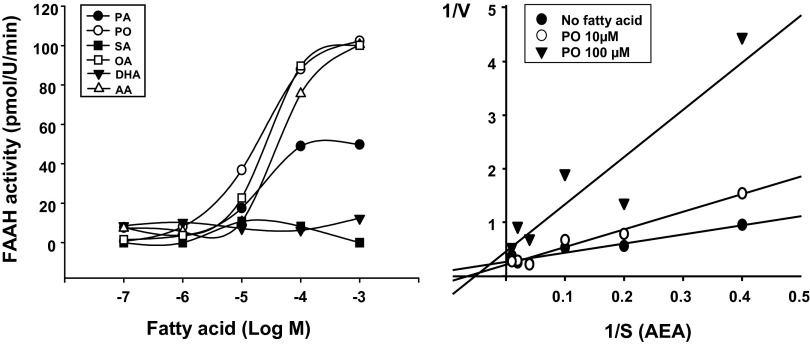
To test the ability of MUFA to inhibit FAAH activity, we measured the activity of human recombinant FAAH in the presence of different concentrations of various fatty acids. As shown in Fig. 3, fatty acids inhibited FAAH activity in a concentration-dependent manner, with the MUFAs palmitoleic acid and oleic acid being the most potent, followed by polyunsaturated arachidonic acid, whereas the saturated fatty acids palmitic acid (C16:0) or stearic acid (C18:0) and the polyunsaturated docosahexaenoic acid had low or no inhibitory activity. Analysis of the relationship between substrate (i.e., AEA) concentration and enzyme activity by reciprocal plots suggested competitive antagonism by an MUFA (Fig. 3).
Fig. 3.
FAAH inhibitory activity of various fatty acids in vitro using recombinant human FAAH. AA, arachidonic acid; DHA, docosahexaenoic acid; OA, oleic acid; PA, palmitic acid; PO, palmitoleic acid; SA, stearic acid. (Left) FAAH activity in the absence and presence of various concentrations of fatty acids was measured as described in Materials and Methods. (Right) FAAH activity was measured at different substrate (i.e., AEA) concentrations to generate Lineweaver–Burke plots (1/V vs. 1/S) in the absence or presence of 10 or 100 μM palmitoleic acid.
Endogenous, but Not Exogenous, MUFAs Inhibit FAAH in Vivo.
Unlike the regular HFD used in most experiments, an HFD with similar fat content but a much higher unsaturated, including monounsaturated, fatty acid composition (HFD¶; Materials and Methods) failed to suppress hepatic FAAH activity or increase AEA content and also failed to increase hepatic SCD1 gene expression and activity relative to values in STD-fed lean mice (Fig. S5). This indicates that endogenously generated but not diet-derived MUFAs possess FAAH inhibitory activity in vivo, and high dietary unsaturated fatty acids may prevent HFD induction of SCD1, in agreement with earlier findings (20). A similar conclusion can be reached from additional experiments in which C57BL/6J mice on STD were gavaged daily with 320 mg oleic acid, and FAAH activity in the brain and liver remained unchanged at 8 and 24 h after a single gavage or after 1 wk of daily gavage with the same dose of oleic acid (Fig. S6).
Inhibition of FAAH Activity in HFD-Fed SCD1−/− Mice Elicits Insulin Resistance.
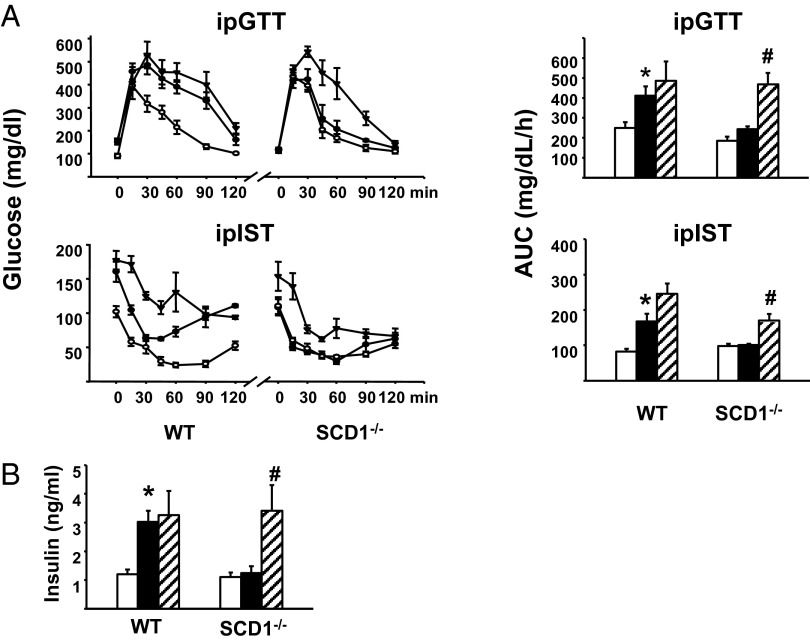
Unlike WT mice, which become glucose-intolerant and insulin-resistant on an HFD, SCD1−/− mice on an HFD retain normal glucose tolerance and insulin sensitivity (Fig. 4A), in agreement with earlier findings (17). To assess whether the absence of FAAH inhibition by endogenous MUFAs plays a role in this phenotype, we tested the effects of chronic in vivo treatment of HFD-fed SCD1−/− mice with URB597 on glucose tolerance and insulin sensitivity. Daily i.p. injection of HFD-fed SCD1−/− mice with 5 mg/kg URB597 for 9 wk caused glucose intolerance and insulin resistance compared with vehicle treatment (Fig. 4A). URB597 treatment also significantly increased plasma insulin (Fig. 4B), whereas the glucose intolerance, insulin resistance, and hyperinsulinemia of WT mice on HFD was not further affected by URB597 treatment (Fig. 4).
Fig. 4.
Inhibition of FAAH activity in HFD-fed SCD1−/− mice induces insulin resistance. (A) SCD1−/− mice on HFD remain glucose-tolerant and insulin-sensitive but develop insulin resistance following chronic treatment with the FAAH inhibitor URB597. Values shown are from STD-fed mice (open circles or columns), vehicle-treated HFD-fed mice (filled circles or columns), or URB597-treated HFD-fed mice (filled triangles or striped columns). URB597 treatment was at 5 mg/kg/d for 9 wk (*P < 0.05 vs. corresponding STD, #P < 0.05 vs. corresponding vehicle-treated HFD value). (B) WT, but not SCD1−/−, mice on an HFD are hyperinsulinemic, and SCD1−/− mice on an HFD become hyperinsulinemic following chronic URB597 treatment. Symbols are as in A.
SCD1 Inhibitor Reverses the Decrease in FAAH Activity in the Liver of DIO Mice.
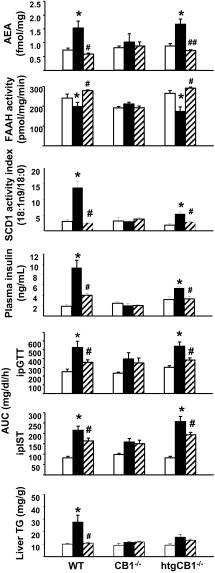
It has been shown that antisense oligonucleotide inhibition of SCD1 expression prevents DIO and hepatic insulin resistance (21, 22). To further investigate the correlation between MUFAs and FAAH activity in the liver, HFD-fed WT, CB1R−/−, and htgCB1R−/− mice were treated with vehicle or 5 mg/kg/d of the SCD1 inhibitor A939572 for 12 wk. A939572 treatment effectively inhibited SCD1 activity in the liver and reversed the HFD-induced decrease in hepatic FAAH activity and the associated increase in hepatic AEA levels in WT and htgCB1R−/− mice, but not in the CB1R−/− mice (Fig. 5). In WT and htgCB1R−/− mice, but not in CB1R−/− mice, the SCD1 inhibitor also normalized plasma insulin levels as well as liver triglyceride content and improved glucose tolerance and insulin sensitivity (Fig. 5). These results clearly support the link between the hepatic endocannabinoid/CB1R system and SCD1 activity.
Fig. 5.
Inhibition of SCD1 activity reverses HFD-induced, CB1R-mediated steatosis, insulin resistance, decreased hepatic FAAH activity, and increased AEA content. WT, CB1R−/−, and htgCB1R−/− mice were maintained on STD (open columns) or HFD and treated for 12 wk with vehicle (filled columns) or the SCD1 inhibitor A939572 5 mg/kg/d (hatched columns). Note that SCD1 blockade reversed the HFD-induced changes in WT and htgCB1R−/−, but not in CB1R−/−, mice (*P < 0.05 vs. STD; #P < 0.05 or ##P < 0.005 vs. HFD vehicle value).
Discussion
In the present study, we investigated the interrelationship between the endocannabinoid AEA and SCD1 activity, two key players in the development of HFD-induced hepatic steatosis and insulin resistance, and identified hepatic MUFAs generated via SCD1 activity as endogenous inhibitors of the AEA degrading enzyme FAAH in the liver, responsible for the elevated hepatic levels of AEA in DIO mice and the resulting CB1R-mediated insulin resistance.
The obligatory role of SCD1 and CB1R in HFD-induced obesity is indicated by the near-complete resistance to DIO and its metabolic complications of mice deficient in SCD1 (17) or CB1R (11, 23). Activation of hepatic CB1R by CB1R agonists promotes de novo lipogenesis via inducing the gene expression of the lipogenic transcription factor SREBP1c and its downstream targets, including SCD1 (11), indicating a functional link between the endocannabinoid/CB1R system and SCD1. Endocannabinoids acting via hepatic CB1R have a similar role, as indicated by the present findings that HFD increased hepatic SCD1 gene expression and enzyme activity in mice with CB1R present in the liver (WT or htgCB1−/− mice), but not in CB1−/− mice. Furthermore, the present findings that MUFAs generated by SCD1 promote CB1R activation by preventing the metabolic degradation of AEA indicate that the link between SCD1 and CB1R is bidirectional via a positive feedback loop.
There are several lines of evidence supporting the functional importance of endogenous MUFA in increasing endocannabinoid tone via this positive feedback loop. First, HFD induced an increase in hepatic AEA and a parallel decrease in the FAAH activity in the liver of WT mice, but not SCD1−/− mice. This is compatible with the role of the HFD-induced increase in hepatic MUFAs being responsible for the aforementioned effects in WT mice. Second, free fatty acids inhibit the activity of recombinant FAAH in a cell-free system, with MUFAs being more potent than saturated or polyunsaturated fatty acids. Fifty percent inhibition of enzyme activity was observed at low micromolar concentrations, which is well below MUFA concentrations in the liver (Fig. S4) and is likely within the range of the much lower levels of fatty acids present in an unbound form. These findings extend early observations that lipids, including fatty acids present in tissue extracts, inhibit FAAH activity (24). The observed inhibition appeared competitive (Fig. 3, Right) and may represent end-product inhibition, in agreement with an earlier observation that oleic acid inhibited the hydrolysis of the preferential FAAH substrate, oleoyl ethanolamide (25). Indeed, arachidonic acid, the end product of the metabolism of anandamide, was also an effective inhibitor of FAAH (Fig. 3, Left), confirming our previous findings in liver homogenates (26). Third, chronic treatment with a FAAH inhibitor restores the ability of HFD to induce insulin resistance in SCD1−/− mice. Fourth, in vivo treatment with an SCD1 inhibitor improved the HFD-induced glucose intolerance and insulin resistance of WT and htgCB1−/− mice, but had no such effects in CB1−/− mice, again indicating the critical requirement for SCD1-generated MUFAs for the development of HFD-induced metabolic impairments and the obligatory role of hepatic CB1R in these effects.
The HFD used in the present study was shown earlier to induce CB1R-mediated DIO, steatosis, and insulin resistance (11), as well as endoplasmic reticulum (ER) stress in the liver (27), associated with increased hepatic AEA levels and decreased FAAH activity (27, 28). Here we show that the HFD-induced twofold increase in AEA and 30% to 40% decrease in FAAH activity in WT mice are associated with dramatic, 7- to 10-fold increases in the hepatic levels of the two primary MUFA products of SCD1, oleic acid (18:1n-9) and palmitoleic acid (16:1n-7), paralleled by a 5.6-fold increase in SCD1 activity, relative to corresponding values in mice on an STD (Fig. S4). Interestingly, HFD induced a similar pattern of changes in htgCB1R−/− mice, whereas it failed to affect these parameters in CB1R−/− mice. This further supports the role of hepatic CB1R in these effects, including the HFD-induced up-regulation of SCD1 activity and MUFA production (Fig. 2).
In agreement with earlier findings (11), HFD feeding did not affect the biosynthetic rate of AEA or the hepatic expression of FAAH (Fig. S3). Thus, MUFAs inhibit the enzyme activity but not the expression of FAAH, which accounts for the increase in hepatic AEA levels, and the inhibition of FAAH by MUFAs is competitive, as illustrated by reciprocal plots of substrate concentration vs. enzyme activity (Fig. 3).
The HFD used in most of the experiments contains 24% MUFAs, suggesting that some of the observed increase in hepatic MUFA content may be of dietary origin. However, increasing the MUFA content of the diet by feeding mice pure oleic acid or an HFD in which 36% of fatty acids are MUFAs failed to affect hepatic MUFA and AEA levels and FAAH activity and suppressed SCD1 expression (Fig. S5). The latter finding is in agreement with an earlier report that feeding mice saturated tristearin robustly increased hepatic SCD1 expression, whereas feeding of the MUFA-containing triolein caused a modest reduction in SCD1 expression (29). This suggests that only endogenous MUFAs generated via SCD1 can act as FAAH inhibitors, whereas MUFAs of dietary origin may actually have the opposite effect by exerting end-product inhibition of SCD1. This, together with increased levels of polyunsaturated fatty acids (PUFAs), which also inhibit SCD1 expression (20), likely account for the reduced de novo hepatic lipogenesis in mice fed an HFD with high MUFA content. This may also provide a plausible explanation for the well known paradox whereby diets rich in MUFAs have been generally found to alleviate the metabolic complications of obesity (30, 31), yet similar or greater improvement is achieved by genetic deletion (17), knockdown (21), or pharmacological blockade of SCD1 (22), and HFD-induced obesity and insulin resistance are associated with increased MUFA content of the ER, contributing to ER stress (32).
As to the preferential role of endogenous vs. diet-derived MUFA, a similar preferential use of endogenous MUFAs for hepatic triglyceride synthesis has been attributed to the close colocalization of SCD1 and diacylglycerol acyltransferase-2 in the ER membrane (33). SCD1 is localized in the ER membrane, and FAAH is an integral plasma membrane protein, with the active sites of both facing the cytosol (34, 35). Lipid transport mechanisms between the ER and plasma membranes are well documented (36), and a similar mechanism may account for the preferential inhibition of FAAH by SCD1-generated as opposed to diet-derived MUFAs. However, direct evidence for such a mechanism is yet to be provided.
Finally, the link between SCD1 and the endocannabinoid system described in the present study is also operational in humans: a recent lipidomic analysis in female human diabetic subjects revealed that diabetes is associated with elevated plasma AEA, but not 2-AG, which positively correlated with increased SCD1 activity and plasma MUFA levels in diabetic vs. control subjects (37). However, under different conditions, 2-AG may have a dominant role, such as the preferential increase in hepatic 2-AG induced by alcohol feeding, which has been linked to alcohol-induced fatty liver (38).
Contrary to the CB1-mediated insulin resistance and abdominal obesity (39), which can be mimicked by short-term exposure to marijuana (40), chronic marijuana use has not been associated with increased cardiometabolic risk, either in terms of being overweight or in the form of insulin resistance (41, 42). Down-regulation of CB1Rs in chronic marijuana smokers (43), and/or inhibition of endocannabinoid action at CB1R by the partial agonist Δ9-tetrahydrocannabinol, may account for these differences.
In summary, the present findings identify endogenous MUFAs as key mediators linking increased SCD1 activity to increased endocannabinoid signaling via CB1Rs in the development of HFD-induced insulin resistance and hepatic steatosis.
Materials and Methods
Animals.
Male C57BL/6J mice and 129/SV mice as well as genetically modified strains backcrossed to the C57BL/6J or 129/SV background were used in this study. Mice with global KO of CB1R (CB1R−/−) or of the SCD1 enzyme (SCD1−/−) were generated as described previously (44, 45). Mice with transgenic expression of CB1R in hepatocytes on a global CB1R−/− background (i.e., htgCB1R−/−) were generated as described previously (27). Mice at the age of 8 to 12 wk were placed on an STD (NIH-31 rodent diet) or an HFD (TD97070; Harlan Teklad) containing 33.5% fat (60% of calories), 26.5% carbohydrate, and 27.4% protein for 12 wk. The fatty acid composition of this HFD is as follows: saturated, 45%; trans-, 24%; cis-MUFA, 24%; and PUFA, 7%. A second HFD referred to as HFD¶ (D12492; Research Diets) had similar total fat content but with higher MUFA (36%) and PUFA (32%) and lower saturated fatty acid content (32%) and no trans fat.
Drugs.
URB597 was purchased from Cayman Chemical. SCD1 inhibitor A939572 was from Biofine International. URB597 and A939572 were dissolved in alkmulphor/EtOH/saline solution (1:1:18) and injected i.p. in volumes of 50 to 100 μL. Alkmulphor is a vegetable oil (Rhodia). Anandamide ([1-3H] ethanolamine) was purchased from American Radiolabeled Chemicals.
Endocannabinoid Levels.
Tissue samples were frozen on dry ice, weighed, and homogenized in 0.5 mL of an ice-cold solution of methanol/Tris buffer (50 mM, pH 8.0), 1:1, containing 7 ng of [2H4]AEA. To each homogenate, 2 mL of ice-cold chloroform/methanol (2:1) and 0.5 mL of 50 mM Tris buffer, pH 8.0, was added. The upper layer was extracted two more times with ice-cold chloroform and deproteinated with 10 volumes of ice-cold acetone. The extract was dried under nitrogen and reconstituted in 50 µL of methanol for analysis by LC/inline MS, using an Agilent 1100 series LC-MS/MS device equipped with a thermostated autosampler and column compartment. Analysis of endocannabinoid levels was performed as described elsewhere (27).
Hepatic FAAH Activity.
FAAH was quantified by the amount of the [3H]ethanolamine released from [3H]AEA labeled on the ethanolamine moiety in various mouse strains fed STD or HFD, and in WT mice acutely treated with various doses of URB597 and killed at the indicated times following treatment. Briefly, mouse livers were homogenized in 10 mM Tris⋅HCl, pH 7.6, containing 1 mM EDTA. Assay mixtures contained 10 µg homogenate protein, radiolabeled AEA (25 µL), and 10 mg/mL fatty acid-free BSA (final assay volume, 200 µL). The mixtures were incubated at 37 °C for 15 min, after which reactions were stopped by placing the tubes in ice and adding 400 µL of chloroform/methanol (1:1, vol/vol). The tubes were vortex-mixed, after which the phases were separated by centrifugation in a bench centrifuge. Aliquots (200 µL) of the methanol/buffer phase were removed for analysis of radioactivity by liquid scintillation spectroscopy with quench correction.
FAAH Inhibitor Screening Assay.
Various substrate (i.e., AEA) concentrations (2.5–100 μM) in the absence or presence of free fatty acids were incubated with 1 U of recombinant human FAAH enzyme (Cayman) for 10 min at 37 °C in an all-glass setup to prevent adhesion of free fatty acids to plastic plates provided for the Cayman assay kit.
Anandamide Biosynthesis.
The activity of NAT, the rate-limiting step in AEA synthesis, was quantified in liver homogenates from mice on an STD or HFD by measuring the conversion of 1,2-phosphatidylcholine-l-α-[1-14C]diarachidonoyl to the AEA precursor N-arachidonoyl phosphatidylethanolamine as described previously (46).
Analysis of SCD1 Activity and Hepatic Fatty Acid Profile.
SCD1 activity index is estimated from the C18:1n-9 to C18:0 fatty acid ratio in the liver (47). Fatty acids in mouse liver were measured by a 7890A gas chromatograph system equipped with a flame ionization detector (Agilent) coupled to a fused-silica narrow-bore DB-FFAP capillary column (Agilent Technologies) to acquire the signal of fatty acid methyl esters (48). Total lipids of mouse liver were extracted by using the same procedure used for endocannabinoid extraction (49), except that extracts were spiked with docosatrienoic ethyl ester (Nu-Chek Prep; Elysian) as the internal standard, 0.7 µmol for the STD group and 1.4 µmol for the HFD group. Total lipids in the extracts were derivatized to the methyl esters using 14% (wt/vol) BF3-methanol (50). Fatty acids were quantified using the internal standard.
i.p. Glucose Tolerance and insulin Sensitivity Test.
Mice fasted overnight were injected i.p. with 2 g/kg glucose. Tail blood was collected at −1, 15, 30, 45, 60, 90, and 120 min, and glucose levels were determined by using a glucometer (Elite; Bayer). One week later, the mice were fasted for 6 h and then received an i.p. injection of insulin (0.75 U/kg; Eli Lilly), and blood glucose levels were determined at 15, 30, 45, 60, 90, and 120 min following the insulin injection.
Blood Chemistry.
Plasma insulin levels (Crystal Chem) were determined by using commercial sandwich ELISAs in accordance with the manufacturers’ instruction.
Hepatic Triglyceride Content.
Triglycerides were extracted as described earlier for endocannabinoids. The combined organic phase was added to 1 mL of a 5% Triton X-100 solution in chloroform and evaporated to dryness under a stream of nitrogen. Samples were reconstituted in 1 mL water for triglyceride quantification by using the EnzyChrom Triglyceride Assay Kit (Bioassays).
Statistical Analyses.
Comparison of the means from two treatment groups was done by using two-way ANOVA followed by Bonferroni post hoc test. For comparing pre- and posttreatment values in the same animals, the paired t test was used. Values with a P value <0.05 were considered statistically significant.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309469110/-/DCSupplemental.
References
- 1.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54(1):1–7. doi: 10.1016/j.neuropharm.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cravatt BF, et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 4.Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 1997;272(43):27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- 5.Dinh TP, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99(16):10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bracey MH, Hanson MA, Masuda KR, Stevens RC, Cravatt BF. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science. 2002;298(5599):1793–1796. doi: 10.1126/science.1076535. [DOI] [PubMed] [Google Scholar]
- 7.McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- 8.Osei-Hyiaman D, et al. Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology. 2005;81(4):273–282. doi: 10.1159/000087925. [DOI] [PubMed] [Google Scholar]
- 9.Ligresti A, Petrosino S, Di Marzo V. From endocannabinoid profiling to ‘endocannabinoid therapeutics’. Curr Opin Chem Biol. 2009;13(3):321–331. doi: 10.1016/j.cbpa.2009.04.615. [DOI] [PubMed] [Google Scholar]
- 10.Cota D, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112(3):423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osei-Hyiaman D, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115(5):1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tam J, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16(2):167–179. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kathuria S, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9(1):76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 14.Petrosino S, Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr Opin Investig Drugs. 2010;11(1):51–62. [PubMed] [Google Scholar]
- 15.Godlewski G, et al. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem Biol. 2010;17(11):1256–1266. doi: 10.1016/j.chembiol.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touriño C, Oveisi F, Lockney J, Piomelli D, Maldonado R. FAAH deficiency promotes energy storage and enhances the motivation for food. Int J Obes (Lond) 2010;34(3):557–568. doi: 10.1038/ijo.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ntambi JM, et al. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA. 2002;99(17):11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen P, et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297(5579):240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 19.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 20.Ntambi JM. Dietary regulation of stearoyl-CoA desaturase 1 gene expression in mouse liver. J Biol Chem. 1992;267(15):10925–10930. [PubMed] [Google Scholar]
- 21.Jiang G, et al. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest. 2005;115(4):1030–1038. doi: 10.1172/JCI23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutiérrez-Juárez R, et al. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006;116(6):1686–1695. doi: 10.1172/JCI26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrié P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28(4):640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 24.Katayama K, et al. Distribution of anandamide amidohydrolase in rat tissues with special reference to small intestine. Biochim Biophys Acta. 1997;1347(2-3):212–218. doi: 10.1016/s0005-2760(97)00078-7. [DOI] [PubMed] [Google Scholar]
- 25.Schmid PC, Zuzarte-Augustin ML, Schmid HH. Properties of rat liver N-acylethanolamine amidohydrolase. J Biol Chem. 1985;260(26):14145–14149. [PubMed] [Google Scholar]
- 26.Mukhopadhyay B, et al. Hyperactivation of anandamide synthesis and regulation of cell-cycle progression via cannabinoid type 1 (CB1) receptors in the regenerating liver. Proc Natl Acad Sci USA. 2011;108(15):6323–6328. doi: 10.1073/pnas.1017689108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology. 2012;142(5):1218–1228, e1. doi: 10.1053/j.gastro.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osei-Hyiaman D, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118(9):3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282(4):2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- 30.Due A, et al. Comparison of the effects on insulin resistance and glucose tolerance of 6-mo high-monounsaturated-fat, low-fat, and control diets. Am J Clin Nutr. 2008;87(4):855–862. doi: 10.1093/ajcn/87.4.855. [DOI] [PubMed] [Google Scholar]
- 31.Marks KA, Kitson AP, Stark KD. Hepatic and plasma sex differences in saturated and monounsaturated fatty acids are associated with differences in expression of elongase 6, but not stearoyl-CoA desaturase in Sprague-Dawley rats. Genes Nutr. 2013;8(3):317–327. doi: 10.1007/s12263-012-0325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu S, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473(7348):528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Man WC, Miyazaki M, Chu K, Ntambi J. Colocalization of SCD1 and DGAT2: Implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. J Lipid Res. 2006;47(9):1928–1939. doi: 10.1194/jlr.M600172-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Man WC, Miyazaki M, Chu K, Ntambi JM. Membrane topology of mouse stearoyl-CoA desaturase 1. J Biol Chem. 2006;281(2):1251–1260. doi: 10.1074/jbc.M508733200. [DOI] [PubMed] [Google Scholar]
- 35.Mileni M, et al. Crystal structure of fatty acid amide hydrolase bound to the carbamate inhibitor URB597: Discovery of a deacylating water molecule and insight into enzyme inactivation. J Mol Biol. 2010;400(4):743–754. doi: 10.1016/j.jmb.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YJ, Hernandez ML, Balla T. Inositol lipid regulation of lipid transfer in specialized membrane domains. Trends Cell Biol. 2013;23(6):270–278. doi: 10.1016/j.tcb.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grapov D, Adams SH, Pedersen TL, Garvey WT, Newman JW. Type 2 diabetes associated changes in the plasma non-esterified fatty acids, oxylipins and endocannabinoids. PLoS ONE. 2012;7(11):e48852. doi: 10.1371/journal.pone.0048852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong WI, et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008;7(3):227–235. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Després JP, Golay A, Sjöström L. Rimonabant in Obesity-Lipids Study Group Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353(20):2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 40.Hollister LE, Reaven GM. Delta-9-tetrahydrocannabinol and glucose tolerance. Clin Pharmacol Ther. 1974;16(2):297–302. doi: 10.1002/cpt1974162297. [DOI] [PubMed] [Google Scholar]
- 41.Rodondi N, Pletcher MJ, Liu K, Hulley SB, Sidney S. Coronary Artery Risk Development in Young Adults (CARDIA) Study Marijuana use, diet, body mass index, and cardiovascular risk factors (from the CARDIA study) Am J Cardiol. 2006;98(4):478–484. doi: 10.1016/j.amjcard.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 42.Muniyappa R, et al. Metabolic effects of chronic cannabis smoking. Diabetes Care. 2013;36(8):2415–2422. doi: 10.2337/dc12-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirvonen J, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17(6):642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96(10):5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131(9):2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, et al. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J Biol Chem. 2003;278(45):45034–45039. doi: 10.1074/jbc.M306062200. [DOI] [PubMed] [Google Scholar]
- 47.Kotronen A, et al. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes. 2009;58(1):203–208. doi: 10.2337/db08-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masood A, Stark KD, Salem N., Jr A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J Lipid Res. 2005;46(10):2299–2305. doi: 10.1194/jlr.D500022-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci USA. 2003;100(3):1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.