Abstract
The town of Fallon within Churchill County, Nevada exhibited an unusually high incidence of childhood leukemia during the years 1997–2003. We examined the temporal and spatial patterning of the leukemia case homes in comparison to the distribution of the general population at risk, other cancer incidence, and features of land use. Leukemia cases were predominantly diagnosed during the early to mid summer, exhibiting a seasonal bias. Leukemia cases lived outside of the “developed/urban” area of Fallon, predominantly in the “agriculture/pasture” region of Churchill County, circumscribing downtown Fallon. This pattern was different from the distribution of the underlying population (p-value < 0.01) and different from the distribution of other cancers, which were evenly distributed when compared to the population (p-value = 0.74). The unusual space-time patterning of childhood leukemia is consistent with the involvement of an infectious disease. A possible mode of transmission for such an infectious disease is by means of a vector, and mosquitoes are abundant in Churchill County outside of the urban area of Fallon. This region harbors a US Navy base, and a temporally concordant increase in military wide childhood leukemia rates suggests the base a possible source of the virus. Taken together, our current understanding of the etiology of childhood leukemia, the rural structure combined with temporal and geospatial patterning of these leukemia cases, and the high degree of population mixing in Fallon, suggest a possible infectious cause.
Keywords: Leukemia, Fallon, Tungsten, Virus, Mosquito, Childhood
1. Introduction
The etiology of most cases of childhood leukemia is unknown. Apart from a higher incidence among children carrying specific predisposition genes, the disease is generally unclustered in families [1] and populations [2,3]. Anecdotal observations of clustering of childhood leukemia have led investigators to hypothesize specific causes of the clusters, ranging from population mixing and unique viruses in naïve populations to chemical exposures from point sources [4–13]. A childhood leukemia cluster observed in Fallon, Churchill County, Nevada is a striking example of such clustering. Fourteen children residing in Churchill County were diagnosed with acute lymphoblastic leukemia (ALL) during the period 1997–2003 with most cases occurring in 1999 and 2000[14]. Based on the population at risk, only one case would be expected every two years, resulting in a risk ratio of 12 for childhood leukemia if a child resided in Churchill County during 1997–2003 [15].
Fallon has a distinctive natural and anthropomorphic environment. The geological basin where Fallon is located contains natural arsenic, tungsten, and radioactive minerals including uranium and polonium [16,17]. This basin also serves as a hydrologic sink, resulting in wetlands and land suitable for abundant agricultural activity. Additionally, Fallon incorporates a Navy training facility and a private hard metal refining facility. While none of these features are by themselves unique, their combination in Fallon focused investigator’s attention on a possible chemical cause for the leukemia cluster and led to the most comprehensive cancer cluster investigation in history by the U.S. Center of Diseases Control (CDC) [14,18]. A total of 205 individuals including the 14 cases of leukemia and their families and 55 comparison families were studied1. Five hundred behavior variables assessed by questionnaires, along with 139 chemicals tested in samples of blood, urine, cheek cells, soil, and house dust, were analyzed. In contrast to the intense scrutiny of chemicals, only 4 viruses with prior associations with B-cell malignancies, human T-lymphotropic virus (HTLV), feline leukemia virus (FeLV), avian leukemia virus (ALV), and Epstein Barr virus (EBV) were examined along with a reverse transcriptase activity assay [14]. A primary finding in the CDC study was elevated tungsten and arsenic levels in the urine of both adults and children, yet these elevated levels were observed equally between case and control series [18].
We examined the temporal and geospatial organization of the Fallon leukemia cases compared to the population of Churchill County and found an unusual patterning of the cases, consistent with a novel hypothesis.
2. Materials and methods
2.1. Data sources
Data on the temporal pattern of leukemia for the 15 cases eligible for the CDC study were extracted from Center for Disease Control (CDC) reports [14]. Incident ALL cases and age-specific population counts were obtained from the Tricare administrative databases for years 1996–2005, these data are billing records from all military service branches from both active and retired service personnel and their dependants. Cancer cases for Churchill County from the years 1999–2004 and their point locations were obtained from the Nevada Cancer Registry. The year 1999 was used as a starting point, as the Nevada Cancer Registry achieved Surveillance, Epidemiology, and End Results (SEER) “gold” status from this year forward. Estimates of underlying the population as well as the population “at risk” were obtained from United States census block data [19]. These counts demonstrated a reasonably homogenous age distribution observed within block groups. In addition to human population data, point (GPS) locations for mosquito traps and the results of West Nile virus tests from those pooled mosquitoes for the year 2004 were obtained from the Churchill County vector control agency. All mapped locations of cancers have been randomly adjusted, or “jittered”, to maintain confidentiality in figures displayed in this paper.
2.2. Analysis
Epidemic curves were plotted by month and compared to published reports on seasonal trends of childhood leukemia [20–23]. Statistical tests of seasonality were not performed due to the small sample size. Incident ALL counts were examined in the Tricare database for age groups 0–19. Rates were calculated using age, branch and year specific populations as the denominator. Rates were age adjusted using a weights calculated from the inverse variance of each observation.
All addresses were geocoded and projected to allow comparisons. The spatial distribution of the Fallon cluster cases was examined using geographic information systems and the statistical package R [24,25]. U.S. census block counts were utilized as estimates of the underlying population. Rates within geographic areas with small numbers are not stable; therefore, special statistical techniques, density equalized map projection (DEMP), sometimes referred to as cartograms, were employed to better represent the underlying population structure [26]. Cluster locations were compared to the Vegetation Synthesis map [27] to assess the land use and vegetation type at each case residential location.
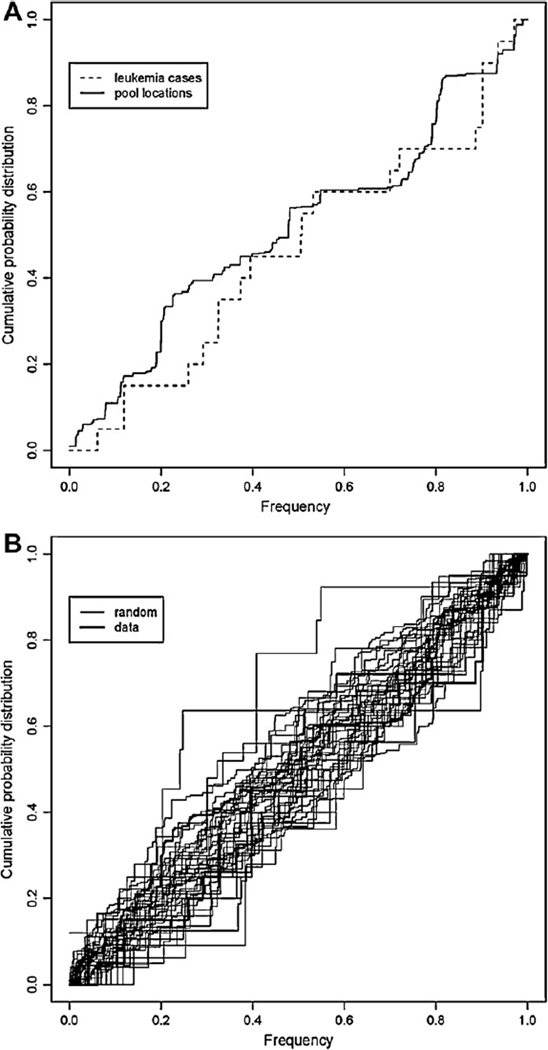
A test of variance was used to examine the spatial distributions of cases [28]. In this study of spatial patterns of disease, the distribution of the population is known and is best represented by census blocks. The observed distribution of cases was directly compared to the population distribution to statistically assess the homogeny of observed disease patterns over the population. Cumulative distribution functions (CDF) of sample points and the underlying population were compared, providing a useful statistical tool to visually identify exceptional patterns among cases of disease. In addition, the mean and variance of the distribution of the sample of cases were compared to the parallel mean and variance from the population providing simple and common statistical measures of observed differences. When the spatial distribution under consideration is transformed to an equal density population (as in DEMP), then, the distribution of the population is uniform, and the sample cumulative distribution is a straight line, with an expected mean value of 0.5 and an estimated mean value with a variance = 1/(12n). In addition, the approximate normal distribution of the estimated sample has a variance = 1/(180n) where n represents the number of cases of disease. The test of the sample mean and variance provides a bivariate evaluation of the likelihood that the observed spatial pattern is not random.
3. Results
3.1. Temporal trends
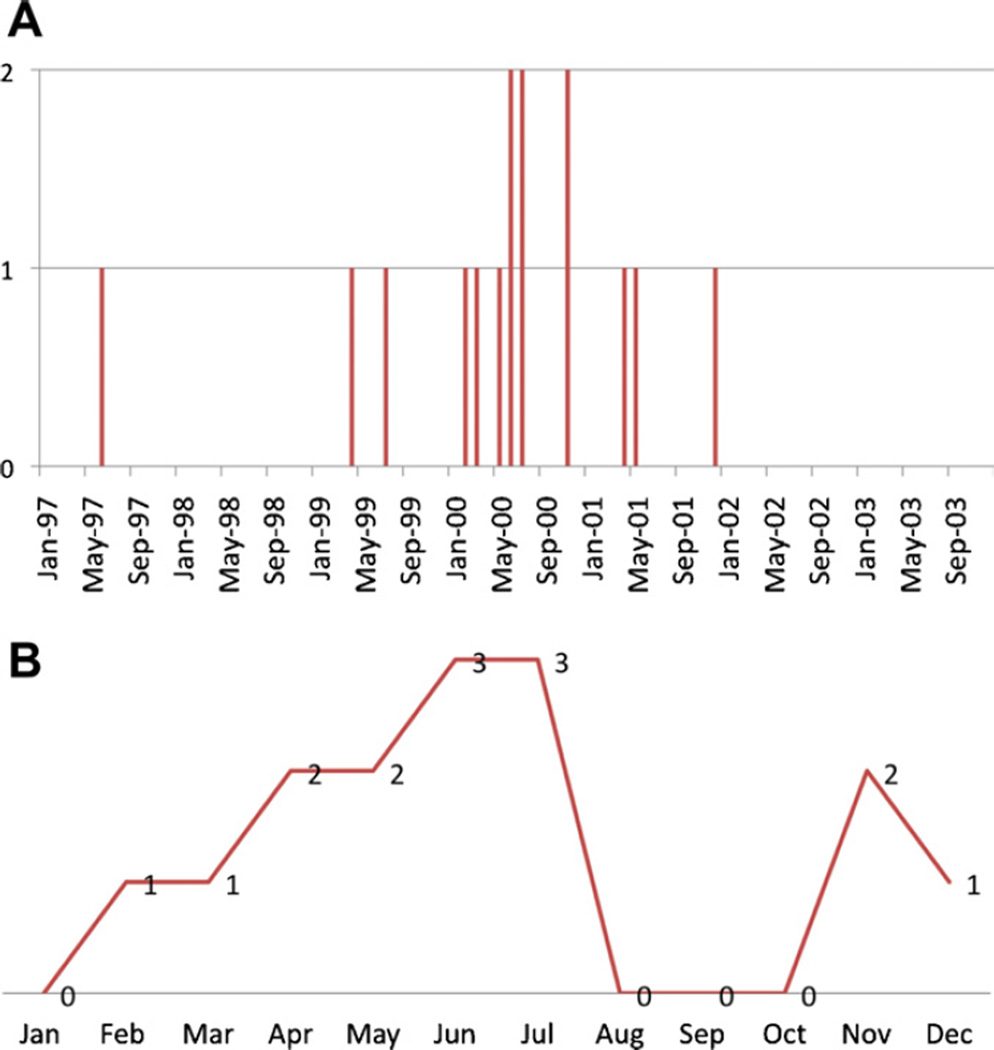
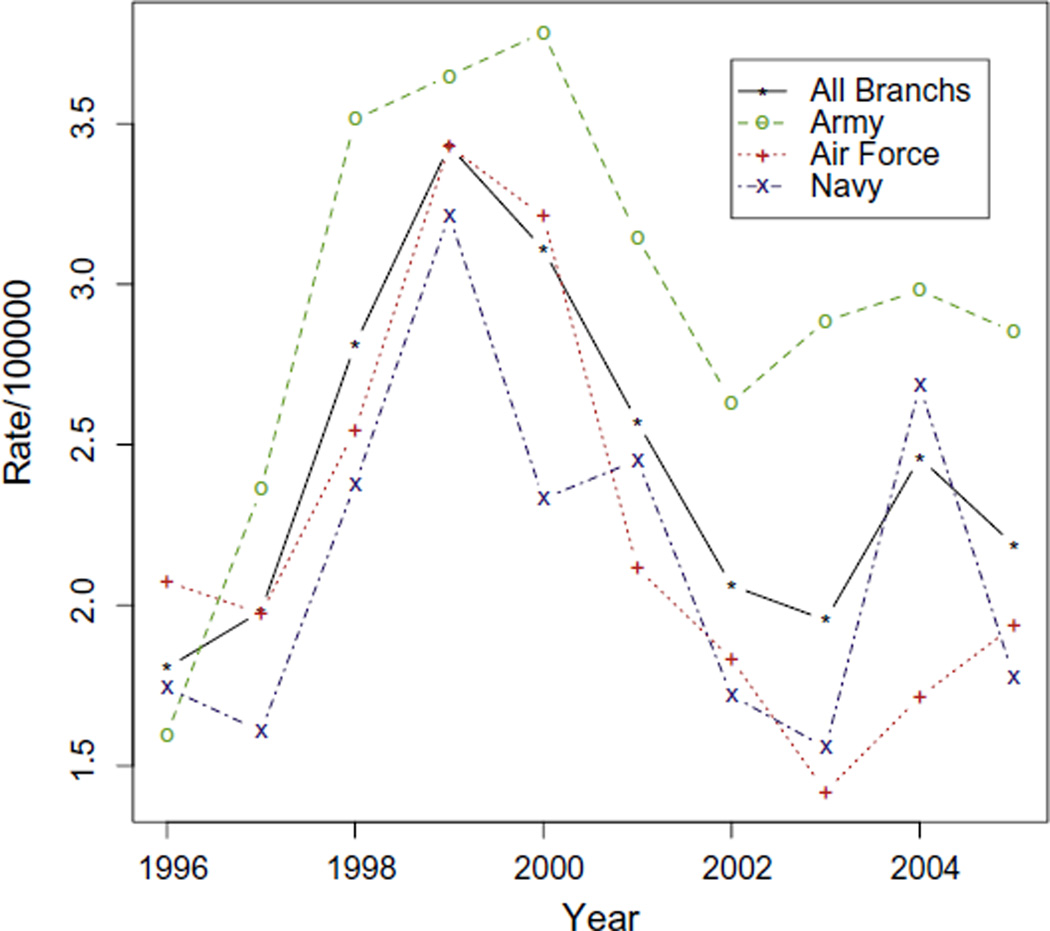
Nine of the 15 CDC defined cases occurred from February 2000– November 2000 [14] (Fig. 1A), a notable increase within the overall cluster period. A seasonal trend is suggested when viewing cases by diagnosis month (Fig. 1B). Small sample size precluded formal statistical testing. Tricare data showed an increase in childhood ALL rates peaking in 1999 in the Navy, with a longer peak observed from 1999–2000 in the Air Force and Army (Fig. 2). Observed rates are stable due to the large sample size and a Poisson distribution analysis rejected the null hypothesis of no change in rates (P < 0.01). This combined with 3 separate concurrent observations of Army, Air Force and Navy increases suggest the observed peak from 1999–2000 is not likely the result of random variation.
Fig. 1.
Temporal pattern of leukemia cases in the Fallon, Nevada leukemia cluster. (A) Timing of leukemia over the five-year period with a notable spike in 2000. (B) Seasonal timing of leukemia cases with most cases between April and July.
Fig. 2.
Age adjusted acute lymphoblastic leukemia (Age 0–19). Case counts and age, branch and year specific denominator populations are from the Tricare data base. Age adjustment was calculated using the inverse variance of the observed rates. There is minimal random variation in these estimates.
3.2. Case distribution
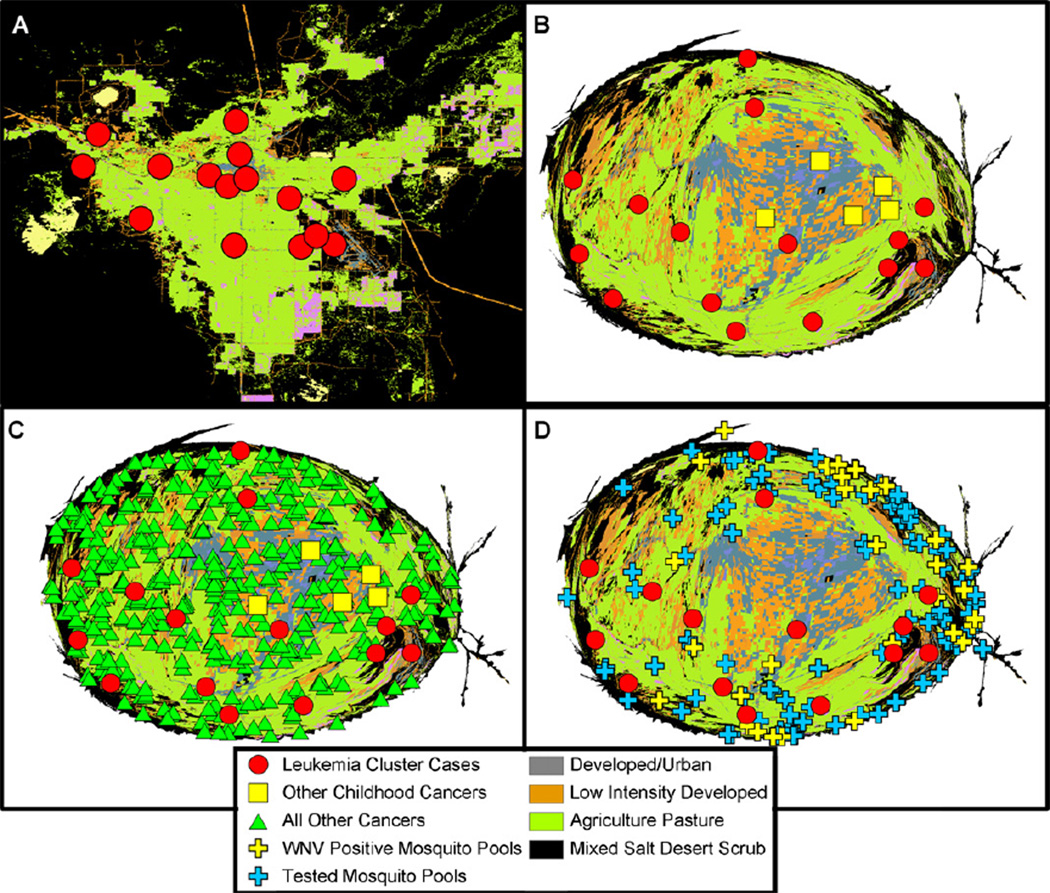
The geographic distribution of Fallon cancer cases exhibits a notable but not obvious annulus surrounding the downtown Fallon area (Fig. 3A). To better visualize these data, a density equalized map (DEMP) projection was utilized (Fig. 3B) [26]. DEMP distributes areal units based on population density, making the density of the population equal across the map. If the distribution of events within a population is random, those events should be evenly distributed at random over a DEMP-generated map.
Fig. 3.
Geospatial organization of cancer cases and environmental features in Churchill County. Vegetation synthesis map of Churchill County (A) Spatial organization of leukemia cases (red circles) over the cluster time period. Locations are enlarged and “jittered” to maintain confidentiality. (B) Density-equalized map of the same information as (A), leukemia cases (red circles). Non-leukemia pediatric cancers (yellow square) are also displayed. On this map, the spatial structure is altered to create an equal distance between members of the population. (C) Location of leukemia cases (red circles), non-leukemia cancer cases (green diamonds), and non-leukemia pediatric cancers (yellow square) on the DEMP projection. Non-leukemia cancer cases appear randomly distributed. The paucity of cancer cases in the lower right of the graph is due to incomplete reporting of cancer to the Nevada registry from the US Navy Air Station. (D) Tested mosquito pools (in the year 2004) are shown by crosses, and those that were negative and positive for WNV are indicated in blue and yellow, respectively. Leukemia cluster cases are indicated (red circles).
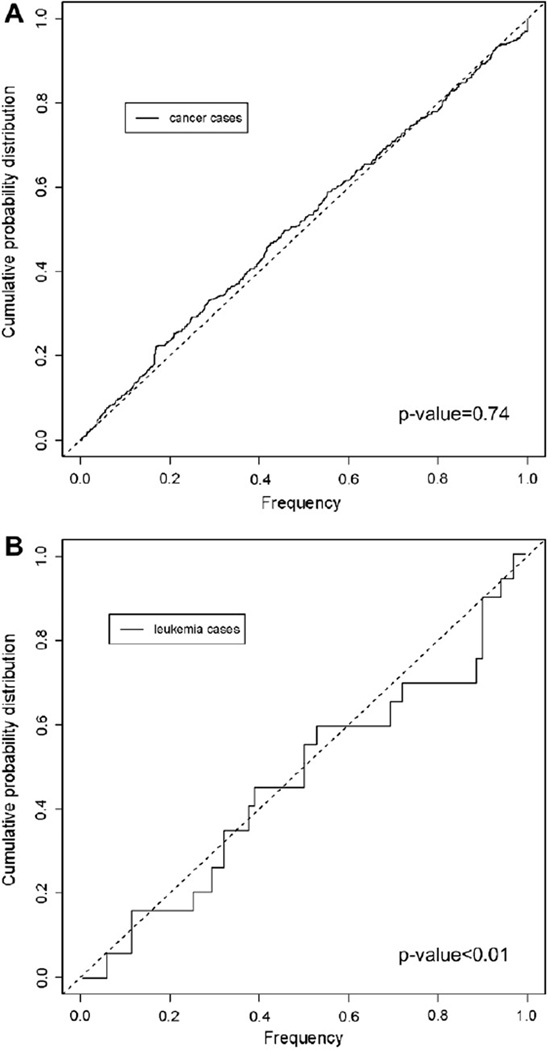
The likelihood that these observed spatial distributions arose by chance alone was tested using the test of variance. The residences of the leukemia cases were compared to the 2000 U.S. Census population and the residences of all cancer (except leukemia) cases, recorded in the Nevada cancer registry during 1999–2004. The goal was to identify any notable pattern that might suggest a common exposure. All non-leukemia cancers in the 1999–2004 period were shown to reside in the same spatial structure as the underlying population (p-value = 0.74; test of variance) (Fig. 3A). Yet the location of the cluster cases was found to be significantly different from that of the underlying population (p-value < 0.01; test of variance; Fig. 4B). Non-leukemia pediatric cancers were also plotted, and it is clear that cases occurred with a different distribution than leukemias and are confined to the Fallon downtown region (Fig. 3B and C). The small sample (n = 5) of non-leukemia pediatric cancers precluded statistical analysis of this group.
Fig. 4.
Comparison of the distribution of cancer cases on the DEMP projection. The graph exhibits a step function of the occurrence of cases, working outwards towards the periphery of the DEMP projection. If cancer cases are randomly distributed, the step function will exhibit a straight line at a 45° angle. The step function can be tested for variance against the observed population, a completely random distribution would be exactly 45°. (A) Non-leukemia cancer cases, and the population at risk are graphed. By the test of variance, these distributions do not differ (p-value = 0.74). (B) Leukemia cases distribution tested against the population at risk are significantly different by the test of variance (p-value < 0.01).
The distribution of cases was also compared to a remotely sensed Nevada Vegetation Synthesis map (Fig. 3D) [27]. One case resided in the “developed/urban” designation, one case resided in “Inter-Mountain Basins Mixed Salt Desert Scrub”, one case resided in “Low Intensity Developed Open Space”, and twelve resided in “Agriculture-Pasture/Hay.”
3.3. Mosquito viral transmission in Nevada
Churchill County vector control officials routinely trap and pool (group) mosquitoes, then test them for a standard variety of pathogens including West Nile Virus (WNV). A sufficient number of mosquitoes is necessary to complete a “test pool.” Test pool locations for the cluster period years were not available for analysis, therefore locations of test pools during 2004 were used as a proxy to represent the presence of mosquito activity. We believe this to be a valid conclusion as in general, sources of mosquito activity do not vary greatly overtime. A map of the 2004 test pools and WNV positive pools shows an annulus outside the urban area of Fallon (Fig. 3D). The area surrounding the urban region of Fallon provides natural and agricultural mosquito-breeding habitat.
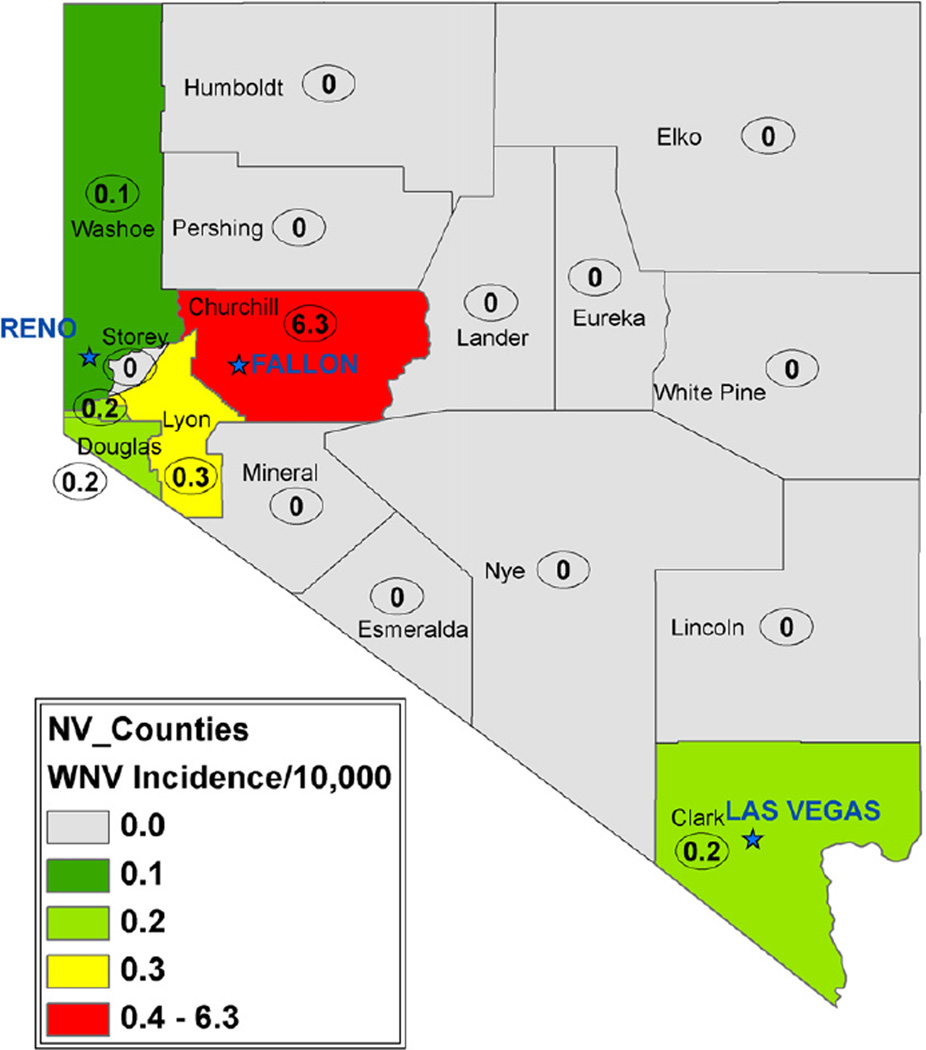
The 2004 mosquito test pool locations and pools testing positive for WNV were examined against the leukemia cluster locations. These distributions are similar with respect to their geographic distribution (Figs. 3D and 5A). The assessment of non-random clustering was also tested using the randomization assumption; samples were combined then resampled at random. The distribution of both the leukemia cases and mosquito pools falls within this bootstrapped distribution (Fig. 5B). No evidence exists that the two distributions differ. Interestingly, during the introductory year, 2004, Churchill County was the epicenter of mosquito-borne WNV cases in Nevada (Fig. 6). To be clear, WNV is not a candidate for a leukemia-causing virus but may serve as a surrogate or biomarker of the rapid facility for arthropod virus transmission in Churchill County.
Fig. 5.
Comparison of the distribution of leukemia cases and tested mosquito pools. (A) Distribution of leukemia cases and mosquito pool testing locations. (B) Fig. 2 shows the same two cumulative distributions (thick lines) and 20 cumulative distribution functions (thin lines) created by a bootstrap sampling of the original data. The “band” of replicate distributions surrounding the data-estimated values provides a sense of the considerable sampling variation associated with these estimates. Therefore, a formal inference from comparing these two frequency distributions (plots) is simply that no evidence exists, that the two distributions differ.
Fig. 6.
2004 West Nile Virus Human Incidence per 10,000. Churchill County had the highest human incidence of WNV in Nevada during the introductory year of the WNV epidemic.
4. Discussion
In this report we present evidence that leukemia cases in Churchill County, Nevada during 1997–2003 were dispersed geographically in rural agricultural wetlands surrounding the city of Fallon, exhibiting a spatial distribution that differed from that of other cancers and non-leukemia pediatric cancers. This geospatial pattern occurred in an area with low population density and high concentrations of mosquitoes. We speculate that patterns of infection may have contributed to leukemogenesis during this period: isolation from early life infectious exposures followed by vector-borne viral transmission resulted in leukemia in susceptible children. Furthermore, the temporally concordant increase in childhood leukemia rates throughout the military suggests the Fallon Naval station as a possible source for the introduction of the virus.
The unusual temporality of the cluster is exceptionally statistically significant [15]. The often-cited Fallon cluster window of 1997–2003 is, however, overly broad as 9 of the 14 CDC investigated cases occurred in a narrow window of 10 months during the year 2000 (Fig. 1A). The temporality and seasonality of the epidemic curve is consistent with a point source exposure or an infectious agent acting out a classic ‘outbreak’ pattern. Many infectious diseases are known to ‘burn’ through areas: as susceptibles in a naïve population become infected, the number of susceptibles wane, leaving fewer and fewer potential hosts. This scenario is especially true with new agents. It is worth noting that the increase in childhood leukemia rates in the military followed a similar curve as the Fallon subjects.
Seasonality of childhood leukemia has been observed in several studies [20–23]. The observed seasonality in the Fallon cluster is earlier than most but not all reported seasonal variations, with most cases occurring in the middle of summer. Although the number of cases is small, the shape of the epidemic curve suggests a seasonal factor (Fig. 1B). Many factors could explain this observed seasonality such as a change in behavior leading to increased chemical exposure. However, seasonality could also be explained by an infectious disease, possibly a vector-borne disease which usually exhibit a highly seasonal pattern peaking in late summer [29,30].
Most research attention to date on the Fallon leukemia cluster has been focused on chemical exposures. While our observations do not preclude a chemical exposure as a potential factor, no plausible exposure has been identified despite extensive testing. In addition, no radical change in use or release of compounds been documented around the time or preceding the cluster. Furthermore all cancers including those with a more probable chemical etiology were randomly distributed spatially during the cluster period despite the non-uniformity of source chemical locations in Churchill County, e.g., the fuel pipeline or Naval Air Station. One notable exception is agricultural chemicals which may be more widely distributed in rural regions, although these were examined in the CDC investigation [14].
When examining the DEMP transformed population distribution, there is a striking annulus or ‘anti urban halo’ of the leukemia patients extending around downtown Fallon. Indeed, almost all cases occurred exclusively around the urban area of Fallon, the most densely populated region of Churchill County. Potential bias may be introduced by utilization of census blocks; however, we believe any error is likely to be minimal due to reasonable population homogeneity of 0–19 year olds versus the general population. This assumption is further supported by examining the non-leukemia pediatric cancers, which center in urban downtown Fallon (Fig. 3B and C). The much larger population residing in the urban environment should also exhibit an excess risk of leukemia if an urban-associated environmental exposure were a cause. One such proposed candidate for a potentially leukemogenic exposure is the hard metal facility at the center of Fallon’s urban area [31]. However, the spatial distribution of leukemia observed here is not compatible with a point source of pollution in the center of town, from which potential emissions decay rapidly in a geometric fashion from the facility [32].
Fallon is in a rural county where lack of protective exposures could increase risk for the entire community. Observed cases occurred outside the downtown Fallon center, which may further increase their isolation. Many of the case children are of school age, and the age and leukemia subtype distribution was not different than encountered among children nationally [33]. It is difficult to speculate on the frequency of regular infectious exposures early in life. It may be true that those children who lived around Fallon were subject to fewer challenges early in life compared to children in the center of Fallon. A lack of community herd immunity to an foreign virus could have resulted in leukemia in certain susceptible children. Such a virus would not induce the same effects in a less isolated population endemic with the same viral illness(es). The co-occurrence of increased incidence of leukemia in military children during the same period is highly suggestive of a specific agent circulating in the highly mobile military community.
The observed geographic pattern, steep epidemic curve and seasonal spike suggest a non-random etiologic driver. The similar location of the observed anti- urban halo of leukemia cases, active mosquito populations, and WNV positive mosquitoes suggest one such driver may be a vector-borne pathogen. During WNV’s introductory year in to Nevada, Churchill County (essentially the city of Fallon) recorded the highest incidence of human cases in Nevada: ~6.3 per 10,000, in stark contrast to the next highest rate in Lyon County with ~0.3 cases per 10,000 (Fig. 5). The residents and vector control officials of Churchill County have known for decades that the area around the city of Fallon is a prime mosquito-breeding habitat. These outlying areas, patchworks of flood-irrigated fields, open irrigation ditches, pasture, and natural sinks which can accumulate standing water and organic matter are ripe breeding grounds for Culex tarsalis and Culex pipiens. These species are versatile, efficient vectors for a range of viruses such as WNV, western equine encephalitis, eastern equine encephalitis and Saint Louis encephalitis. The geographic co-occurrence of mosquito habitat and leukemia cluster residences suggests the possibility of a relationship. Weather can play a role in breeding mosquito populations. Warm, still air and abundant standing water are necessary for feeding and breeding. In 20 years prior to 2002, the wettest season for the greater Fallon area was observed during the 1995–1996 winter. It is not clear whether this wet season preceding the cluster could have changed mosquito behavior. Given that 50,000+ acres of land surrounding Fallon with miles of irrigation ditches are permanent farmland, changing weather patterns may play less of a role than farming practices in determining mosquito populations. It is clear that Fallon has large active breeding mosquito populations every year.
There is no logical or implied link between WNV and the Fallon childhood leukemia cluster. However, WNV positive mosquito pools in Churchill County provide a proxy for a potential vector-borne disease given that at a large-scale mosquito habitat is reasonably stable over time. Although a bacterial or parasitic agent is also possible, only viruses have been causally associated with leukemia in humans [34]. A candidate viral disease outbreak in military services military with a temporal concordance to the Fallon cluster is adenovirus due to vaccine discontinuation in the 1990’s [35]. While gene therapy constructs using adenoviral vectors caused leukemia in some recipient patients [36], no link to leukemia with natural adenovirus, nor natural vector transmission of this virus, has been reported.
Both Kinlen’s ‘population-mixing’ hypothesis [37,38] and Greaves ‘delayed infection’ hypothesis [39] summarize the considerable evidence that childhood leukemia may be the consequence of an abnormal response to a common or uncommon infection(s). While a specific mechanism has not been established, a large body of literature supports one or both of these hypotheses [37,39,40]. While it has been shown that a pre-leukemic state begins in utero [41,42], the 10% concurrence of disease in monozygotic twins obligates the involvement of a secondary trigger [43]. Despite the fact that environmental triggers have been proposed as part of the etiology for childhood leukemia for quite sometime, limited supportive data exist [44]. Investigations of viral infection in sporadic, unclustered childhood leukemia cases have not implicated any specific infections in such populations [45–49], but specific infections have not been adequately addressed in any leukemia cluster investigation. The spike in incident military childhood ALL cases during the same time period as the Fallon cluster support the hypothesis that a specific virus was introduced into the community by the large yearly influx of military personnel training at the base that was then transmitted/amplified by the mosquito population. The subsequent spread of infection could have been mild or asymptomatic for most, but for those children who were genetically susceptible to leukemia and carrying a preleukemic mutation, the infection could have been the ‘2nd hit’ or the causal infection in a population otherwise naïve. Such is the essence of the proposal by Kinlen in a 2004 editorial regarding the Fallon cluster [50], augmented by us to include a novel means of community viral dispersal.
The agent proposed here is speculative. While an unknown agent is equally plausible, known candidates currently exist. Historically, viruses have been thought to play a large role in human cancer, particularly retroviruses. A wealth of literature has been developed since the 70’s on the transmission of a variety of endogenous and exogenous retroviruses in animals including avian leukemia virus (ASLV), friend leukemia virus (FLV), feline leukemia virus (FeLV), bovine leukemia virus (BLV) and murine leukemia virus (MLV) (reviewed in [51]). Retroviruses can be transmitted in humans by every route except droplets. Arthropod-borne retrovirus infection is prevalent in the animal world. Equine infectious anemia is primarily transmitted to horses through mechanical transmission by horse flies and deer flies. Murine leukemia virus (Friend) has been observed to be transmissible (though not amplified) by a mosquito bite [52]. Horse flies have been shown to transmit BLV in 10% of bites, through mechanical transmission [53]. Evidence also suggests that insect vector modes of transmission of HTLV1, a virus associated with T-cell leukemia, may occur in humans [54].
The discovery of new viral etiologies of human disease, including cancer, continues [55]. The recent discovery of a novel polyomavirus causal for Merkel cell carcinoma [56,57] is such an example, and xenotropic murine leukemia related virus is another recent but controversial example [58–61]. Moreover, other known vector-borne viruses, such as western equine encephalitis, and Saint Louis encephalitis are known to circulate in Nevada and have been detected in Churchill County. None of these vector-borne viruses were tested by the CDC viral panel and may also represent possible candidates.
5. Conclusion
The Fallon cluster may have been set in motion by a confluence of factors: transiently elevated community viral load as a result of population mixing from the military base, an immunologically naïve population of children, an extremely active breeding mosquito population, and other contributing environmental factors including chemicals. The observed cluster appears highly unique but may have been perceptible only because of the geographic isolation of the community. An apparent increase in vector control activities in 2001–2003 with the impending introduction of WNV may have ended the already waning transmission cycle that was responsible for the cluster. The steep epidemic curve, concordant military increase and anti-urban halo are details of the Fallon cluster that are worth further investigation and could be a key element in establishing a cause of the Fallon leukemia cluster. The proximity of habitat, active mosquito populations, and identity of viruses warrant further consideration. The generation of a new hypotheses and supporting evidence will guide future inquiry into the etiology of the most common childhood cancer.
Acknowledgements
We thank the Churchill County Vector Control District for data on mosquito control; Dr. Jeffery Underwood, Nevada State Climatologist, and the Tricare Management Authority and the SIDR and SADR Military Health System administrative databases for sharing data. We also thank Ivan Smirnov with database support, and Howard Foster and Lloyd Morgan for helpful discussions.
Funding source: Funding provided by the Environment Protection Agency (subcontract from the University of Nevada, Reno), and the Children with Leukaemia Foundation (JLW), and by NIH P42ES0470 and R01ES09137 (PAB).
Footnotes
CDC case definition was comprised of 2 factors: Pre-B ALL and 6 month of residence in Churchill County prior to diagnosis. Using this case definition 15 cases were eligible (14 enrolled).
Conflict of interest statement
None declared.
References
- 1.Schmiegelow K, Hjalgrim H. Is the risk of acute lymphoblastic leukemia reduced in siblings to children with the disease? A novel hypothesis explored by international collaboration. Leukemia. 2006;20(7):1206–1208. doi: 10.1038/sj.leu.2404250. [DOI] [PubMed] [Google Scholar]
- 2.Schmiedel S, Blettner M, Kaatsch P, Schüz J. Spatial clustering and space – time clusters of leukemia among children in Germany, 1987–2007. Eur. J. Epidemiol. 2010:1–7. doi: 10.1007/s10654-010-9488-7. [DOI] [PubMed] [Google Scholar]
- 3.Selvin S, Ragland KE, Chien EY-L, Buffler PA. Spatial analysis of childhood leukemia in a case/control study. Int. J. Hygiene Environ. Health. 2004;207(6):555–562. doi: 10.1078/1438-4639-00327. [DOI] [PubMed] [Google Scholar]
- 4.Hjalmars U, Kulldorff M, Gustafsson G, Nagarwalla N. Childhood leukaemia in Sweden: using GIS and a spatial scan-statistic for cluster detection. Stat. Med. 1996;15(7–9):707–715. doi: 10.1002/(sici)1097-0258(19960415)15:7/9<707::aid-sim242>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Mosavi-Jarrahi A, Moini M, Mohagheghi MA, Alebouyeh M, Yazdizadeh B, Shahabian A, Nahvijo A, Alizadeh R. Clustering of childhood cancer in the inner city of Tehran metropolitan area: a GIS-based analysis. Int. J. Hygiene Environ. Health. 2007;210(2):113–119. doi: 10.1016/j.ijheh.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Knox EG, Gilman EA. Spatial clustering of childhood cancers in Great Britain. J. Epidemiol. Commun. Health. 1996;50(3):313–319. doi: 10.1136/jech.50.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petridou E, Alexander FE, Trichopoulos D, Revinthi K, Dessypris N, Wray N, Haidas S, Koliouskas D, Kosmidis H, Piperopoulou F, Tzortzatou F. Aggregation of childhood leukemia in geographic areas of Greece. Cancer Causes Control. 1997;8(2):239–245. doi: 10.1023/a:1018480515690. [DOI] [PubMed] [Google Scholar]
- 8.Alexander FE, Chan LC, Lam TH, Yuen P, Leung NK, Ha SY, Yuen HL, Li CK, Lau YL, Greaves MF. Clustering of childhood leukaemia in Hong Kong: association with the childhood peak and common acute lymphoblastic leukaemia and with population mixing. Br. J. Cancer. 1997;75(3):457–463. doi: 10.1038/bjc.1997.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilman EA, Knox EG. Childhood cancers: space-time distribution in Britain. J. Epidemiol. Commun. Health. 1995;49(2):158–163. doi: 10.1136/jech.49.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafsson B, Carstensen J. Space-time clustering of childhood lymphatic leukaemias and non-Hodgkin’s lymphomas in Sweden. Eur. J. Epidemiol. 2000;16(12):1111–1116. doi: 10.1023/a:1010953713048. [DOI] [PubMed] [Google Scholar]
- 11.Gustafsson B, Carstensen J. Evidence of space-time clustering of childhood acute lymphoblastic leukaemia in Sweden. Br. J. Cancer. 1999;79(2–4):655–657. doi: 10.1038/sj.bjc.6690103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNally RJ, Alexander FE, Birch JM. Space-time clustering analyses of childhood acute lymphoblastic leukaemia by immunophenotype. Br. J. Cancer. 2002;87(5):513–515. doi: 10.1038/sj.bjc.6600498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickinson HO, Parker L. Quantifying the effect of population mixing on childhood leukaemia risk: the Seascale cluster. Br. J. Cancer. 1999;81(1):144–151. doi: 10.1038/sj.bjc.6690664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.C.f.D.C.a.P. CDC. Cross-Sectional Exposure Assessment of Environmental Contaminants in Churchill County. Nevada: 2003. [Google Scholar]
- 15.Steinmaus C, Lu M, Todd RL, Smith AH. Probability estimates for the unique childhood leukemia cluster in Fallon, Nevada, and risks near other U.S. Military aviation facilities. Environ. Health Perspect. 2004;112(6):766–771. doi: 10.1289/ehp.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seiler RL. Temporal changes in water quality at a childhood leukemia cluster. Ground Water. 2004;42(3):446–455. doi: 10.1111/j.1745-6584.2004.tb02692.x. [DOI] [PubMed] [Google Scholar]
- 17.Seiler RL. (210)Po in Nevada groundwater and its relation to gross alpha radioactivity. Ground Water. 2010 doi: 10.1111/j.1745-6584.2010.00688.x. [DOI] [PubMed] [Google Scholar]
- 18.Rubin CS, Holmes AK, Belson MG, Jones RL, Flanders WD, Kieszak SM, Osterloh J, Luber GE, Blount BC, Barr DB, Steinberg KK, Satten GA, McGeehin MA, Todd RL. Investigating childhood leukemia in Churchill County, Nevada. Environ. Health Perspect. 2007;115(1):151–157. doi: 10.1289/ehp.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S.C. Bureau. Census 2000 Summary File 1 [United States] 2002 [Google Scholar]
- 20.Hayes DM. The seasonal incidence of acute leukemia. A contribution to the epidemiology of the disease. Cancer. 1961;14:1301–1305. doi: 10.1002/1097-0142(196111/12)14:6<1301::aid-cncr2820140620>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Knox G. Epidemiology of Childhood Leukaemia in Northumberland and Durham. Br. J. Prev. Soc. Med. 1964;18:17–24. doi: 10.1136/jech.18.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badrinath P, Day NE, Stockton D. Seasonality in the diagnosis of acute lymphocytic leukaemia. Br. J. Cancer. 1997;75(11):1711–1713. doi: 10.1038/bjc.1997.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorensen HT, Pedersen L, Olsen JH, Rothman KJM. Seasonal variation in month of birth and diagnosis of early childhood acute lymphoblastic leukemia. JAMA. 2001;285(2):168–169. [PubMed] [Google Scholar]
- 24.ESRI. ArcGIS. Redlands, CA: 2008. [Google Scholar]
- 25.R.D.C. Team. R Foundation for Statistical Computing. Vienna, Austria: 2010. R: A language and environment for statistical computing. [Google Scholar]
- 26.Merrill DW, Selvin S, Close ER, Holmes HH. Use of density equalizing map projections (DEMP) in the analysis of childhood cancer in four California counties. Stat. Med. 1996;15(17–18):1837–1848. doi: 10.1002/(SICI)1097-0258(19960915)15:17<1837::AID-SIM395>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 27.Peterson DL. Documentation and geospatial data. Carson City, Nevada: Nevada Natural Heritage Program; 2008. A Synthesis of Vegetation Maps for Nevada (Initiating a ‘Living’ Vegetation Map) [Google Scholar]
- 28.Cochran WG. Some methods for strengthening the common X2 tests. Biometrics. 1954;10(4):417–451. [Google Scholar]
- 29.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect. Dis. 2002;2(9):519–529. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 30.Nicholas K. West Nile Virus: Epidemiology and Ecology in North America. In: Karl Maramorosch FAMAJSTJC, Thomas PM, editors. Advances in Virus Research. Academic Press; 2003. pp. 185–234. [DOI] [PubMed] [Google Scholar]
- 31.Sheppard PR, Speakman RJ, Ridenour G, Witten ML. Temporal variability of tungsten and cobalt in Fallon, Nevada. Environ. Health Perspect. 2007;115(5):715–719. doi: 10.1289/ehp.9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheppard PR, Ridenour G, Speakman RJ, Witten ML. Elevated tungsten and cobalt in airborne particles in Fallon, Nevada: possible implications for the childhood leukemia cluster. Appl. Geochem. 2006;21:152–165. [Google Scholar]
- 33.Steinberg KK, Relling MV, Gallagher ML, Greene CN, Rubin CS, French D, Holmes AK, Carroll WL, Koontz DA, Sampson EJ, Satten GA. Genetic studies of a cluster of acute lymphoblastic leukemia cases in Churchill County, Nevada. Environ. Health Perspect. 2007;115(1):158–164. doi: 10.1289/ehp.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Martel C, Franceschi S. Infections and cancer: established associations and new hypotheses. Crit. Rev. Oncol. Hematol. 2009;70(3):183–194. doi: 10.1016/j.critrevonc.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 35.Russell KL, Hawksworth AW, Ryan MA, Strickler J, Irvine M, Hansen CJ, Gray GC, Gaydos JC. Vaccine-preventable adenoviral respiratory illness in US military recruits, 1999–2004. Vaccine. 2006;24(15):2835–2842. doi: 10.1016/j.vaccine.2005.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 37.Kinlen LJ. Infection and childhood leukemia. Cancer Causes Control. 1998;9(3):237–239. doi: 10.1023/a:1017149601195. [DOI] [PubMed] [Google Scholar]
- 38.Kinlen LJ. Infection and childhood leukaemia near nuclear sites. Lancet. 1997;349(9066):1702. doi: 10.1016/s0140-6736(05)62679-7. [DOI] [PubMed] [Google Scholar]
- 39.Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nat. Rev. Cancer. 2006;6(3):193–203. doi: 10.1038/nrc1816. [DOI] [PubMed] [Google Scholar]
- 40.Urayama KY, Buffler PA, Gallagher ER, Ayoob JM, Ma X. A meta-analysis of the association between day-care attendance and childhood acute lymphoblastic leukaemia. Int. J. Epidemiol. 2010;39(3):718–732. doi: 10.1093/ije/dyp378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greaves M. Pre-natal origins of childhood leukemia. Rev. Clin. Exp. Hematol. 2003;7(3):233–245. [PubMed] [Google Scholar]
- 42.Wiemels JL, Cazzaniga G, Daniotti M, Eden OB, Addison GM, Masera G, Saha V, Biondi A, Greaves MF. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354(9189):1499–1503. doi: 10.1016/s0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- 43.Greaves MF, Maia AT, Wiemels JL, Ford AM. Leukemia in twins: lessons in natural history. Blood. 2003;102(7):2321–2333. doi: 10.1182/blood-2002-12-3817. [DOI] [PubMed] [Google Scholar]
- 44.Buffler PA, Kwan ML, Reynolds P, Urayama KY. Environmental and Genetic Risk Factors for Childhood Leukemia: Appraising the Evidence. Cancer Invest. 2005;23(1):60–75. [PubMed] [Google Scholar]
- 45.Bender AP, Robison LL, Kashmiri SV, McClain KL, Woods WG, Smithson WA, Heyn R, Finlay J, Schuman LM, Renier C, et al. No involvement of bovine leukemia virus in childhood acute lymphoblastic leukemia and non-Hodgkin’s lymphoma. Cancer Res. 1988;48(10):2919–2922. [PubMed] [Google Scholar]
- 46.MacKenzie J, Perry J, Ford AM, Jarrett RF, Greaves M. JC and BK virus sequences are not detectable in leukaemic samples from children with common acute lymphoblastic leukaemia. Br. J. Cancer. 1999;81(5):898–899. doi: 10.1038/sj.bjc.6690783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Priftakis P, Dalianis T, Carstensen J, Samuelsson U, Lewensohn-Fuchs I, Bogdanovic G, Winiarski J, Gustafsson B. Human polyomavirus DNA is not detected in Guthrie cards (dried blood spots) from children who developed acute lymphoblastic leukemia. Med. Pediatr. Oncol. 2003;40(4):219–223. doi: 10.1002/mpo.10246. [DOI] [PubMed] [Google Scholar]
- 48.Ong V, Liem NL, Schmid MA, Verrills NM, Papa RA, Marshall GM, Mackenzie KL, Kavallaris M, Lock RB. A role for altered microtubule polymer levels in vincristine resistance of childhood acute lymphoblastic leukemia xenografts. J. Pharmacol. Exp. Ther. 2008;324(2):434–442. doi: 10.1124/jpet.107.128926. [DOI] [PubMed] [Google Scholar]
- 49.Vasconcelos GM, Kang M, Pombo-de-Oliveira MS, Schiffman JD, Lorey F, Buffler P, Wiemels JL. Adenovirus detection in Guthrie cards from paediatric leukaemia cases and controls. Br. J. Cancer. 2008;99(10):1668–1672. doi: 10.1038/sj.bjc.6604714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinlen L, Doll R. Population mixing and childhood leukaemia: Fallon and other US clusters. Br. J. Cancer. 2004;91(1):1–3. doi: 10.1038/sj.bjc.6601982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foil LD, Issel CJ. Transmission of retroviruses by arthropods. Annu. Rev. Entomol. 1991;36:355–381. doi: 10.1146/annurev.en.36.010191.002035. [DOI] [PubMed] [Google Scholar]
- 52.Ferrer JF. Bovine leukosis: natural transmission and principles of control. J. Am. Vet. Med. Assoc. 1979;175(12):1281–1286. [PubMed] [Google Scholar]
- 53.Hawkins JA, Adams WV, Jr, Wilson BH, Issel CJ, Roth EE. Transmission of equine infectious anemia virus by Tabanus fuscicostatus. J. Am. Vet. Med. Assoc. 1976;168(1):63–64. [PubMed] [Google Scholar]
- 54.Miller GJ, Lewis LL, Colman SM, Cooper JA, Lloyd G, Scollen N, Jones N, Tedder RS, Greaves MF. Clustering of human T lymphotropic virus type I seropositive in Montserrat, West Indies: evidence for an environmental factor in transmission of the virus. J. Infect. Dis. 1994;170(1):44–50. doi: 10.1093/infdis/170.1.44. [DOI] [PubMed] [Google Scholar]
- 55.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat. Rev. Cancer. 2010;10(12):878–889. doi: 10.1038/nrc2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carter JJ, Paulson KG, Wipf GC, Miranda D, Madeleine MM, Johnson LG, Lemos BD, Lee S, Warcola AH, Iyer JG, Nghiem P, Galloway DA. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J. Natl. Cancer Inst. 2009;101(21):1510–1522. doi: 10.1093/jnci/djp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlaberg R, Choe DJ, Brown KR, Thaker HM, Singh IR. XMRV is present in malignant prostatic epithelium and is associated with prostate cancer, especially high-grade tumors. Proc. Natl. Acad. Sci. U.S.A. 2009;106(38):16351–16356. doi: 10.1073/pnas.0906922106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Lombardi VC, Ruscetti FW, Das Gupta J, Pfost MA, Hagen KS, Peterson DL, Ruscetti SK, Bagni RK, Petrow-Sadowski C, Gold B, Dean M, Silverman RH, Mikovits JA. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science. 2009;326(5952):585–589. doi: 10.1126/science.1179052. [DOI] [PubMed] [Google Scholar]
- 60.Lo S-C, Pripuzova N, Li B, Komaroff AL, Hung G-C, Wang R, Alter HJ. Detection of MLV-related virus gene sequences in blood of patients with chronic fatigue syndrome and healthy blood donors. Proc. Natl. Acad. Sci. U.S.A. doi: 10.1073/pnas.1006901107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Kermode-Scott B. Canada bans blood donations from people with history of chronic fatigue syndrome. BMJ. 2010;340:c1974. doi: 10.1136/bmj.c1974. (April 09_2) [DOI] [PubMed] [Google Scholar]