Abstract
Acute leukemia is the most common cancer in children but the causes of the disease in the majority of cases are not known. About 80% are precursor-B cell in origin (CD19+, CD10+), and this immunophenotype has increased in incidence over the past several decades in the Western world. Part of this increase may be due to the introduction of new chemical exposures into the child's environment including parental smoking, pesticides, traffic fumes, paint and household chemicals. However, much of the increase in leukemia rates is likely linked to altered patterns of infection during early childhood development, mirroring causal pathways responsible for a similarly increased incidence of other childhood-diagnosed immune-related illnesses including allergy, asthma, and type 1 diabetes. Factors linked to childhood leukemia that are likely surrogates for immune stimulation include exposure to childcare settings, parity status and birth order, vaccination history, and population mixing. In case-control studies, acute lymphoblastic leukemia (ALL) is consistently inversely associated with greater exposure to infections, via daycare and later birth order. New evidence suggests also that children who contract leukemia may harbor a congenital defect in immune responder status, as indicated by lower levels of the immunosuppressive cytokine IL-10 at birth in children who grow up to contract leukemia, as well as higher need for clinical care for infections within the first year of life despite having lower levels of exposure to infections. One manifestation of this phenomenon may be leukemia clusters which tend to appear as a leukemia “outbreak” among populations with low herd immunity to a new infection. Critical answers to the etiology of childhood leukemia will require incorporating new tools into traditional epidemiologic approaches – including the classification of leukemia at a molecular scale, better exposure assessments at all points in a child's life, a comprehensive understanding of genetic risk factors, and an appraisal of the interplay between infectious exposures and the status of immune response in individuals.
Keywords: childhood, leukemia, review, epidemiology, etiology, cluster
1. Introduction
Our objective with this report is to highlight recent advances and provide a perspective on the current understanding of the etiology of childhood leukemia rather than being an all-inclusive review. Childhood leukemia is a group of diseases with varied immunophenotypes and genetic changes. By all measures, the leukemia cases that comprise the Fallon leukemia cluster do not differ from a typical set of leukemia cases found anywhere in the US [1]. As this review paper consists of a part of a special issue on the Fallon cluster, we comment on all subtypes of childhood leukemia with a focus on the most common – acute lymphoblastic leukemia.
Childhood acute leukemia is the most common cancer in children representing 31% of all cancers and about 3,250 new cases per year in the US [2]. Numerous breakthroughs in the past 50 years have increased survivability of the disease to greater than 80%, but survivors face long term morbidities [3]. The identification of causes and prevention/early intervention is clearly a worthwhile goal. Known causes include ionizing radiation and congenital genetic syndromes such as Down's, neurofibromatosis, Fanconi's anemia, and Bloom's Syndrome, all of which together explain less than 10% of cases. Incidence of the disease has increased close to 1% per year in the past two decades [4] with similar rates of increase decades earlier [5, 6], indicating that causal factors for the disease are likely to have become more prevalent in the population in the past several decades. The most notable feature of this period of time is the rise of the “common acute lymphocytic leukemia” (or CD19+, CD10+, B-cell cALL) peak in 2-6 year olds, a peak absent in some populations such as Africa and India, and in an ecological sense associated with countries that have higher socioeconomic status [7]. It is also notable that this peak may vary by ethnicity within the United States, with Hispanic:white:black ratio being 1.2:1.0:0.6 [8, 9]. Whether this is related to genetic or environmental reasons is unknown.
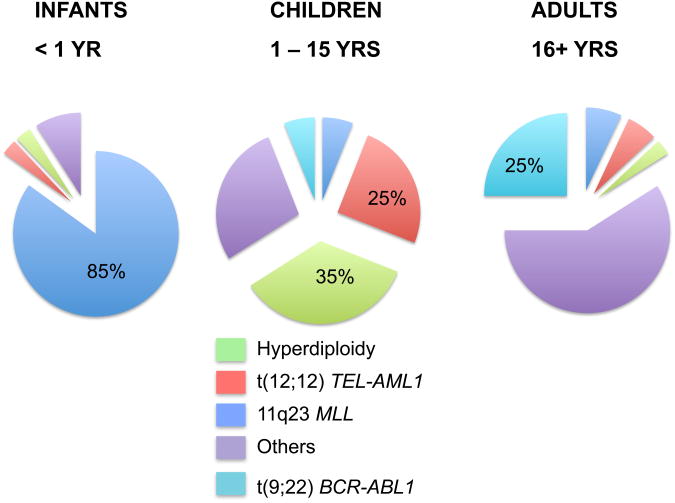
The disease is comprised of several subtypes that vary in phenotype and age incidencel patterns (Figure 1). The broadest division for phenotyping leukemia is the lymphoid and myeloid split, with about 80% of leukemias being lymphocytic. Infant leukemias (<1 yr of age) predominantly exhibit 11q23 MLL gene rearrangements and may have lymphocytic (pro-B), myeloid, or undifferentiated features (Figure 2). Leukemia among young children (2-10 yrs) are dominated by a pre-B lymphocytic phenotype, mostly the cALL subtype. Teenagers tend to trend towards adult-like leukemias, with an increasing frequency of myeloid types and a disappearance of cALL with increasing age. An appreciation of these phenotypes is critical as risk factors and immune dysfunction in particular are likely to affect their relative incidence differentially. Furthermore, within these subgroups are further groups defined by specific genetic features such as particular translocations, the presence of extra chromosomes, and gene mutations; the formation of each of these mutations appear to occur within specific time periods during the child's life with the mechanistic formation likely having distinct causes.
Figure 1. Distribution of acute lymphoblastic leukemia subtypes by age.
The figure demonstrates remarkable differences in the frequencies of cytogenetic features of leukemias by age.
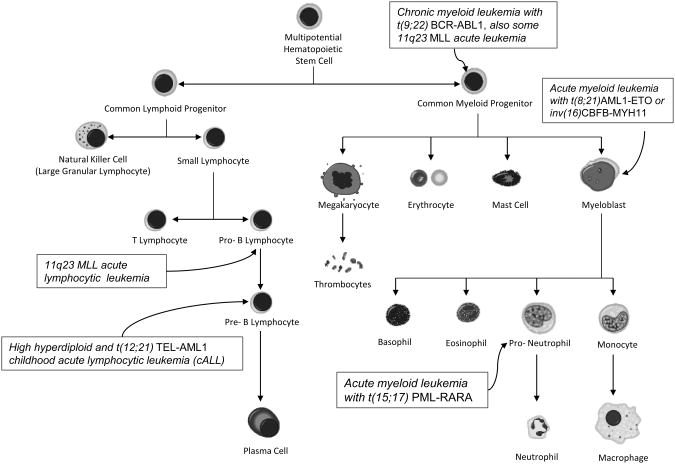
Figure 2. Hematopoiesis.
Blood cells are derived from multipotential progenitors. Nearly every cell noted here has a neoplastic counterpart which exhibits morphological features of its cell of origin. Those leukemia subtypes discussed extensively in the text are noted.
The genetic and epigenetic aberrations frequent in the childhood leukemias are often important prognostic indicators, and identification of several of these are critical to modern disease classification protocols [10]. The uniform characterization of childhood leukemias under modern clinical protocols also provides relevant subgroup information for etiologic studies and will increasingly become a critical component of leukemia epidemiology studies.
2. The timing of initiating events in leukemia
Childhood leukemia like all cancers is a product of two or more molecular changes in stem-like cells that have the ability to divide while maintaining an immature state. The of leukemias are pre-B cell phenotype, meaning that they exhibit cell surface markers of normal pre-B cells, and appear to be clonal outgrowths of normal pre-B cells “frozen” at a particular differentiation stage (Figure 2). Less common are leukemias with myeloid or T-cell lineage. Being blood cells, leukemias have an inherent capacity for mobilization in the bloodstream and extravasation. Precursor blood cells also have an enormous capacity for “blast-like” growth with their normal function to produce 1011 cells per organism per day. These attributes are among the six “hallmarks of cancer” [11], and the fact that hematopoietic precursors harbor these “cancer-like” attributes may be a reason by which leukemias seem to need far fewer genetic aberrations compared to solid tumors, which need to evolve these metastatic capacities through genetic mutations. The genetic simplicity of leukemia combined with the young age of the childhood leukemias and the availability of archived biological resources has allowed researchers to delineate the timing of the formation of genetic aberrations within the lifetime of the child.
Most of the common genetic mutations in leukemia have been assessed for their formation during the fetal period using unique genetic tools and archived patient materials [12]. This research was made possible by the availability of neonatal heel-prick blood spots, or archived newborn blood spots (ANB cards) for children with leukemia. Several common translocations assessed on Guthrie cards, including t(12;21)TEL-AML1, t(8;21)AML1-ETO, inv(16)CBFB-MYH11, have indicated a clear presence of the mutations on neonatal blood spots at birth in children who contract leukemia later [13-16]. Several other mutations, including t(1;19)E2A-PBX1, FLT3, and RAS mutations are clearly postnatal [17-19]. MLL translocations (11q23) appear to occur within temporal proximity to diagnosis, meaning that infants (<1 yr old) have prenatal translocations, and children beyond 2 years of age at diagnosis have postnatal translocations [20, 21].
The mutations associated with leukemia are insufficient to cause disease by themselves. This is the case for TEL-AML1 and AML1-ETO, the most common translocations for ALL and AML, respectively. Studies using cord bloods from normal born children indicate that these translocations may occur at a rate of 1% or more in the normal population [22, 23]. This result suggests that a significant proportion of the population carries preleukemic clones, and the vast majority of these clones are self-limiting and do not result in disease. This also suggests that critical rate-limiting step in the genesis of leukemia occurs during childhood (ie., after birth), and that harboring initiating genetic translocations does not result in an inevitable diagnosis of leukemia; hence, interventions may be possible.
3. Causes of mutations in leukemia
Fetal and child hematopoiesis exhibits a remarkably high rate of cellular kinetics, and blood cell formation takes place within several organs – the aorta-gonad-mesonephros region, followed by the liver, spleen, and ultimately the bone marrow. The high degree of cellular proliferation creates a situation whereby perturbation via environmental insults including chemicals may induce mutations. The epidemiology of mutation-specific subtypes of leukemia is barely beginning, but there is evidence of progress. Children with t(12;21)TEL-AML1 translocations were more than 4-fold more likely to be born from mothers who were exposed to paints during their pregnancy when compared to controls; this increased risk was not noted for other cytogenetic subtypes of leukemia [24]. Future leukemia epidemiology studies should consider genetic and cytogenetic subsets and combine cases of rare subgroups from multiple studies worldwide to gain statistical power.
While the incorporation of genetic classifications in epidemiology studies is only just beginning, the causes of broadly classified childhood leukemia have been under investigation for decades as reflected by dozens of studies. The only consistent and confirmed environmental cause is ionizing radiation from sources such as diagnostic imaging during pregnancy or atomic bomb exposure during childhood and young adulthood [25]. Other suspected contributors of leukemogenesis include diet of the mother and child, parental smoking, pesticides and household chemicals, traffic fumes, and immunologic modifiers, which we will cover in the next section. Smoking and pesticides have been recently and comprehensively reviewed and will not be further covered here [26, 27].
As we are currently in the age of the genome-wide association study (GWAS), the contribution of genetic modifiers is of great current interest. To date, variation in several genes implicated in B-cell development (ARID5B, IKZF1, CEBPA) and cell cycle regulation/DNA repair (CDKN2A/B) are confirmed genetic risk factors for childhood ALL [28-31], but published GWAS studies are small and dozens of other genetic risk factors are certain to emerge in the coming years. Besides these confirmed risk factors, candidate gene studies have implicated dozens of other genetic risk factors in candidate gene studies assessing pathways such as DNA repair, folic acid metabolism, and carcinogen detoxification. A recent meta-analysis of the multitude of studies has been published [32], but results of candidate gene studies must be taken with caution since publication bias and influence of underpowered studies may lead to false conclusions. Besides the standard GWAS, additional fine scale genotyping efforts in the major histocompatibility genes are of particular interest because of associations between patterns of infection and leukemia risk. Several HLA haplotype associations with childhood leukemia been noted (eg.,[33-36]); however, a recent SNP-based analysis suggested no associations within the greater HLA region [37]. HLA alleles are complex and different genotypes can the same peptide binding pockets, requiring nonconventional analytic approaches. Also, the capacity of HLA alleles to support immune reactions against particular epitopes varies greatly, so the ultimate HLA-leukemia association study should account for specific infectious exposures as well as tumor genetic profiles of leukemias.
4. Patterns of infection and acute lymphocytic leukemia
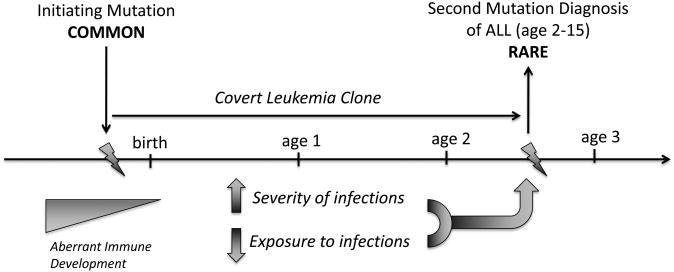
As noted above, most subtypes of childhood leukemia have their initial genetic mutations before birth, but only a fraction of those “initiated” preleukemic clones will progress to leukemia. By far, the most critical epidemiologic modifiers identified to date for the majority of childhood leukemias that will influence the progression of leukemia pertain to immunological development. The idea that exposure to infections and the development of the immune system might influence the etiology of leukemia in children grew out of two observations and led to two related hypotheses. Kinlen noted that leukemia incidence was often increased when children and families were moved and mixed in a new setting, such as the removal of children from central London during World War II, and the creation of “new towns” in Britain in the decades following that war [38, 39]. Kinlen proposed a “population mixing” hypothesis on the origins of leukemia and posited a specific viral infection as potentially causative in the leukemia “outbreaks” which occurred soon after mixing. In examining data from the perspective of individuals rather than the population as a whole, Greaves noticed that children who received lower levels of immune stimulation during childhood developed a higher risk for leukemia and posited that a normal course of infections via early childhood contacts were protective [40, 41]. A specific viral cause is not involved in this hypothesis; rather, the lack of immune stimulation in children who are relatively isolated followed by an aberrant over-response to common infections later in childhood is thought to induce leukemia in children who harbor preleukemic mutations. Several common childhood leukemia aberrations are prenatal in origin and occur at a much higher frequency than the disease [15, 23], and those children who have a preleukemic mutation combined with aberrant immune development will be at the greatest risk. This is the essence of the “two-hit” natural history of leukemia elucidated by Greaves (Figure 3) [40]. Given the generally sporadic patterning of leukemia incidence among populations, one could argue that prevailing evidence favors Greaves' hypothesis of abnormal immune development rather than the involvement of a specific leukemia virus for the induction of the second “hit.”
Figure 3. A model illustrating key events in the development of childhood acute lymphoblastic leukemia.
Genetic events are shown above the horizontal line, and immune development below the line. Initiating mutations are quite common, perhaps in 1% of normal live births, but among these births a second mutation after birth in the same initiated cell is rare. Epidemiologic exposures linked to the initiating mutation are not well established but chemical causes are a possibility (Scelo paper cite). Occurrence of the secondary post-natal mutation is enhanced by features of immune development - namely, a higher propensity to overreact to common infections early in life, and a lower overall exposure to infections and other immune stimuli. Figure modeled after Figure 1, Greaves et al., 2006.
Since it is a rare disease, nearly all childhood leukemia studies gather information on cases and controls after children are diagnosed with leukemia. Immunological development is quite difficult to assess in these retrospective studies. Data is typically questionnaire-based and variables include those that are easily remembered without bias by families enrolled in such studies. These include: childcare history, vaccinations, infections, and allergies/asthma. Childcare history, or daycare, is considered a proxy measure for exposure to infection – the more contacts a child has in daycare setting, the better chance for exposure to new infections. Studies of daycare and leukemia sometimes demonstrated no difference in frequency, or more often a reduced level of daycare in children who contract leukemia compared to controls. A recent meta-analysis by Urayama and colleagues compiled evidence from 15 studies and yielded a combined OR = 0.76, 95% confidence interval (CI): 0.67-0.87, indicating a reduced level of prior childhood contacts in leukemia patients via daycare setting in the majority of studies, which is interpreted as a reduced level of immune stimulation/modulation in those children [42]. This epidemiologic result supports Greaves' “delayed infection” hypothesis elucidated above [40]. Other support for Greaves' hypothesis has come from studies showing an inverse relationship between childhood ALL and higher birth order [43, 44] along with the daycare attendance studies, which both indicate that an increased opportunity for early childhood infections protects against leukemia. Additional support is provided by studies of normal childhood vaccinations. While vaccinations are largely ubiquitous making it difficult to assess their role in a population (very few unexposed persons) studies of Haemophilis Influenza (Hib), a vaccination phased in over the past two decades, indicates that vaccination reduces risk to leukemia [45, 46]. This is not likely to be a specific effect; rather, the Hib vaccination is another example of an immunomodulatory stimulation which will help moderate future responses to new infections.
Greaves' hypothesis is similar to the “hygiene hypothesis” proposed by Strachan to explain the rising prevalence of allergy in the western population [47]. Strachan hypothesized that childhood infections may be protective against allergy, but that declining family size and improved sanitation may have reduced exposure to infectious agents during early childhood thus resulting in the rising prevalence of allergy [47]. The hygiene hypothesis has been supported epidemiologic studies that reported an inverse association between allergy and higher birth order [48-50] or early daycare attendance [51, 52], similar to the associations seen with childhood leukemia. Allergies and asthma have generally been associated in an inverse relationship with leukemia as detailed in a recent meta-analysis on the subject [53]. On the face of it, this contradicts the data above on allergies; if allergies and leukemia share the same risk factors related to immune development, shouldn't their incidence patterns be similar? The case-control studies that assess allergy do so by assessing parental recall of allergies. Case parents may be more likely to ruminate about factors that may have affected their child's risk to leukemia leading to false positive associations. In addition, control families tend to misreport allergies that may have occurred after a “reference date” (diagnostic date for the corresponding leukemic children), therefore, over-reporting exposures, infections, and medical conditions [54]. The two studies that utilized medical record abstraction rather than patient interview found that allergy was a risk factor for leukemia [55, 56], which is more plausible given the similar risk factors for these two diseases. This illustrates a situation whereby systematic bias may affect a series of case-control studies and produce an aberrant conclusion.
The leukemia cluster at Fallon exhibited a similar age and immunophenotype profile as any typical cohort of childhood leukemias, and it is likely that infection patterns affected the incidence and timing of the cluster. Given the geographic patterning and narrow time window of the cluster, it is likely that a specific infection was involved, closely emulating Kinlen hypothesis [57]. Further details are outlined below in Section 7 and in an accompanying paper in this issue [58].
5. Fetal immune development
Leukemia is a cancer of the immune system, and while immune stimulation by a normal course of infections and vaccinations appears to be protective, there are other data suggesting that children who contract leukemia may be born with an aberrant immunity that makes them respond to infections more vigorously. The United Kingdom Childhood Cancer Study (UKCCS) reported that children diagnosed with ALL had significantly more clinically diagnosed infectious episodes in the first year of life compared to controls [59]; this result was replicated in an independent study [60]. The number of infectious episodes in children with ALL increased with increasing indices of infectious exposure (birth order, regular social activity outside the home, and social deprivation at birth), a phenomenon not observed among healthy control children [61]. The UKCCS also clearly demonstrated fewer social contacts for children contracting leukemia, indicating that overall exposure to infections were likely lower than controls [62]. This suggests that there may be two separate phenomena influencing leukemia risk: (i) a lower repertoire of infections during early immune development which will increase risk (ie., daycare, see the last section); (ii) an altered congenital responder status to infection resulting in a functionally aberrant clinical presentation of occasional infections in these same children (ie., greater propensity to need clinical care when contracting infections). In support of this, we recently discovered that a key cytokine is severely deficient among children who grow up to contract leukemia, a greater than 10-fold difference when comparing the highest tertile to the lowest [63]. Interleukin-10 (IL-10) is a key regulator for modulating the intensity and duration of immune responses to infections [63]. IL-10 is a strong anti-inflammatory cytokine secreted by monocytes and lymphocytes and is critical in limiting cell-mediated immune reactions. It has been reported that an increased risk for repeated respiratory infections during infancy and childhood associated with an elevated production of IL-5 by T cells at birth could be attenuated by IL-10 production [64]. It is possible that children with dysregulated immune function at birth are at higher risk for developing leukemia due to constitutively lower expression of IL-10, a cytokine that is critical to prevent an overactive inflammatory response to pathogenic infectns. Biological stress from postnatal infection in combination with a dysregulated immune response may confer a growth advantage for a preleukemic clone leading to its rapid expansion and an increased opportunity for the occurrence of a second mutation required for the development of childhood leukemia [40]. The mechanism behind this constitutive lower IL-10 expression at birth will be a critical research question in discovering the causes of childhood leukemia.
6. Infant leukemias and MLL translocation
The infants are quite marked among childhood leukemias due to their nearly singular association with one particular genetic mutation – translocations at the MLL gene in chromosome 11q23. Mutations in this gene occur in more than 85% of infant leukemias and close to 100% of prenatal and congenital leukemias. These leukemias can harbor lymphoid or myeloid and occasionally undefined lineage characteristics but share a common transcription profile [65]. MLL has been termed a “promiscuous” gene as its translocation to nearly 100 different partners has been associated with leukemia [66]. As noted above, MLL translocations in infants are typically prenatal events. One may ask whether MLL translocation is simply more common than other translocations in the fetus, resulting in its more frequent presence in infants. Several observations counter this, however. First, infant leukemias with MLL appear to have more mature immunoglobulin gene rearrangement pattern than the common childhood ALLs of older children, indicating that the leukemia cells actually were initiated later, possibly in the third trimester of pregnancy [67-69]. Second, MLL translocations are highly transforming and the human disease has an extremely short latency, indicating that secondary mutations in this leukemia subtype may not be the rate-limiting event for full disease, and the leukemia incidence rate may mirror the translocation frequency [70]. TEL-AML1 translocations, for instance, are present at a 1% frequency among neonates and the vast majority of translocation-positive cells do not progress to leukemia [22, 23], compared to 10-5 frequency for infant leukemia with MLL translocations.
MLL translocations are common in another set of leukemia patients - iatrogenic leukemias induced by cancer chemotherapy of other cancer sites. Interestingly, 11q23/MLL translocations were first reported in adult therapy-related leukemias only after the introduction of cancer chemotherapy drugs which target topoisomerase II, including the epidophyllotoxins, during the 1980s [71]. This observation resulted in a theory that MLL translocations in idiopathic leukemias may be a product of dietary, medicinal, or environmental chemicals which target topoisomerase II [72]. The intense research interest that followed described many possible mechanisms by which drugs and other conditions might enhance breakage of MLL gene. Specific topoisomerase II sequences in the MLL gene, chromosomal scaffold attachment sites, apoptotic nucleases, and unique structures derived from transcriptional torsional forces may contribute to MLL breakage [73-79]. The mechanism is still not fully clear and the exact chemical constituents that might contribute to the formation of MLL translocations in infants has not been fully elucidated. An intriguing report from Brazil and another international study implicated a type of insecticide and a therapeutic analgesic as potential agents [80, 81]. While the epidemiology of de novo (ie., not treatment-related) infant leukemias in the US has not yet found consistent culprits [82, 83], the strong epidemiologic associations with specific clinical therapies in iatrogenic leukemias continues to focus interest on topoisomerases in the causes of infant ALLs. Efforts at examination of MLL breakpoints at the molecular scale have identified a translocation hotspot in therapy-related leukemias [73, 74]; interestingly about half of infant breakpoints have the same molecular phenotype as therapy-related breakpoints suggesting that diverse mechanisms of formation (more than simply topoisomerase II inhibition) may play a role in infants [84-86].
MLL leukemias are not alone in harboring a chemical etiology. Certain myeloid subtypes are also suggestive for this. The AML1 gene is involved in many translocations and has also been linked to anti-topoisomerase II agents, similar to the MLL gene [87, 88]. Additional chemicals Classical clastogenic chemotherapy and environmental alkylating agents are associated with leukemias that have less precise breakpoints but commonly exhibit broken chromosomes and aneuploidy [89].
7. Leukemia clusters
Perhaps no other cancer type has captured both public and scientific attention in the same way that childhood leukemias have in the topic of cancer clusters. The most famous clusters include the Seascale cluster in the Lake District, UK; the Woburn cluster in Massachusetts, and the Fallon cluster in Nevada. Typically, chemico-physical “causes” are blamed with local culprits being ionizing radiation from a new nuclear plant (Seascale), trichloroethylene (Woburn) and tungsten alloy dust or petroleum fuels (Fallon). Only one of these factors approached a legal standard of proof (Woburn) at this writing, but scientific proof has been elusive. Studies of radiation exposure in the Seascale cluster were negative; however, it has been noted that the children who got leukemia were preferentially migrants to the area and experienced the most population mixing, providing credence to the population mixing hypothesis or infectious causes of this cluster [90, 91]. In Fallon, the unique exposure environment includes arsenic and 210Polonium in the water [92](in this issue), a tungsten/cobalt refinery [93], and a Navy flight and aircraft carrier base with its attendant exposures including jet fuel and other organics [94]. The leukemias from the Fallon cluster share a unique profile – nearly all cases were from children who lived near agriculture fields and open irrigation ditches forming an annulus around the town [58](in this issue). This indicates a possible scenario whereby an infection transmitted through a water-borne means might influence the disease. The US armed forces exhibited a similar peak incidence of leukemia at the same time suggesting transmission of an infectious agent throughout the armed services branches [58]. The timing was coincident with an adenovirus epidemic throughout the military [95, 96]; however, no concrete link between adenovirus and leukemia is currently available.
8. Adult Leukemias
Any discussion about the causes of childhood leukemias should consider adults, who represent about 90% of all leukemias diagnosed or about 40,000 individuals per year in the US. Adult leukemias are different diseases from their childhood counterparts. First of all, some subtypes exist in adults and not children and vice versa. The most common leukemia in adults is chronic lymphocytic leukemia (CLL), which is not seen in children [97]. Adults also display a wider range of leukemia subtypes with lower incidence of B and T-cell ALL but much higher chronic lymphocytic leukemia (CML), AML, and myelodysplastic and myleoproliferative syndromes [98]. These latter four subtypes are myeloid in nature, meaning that they are derived from the precursors to cells critical in innate immunity rather than adaptive immunity. These cells, particularly neutrophils and their precursors, produce large amounts of enzymes that can both produce cytotoxic mediators as part of their normal function (eg., myeloperoxidase), but also can activate environmental chemicals that reach the bone marrow, producing genotoxic intermediates [99]. A prime example of this phenomenon is exposure to benzene, which is metabolized in the liver to produce various phenols and quinone intermediates (which themselves are not leukemogenic when ingested). The zonal localization of Phase I and II enzymes in the liver facilitate the formation of these benzene metabolites, which enter the hepatic vein and general circulation; whereas phenol and hydroquinones themselves are detoxified by conjugation in the liver prior to entering general circulation [100]. Benzene metabolites are activated to nucleophilic compounds by myeloperoxidase in the bone marrow causing DNA damage and also may be subsequently reduced by quinone oxidoreductases [101, 102]. Benzene is a confirmed cause of both leukemia and lymphoma [103, 104] and has been linked to the formation of chromosome rearrangements typical of leukemia even in normal healthy, exposed persons as well as hematopoietic defects [105-107]. Other causes of leukemia in adults are chemicals associated with the rubber industry (butadiene, etc.), radiation, smoking, and chemotherapies for cancer [108].
A second difference between adult and childhood cancers is the molecular phenotype of some of the same disease classifications. The TEL-AML1 and hyperdiploid subtypes, which together account for half of childhood leukemias, are rare if not absent among adult ALLs, the latter of which are dominated by more complex chromosome aberrations as well as a different dominant repertoire of translocations including BCR-ABL1 (the “Philadelphia chromosome”) [109]. Unlike the childhood leukemias, there is some specific viral involvement of some types of adult leukemias. HTLV1 causes T-cell leukemia, rarely, in individuals infected in endemic areas [110].
9. Environmental causes: synthesis of the evidence
Like most cancers, leukemia has been linked to certain environmental chemicals and ionizing radiation. Chemicals with specific capacity to target the bone marrow via selective accumulation and metabolic activation are the prime candidates. The myeloid series harbors higher levels of enzymes with metabolic activation and are more consistently linked to chemical exposures such as cigarette smoking, occupational solvent exposures, and alkylator chemotherapies in therapy-related leukemias [111]. The 11q23 MLL translocation is linked to chemicals with topoisomerase-II inhibition activity and result in leukemias of both myeloid and lymphoid subtypes. Benzene is a confirmed cause of myeloid leukemias with some evidence of causing lymphoid hematologic neoplasms [112], and there is some epidemiologic evidence that chemicals may impact lymphoid subtypes of childhood leukemia as well [24, 113].
As leukemia is a neoplasm of the immune system, it is highly plausible that immune modulators will also modulate risk, particularly for the leukemias derived from adaptive immune branches including pre-B and pre-T leukemias. Infections, vaccinations, population mixing, and birth order are linked to lymphocytic leukemias, in particular the pre-B cell childhood ALLs. The discovery of causes of childhood leukemia clusters has been vexing to the research community, but evidence best implicates patterns of infections, possibly specific infections, spreading through naïve communities as an ultimate cause. Prior to an infection being the ultimate stimulus, a normal series of early infections appears to be protective for leukemia. Recent evidence from epidemiology and the laboratory suggests that newborns who grow up to get leukemia may be born with low IL-10 levels and a propensity to react to normal infections in a hyper-responsive manner during their first year of life, which may induce a progression of preleukemic clones via inflammatory processes related to infection.
10. Conclusion
Childhood leukemia like all cancers is currently being subjected to intensive analysis by the “new genetics,” meaning genome-wide association studies (GWAS), high dimension expression and copy number variation, mutation analysis, and array-based intensive DNA methylation analyses. The power of GWAS studies is clear but limited due to the modest effect sizes of “genome-wide significant” SNPs. The small effect sizes of the handful of GWAS genes discovered so far limit the potential for the genes to be useful in prevention or clinical modalities; however, the identities of such genes illuminate cellular pathways that may otherwise not be known to be involved in these diseases. Just as environmental epidemiology studies will become more powerful with precise disease classifications, GWAS studies will become more powerful when larger numbers of diseases in precise classifications are studied, such as those described by recurrent chromosomal abnormalities such as TEL-AML1, high hyperdiploidy, and PML-RARA. Indeed a TEL-AML1 GWAS has just been completed and shows larger effect sizes than the original, mixed phenotype GWAS studies [114]. Additional GWAS studies will require consortiums involving international pooling of samples to obtain required sample sizes.
Next generation sequencing analyses will certainly discover new genes and pathways in leukemia. The transformative potential of this technology is evident in the discovery of IDH1/2 and TET2 mutations in adult acute myeloid leukemia and brain cancer and the discovery of the critical nature of DNA methylation pathways in these two diseases [115, 116]. Mutations in these genes are not present in childhood AML or ALL, which, besides having several common translocations, mutations, and deletions, have not been fully vetted for genetic and epigenetic architecture. Coordinated analyses involving gene expression, gene copy number change, and epigenetic modifications such as the TARGET initiative will help to complete knowledge of the range of modifications in leukemia cells [117].
These high technology initiatives must ultimately be linked up to population-based epidemiologically-derived data on infections, exposures, diet, and other environmental factors for true clarity to be reached on the causes of childhood leukemias. Clearly these studies must be very large to support the investigation of infrequent genetically-described subtypes of leukemia. This is not possible for investigations of leukemia clusters which are finite in space and time, sometimes statistically remarkable like the Fallon leukemia cluster but in practical terms very small. The Fallon cluster is a situation which demands a fresh study design that incorporates investigation of individual samples to assess viral or chemical involvement. Traditional case-control surveys do not make sense for small clusters unless they focus on the overall community from which the leukemia cases are derived. Future studies would benefit from earlier identification of cancer clusters via active surveillance at cancer registries, the capture of complete residential histories for cancer patients, and prospective population-level archiving of biological materials to permit accurate environmental and viral testing.
Key Points.
We reviewed the epidemiology of the most common cancer in children -leukemia.
Mutational events in childhood leukemia can often be traced to a fetal origin.
Childhood leukemia is inversely associated with surrogate markers of infection.
Children born with an altered responder status to infections have a higher risk.
Myeloid and infant leukemias can originate from chemical exposures.
Acknowledgments
We thank the Environmental Protection Agency (UNR/Reno) for funding, along with the Children with Cancer Foundation (UK), the Leukemia and Lymphoma Society of America, and the National Institutes of Health R01CA155461.
Footnotes
Conflict of Interest Statement: None Declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinberg KK, Relling MV, Gallagher ML, Greene CN, Rubin CS, French D, Holmes AK, Carroll WL, Koontz DA, Sampson EJ, Satten GA. Genetic studies of a cluster of acute lymphoblastic leukemia cases in Churchill County, Nevada. Environ Health Perspect. 2007;115(1):158–164. doi: 10.1289/ehp.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ries LAG, Smith MA, J G, Linet M, Tamra T, Young JL, Bunin GRe. In: Cancer Incidence and Survival among Children and Adolescents: United States SEER Progran 1975-1995. National Cancer Institute, editor. Bethesda, MD: 1999. [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, Schwartz CL, Leisenring W, Robison LL. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 4.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992-2004) Cancer. 2008;112(2):416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 5.Kaatsch P, Mergenthaler A. Incidence, time trends and regional variation of childhood leukaemia in Germany and Europe. Radiat Prot Dosimetry. 2008;132(2):107–113. doi: 10.1093/rpd/ncn259. [DOI] [PubMed] [Google Scholar]
- 6.Gurney JG, Davis S, Severson RK, Fang JY, Ross JA, Robison LL. Trends in cancer incidence among children in the U.S. Cancer. 1996;78(3):532–541. doi: 10.1002/(SICI)1097-0142(19960801)78:3<532::AID-CNCR22>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Greaves MF, Alexander FE. An infectious etiology for common acute lymphoblastic leukemia in childhood? Leukemia. 1993;7(3):349–360. [PubMed] [Google Scholar]
- 8.Chow EJ, Puumala SE, Mueller BA, Carozza SE, Fox EE, Horel S, Johnson KJ, McLaughlin CC, Reynolds P, Von Behren J, Spector LG. Childhood cancer in relation to parental race and ethnicity: a 5-state pooled analysis. Cancer. 2010;116(12):3045–3053. doi: 10.1002/cncr.25099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker SL, Davis KJ, Wingo PA, Ries LA, Heath CW., Jr Cancer statistics by race and ethnicity. CA Cancer J Clin. 1998;48(1):31–48. doi: 10.3322/canjclin.48.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29(5):551–565. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 12.Wiemels J. Chromosomal translocations in childhood leukemia: natural history, mechanisms, and epidemiology. J Natl Cancer Inst Monogr. 2008;(39):87–90. doi: 10.1093/jncimonographs/lgn006. [DOI] [PubMed] [Google Scholar]
- 13.McHale CM, Wiemels JL, Zhang L, Ma X, Buffler PA, Feusner J, Matthay K, Dahl G, Smith MT. Prenatal origin of childhood acute myeloid leukemias harboring chromosomal rearrangements t(15;17) and inv(16) Blood. 2003;101(11):4640–4641. doi: 10.1182/blood-2003-01-0313. [DOI] [PubMed] [Google Scholar]
- 14.McHale CM, Wiemels JL, Zhang L, Ma X, Buffler PA, Guo W, Loh ML, Smith MT. Prenatal origin of TEL-AML1-positive acute lymphoblastic leukemia in children born in California. Genes Chromosomes Cancer. 2003;37(1):36–43. doi: 10.1002/gcc.10199. [DOI] [PubMed] [Google Scholar]
- 15.Wiemels JL, Cazzaniga G, Daniotti M, Eden OB, Addison GM, Masera G, Saha V, Biondi A, Greaves MF. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354(9189):1499–1503. doi: 10.1016/s0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- 16.Wiemels JL, Xiao Z, Buffler PA, Maia AT, Ma X, Dicks BM, Smith MT, Zhang L, Feusner J, Wiencke J, Pritchard-Jones K, Kempski H, Greaves M. In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia. Blood. 2002;99(10):3801–3805. doi: 10.1182/blood.v99.10.3801. [DOI] [PubMed] [Google Scholar]
- 17.Wiemels JL, Leonard BC, Wang Y, Segal MR, Hunger SP, Smith MT, Crouse V, Ma X, Buffler PA, Pine SR. Site-specific translocation and evidence of postnatal origin of the t(1;19) E2A-PBX1 fusion in childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2002;99(23):15101–15106. doi: 10.1073/pnas.222481199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiemels JL, Kang M, Chang JS, Zheng L, Kouyoumji C, Zhang L, Smith MT, Scelo G, Metayer C, Buffler P, Wiencke JK. Backtracking RAS mutations in high hyperdiploid childhood acute lymphoblastic leukemia. Blood Cells Mol Dis. 2010;45(3):186–191. doi: 10.1016/j.bcmd.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang P, Kang M, Xiao A, Chang J, Feusner J, Buffler P, Wiemels J. FLT3 mutation incidence and timing of origin in a population case series of pediatric leukemia. BMC Cancer. 2010;10:513. doi: 10.1186/1471-2407-10-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maia AT, Koechling J, Corbett R, Metzler M, Wiemels JL, Greaves M. Protracted postnatal natural histories in childhood leukemia. Genes Chromosomes Cancer. 2004;39(4):335–340. doi: 10.1002/gcc.20003. [DOI] [PubMed] [Google Scholar]
- 21.Gale KB, Ford AM, Repp R, Borkhardt A, Keller C, Eden OB, Greaves MF. Backtracking leukemia to birth: identification of clonotypic gene fusion sequences in neonatal blood spots. Proc Natl Acad Sci U S A. 1997;94(25):13950–13954. doi: 10.1073/pnas.94.25.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuna J, Madzo J, Krejci O, Zemanova Z, Kalinova M, Muzikova K, Zapotocky M, Starkova J, Hrusak O, Horak J, Trka J. ETV6/RUNX1 (TEL/AML1) is a frequent prenatal first hit in childhood leukemia. Blood. 2011;117(1):368–369. doi: 10.1182/blood-2010-09-309070. author reply 370-361. [DOI] [PubMed] [Google Scholar]
- 23.Mori H, Colman SM, Xiao Z, Ford AM, Healy LE, Donaldson C, Hows JM, Navarrete C, Greaves M. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci U S A. 2002;99(12):8242–8247. doi: 10.1073/pnas.112218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scelo G, Metayer C, Zhang L, Wiemels JL, Aldrich M, Selvin S, DuCore J, Smith MT, Buffler PA. Home use of paint and petroleum solvents before birth and early childhood and risk of childhood leukemia. submitted. 2008 doi: 10.1289/ehp.11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little MP. Leukaemia following childhood radiation exposure in the Japanese atomic bomb survivors and in medically exposed groups. Radiat Prot Dosimetry. 2008;132(2):156–165. doi: 10.1093/rpd/ncn264. [DOI] [PubMed] [Google Scholar]
- 26.Metayer C, Buffler PA. Residential exposures to pesticides and childhood leukaemia. Radiat Prot Dosimetry. 2008;132(2):212–219. doi: 10.1093/rpd/ncn266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang JS. Parental smoking and childhood leukemia. Methods Mol Biol. 2009;472:103–137. doi: 10.1007/978-1-60327-492-0_5. [DOI] [PubMed] [Google Scholar]
- 28.Trevino LR, Yang W, French D, Hunger SP, Carroll WL, Devidas M, Willman C, Neale G, Downing J, Raimondi SC, Pui CH, Evans WE, Relling MV. Germline genomic variants associated with childhood acute lymphoblastic leukemia. Nat Genet. 2009;41(9):1001–1005. doi: 10.1038/ng.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papaemmanuil E, Hosking FJ, Vijayakrishnan J, Price A, Olver B, Sheridan E, Kinsey SE, Lightfoot T, Roman E, Irving JA, Allan JM, Tomlinson IP, Taylor M, Greaves M, Houlston RS. Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat Genet. 2009;41(9):1006–1010. doi: 10.1038/ng.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherborne AL, Hosking FJ, Prasad RB, Kumar R, Koehler R, Vijayakrishnan J, Papaemmanuil E, Bartram CR, Stanulla M, Schrappe M, Gast A, Dobbins SE, Ma Y, Sheridan E, Taylor M, Kinsey SE, Lightfoot T, Roman E, Irving JA, Allan JM, Moorman AV, Harrison CJ, Tomlinson IP, Richards S, Zimmermann M, Szalai C, Semsei AF, Erdelyi DJ, Krajinovic M, Sinnett D, Healy J, Gonzalez Neira A, Kawamata N, Ogawa S, Koeffler HP, Hemminki K, Greaves M, Houlston RS. Variation in CDKN2A at 9p21.3 influences childhood acute lymphoblastic leukemia risk. Nat Genet. 2010;42(6):492–494. doi: 10.1038/ng.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad RB, Hosking FJ, Vijayakrishnan J, Papaemmanuil E, Koehler R, Greaves M, Sheridan E, Gast A, Kinsey SE, Lightfoot T, Roman E, Taylor M, Pritchard-Jones K, Stanulla M, Schrappe M, Bartram CR, Houlston RS, Kumar R, Hemminki K. Verification of the susceptibility loci on 7p12.2, 10q21.2, and 14q11.2 in precursor B-cell acute lymphoblastic leukemia of childhood. Blood. 2010;115(9):1765–1767. doi: 10.1182/blood-2009-09-241513. [DOI] [PubMed] [Google Scholar]
- 32.Vijayakrishnan J, Houlston RS. Candidate gene association studies and risk of childhood acute lymphoblastic leukemia: a systematic review and meta-analysis. Haematologica. 2010;95(8):1405–1414. doi: 10.3324/haematol.2010.022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorak MT, Lawson T, Machulla HK, Darke C, Mills KI, Burnett AK. Unravelling an HLA-DR association in childhood acute lymphoblastic leukemia. Blood. 1999;94(2):694–700. [PubMed] [Google Scholar]
- 34.Taylor GM, Dearden S, Payne N, Ayres M, Gokhale DA, Birch JM, Blair V, Stevens RF, Will AM, Eden OB. Evidence that an HLA-DQA1-DQB1 haplotype influences susceptibility to childhood common acute lymphoblastic leukaemia in boys provides further support for an infection-related aetiology. Br J Cancer. 1998;78(5):561–565. doi: 10.1038/bjc.1998.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor GM, Dearden S, Ravetto P, Ayres M, Watson P, Hussain A, Greaves M, Alexander F, Eden OB. Genetic susceptibility to childhood common acute lymphoblastic leukaemia is associated with polymorphic peptide-binding pocket profiles in HLA-DPB1*0201. Hum Mol Genet. 2002;11(14):1585–1597. doi: 10.1093/hmg/11.14.1585. [DOI] [PubMed] [Google Scholar]
- 36.Taylor GM, Hussain A, Verhage V, Thompson PD, Fergusson WD, Watkins G, Lightfoot T, Harrison CJ, Birch JM. Strong association of the HLA-DP6 supertype with childhood leukaemia is due to a single allele, DPB 1*0601. Leukemia. 2009;23(5):863–869. doi: 10.1038/leu.2008.374. [DOI] [PubMed] [Google Scholar]
- 37.Hosking FJ, Leslie S, Dilthey A, Moutsianas L, Wang Y, Dobbins SE, Papaemmanuil E, Sheridan E, Kinsey SE, Lightfoot T, Roman E, Irving JA, Allan JM, Taylor M, Greaves M, McVean G, Houlston RS. MHC variation and risk of childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2011;117(5):1633–1640. doi: 10.1182/blood-2010-08-301598. [DOI] [PubMed] [Google Scholar]
- 38.Kinlen L. Evidence for an infective cause of childhood leukaemia: comparison of a Scottish new town with nuclear reprocessing sites in Britain. Lancet. 1988;2(8624):1323–1327. doi: 10.1016/s0140-6736(88)90867-7. [DOI] [PubMed] [Google Scholar]
- 39.Kinlen LJ. Epidemiological evidence for an infective basis in childhood leukaemia [editorial] Br J Cancer. 1995;71(1):1–5. doi: 10.1038/bjc.1995.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer. 2006;6(3):193–203. doi: 10.1038/nrc1816. [DOI] [PubMed] [Google Scholar]
- 41.Greaves MF. Speculations on the cause of childhood acute lymphoblastic leukemia. Leukemia. 1988;2(2):120–125. [PubMed] [Google Scholar]
- 42.Urayama KY, Buffler PA, Gallagher ER, Ayoob JM, Ma X. A meta-analysis of the association between day-care attendance and childhood acute lymphoblastic leukaemia. Int J Epidemiol. 2010;39(3):718–732. doi: 10.1093/ije/dyp378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dockerty JD, Draper G, Vincent T, Rowan SD, Bunch KJ. Case-control study of parental age, parity and socioeconomic level in relation to childhood cancers. Int J Epidemiol. 2001;30(6):1428–1437. doi: 10.1093/ije/30.6.1428. [DOI] [PubMed] [Google Scholar]
- 44.Westergaard T, Andersen PK, Pedersen JB, Olsen JH, Frisch M, Sorensen HT, Wohlfahrt J, Melbye M. Birth characteristics, sibling patterns, and acute leukemia risk in childhood: a population-based cohort study. J Natl Cancer Inst. 1997;89(13):939–947. doi: 10.1093/jnci/89.13.939. [DOI] [PubMed] [Google Scholar]
- 45.Groves F, Auvinen A, Hakulinen T. Haemophilus influenzae type b vaccination and risk of childhood leukemia in a vaccine trial in finland. Ann Epidemiol. 2000;10(7):474. doi: 10.1016/s1047-2797(00)00110-1. [DOI] [PubMed] [Google Scholar]
- 46.Ma X, Does MB, Metayer C, Russo C, Wong A, Buffler PA. Vaccination history and risk of childhood leukaemia. Int J Epidemiol. 2005;34(5):1100–1109. doi: 10.1093/ije/dyi113. [DOI] [PubMed] [Google Scholar]
- 47.Strachan DP. Hay fever, hygiene, and household size. Bmj. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernsen RM, de Jongste JC, van der Wouden JC. Birth order and sibship size as independent risk factors for asthma, allergy, and eczema. Pediatr Allergy Immunol. 2003;14(6):464–469. doi: 10.1046/j.0905-6157.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 49.Lewis SA, Britton JR. Consistent effects of high socioeconomic status and low birth order, and the modifying effect of maternal smoking on the risk of allergic disease during childhood. Respir Med. 1998;92(10):1237–1244. doi: 10.1016/s0954-6111(98)90427-9. [DOI] [PubMed] [Google Scholar]
- 50.Westergaard T, Rostgaard K, Wohlfahrt J, Andersen PK, Aaby P, Melbye M. Sibship characteristics and risk of allergic rhinitis and asthma. Am J Epidemiol. 2005;162(2):125–132. doi: 10.1093/aje/kwi169. [DOI] [PubMed] [Google Scholar]
- 51.Haby MM, Marks GB, Peat JK, Leeder SR. Daycare attendance before the age of two protects against atopy in preschool age children. Pediatr Pulmonol. 2000;30(5):377–384. doi: 10.1002/1099-0496(200011)30:5<377::aid-ppul3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Kramer U, Heinrich J, Wjst M, Wichmann HE. Age of entry to day nursery and allergy in later childhood. Lancet. 1999;353(9151):450–454. doi: 10.1016/S0140-6736(98)06329-6. [DOI] [PubMed] [Google Scholar]
- 53.Linabery AM, Jurek AM, Duval S, Ross JA. The association between atopy and childhood/adolescent leukemia: a meta-analysis. Am J Epidemiol. 2010;171(7):749–764. doi: 10.1093/aje/kwq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuz J, Morgan G, Bohler E, Kaatsch P, Michaelis J. Atopic disease and childhood acute lymphoblastic leukemia. Int J Cancer. 2003;105(2):255–260. doi: 10.1002/ijc.11054. [DOI] [PubMed] [Google Scholar]
- 55.Chang JS, Tsai CR, Wiemels JL. Allergy and risk of childhood leukemia: a population-based and record based study. Amer J Epidem. 2011 doi: 10.1093/aje/kws263. in press. [DOI] [PubMed] [Google Scholar]
- 56.Spector L, Groves F, DeStefano F, Liff J, Klein M, Mullooly J, Black S, Shinefield H, Ward J, Marcy M. Medically recorded allergies and the risk of childhood acute lymphoblastic leukaemia. Eur J Cancer. 2004;40(4):579–584. doi: 10.1016/j.ejca.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 57.Kinlen L, Doll R. Population mixing and childhood leukaemia: Fallon and other US clusters. Br J Cancer. 2004;91(1):1–3. doi: 10.1038/sj.bjc.6601982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Francis SS, Selvin S, Yang W, Buffler PA, Wiemels JL. Unusual space-time patterning of the Fallon, Nevada leukemia cluster: Evidence of an infectious etiology. Chem Biol Interact. 2012 doi: 10.1016/j.cbi.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roman E, Simpson J, Ansell P, Kinsey S, Mitchell CD, McKinney PA, Birch JM, Greaves M, Eden T. Childhood acute lymphoblastic leukemia and infections in the first year of life: a report from the United Kingdom Childhood Cancer Study. Am J Epidemiol. 2007;165(5):496–504. doi: 10.1093/aje/kwk039. [DOI] [PubMed] [Google Scholar]
- 60.Cardwell CR, McKinney PA, Patterson CC, Murray LJ. Infections in early life and childhood leukaemia risk: a UK case-control study of general practitioner records. Br J Cancer. 2008;99(9):1529–1533. doi: 10.1038/sj.bjc.6604696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simpson J, Smith A, Ansell P, Roman E. Childhood leukaemia and infectious exposure: a report from the United Kingdom Childhood Cancer Study (UKCCS) Eur J Cancer. 2007;43(16):2396–2403. doi: 10.1016/j.ejca.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 62.Gilham C, Peto J, Simpson J, Roman E, Eden TO, Greaves MF, Alexander FE. rDay care in infancy and risk of childhood acute lymphoblastic leukaemia: findings from UK case-control study. Bmj. 2005;330(7503):1294. doi: 10.1136/bmj.38428.521042.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang JS, Zhou M, Buffler PA, Chokkalingam AP, Metayer C, Wiemels JL. Profound Deficit of IL10 at Birth in Children Who Develop Childhood Acute Lymphoblastic Leukemia. Cancer Epidemiol Biomarkers Prev. 2011 doi: 10.1158/1055-9965.EPI-11-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang G, Rowe J, Kusel M, Bosco A, McKenna K, de Klerk N, Sly PD, Holt PG. Interleukin-10/interleukin-5 responses at birth predict risk for respiratory infections in children with atopic family history. Am J Respir Crit Care Med. 2009;179(3):205–211. doi: 10.1164/rccm.200803-438OC. [DOI] [PubMed] [Google Scholar]
- 65.Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, Sallan SE, Lander ES, Golub TR, Korsmeyer SJ. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30(1):41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 66.Burmeister T, Meyer C, Schwartz S, Hofmann J, Molkentin M, Kowarz E, Schneider B, Raff T, Reinhardt R, Gokbuget N, Hoelzer D, Thiel E, Marschalek R. The MLL recombinome of adult CD10-negative B-cell precursor acute lymphoblastic leukemia: results from the GMALL study group. Blood. 2009;113(17):4011–4015. doi: 10.1182/blood-2008-10-183483. [DOI] [PubMed] [Google Scholar]
- 67.Fasching K, Panzer S, Haas OA, Borkhardt A, Marschalek R, Griesinger F, Panzer-Grumayer ER. Presence of N regions in the clonotypic DJ rearrangements of the immunoglobulin heavy-chain genes indicates an exquisitely short latency in t(4;11)-positive infant acute lymphoblastic leukemia. Blood. 2001;98(7):2272–2274. doi: 10.1182/blood.v98.7.2272. [DOI] [PubMed] [Google Scholar]
- 68.Steenbergen EJ, Verhagen OJ, van Leeuwen EF, Behrendt H, Merle PA, Wester MR, von dem Borne AE, van der Schoot CE. B precursor acute lymphoblastic leukemia third complementarity- determining regions predominantly represent an unbiased recombination repertoire: leukemic transformation frequently occurs in fetal life. Eur J Immunol. 1994;24(4):900–908. doi: 10.1002/eji.1830240418. [DOI] [PubMed] [Google Scholar]
- 69.Wasserman R, Galili N, Ito Y, Reichard BA, Shane S, Rovera G. Predominance of fetal type DJH joining in young children with B precursor lymphoblastic leukemia as evidence for an in utero transforming event. J Exp Med. 1992;176(6):1577–1581. doi: 10.1084/jem.176.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim-Rouille MH, MacGregor A, Wiedemann LM, Greaves MF, Navarrete C. MLL-AF4 gene fusions in normal newborns [letter; comment] Blood. 1999;93(3):1107–1108. [PubMed] [Google Scholar]
- 71.Larson RA, Le Beau MM, Ratain MJ, Rowley JD. Balanced translocations involving chromosome bands 11q23 and 21q22 in therapy-related leukemia [letter] Blood. 1992;79(7):1892–1893. [PubMed] [Google Scholar]
- 72.Ross JA, Potter JD, Robison LL. Infant leukemia, topoisomerase II inhibitors, and the MLL gene. J Natl Cancer Inst. 1994;86(22):1678–1680. doi: 10.1093/jnci/86.22.1678. [DOI] [PubMed] [Google Scholar]
- 73.Aplan PD, Chervinsky DS, Stanulla M, Burhans WC. Site-specific DNA cleavage within the MLL breakpoint cluster region induced by topoisomerase II inhibitors [see comments] Blood. 1996;87(7):2649–2658. [PubMed] [Google Scholar]
- 74.Broeker PL, Super HG, Thirman MJ, Pomykala H, Yonebayashi Y, Tanabe S, Zeleznik-Le N, Rowley JD. Distribution of 11q23 breakpoints within the MLL breakpoint cluster region in de novo acute leukemia and in treatment-related acute myeloid leukemia: correlation with scaffold attachment regions and topoisomerase II consensus binding sites. Blood. 1996;87(5):1912–1922. [PubMed] [Google Scholar]
- 75.Strissel PL, Strick R, Rowley JD, Zeleznik-Le NJ. An in vivo topoisomerase II cleavage site and a DNase I hypersensitive site colocalize near exon 9 in the MLL breakpoint cluster region. Blood. 1998;92(10):3793–3803. [PubMed] [Google Scholar]
- 76.Strick R, Strissel PL, Borgers S, Smith SL, Rowley JD. Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia. Proc Natl Acad Sci U S A. 2000 doi: 10.1073/pnas.070061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strissel PL, Strick R, Tomek RJ, Roe BA, Rowley JD, Zeleznik-Le NJ. DNA structural properties of AF9 are similar to MLL and could act as recombination hot spots resulting in MLL/AF9 translocations and leukemogenesis. Hum Mol Genet. 2000;9(11):1671–1679. doi: 10.1093/hmg/9.11.1671. [DOI] [PubMed] [Google Scholar]
- 78.Sim SP, Liu LF. Nucleolytic cleavage of the mixed lineage leukemia breakpoint cluster region during apoptosis. J Biol Chem. 2001;276(34):31590–31595. doi: 10.1074/jbc.M103962200. [DOI] [PubMed] [Google Scholar]
- 79.Vaughan AT, Betti CJ, Villalobos MJ. Surviving apoptosis. Apoptosis. 2002;7(2):173–177. doi: 10.1023/a:1014374717773. [DOI] [PubMed] [Google Scholar]
- 80.Alexander FE, Patheal SL, Biondi A, Brandalise S, Cabrera ME, Chan LC, Chen Z, Cimino G, Cordoba JC, Gu LJ, Hussein H, Ishii E, Kamel AM, Labra S, Magalhaes IQ, Mizutani S, Petridou E, de Oliveira MP, Yuen P, Wiemels JL, Greaves MF. Transplacental chemical exposure and risk of infant leukemia with MLL gene fusion. Cancer Res. 2001;61(6):2542–2546. [PubMed] [Google Scholar]
- 81.Pombo-de-Oliveira MS, Koifman S. Infant acute leukemia and maternal exposures during pregnancy. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2336–2341. doi: 10.1158/1055-9965.EPI-06-0031. [DOI] [PubMed] [Google Scholar]
- 82.Slater ME, Linabery AM, Spector LG, Johnson KJ, Hilden JM, Heerema NA, Robison LL, Ross JA. Maternal exposure to household chemicals and risk of infant leukemia: a report from the Children's Oncology Group. Cancer Causes Control. 2011;22(8):1197–1204. doi: 10.1007/s10552-011-9798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ognjanovic S, Blair C, Spector LG, Robison LL, Roesler M, Ross JA. Analgesic use during pregnancy and risk of infant leukaemia: a Children's Oncology Group study. Br J Cancer. 2011;104(3):532–536. doi: 10.1038/sj.bjc.6606046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jung R, Jacobs U, Krumbholz M, Langer T, Keller T, De Lorenzo P, Valsecchi MG, van der Velden VH, Moericke A, Stanulla M, Teigler-Schlegel A, Panzer-Gruemayer ER, van Dongen JJ, Schrappe M, den Boer ML, Pieters R, Rascher W, Metzler M. Bimodal distribution of genomic MLL breakpoints in infant acute lymphoblastic leukemia treatment. Leukemia. 2010;24(4):903–907. doi: 10.1038/leu.2010.14. [DOI] [PubMed] [Google Scholar]
- 85.Cimino G, Rapanotti MC, Biondi A, Elia L, Lo Coco F, Price C, Rossi V, Rivolta A, Canaani E, Croce CM, Mandelli F, Greaves M. Infant acute leukemias show the same biased distribution of ALL1 gene breaks as topoisomerase II related secondary acute leukemias. Cancer Res. 1997;57(14):2879–2883. [PubMed] [Google Scholar]
- 86.Felix CA, Hosler MR, Slater DJ, Parker RI, Masterson M, Whitlock JA, Rebbeck TR, Nowell PC, Lange BJ. MLL genomic breakpoint distribution within the breakpoint cluster region in de novo leukemia in children. J Pediatr Hematol Oncol. 1998;20(4):299–308. doi: 10.1097/00043426-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 87.Rowley JD, Olney HJ. International workshop on the relationship of prior therapy to balanced chromosome aberrations in therapy-related myelodysplastic syndromes and acute leukemia: Overview report. Genes Chromosomes Cancer. 2002;33(4):331–345. doi: 10.1002/gcc.10040. [DOI] [PubMed] [Google Scholar]
- 88.Roulston D, Espinosa R, 3rd, Nucifora G, Larson RA, Le Beau MM, Rowley JD. CBFA2(AML1) translocations with novel partner chromosomes in myeloid leukemias: association with prior therapy. Blood. 1998;92(8):2879–2885. [PubMed] [Google Scholar]
- 89.Larson rA, LeBeau MM, Vardiman JW, Rowley JD. Myeloid leukemia after hematotoxins. Environ Health Perspect. 1996;104(6):1303–1307. doi: 10.1289/ehp.961041303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dickinson HO, Parker L. Quantifying the effect of population mixing on childhood leukaemia risk: the Seascale cluster [see comments] Br J Cancer. 1999;81(1):144–151. doi: 10.1038/sj.bjc.6690664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Greaves MF. Aetiology of acute leukaemia. Lancet. 1997;349(9048):344–349. doi: 10.1016/s0140-6736(96)09412-3. [DOI] [PubMed] [Google Scholar]
- 92.Seiler R. Physical setting and natural sources of exposure to carcinogenic trace elements and radionuclides in Lahontan Valley, Nevada. Chem Biol Interact. 2011 doi: 10.1016/j.cbi.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Sheppard PR, Speakman RJ, Ridenour G, Witten ML. Temporal variability of tungsten and cobalt in Fallon, Nevada. Environ Health Perspect. 2007;115(5):715–719. doi: 10.1289/ehp.9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rubin CS, Holmes AK, Belson MG, Jones RL, Flanders WD, Kieszak SM, Osterloh J, Luber GE, Blount BC, Barr DB, Steinberg KK, Satten GA, McGeehin MA, Todd RL. Investigating childhood leukemia in Churchill County, Nevada. Environ Health Perspect. 2007;115(1):151–157. doi: 10.1289/ehp.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Metzgar D, Osuna M, Kajon AE, Hawksworth AW, Irvine M, Russell KL. Abrupt emergence of diverse species B adenoviruses at US military recruit training centers. J Infect Dis. 2007;196(10):1465–1473. doi: 10.1086/522970. [DOI] [PubMed] [Google Scholar]
- 96.Russell KL, Broderick MP, Franklin SE, Blyn LB, Freed NE, Moradi E, Ecker DJ, Kammerer PE, Osuna MA, Kajon AE, Morn CB, Ryan MA. Transmission dynamics and prospective environmental sampling of adenovirus in a military recruit setting. J Infect Dis. 2006;194(7):877–885. doi: 10.1086/507426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Washburn L. Chronic lymphocytic leukemia: the most common leukemia in adults. Jaapa. 2011;24(5):54–58. doi: 10.1097/01720610-201105000-00009. Quiz 59. [DOI] [PubMed] [Google Scholar]
- 98.Linet MS, Devesa SS, Morgan GJ. The Leukemias. In: Shottenfeld D, F JF, editors. Cancer Epidemiology and Prevention. Third. Oxford University Press; Oxford: 2006. [Google Scholar]
- 99.Lonkar P, Dedon PC. Reactive species and DNA damage in chronic inflammation: reconciling chemical mechanisms and biological fates. Int J Cancer. 2011;128(9):1999–2009. doi: 10.1002/ijc.25815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Medinsky MA, Kenyon EM, Schlosser PM. Benzene: a case study in parent chemicaland metabolite interactions. Toxicology. 1995;105(2-3):225–233. doi: 10.1016/0300-483x(95)03217-4. [DOI] [PubMed] [Google Scholar]
- 101.Smith MT. The mechanism of benzene-induced leukemia: a hypothesis and speculations on the causes of leukemia. Environ Health Perspect. 1996;104(6):1219–1225. doi: 10.1289/ehp.961041219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wiemels J, Wiencke JK, Varykoni A, Smith MT. Modulation of the toxicity and macromolecular binding of benzene metabolites by NAD(P)H:Quinone oxidoreductase in transfected HL-60 cells. Chemical Research in Toxicology. 1999;12(6):467–475. doi: 10.1021/tx9800811. [DOI] [PubMed] [Google Scholar]
- 103.Hayes RB, Yin S, Rothman N, Dosemeci M, Li G, Travis LT, Smith MT, Linet MS. Benzene and lymphohematopoietic malignancies in China. J Toxicol Environ Health A. 2000;61(5-6):419–432. doi: 10.1080/00984100050166442. [DOI] [PubMed] [Google Scholar]
- 104.Steinmaus C, Smith AH, Jones RM, Smith MT. Meta-analysis of benzene exposure and non-Hodgkin lymphoma: biases could mask an important association. Occup Environ Med. 2008;65(6):371–378. doi: 10.1136/oem.2007.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lan Q, Zhang L, Li G, Vermeulen R, Weinberg RS, Dosemeci M, Rappaport SM, Shen M, Alter BP, Wu Y, Kopp W, Waidyanatha S, Rabkin C, Guo W, Chanock S, Hayes RB, Linet M, Kim S, Yin S, Rothman N, Smith MT. Hematotoxicity in workers exposed to low levels of benzene. Science. 2004;306(5702):1774–1776. doi: 10.1126/science.1102443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang L, Lan Q, Guo W, Hubbard AE, Li G, Rappaport SM, McHale CM, Shen M, Ji Z, Vermeulen R, Yin S, Rothman N, Smith MT. Chromosome-wide aneuploidy study (CWAS) in workers exposed to an established leukemogen, benzene. Carcinogenesis. 2011;32(4):605–612. doi: 10.1093/carcin/bgq286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang L, Rothman N, Wang Y, Hayes RB, Li G, Dosemeci M, Yin S, Kolachana P, Titenko-Holland N, Smith MT. Increased aneusomy and long arm deletion of chromosomes 5 and 7 in the lymphocytes of Chinese workers exposed to benzene. Carcinogenesis. 1998;19(11):1955–1961. doi: 10.1093/carcin/19.11.1955. [DOI] [PubMed] [Google Scholar]
- 108.Bowen DT. Etiology of acute myeloid leukemia in the elderly. Semin Hematol. 2006;43(2):82–88. doi: 10.1053/j.seminhematol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 109.Liu XP, Zhu XF, Wang JX, Mi YC, Zou Y, Chen YM, Li CW, Dai Y, Qin S, Xiao JG, Xu FY, Gong JY, Wang SP, Yu CL, Fan J. [A comparative cytogenetic analysis in large scale between adult and childhood patients with acute lymphoblastic leukemia] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17(6):1399–1404. [PubMed] [Google Scholar]
- 110.Goncalves DU, Proietti FA, Ribas JG, Araujo MG, Pinheiro SR, Guedes AC, Carneiro-Proietti AB. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010;23(3):577–589. doi: 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107(9):2099–2107. doi: 10.1002/cncr.22233. [DOI] [PubMed] [Google Scholar]
- 112.Cogliano VJ, Baan R, Straif K. Updating IARC's carcinogenicity assessment of benzene. Am J Ind Med. 2011;54(2):165–167. doi: 10.1002/ajim.20916. [DOI] [PubMed] [Google Scholar]
- 113.Ma X, Buffler PA, Gunier RB, Dahl G, Smith MT, Reinier K, Reynolds P. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environ Health Perspect. 2002;110(9):955–960. doi: 10.1289/ehp.02110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ellinghaus E, Stanulla M, Richter G, Ellinghaus D, Te Kronnie G, Cario G, Cazzaniga G, Horstmann M, Panzer Grumayer R, Cave H, Trka J, Cinek O, Teigler-Schlegel A, Elsharawy A, Hasler R, Nebel A, Meissner B, Bartram T, Lescai F, Franceschi C, Giordan M, Nurnberg P, Heinzow B, Zimmermann M, Schreiber S, Schrappe M, Franke A. Identification of germline susceptibility loci in ETV6-RUNX1-rearranged childhood acute lymphoblastic leukemia. Leukemia. 2011 doi: 10.1038/leu.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abdel-Wahab O, Mullally A, Hedvat C, Garcia-Manero G, Patel J, Wadleigh M, Malinge S, Yao J, Kilpivaara O, Bhat R, Huberman K, Thomas S, Dolgalev I, Heguy A, Paietta E, Le Beau MM, Beran M, Tallman MS, Ebert BL, Kantarjian HM, Stone RM, Gilliland DG, Crispino JD, Levine RL. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood. 2009;114(1):144–147. doi: 10.1182/blood-2009-03-210039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Langemeijer SM, Aslanyan MG, Jansen JH. TET proteins in malignant hematopoiesis. Cell Cycle. 2009;8(24):4044–4048. doi: 10.4161/cc.8.24.10239. [DOI] [PubMed] [Google Scholar]
- 117.Zhang J, Mullighan CG, Harvey RC, Wu G, Chen X, Edmonson M, Buetow KH, Carroll WL, Chen IM, Devidas M, Gerhard DS, Loh ML, Reaman GH, Relling MV, Camitta BM, Bowman WP, Smith MA, Willman CL, Downing JR, Hunger SP. Key pathways are frequently mutated in high risk childhood acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2011;118(11):3080–7. doi: 10.1182/blood-2011-03-341412. [DOI] [PMC free article] [PubMed] [Google Scholar]