Abstract
BACKGROUND
The eastern equine encephalitis (EEE) and Venezuelan equine encephalitis (VEE) viruses are pathogens that infect humans and horses in the Americas. Outbreaks of neurologic disease in humans and horses were reported in Panama from May through early August 2010.
METHODS
We performed antibody assays and tests to detect viral RNA and isolate the viruses in serum samples from hospitalized patients. Additional cases were identified with enhanced surveillance.
RESULTS
A total of 19 patients were hospitalized for encephalitis. Among them, 7 had confirmed EEE, 3 had VEE, and 1 was infected with both viruses; 3 patients died, 1 of whom had confirmed VEE. The clinical findings for patients with EEE included brain lesions, seizures that evolved to status epilepticus, and neurologic sequelae. An additional 99 suspected or probable cases of alphavirus infection were detected during active surveillance. In total, 13 cases were confirmed as EEE, along with 11 cases of VEE and 1 case of dual infection. A total of 50 cases in horses were confirmed as EEE and 8 as VEE; mixed etiologic factors were associated with 11 cases in horses. Phylogenetic analyses of isolates from 2 cases of equine infection with the EEE virus and 1 case of human infection with the VEE virus indicated that the viruses were of enzootic lineages previously identified in Panama rather than new introductions.
CONCLUSIONS
Cases of EEE in humans in Latin America may be the result of ecologic changes that increased human contact with enzootic transmission cycles, genetic changes in EEE viral strains that resulted in increased human virulence, or an altered host range. (Funded by the National Institutes of Health and the Secretaría Nacional de Ciencia, Tecnología e Innovación, Panama.)
EASTERN EQUINE ENCEPHALITIS (EEE) and Venezuelan equine encephalitis (VEE) viruses, alphaviruses that are members of the Togaviridae family, are important causes of febrile illness and encephalitis in the Americas.1 The VEE virus occupies sylvatic, rodent–mosquito enzootic cycles that spill over to infect people; equine-adaptive or mosquito-adaptive mutations result in amplification, causing major epidemics.2 On average, only 5 to 6 cases of human infection with the EEE virus are reported each year in North America. More cases occur in equids and other domesticated animals by means of spillover from avian–mosquito swamp cycles, with case fatality rates of more than 50% in humans and equids.1 In Latin America, EEE is common in equids,3,4 but only 3 cases have been recognized in humans,5,6 despite extensive exposure to enzootic cycles and concerted surveillance during equine outbreaks.7 Laboratory studies suggest virulence differences between the North American and South American EEE viral strains, which are related to their ability to counteract interferon,8-10 as well as differences in cell tropism and translational efficiency.11
In Panama, the VEE virus was first associated with fatal human disease in 1960,12 and there have been additional cases since then.13,14 However, equine VEE has not been detected in Panama, despite widespread enzootic circulation,2 probably because of the avirulence of Panamanian strains in equids.15 Conversely, whereas the EEE virus has caused equine outbreaks in Panama since the 1930s, it has rarely been associated with human disease there,3,16 despite widespread mosquito isolations and human surveillance during outbreaks.2,3,17
In May 2010, human encephalitis was reported in Darién, a province in eastern Panama. Cases of infection in horses were reported from the same region and from the provinces of Panama and Colón through early August. We report the results of the outbreak investigation.
METHODS
CASES IN HUMANS
Human encephalitis was first reported on May 29 and May 31, 2010, in Darién. After the confirmation of two cases of alphavirus infection, the Panama Ministry of Health implemented active surveillance from June 4 to September 15, 2010, by alerting the public in two eastern provinces, Panama and Darién, and urging people with fever to go to medical centers, by visiting households with confirmed cases in humans or equids and obtaining samples from febrile household members and healthy contacts, and by requesting medical personnel at primary care centers and hospitals to notify the Ministry of Health and submit serum samples (and, when available, samples of cerebrospinal fluid [CSF] from patients meeting the criteria for suspected or probable cases). The criteria for suspected cases included a temperature of at least 38°C and headache in adults or children or irritability in children 1 year of age or younger. The criteria for probable cases included the criteria for suspected cases plus an altered state of consciousness, behavioral or neurologic signs (seizures, disorientation, drowsiness, lethargy, loss of hearing, or loss of consciousness), and residence in an area with confirmed alphaviral encephalitis in humans or equids.
SEROLOGIC TESTING
Serum samples were initially tested for antibodies against three neurotropic Panamanian alphaviruses — EEE virus, VEE virus, and western equine encephalitis (WEE) virus — with the use of hemagglutination-inhibition assays18 and for the West Nile, St. Louis encephalitis, and Ilheus flaviviruses. Serum and CSF samples were tested for EEE virus and VEE virus IgM antibodies with the use of an enzyme-linked immunosorbent assay, and infection was confirmed in a subset of these samples with the use of a plaque-reduction neutralization test (PRNT).18 The EEE viral strains used were 435731, a 1986 Panamanian horse isolate, or a chimeric Sindbis–EEE viral strain that includes structural proteins from the Brazilian EEE viral strain BeAn436087.19 The Panamanian VEE viral subtype ID strain 212908 was used for PRNT.
VIRUS ISOLATION
Human serum samples obtained during the acute stage of disease (duration of symptoms, ≤3 days), human CSF samples, or 10% horse brain homogenates were used to inoculate Vero cells and observed for cytopathic effects. RNA from clinical samples and isolates was extracted with the use of TRIzol (Invitrogen) or the QIAamp RNA viral extraction kit (Qiagen) and tested with the use of genus-specific reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assays.20
VIRAL GENOME SEQUENCING AND PHYLOGENETICS
PCR amplicons were sequenced with the use of the Applied Biosystems 3500 Series Genetic Analyzer. After searching the GenBank database with the use of Basic Local Alignment Search Tool (BLAST) software, we performed RT-PCR assays specific for the EEE virus and the VEE virus to amplify the structural polyprotein open reading frame (ORF). Alignment with homologous GenBank sequences was performed with the use of the MUSCLE algorithm.21 A maximum-likelihood phylogenetic tree was constructed with PAUP, version 4.0.22
RESULTS
INITIAL HUMAN CASES OF VIRAL ENCEPHALITIS
From May 29 to July 4, 2010, a total of 18 patients from Darién were hospitalized with encephalitis. Among these patients, there were 7 confirmed cases of EEE, 3 confirmed cases of VEE, and 1 confirmed case of coinfection with these viruses. Three patients died: 1 patient was positive for infection with the VEE virus and 1 patient was negative for alphavirus infection; diagnostic samples were not available for the third patient. No autopsies were performed.
Among the hospitalized patients with confirmed infection, all 11 had fever, 8 had vomiting, and 6 had seizures (Table 1). CSF analysis revealed pleocytosis in 4 patients (3 to 387 cells per milliliter) and elevated protein levels in 1 patient; the results of both tests were normal in 2 patients (Table 1). Three patients were discharged with sequelae ranging from seizures that responded to anticonvulsant treatment to left hemiparesis and psychomotor retardation. Five of the patients with the most severe signs and symptoms are described in the Supplementary Appendix, available with the full text of this article at NEJM.org. Among the 7 patients hospitalized with EEE (including 1 patient with dual EEE and VEE virus infection), 3 had sequelae at discharge: 1 each with recurrent seizures, psychomotor retardation, and a vegetative state (Table 1).
Table 1.
Clinical and Laboratory Findings in 11 Patients Hospitalized with Encephalitis in Panama, 2010.*
| Patient No. |
Location | Age | Sex | Date at Illness Onset |
Signs and Symptoms |
Cause | Days Hospitalized |
White-Cell Count | CSF | Sample Day and Type† |
IgM ELISA | PRNT Titer‡ | RT-PCR | Viral Culture |
Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein | Glucose | White Cells | |||||||||||||||
| yr | no. |
per mm2
(% neutrophils) |
mg/dl |
per mm2
(% monocytes) |
|||||||||||||
| 247134 | Rio Iglesias | 9 | Male | May 29 | Fever, headache, vomit ing, seizures, status epilepticus |
EEEV | 12 | 19,500 (96) | 274 | 69 | 146 (97) | 6, serum |
Positive for EEEV, negative for VEEV |
EEEV = 40; VEEV <20 |
ND | ND | Left hemiparesis |
| 12, serum |
Positive for EEEV, negative for VEEV |
ND | ND | ND | |||||||||||||
| 44, serum |
Negative for EEEV and VEEV |
ND | ND | ND | |||||||||||||
| 247135 | Meteti | 2 | Male | May 31 | Fever, seizures, status epilepticus |
EEEV | 22 | 19,100 (82) | 67 | 62 | 387 (72) | 6, serum |
Positive for EEEV, negative for VEEV |
EEEV = 80, VEEV <20 |
ND | ND | Right hemiparesis, psy chomotor retarda tion, impaired exec utive functioning |
| 23, serum |
Positive for EEEV, negative for VEEV |
ND | ND | ND | |||||||||||||
| 55, serum |
Negative for EEEV and VEEV |
ND | ND | ND | |||||||||||||
| 247220 | Rio Iglesias | 2 | Female | June 6 | Fever, headache, vomiting, somnolence or altered mental status |
EEEV | 8 | NA | NA | NA | NA | 4, serum |
Positive for EEEV, negative for VEEV |
ND | ND | ND | NA |
| 49, serum |
Negative for EEEV andVEEV | ND | ND | ND | |||||||||||||
| 247550 | Santa Rosa | 2 | Female | June 6 | Fever, vomiting, seizures, status epilepticus, som nolence or altered men tal status, dehydration |
EEEV | 21 | 9000 (75) | 30 | 57 | 30 (72) | 3, CSF |
Negative for EEEV and VEEV |
ND | Negative | Negative¶ | Seizures, psychomotor retardation, spastic quadriparesis |
| 3, serum |
Positive for EEEV, negative for VEEV |
ND | Negative | Negative | |||||||||||||
| 15, serum |
Positive for EEEV, negative for VEEV |
EEEV = 320, VEEV <20 |
ND | ND | |||||||||||||
| 44, serum§ |
Negative for EEEV and VEEV |
EEEV = 320, VEEV <20 |
ND | ND | |||||||||||||
| 247551 | Yaviza | 1 | Male | June 6 | Fever, vomiting, seizures, diarrhea, status epilep ticus, dehydration |
EEEV | 20 | 24,000 (11) | >300 | 141 | 0 | 6, serum |
Positive for EEEV, negative for VEEV |
ND | ND | ND | Seizures |
| 21, serum |
Positive for EEEV, negative for VEEV |
EEEV = 640, VEEV = 0 |
ND | ND | |||||||||||||
| 54, serum |
Positive for EEEV, negative for VEEV |
EEEV = 640, VEEV = 0 |
ND | ND | |||||||||||||
| 247419 | Yaviza | 13 | Male | June 15 | Fever, headache, vomiting, somnolence or altered mental status |
EEEV and VEEV |
5 | 4100 (80) | 19 | 56 | 3 (NA) | 1, serum |
Negative for EEEV and VEEV |
EEEV = 0, VEEV = 0 |
Negative | VEE virus |
None |
| 2, serum |
Negative for EEEV and VEEV |
EEEV = 40, VEEV = 0 |
Alphavirus | VEE virus |
|||||||||||||
| 40, serum |
Positive for EEEV, negative for VEEV |
EEEV = 40, VEEV = 80 |
ND | ND | |||||||||||||
| 247801 | Chepigana | 3 | Male | June 19 | Fever, headache, vomiting, somnolence or altered mental status |
VEEV | 5 | 8700 (88) | 21 | 80 | 2 (100) | 1, serum |
Negative for EEEV and VEEV |
ND | ND | ND | None |
| 12, serum |
Negative for EEEV, positive for VEEV |
ND | ND | ND | |||||||||||||
| 36, serum |
Negative for EEEV, positive for VEEV |
EEEV = 0, VEEV = 80 |
ND | ND | |||||||||||||
| 247747 | El Real | 10 | Male | June 22 | Fever, headache, vomiting, seizures, diarrhea |
EEEV | 3 | NA | NA | NA | NA | 1, serum |
ND | EEEV = 0, VEEV = 0 |
ND | ND | None |
| 6, serum |
Positive for EEEV, negative for VEEV |
ND | ND | ND | |||||||||||||
| 247752 | Meteti | 18 | Male | June 26 | Fever, headache, somno lence or altered mental status, cough, he- matemesis, shock |
VEEV | NA | ND | ND | ND | ND | 1, serum |
ND | ND | Alphavirus | VEEV | Death |
| 247921 | Santa Fe | 2 | Male | June 26 | Fever, seizures | EEEV | 8 | 17,800 (72) | 53 | 66 | 272 (75) | 4, serum |
Positive for EEEV, negative for VEEV |
EEEV = 20, VEEV = 0 |
ND | ND | None |
| 18, serum§ |
Positive for EEEV, negative for VEEV |
EEEV >640, VEEV=0 |
ND | ND | |||||||||||||
| 29, serum§ |
Negative for EEEV andVEEV |
ND | ND | ND | |||||||||||||
| 248036 | Yaviza | 4 | Male | June 29 | Fever, headache, vomiting, seizures |
VEEV | 3 | 12,400 (29) | ND | ND | ND | 1, serum§ |
Negative for EEEV andVEEV |
ND | ND | ND | Seizures |
| 7, serum§ |
Negative for EEEV andVEEV |
ND | ND | ND | |||||||||||||
| 26, serum§ |
Negative for EEEV, positive for VEEV |
EEEV = 0, VEEV = 80 |
ND | ND | |||||||||||||
CSF denotes cerebrospinal fluid, EEEV eastern equine encephalitis virus, ELISA enzyme-linked immunosorbent assay, NA not available, ND not done, PRNT plaque-reduction neutralization test, RT-PCR reverse-transcriptase–polymerase chain reaction, and VEEV Venezuelan equine encephalitis virus. To convert the values for glucose to millimoles per liter, multiply by 0.05551.
Numbers indicate the number of days from the onset of illness to sample collection.
The titer is the reciprocal of the end-point dilution yielding 80% plaque reduction.
Seroconversion from IgG-negative to IgG-positive.
In this patient there was a cytopathic effect due to a virus other than an alphavirus.
ADDITIONAL CASES DETECTED BY SURVEILLANCE
After the confirmation of alphaviral encephalitis in 2 hospitalized patients on May 29 and 31, 2010, active surveillance for encephalitis was implemented on June 4 and ended September 15. During this period, samples from 190 patients were collected, and 174 samples were assayed. Among the 190 patients, 99 met the criteria for suspected infection, 19 met the criteria for probable infection, and 59 did not meet the criteria for either suspected or probable infection; complete clinical data were missing for 13 patients (Fig. S1 in the Supplementary Appendix). A total of 25 patients had confirmed alphavirus infection (including 5 described in the Supplementary Appendix): 13 patients with EEE, 11 patients with VEE, and 1 patient with dual infection (Tables 1 and 2, and Fig. S1 in the Supplementary Appendix). Serum and CSF samples from 7 of these patients yielded VEE virus (Tables 1 and 2); EEE virus was not isolated.
Table 2.
Clinical Findings in 14 Cases of Nonspecific Alphaviral Illness in Humans in Panama, 2010.
| Patient No. |
Location | Age | Sex | Date of Onset |
Signs and Symptoms |
Days after Onset |
IgM ELISA | PRNT Titer* |
RT-PCR | Viral Detection in Culture† |
Cause |
|---|---|---|---|---|---|---|---|---|---|---|---|
| yr | no. | ||||||||||
| 247223 | Metetί | 35 | Female | June 2 | Fever, headache, arthralgia |
8 | Positive for EEEV, negative for VEEV |
ND | ND | ND | EEEV |
| 247320 | Chepigana | 38 | Female | June 8 | Fever, malaise | 2 | Negative for EEEV, positive for VEEV |
EEEV <20, VEEV = 640 |
ND | ND | VEEV |
| 247268 | Pinogana | 53 | Male | June 11 | Fever, headache | 1 | Negative for EEEV and VEEV |
ND | ND | VEEV | VEEV |
| 247852 | Santa Rosa | 39 | Female | June 16 | Fever, headache, malaise |
15 | Positive for EEEV, negative for VEEV |
EEEV = 80, VEEV = 0 |
ND | Negative | EEEV |
| 35 | Positive for EEEV, negative for VEEV |
EEEV = 320, VEEV = 0 |
|||||||||
| 247696 | Metetί | 43 | Male | June 17 | Fever, headache, seizures, retro-orbital pain |
6 | Positive for EEEV, negative for VEEV |
EEEV = 0, VEEV = 0‡ |
ND | ND | EEEV |
| 247528 | Yaviza | 27 | Female | June 17 | Fever, headache | 1 | Negative for EEEV and VEEV |
ND | Positive | VEEV | VEEV |
| 247507 | Metetί | 19 | Male | June 17 | Fever, headache, vomiting |
1 | Negative for EEEV and VEEV |
ND | Negative | VEEV | VEEV |
| 247509 | Rio Iglesia | 41 | Female | June 18 | Fever | 1 | Negative for EEEV and VEEV |
ND | Positive | VEEV | VEEV |
| 247566 | Rio Iglesia | 4 | Female | June 19 | Fever, headache | 1 | Positive for EEEV and VEEV |
EEEV = 40, VEEV = 0 |
Negative | ND | EEEV |
| 247768 | Chepigana | 73 | Male | June 23 | Fever, headache | 1 | Negative for EEEV, positive for VEEV |
EEEV = 0, VEEV = 320 |
ND | ND | VEEV |
| 247603 | El Real de Santa Maria |
8 | Female | June 23 | Fever, headache vomiting |
1 | Positive for EEEV, negative for VEEV |
EEEV = 40, VEEV = 0 |
ND | ND | EEEV |
| 247600 | Santa Fe | 80 | Male | June 24 | Fever, confusion, retro- orbital pain, abdominal pain, diarrhea |
7 | Negative for EEEV and VEEV |
EEEV = 0, VEEV = 40 |
ND | ND | VEEV |
| 247770 | Chepigana | 16 | Male | June 28 | Fever, confusion, malaise | 1 | Positive for EEEV, negative for VEEV |
ND | Negative | ND | EEEV |
| 248025 | Lajas Blancas | NA | Male | July 19 | Fever, headache, arthralgia |
3 | Negative for EEEV and VEEV |
ND | Negative | VEEV | VEEV |
The titer is the reciprocal of the end-point dilution yielding 80% plaque reduction.
Vero cell cultures were used to isolate the virus from serum.
The discrepancy between the results on the IgM ELISA assay and the PRNT titer in this case may be due to the formation of non-neutralizing antibodies, as described by Aguilar et al.7
Despite efforts to identify all patients who met the criteria for suspected or probable infection, the 190 patients from whom serum samples were obtained included 59 who did not meet these criteria. In this group, 1 patient had confirmed EEE (2%) and 2 patients had confirmed VEE (3%) (Fig. S1 in the Supplementary Appendix). The patient with EEE had headache and arthralgia but no fever, and the 2 patients with VEE were febrile but reported no headache and had no other neurologic signs or symptoms. Among the 99 cases of suspected infection, 5 (5%) were confirmed as EEE and 6 (6%) as VEE. Among the 19 probable cases, 7 (37%) were EEE, 3 (16%) were VEE, and 1 (5%) was dual infection (Tables 1 and 2, and Fig. S1 in the Supplementary Appendix).
Concurrently, among 75 healthy people living near persons with confirmed alphaviral encephalitis, 2 (3%) had positive test results for the EEE virus and 20 (27%) had positive results for the VEE virus. The low proportion of persons with confirmed EEE virus is consistent with the preponderance of asymptomatic infections in North America.1 Although most patients infected with the VEE virus are symptomatic, the infections are often misdiagnosed as dengue because of the characteristic absence of obvious neurologic disease, which is consistent with our findings.2 Incidence rates for confirmed cases of EEE within the townships reporting cases ranged from 0.1 to 0.7 per 1000 persons (Fig. 1 and 2), whereas incidence rates for confirmed cases of VEE ranged from 0.1 to 2.6 per 1000 persons (Fig. 1 and 2); the case fatality rate for confirmed EEE was 0% and that for confirmed VEE was 8%.
Figure 1. Incidence Rates of Eastern Equine Encephalitis (EEE) and Venezuelan Equine Encephalitis (VEE) Virus Infections in Panama in 2010.
Panel A shows a map of Panama, with the areas of interest outlined in red and enlarged in Panels B and C. Panel B shows the incidence rates of EEE on the basis of 14 confirmed cases (13 cases of EEE and 1 case of dual EEE–VEE virus infection). Panel C shows the incidence rates of VEE virus infection on the basis of 12 confirmed cases (11 cases of VEE virus infection and 1 case of dual infection). Incidence rates have been rounded to the nearest 10th and were calculated on the basis of the respective township population available from the 2010–2011 Panamanian census.
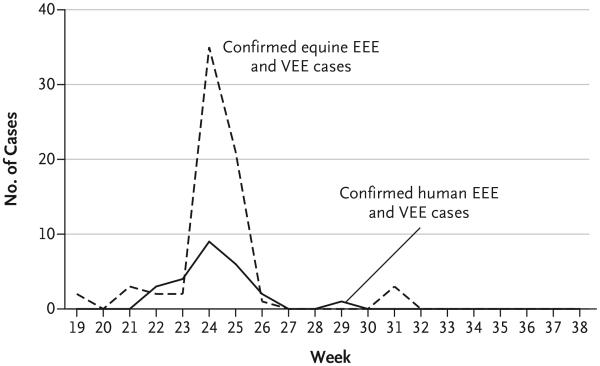
Figure 2. Epidemic Curve for Equine and Human Cases Compatible with Infection with the EEE or VEE Virus.
Confirmed cases in humans that were detected during the outbreak and during enhanced surveillance in the provinces of Darién and Panama included 14 cases of infection with the EEE virus (13 cases plus 1 case of dual EEE–VEE virus infection) and 12 cases of infection with the VEE virus (11 cases plus 1 case of dual EEE–VEE virus infection). Confirmed equine cases in the provinces of Panama, Darién, and Colón totaled 69, with neurologic and febrile disease that was consistent with infection with the EEE or VEE virus.
EQUINE CASES
The first case of equine encephalitis was reported on May 10 in Metetí, Darién Province. Shortly thereafter, the Ministry of Agriculture issued a national health alert. Between May 10 and August 8, a total of 161 horses with acute febrile illness or neurologic disease consistent with EEE or VEE were identified in the provinces of Darién (122 cases), Panama (36), and Colón (3) (Fig. 2). The initial cases were confirmed on May 31 as infections with the EEE virus. Mandatory reporting of equine neurologic disease (characterized by prostration, lethargy, incoordination, excessive chewing, and hanging of the head) was instituted. The Ministry of Agriculture also alerted horse owners of the outbreak, and ministry veterinarians vaccinated all equids in the three provinces with VEE viral strain TC-83, which was followed by administration of the inactivated VEE–WEE–EEE vaccine in June and July.
Blood samples were collected from febrile equids with or without neurologic signs, and brain tissue was harvested from dead horses. Among 155 horses tested (all symptomatic cases), 50 had confirmed EEE, 8 had VEE, and the findings for 11 were mixed. Case fatality rates for confirmed cases were 76% in Darién and 83% in Panama, with no fatal cases in Colón. Incidence rates for confirmed cases were 0.3 to 3.1 cases per 1000 horses for EEE virus, 0.1 to 0.3 per 1000 for VEE virus, and 0.2 to 0.3 per 1000 for dual infection. The most common signs were prostration, lethargy, incoordination, excessive chewing, and hanging of the head. Another 19 cases of neurologic equine disease occurred outside the main outbreak area, in the provinces of Coclé (11 cases) and Veraguas (8 cases); case fatality rates were 18% and 100%, respectively. Serum samples from 2 of 6 horses in Coclé had high hemagglutination-inhibition titers against West Nile virus, but no samples from horses in Veraguas were available for testing. The EEE virus was isolated from the brains of 2 horses and detected in both with the use of an RT-PCR assay.
CHARACTERIZATION OF VIRAL ISOLATES
Seven cultures of serum samples from 104 patients yielded cytopathic effects after inoculation with Vero cells, and all seven were positive for the VEE virus. Brain homogenates from 2 horses contained the EEE virus. Phylogenetic analyses placed the equine EEE viral isolates (247188 and 247168) within subtype III, along with Panamanian equine isolates from 1984 and 1986 (Fig. 3A). Within the structural poly-protein ORF, the 2010 EEE viral strains differed from each other by 19 nucleotides (0.2%); isolate 247188 differed from the 1984 and 1986 isolates by 28 and 36 nucleotides (0.2 and 0.3%), respectively, and isolate 247168 differed from the 1984 and 1986 isolates by 4 and 10 nucleotides (0.3 and 0.8%), respectively. None of these differences are known to affect virulence. A human VEE viral isolate (247268) occupied a subtype ID lineage associated with prior human disease in Panama (Fig. 3B).14 RT-PCR amplicons from other human VEE viral isolates were nearly identical in sequence. No mutations associated with equine amplification or adaptations to mosquito vectors were identified.23,24
Figure 3. Phylogenetic Trees of Alphaviruses Isolated during the EEE and VEE Outbreaks in Panama in 2010.
Shown are the results of a maximum-likelihood estimation of the phylogenetic trees for 41 strains of the EEE virus (Panel A) and 46 strains of the VEE virus (Panel B), based on the strains isolated in the Panama outbreaks (shown in red). Viral strains are listed according to subtype, country or state of collection, and year of collection. Bootstraps are shown on major branches and were determined in GARLI (Genetic Algorithm for Rapid Likelihood Inference) with 100 replicates. Scale bars show nucleotide sequence divergence.
DISCUSSION
Although equine cases of EEE have been documented throughout Latin America, reports of symptomatic human infections are limited to 2 cases in Trinidad6 and 1 case in Brazil,5 despite many fatal cases in North America.1 The reasons for this geographic difference in virulence or human infectivity remain unclear, but they may include intrinsic differences among EEE viral strains.7
In the period from May through July 2010, we documented an outbreak of encephalitis in eastern Panama, with 7 hospitalized patients who had laboratory-confirmed cases of EEE virus infection, 1 who had infection with both EEE and VEE viruses, and 1 patient with suspected EEE virus infection who died. Clinical findings and the results of brain imaging studies were indistinguishable in 2 cases from those described in North America.1,25 Incidence rates ranged from 0.1 to 0.7 cases per 1000 persons, and the case fatality rate, at 0%, was lower than that reported in North America (50 to 70%), although 1 of the 3 fatal cases of encephalitis in Panama, for which diagnostic samples were not available, may have been EEE.1 The age distribution of confirmed Panamanian cases — all of which occurred in children younger than 10 years of age — also differed from the North American cases, which are characterized by severe disease occurring in all age groups.
All 7 Panamanian patients with severe EEE underwent a prodromal phase, with fever, headache, vomiting and diarrhea, followed by progression to neurologic disease similar to that observed in severe North American cases.25-27 A notable difference was the high frequency of seizures that evolved to status epilepticus among the Panamanian patients (in 6 of the 7 patients [86%]), as compared with the frequency in North America (25%).25 Findings on brain imaging also differed from those in the North American cases25,26; the predominant basal ganglia and thalamic lesions seen in the North American cases were not evident in the Panamanian EEE cases, but other regions, such as the temporal lobes, were involved.
At the time of the EEE outbreak in Panama, VEE virus was also circulating in Darién Province and accounted for at least 2 cases of hospitalization for mild neurologic illness and 1 death. Although we confirmed both the EEE and VEE viruses as etiologic agents, it is possible that other viral agents (not alphaviruses) were cocirculating, as suggested by the isolation of unidentified viruses (with cytopathic effects on Vero cells) in 11 cases.
In addition to the cases in humans, 161 cases of neurologic equine disease were detected in the provinces of Darién, Panama, and Colón, including 50 confirmed cases of EEE. Panamanian VEE viral strains are not known to cause neurologic disease in equids,15 so other infections or even noninfectious causes of disease are possible. The clustering of cases of EEE and VEE in humans and horses probably reflects their similar enzootic habitats — in particular, their vectors in the culex (melanoconion) species.2,17 Although we did not isolate the EEE virus in the cases in humans and our genetic characterization was based on equine isolates, it is likely that these strains were nearly identical to those that infected humans because host-specific lineages of the EEE virus and other arboviruses that infect humans through enzootic spillover have never been identified.28
The reason for the appearance of human EEE concurrently with VEE in 2010 is unknown. The results of phylogenetic analyses suggest that the introduction of a new EEE viral strain is unlikely. Other possible explanations include detection of cases of EEE, through surveillance, that would previously have gone unnoticed; increased exposure of people to the EEE virus as a result of ecologic changes or enhanced enzootic circulation; and increased virulence or an altered host range of the Panamanian EEE virus. The identification of EEE in remote indigenous populations with limited health care suggests that surveillance of such populations for encephalitis is incomplete. However, neurologic VEE has been detected in these same populations in the past, without detection of EEE.14 Human immigration into the affected regions has resulted in considerable deforestation, which may increase contact with enzootic EEE virus or enhance secondary amplification.
The known overlap in the EEE virus and VEE virus mosquito vectors, and possibly in enzootic hosts, suggests that the coincidental appearance of cases of EEE and VEE in 2010 resulted from a common increase in the enzootic circulation of the two viruses. However, the VEE cases we detected may represent a normal endemic level of infection that is typically unrecognized because cases are misdiagnosed as dengue.2,14 This high level of endemic VEE, along with the regular occurrence of equine EEE epizootics in Panama since the 1930s,3 argues against major ecologic changes in the circulation of EEE virus in 2010. Furthermore, extensive human serologic surveillance in Panamanian regions affected by a 1973 EEE outbreak in equids revealed little evidence of human infection,3 a finding that is inconsistent with the concurrent human and equine infections identified in 2010. This difference between the 1973 and 2010 outbreaks suggests a fundamental change in human infectivity since 1973.
A recent change in the virulence or host range of the EEE virus in Panama is thus an alternative hypothesis to increased circulation. Alphaviruses, including the VEE virus23,24 and the chikungunya virus,29,30 can adapt to new vectors and hosts through mutations in genes encoding envelope glycoproteins. The determinants of virulence and the host range of the EEE virus are poorly understood,8 and studies with typical animal models have not proved useful, although cotton rats show promise as animal models for human virulence.28 Comparisons in these animals of the Panamanian EEE viral isolates from the 1980s and those from 2010 may be useful in the evaluation of hypothetically increased virulence.
Because the EEE virus is typically cleared from serum by the time neurologic disease appears, diagnosis is usually based on antibody detection. The superimposition of epidemic EEE on endemic VEE in Panama underscores the diagnostic challenge during an outbreak involving two sources of encephalitis. In this instance, the detection of IgM antibodies against the EEE or VEE virus without the use of assays to rule out one or the other could yield a misdiagnosis. Because the alphavirus-induced IgM antibody response typically lasts 2 to 3 months, the presence of these antibodies in serum during the acute phase of infection may reflect a prior infection if sero-conversion for the current infection has not yet occurred. Therefore, samples of serum and CSF obtained during the acute phase should also be evaluated by means of viral isolation, RT-PCR assay, or both, and serum samples obtained during the convalescent phase of disease should be tested to ensure that two infections have not occurred during this window of 2 to 3 months. During the 2010 outbreak, seroconversion for both the EEE virus and the VEE virus occurred in only one person. Postmortem examinations are also important to allow for isolation of the EEE virus from the brain, which is typically the only infected organ at the time of death.1
Supplementary Material
Acknowledgments
Supported by contracts from the National Institutes of Health (U54-AI057156, to Dr. Weaver; HHSN27220100004OI and HHSN27200004/DO4, to Dr. Tesh), and by a grant from Secretaría Nacional de Ciencia, Tecnología e Innovación, Panama (60-4-FID09-103, to Mr. Carrera).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Celedonio Castillo, Ruth Helleby, Danilo Franco, Brechla Moreno, Ilka Guerra, Melissa Gaitan, Jose Valenzuela, Maria Cano, Mariana Garcia, Marlene Castillo, Leyda Abrego, Yamitzel Zaldivar, Yaxelis Mendoza, and Anayansi Valderrama for technical assistance; Stanley Chen, Robert Seymour, and Nicole Arrigo for suggestions during the preparation of the manuscript; Bredio Velazco from the Ministry of Agriculture and Development of Panama for providing equine data; and Rosalba Salas from the Pan American Foot and Mouth Disease Center for technical assistance.
References
- 1.Smith DW, Mackenzie JS, Weaver SC. Alphaviruses. In: Richman DD, Whitley RJ, Hayden FG, editors. Clinical virology. ASM Press; Washington, DC: 2009. pp. 1241–74. [Google Scholar]
- 2.Aguilar PV, Estrada-Franco JG, Navarro-Lopez R, Ferro C, Haddow AD, Weaver SC. Endemic Venezuelan equine encephalitis in the Americas: hidden under the dengue umbrella. Future Virol. 2011;6:721–40. doi: 10.2217/FVL.11.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietz WH, Jr, Galindo P, Johnson KM. Eastern equine encephalomyelitis in Panama: the epidemiology of the 1973 epizootic. Am J Trop Med Hyg. 1980;29:133–40. doi: 10.4269/ajtmh.1980.29.133. [DOI] [PubMed] [Google Scholar]
- 4.Sabattini MS, Daffner JF, Monath TP, et al. Localized eastern equine encephalitis in Santiago del Estero Province, Argentina, without human infection. Medicina (B Aires) 1991;51:3–8. [PubMed] [Google Scholar]
- 5.Alice FJ. Infeccao humana pelo virus “leste” da encefalite equina. Bol Inst Biol da Bahia (Brazil) 1956;3:3–9. [Google Scholar]
- 6.Corniou B, Ardoin P, Bartholomew C, Ince W, Massiah V. First isolation of a South American strain of eastern equine virus from a case of encephalitis in Trinidad. Trop Geogr Med. 1972;24:162–7. [PubMed] [Google Scholar]
- 7.Aguilar PV, Robich RM, Turell MJ, et al. Endemic eastern equine encephalitis in the Amazon region of Peru. Am J Trop Med Hyg. 2007;76:293–8. [PubMed] [Google Scholar]
- 8.Aguilar PV, Paessler S, Carrara AS, et al. Variation in interferon sensitivity and induction among strains of eastern equine encephalitis virus. J Virol. 2005;79:11300–10. doi: 10.1128/JVI.79.17.11300-11310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguilar PV, Adams AP, Wang E, et al. Structural and nonstructural protein genome regions of eastern equine encephalitis virus are determinants of interferon sensitivity and murine virulence. J Virol. 2008;82:4920–30. doi: 10.1128/JVI.02514-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner CL, Yin J, Burke CW, Klimstra WB, Ryman KD. Type I interferon induction is correlated with attenuation of a South American eastern equine encephalitis virus strain in mice. Virology. 2009;390:338–47. doi: 10.1016/j.virol.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner CL, Burke CW, Tesfay MZ, Glass PJ, Klimstra WB, Ryman KD. Eastern and Venezuelan equine encephalitis viruses differ in their ability to infect dendritic cells and macrophages: impact of altered cell tropism on pathogenesis. J Virol. 2008;82:10634–46. doi: 10.1128/JVI.01323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson KM, Shelokov A, Peralta PH, Dammin GJ, Young NA. Recovery of Venezuelan equine encephalomyelitis virus in Panama: a fatal case in man. Am J Trop Med Hyg. 1968;17:432–40. doi: 10.4269/ajtmh.1968.17.432. [DOI] [PubMed] [Google Scholar]
- 13.Franck PT, Johnson KM. An outbreak of Venezuelan encephalitis in man in the Panama Canal Zone. Am J Trop Med Hyg. 1970;19:860–5. doi: 10.4269/ajtmh.1970.19.860. [DOI] [PubMed] [Google Scholar]
- 14.Quiroz E, Aguilar PV, Cisneros J, Tesh RB, Weaver SC. Venezuelan equine encephalitis in Panama: fatal endemic disease and genetic diversity of etiologic viral strains. PLoS Negl Trop Dis. 2009;3(6):e472. doi: 10.1371/journal.pntd.0000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walton TE, Alvarez O, Jr, Buckwalter RM, Johnson KM. Experimental infection of horses with enzootic and epizootic strains of Venezuelan equine encephalomyelitis virus. J Infect Dis. 1973;128:271–82. doi: 10.1093/infdis/128.3.271. [DOI] [PubMed] [Google Scholar]
- 16.Obaldia N, Dutary B, Clavel F, et al. Encefalomielitis equina del este, epizootic de 1986 en Panama. Notas Veterinarias. Assoc Panameña de Medicos Veterinarios. 1991;1:4–7. [Google Scholar]
- 17.Srihongse S, Galindo P. The isolation of eastern equine encephalitis virus from Culex (Melanoconion) taeniopus Dyar and Knab in Panama. Mosq News. 1967;27:74–6. [Google Scholar]
- 18.Beaty BJ, Calisher CH, Shope RE. Arbo-viruses. Diagnostic procedures for viral, rickettsial and chlamydial infections. In: Lennete ET, Lennete DA, editors. 7th American Public Health Association; Washington, DC: 1995. pp. 189–212. [Google Scholar]
- 19.Johnson BW, Kosoy O, Wang E, et al. Use of sindbis/eastern equine encephalitis chimeric viruses in plaque reduction neutralization tests for arboviral disease diagnostics. Clin Vaccine Immunol. 2011;18:1486–91. doi: 10.1128/CVI.05129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez-Seco MP, Rosario D, Quiroz E, Guzmán G, Tenorio A. A generic nested-RT-PCR followed by sequencing for detection and identification of members of the alphavirus genus. J Virol Methods. 2001;95:153–61. doi: 10.1016/s0166-0934(01)00306-8. [DOI] [PubMed] [Google Scholar]
- 21.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swofford DL. Sinauer Associates; Sunderland, MA: 1998. PAUP*: Phylogenetic Analysis Using Parsimony (and Other Methods) Version 4. [Google Scholar]
- 23.Anishchenko M, Bowen RA, Paessler S, Austgen L, Greene IP, Weaver SC. Venezuelan encephalitis emergence mediated by a phylogenetically predicted viral mutation. Proc Natl Acad Sci U S A. 2006;103:4994–9. doi: 10.1073/pnas.0509961103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brault AC, Powers AM, Ortiz D, Estrada-Franco JG, Navarro-Lopez R, Weaver SC. Venezuelan equine encephalitis emergence: enhanced vector infection from a single amino acid substitution in the envelope glycoprotein. Proc Natl Acad Sci U S A. 2004;101:11344–9. doi: 10.1073/pnas.0402905101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med. 1997;336:1867–74. doi: 10.1056/NEJM199706263362604. [DOI] [PubMed] [Google Scholar]
- 26.Lury KM, Castillo M. Eastern equine encephalitis: CT and MRI findings in one case. Emerg Radiol. 2004;11:46–8. doi: 10.1007/s10140-004-0350-7. [DOI] [PubMed] [Google Scholar]
- 27.Piliero PJ, Brody J, Zamani A, Deresiewicz RL. Eastern equine encephalitis presenting as focal neuroradiographic abnormalities: case report and review. Clin Infect Dis. 1994;18:985–8. doi: 10.1093/clinids/18.6.985. [DOI] [PubMed] [Google Scholar]
- 28.Arrigo NC, Adams AP, Watts DM, Newman PC, Weaver SC. Cotton rats and house sparrows as hosts for North and South American strains of eastern equine encephalitis virus. Emerg Infect Dis. 2010;16:1373–80. doi: 10.3201/eid1609.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsetsarkin KA, Weaver SC. Sequential adaptive mutations enhance efficient vector switching by chikungunya virus and its epidemic emergence. PLoS Pathog. 2011;7(12):e1002412. doi: 10.1371/journal.ppat.1002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.