Abstract
Objective
The Child Behavior Checklist–Dysregulation Profile (CBCL/DP) identifies youth at increased risk for significant psychopathology. Although the genetic architecture and several biological correlates of the CBCL/DP have been described, little work has elucidated its underlying neurobiology. We examined the potential utility of electroencephalography (EEG), along with behavioral and cognitive assessments, in differentiating individuals based on the CBCL/DP.
Method
Participants aged 7–14 years were categorized into three age- and sex-matched groups based on clinical assessment and CBCL/DP: typically developing non-attention-deficit/hyperactivity disorder (ADHD) controls (n=38), ADHD without the CBCL/DP (ADHD/DP−)(n=38), and individuals with the CBCL/DP (CBCL/DP+) (n=38). Groups were compared with EEG and measures of clinical phenomenology and cognition.
Results
ADHD/DP− and CBCL/DP+ groups had increased inattention, but the CBCL/DP+ group had increased hyperactive/impulsive symptoms, disruptive behavior, mood, and anxiety comorbidities compared with ADHD alone. Cognitive profiles suggested that ADHD/DP− participants had fast impulsive responses, while CBCL/DP+ participants were slow and inattentive. On EEG, CBCL/DP+ had a distinct profile of attenuated delta and elevated alpha band spectral power in central and parietal regions compared to ADHD/DP− and controls. The low delta/high alpha profile was correlated with measures of emotion and behavior problems and not with inattentive symptomatology or cognitive measures. There were no EEG differences between ADHD/DP− and control groups.
Conclusions
An EEG/cognitive profile suggests a distinct pattern of underlying neural dysfunction with the CBCL/DP that might ultimately serve as a biosignature. Further work is required to identify potential relationships with clinically defined psychiatric disorders, particularly those of dysregulated mood.
Keywords: attention-deficit/hyperactivity disorder (ADHD), biological markers, brain imaging techniques, cognitive neuroscience, mood dysregulation
INTRODUCTION
There is ongoing interest in the Child Behavior Checklist–Dysregulation Profile (CBCL/DP) as a measure of pediatric psychopathology.1–3 The CBCL/DP is defined categorically in youth by clinical elevations on each of the standard CBCL Attention Problems, Aggression, and Anxious/Depressed subscales, or dimensionally as the sum of raw scores on the same.4–6 The CBCL/DP has become widely regarded as a measure of emotional and behavioral dysregulation,3, 6–10 with possible prognostic significance within heterogeneous groups of children with emotional and disruptive behavior disorders. The profile has been described as highly heritable,11 and suggestive genetic associations support its potential validity as a distinct phenotype. Little research, however, has examined biological correlates of the CBCL/DP that might aid in its interpretation and further improve its predictive validity. This study attempts to identify associations of the profile with measures of cortical activation using electroencephalography (EEG), as well as assessments of behavior and cognition.
The CBCL/DP has demonstrated predictive value in phenomenological and longitudinal studies. Youth with the CBCL/DP show increased risk for anxiety,12 mood disorders,12, 13 disruptive behaviors,13 substance abuse,9, 12 personality disorders,12 suicidality,8, 9, 12 psychiatric hospitalizations,13 overall impairment,8, 9, 14 and increased levels of psychosocial adversity.14 In a family genetics study of attention-deficit/hyperactivity disorder (ADHD)–affected sibling pairs, individuals with the CBCL/DP had increased rates of lifetime anxiety and disruptive behavior disorders, as well as parental histories of substance abuse.5 In preschoolers, the CBCL/DP was associated with significant behavioral and emotional dysregulation and maladaptive parenting.10 The CBCL/DP predicts high novelty seeking, high harm avoidance, low reward dependence, and low persistence—traits associated with adult disorders of self-regulation such as those seen in cluster B personalities.6 One longitudinal study utilizing the CBCL/DP revealed that identified youth demonstrated increased rates of anxiety and disruptive behavior disorders as adults.7
Numerous studies suggest that the increased risks associated with the CBCL/DP are strongly mediated by genetic factors. Individuals with the CBCL/DP have increased likelihoods of having other siblings with similarly elevated scores.13 The genetic architecture of the CBCL/DP has been described and reveals additive genetic effects with heritability estimates ranging from 59%–68%.11 Candidate gene studies reveal possible associations with the dopamine transporter (SLC6A3) and brain-derived neurotrophic factor (BDNF).15 Genome-wide linkage studies suggest potential loci (LOD scores > 2.5) on chromosomes 2q23,5 and 1p21.1, 6p21.3, and 8q21.13.16 One genome-wide association study found suggested evidence of a role for the CBCL/DP and genes implicated with hippocampal-dependent memory and learning.15
Despite some progress in describing the characteristics, family patterns, and heritability of the CBCL/DP, little is known about its possible neurobiological underpinnings. One small study using a tryptophan depletion paradigm suggested that individuals with higher scores on the CBCL/DP have alterations in serotonin functioning compared to those with lower scores, and that this is primarily mediated by the Aggression subscale.17 A second preliminary report suggested that higher CBCL/DP scores are associated with increased basal levels of thyroid-stimulating hormone,18 although another investigation found no relationship between the CBCL/DP and thyroid functioning.17 A recent pilot investigation in 37 individuals using proton magnetic resonance spectroscopy revealed a significant correlation between glutamate concentrations in the anterior cingulate cortex (ACC) and CBCL/DP scores in the high CBCL/DP group, suggesting that impaired glutamatergic functioning in the ACC might underlie aspects of emotional dysregulation.19
EEG is a well-established noninvasive method of brain imaging that measures activity in functional neural systems and has been proposed as a potential biomarker for several disorders associated with impaired cognition.20 EEG profiles are highly heritable and have proven successful as endophenotypes for genetic investigations of several psychiatric disorders.21 A large literature on EEG differences between individuals with and without ADHD describes a predominant finding of increased frontocentral theta band activity, which is thought to arise in part from the anterior cingulate.22, 23 A meta-analysis of nine studies with a collective sample of 1,498 participants found an average excess of 32% theta band power for children with ADHD relative to controls.23 EEG spectral power differences in other frequency bands, such as elevated alpha and attenuated beta band power, have been reported, although considerable variability in findings can be found throughout the literature. This variability has been attributed to sample characteristics such as age, sex, and ADHD subtype; however, emotional dysregulation has not been systematically studied.
EEG findings in the mood literature have focused on regional differences in alpha band power. Previous studies have found higher alpha band power synchrony, and connectivity among individuals with major depression24–26 and emotion dysregulation27 when compared with controls. Additionally, studies have found increased central and parietal alpha band power in depression28, 29 and melancholic temperament30 suggesting that alpha band power might reflect affective dysregulation arising from the thalamocortical circuit.
In this current investigation, we were interested in determining whether the CBCL/DP might be associated with unique EEG, behavioral, and/or cognitive profiles that reveal additional information on brain functioning in identified individuals. Because the vast majority of children with the CBCL/DP have ADHD, it is of interest to determine whether distinct EEG profiles will be associated with ADHD, prominent mood features, or both. Specifically, we hypothesize that there will be a dissociation between groups such that the ADHD group lacking the CBCL/DP will be associated higher theta and lower beta band power, as previously shown, 31 while the group positive for the CBCL/DP will demonstrate higher alpha band power, as has been long demonstrated with depression and anxiety.
METHOD
Participants
The study includes male and female participants ranging from 7–14 years of age recruited from 2 concurrently running research studies of ADHD and related disorders that used identical diagnostic, cognitive, and EEG procedures. The first study was an intervention project on cognitive control that assessed individuals with both internalizing and externalizing disorders, as well as typically developing controls without ADHD. The second was a family genetics study that enrolled families with affected sibling pairs with ADHD. From this latter study, we used 1 child per family to maintain the independence of each participant. Using baseline data available on this total set of combined participants, we created 3 study groups matched for age and sex—a typically developing non-ADHD control sample without the CBCL/DP profile (n=38), a sample with ADHD also lacking the DP profile (ADHD/DP-) (n=38), and a sample of youth with the DP profile (CBCL/DP+) (n=38). Typically developing non-ADHD controls were excluded for lifetime histories of any Axis I psychiatric disorder except oppositional defiant disorder (ODD) or simple phobia. Exclusion criteria for all groups included an estimated full scale intelligence <80 and lifetime histories of seizure disorder, head injury with loss of consciousness, psychosis, autism spectrum disorders, narrow phenotype bipolar disorder, or any current (past 6 months) history of major depression or panic disorder. All families were English speaking. Mean socioeconomic status as defined by Hollingshead was 2.4 (SD 0.9) (Hollingshead range I–V).32 All parent and participants received thorough verbal and written explanations of study requirements and provided written permission/assent prior to initiation of any study procedures as approved by the University of California–Los Angeles Institutional Review Board.
Diagnostic Assessments
Participants underwent extensive clinical assessment including diagnostic interviews, questionnaire administration, and EEG. Presence or absence of ADHD and other DSM-IV disorders was based on the Schedule for Affective Disorders and Schizophrenia for School-age Children (KSADS-PL),33 administered to the parent (usually mother) and child (if 8 years of age or older) by clinical psychologists or other highly trained interviewers following procedures that have previously been described in detail.5, 34, 35 Teacher and parent reports on ADHD symptom rating scales were used to supplement data obtained from clinical interviews in arriving at final diagnoses. “Best estimate” final diagnoses were based on review of all available data using standard approaches, 36 and were supervised by senior research clinicians (J.J.M., J.P., J.T.M.). Inter-rater reliabilities were computed with a mean weighted kappa of 0.95 (SD=.03) across all diagnoses occurring in more than 5% in the overall sample.
Parent informants completed the Child Behavior Checklist for each participant.1 The CBCL/DP was defined categorically in individuals with elevated T scores ≥ 70 on each of the Attention Problems, Aggression, and Anxious/Depressed subscales, following previous work.2, 4, 5, 37 All participants meeting or exceeding this threshold were designated as CBCL/DP+. ADHD participants below this threshold were designated as ADHD/DP−.
Cognitive Tests
General cognitive function was assessed using the Wechsler scales (the Wechsler Intelligence Scale for Children [WISC], the Wechsler Abbreviated Scale of Intelligence [WASI]).38, 39 Vocabulary, Matrix Reasoning, Digit Span, and Coding subtests were administered—the first 2 subtests to generate an estimate of Full Scale intelligence (IQ) and the latter 2 to measure cognitive processes reportedly deficient in ADHD. During EEG recording, a computerized version of the Go/No-go Task was administered to assess response inhibition (based on omission errors to measure inattention and commission errors to measure impulsivity) and reaction time (an index of processing speed).40
Electroencephalography
EEG recording was conducted using 40 Ag/AgCL surface electrodes that were embedded in an electrode cap utilizing an extended international 10/20–location system (ElectroCap, Eaton, OH) and referenced to linked ears. Impedance was set below 10 kOhms and EEG signal was recorded with MANSCAN (Sam Technology, San Francisco, CA) hardware and software. EEG data were assessed at a rate of 256 samples per second. Eye movements were tracked with electrodes placed on the outer canthus of each eye for horizontal movements and above the eye for vertical movements. Continuous EEG data were recorded while subjects performed the 14-minute Go/No-go task.
An experienced technician reviewed continuous EEG data offline, and all segments containing eye, head movement, or muscle artifact were removed prior to further analysis. EEG spectral power (µV2) data were obtained using a Fast Fourier Transform, exported, and averaged into the following bandwidths: Delta (1–3 Hz), Theta (4–7 Hz), Alpha (8–12 Hz), and Beta (13–21 Hz). Electrodes within the same region were averaged together to reduce the number of comparisons; these formed regional estimates for frontal (F3, F4, Fz), central (C3, C4, Cz), and parietal (P3, P4, Pz) areas. The primary dependent variables were spectral power estimates in each of the frequency bandwidths (i.e. delta, theta, alpha, and beta) in frontal, central, and parietal regions. All data review, transformation, and analyses were conducted blind to ADHD and CBCL status.
Statistical Analysis
Statistical tests were run in SPSS 17.0. Age was used as a covariate in all analyses. Separate univariate analyses of variance (ANOVAs) were conducted using group as the independent variable and EEG spectral power in each frequency band within each region as dependent variables. Post-hoc comparisons (Tukey’s honestly significant difference test [HSD]) were used when the univariate ANOVA was significant (p < .05) to determine pairwise differences between groups. To test the relationship between EEG measures and behavioral and cognitive measures, a partial Pearson product moment correlation, controlling for age was used. To reduce the number of comparisons, only behavioral, cognitive, and EEG measures that significantly differentiated between groups were used in the correlation analysis.
RESULTS
Participant characteristics are summarized in Table 1. There were no differences in age or percentage of males in any of the 3 groups. Both the ADHD/DP− and CBCL/DP+ groups had similar mean numbers of inattentive symptoms, but the CBCL/DP+ group had increased rates of hyperactive/impulsive symptoms, comorbid disruptive behavior, mood, and anxiety disorders. The CBCL/DP+ group had significantly higher scores on other CBCL subscales compared to ADHD/DP- group, while both of these had significantly higher scores than controls (all differences p < .05).
Table 1.
Participant Characteristics (Total N = 114)
| Control | ADHD/DP− | CBCL/DP+ | F or χ2 | p | |
|---|---|---|---|---|---|
| n=38 | n=38 | n=38 | |||
| Age (SD) in years, mean (SD) | 10.4 (2.5) | 10.6 (3.0) | 10.8 (3.1) | <1 | NS |
| Sex, % male | 54 | 67 | 60 | 1.28 | NS |
| Number ADHD Symptoms, mean (SD) | |||||
| Inattentive | 0.1 (0.4)a | 7.7 (1.5)b | 8.0 (1.9)b | 398.3 | <.001 |
| Hyperactive/Impulsive | 0.2 (0.9)a | 3.3 (2.5)b | 5.5 (2.3)c | 45.5 | <.001 |
| Psychiatric Comorbidities, % | |||||
| ADHD | 0a | 100b | 90b | 99.6 | <.001 |
| ODD/Conduct Disorder | 0a | 15b | 76c | 57.5 | <.001 |
| Mood Disorders | 0a | 0a | 22b | 17.9 | <.001 |
| Anxiety Disorders | 0a | 21b | 61c | 36.6 | <.001 |
| CBCL Subscale Scores, mean (SD) | |||||
| Withdrawn | 53 (4.0)a | 56 (7.3)b | 69 (10.3)c | 50.8 | <.001 |
| Somatic Complaints | 54 (4.0)a | 56 (6.7)b | 63 (9.8)b | 18.9 | <.001 |
| Thought Problems | 52 (2.7)a | 56 (6.6)b | 72 (8.5)c | 111.9 | <.001 |
| Delinquent Behavior | 52 (2.8)a | 55 (6.6)b | 68 (8.0)c | 80.3 | <.001 |
Note: ADHD = attention-deficit/hyperactivity disorder; ADHD/DP− = ADHD without the Child Behavior Checklist–Dysregulation Profile (CBCL/DP); CBCL = Child Behavior Checklist; CBCL/DP+ = with the CBCL/DP (CBCL/DP+); NS = nonsignificant; ODD = oppositional defiant disorder.
Numbers with different superscripts within a row are significantly different from each other p<.05.
Cognitive Functions
Scores on cognitive tests for each group are presented in Table 2. Control participants had significantly higher scores on Full Scale IQ and Digit Span compared with ADHD/DP− and CBCL/DP+ groups, however mean scores for all groups were in the average range. Several group effects emerged on the Go/No-go. A main effect on omission errors suggested that controls exhibited better-sustained attention than ADHD/DP− and CBCL/DP+ groups. Control and CBCL/DP+ groups made fewer commission errors than the ADHD/DP− group, suggesting that the ADHD/DP− group is more impulsive than the other 2. Finally, the CBCL/DP+ group had a significantly slower reaction time compared to ADHD/DP−, whereas controls did not differ from either.
Table 2.
Cognitive Functioning by Group
| Control | ADHD/DP− | CBCL/DP+ | F | p | |
|---|---|---|---|---|---|
| Wechsler scores, m (SD) | |||||
| Full Scale IQ | 108 (14.6)a | 102 (12.4)b | 100 (14.7)b | 4.0 | .02 |
| Digit Span | 11.0 (3.2)a | 8.8 (2.7)b | 8.3 (3.2)b | 7.9 | .001 |
| Coding | 9.4 (3.1) | 7.8 (2.4) | 7.9 (3.9) | 2.7 | .07 |
| Go/No-go, m (SD) | |||||
| Omission Errors | 35.8 (37.8)a | 47.1 (35.2)b | 62.2 (50.8)b | 4.0 | .02 |
| Commission Errors | 22.2 (6.7)a | 26.2 (5.6)b | 21.8 (6.1)a | 5.3 | .006 |
| Hit Reaction Time | 386.5 (78.4)a,b | 358.9 (74.8)a | 408.7 (93.0)b | 4.5 | .01 |
Note: ADHD/DP− = attention-deficit/hyperactivity disorder without the Child Behavior Checklist–Dysregulation Profile (CBCL/DP); CBCL/DP+ = with the CBCL/DP (CBCL/DP+).
Numbers with different superscripts within a row are significantly different from each other p<.05.
Neurophysiological Functions
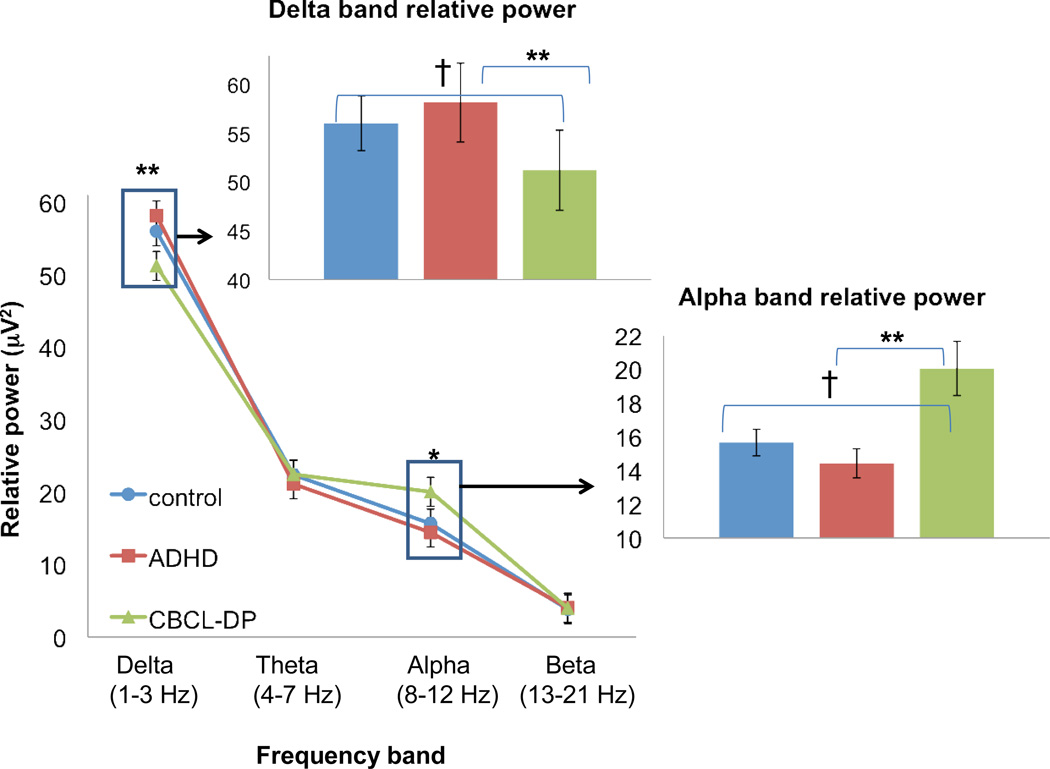
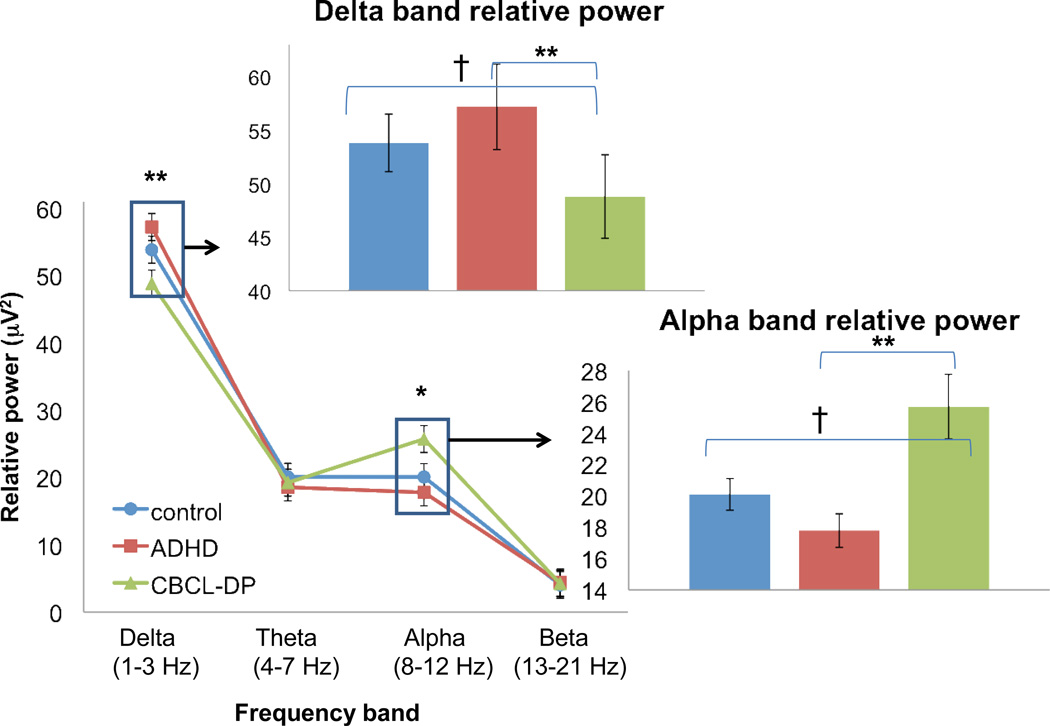
Analyses revealed a distinct EEG profile in CBCL/DP+ participants compared with controls or ADHD/DP− (see Figures 1 and 2). Specifically, the CBCL/DP+ group exhibited EEG differences in central and parietal regions with lower delta band power (central: F2, 110 = 4.6, p =.01; parietal: F2, 110 = 5.2, p =.007) and higher alpha band power (central: F2, 110 = 4.2, p =.02; parietal: F2, 110 = 3.8, p =.03). Post-hoc pairwise comparisons indicate that in both regions (central and parietal) and both frequency bands (delta and alpha) the CBCL/DP+ group was significantly different from the ADHD/DP- (p < .01) and marginally different from controls (p < .10). There were no significant differences between study groups on theta band or beta band spectral power.
Figure 1.
Electroencephalography (EEG) spectral power in central region. Note: Spectral power is presented for all frequency bands in the central region in the lower line graph. Inset bar graphs show pairwise differences for frequency bands where the omnibus statistic for group effect is significant: †p < .10, *p < .05, **p < .01.
Figure 2.
Electroencephalography (EEG) spectral power in Parietal Region. Note: Spectral power is presented for all frequency bands in the parietal region in the lower line graph. Inset bar graphs show pairwise differences for frequency bands where the omnibus statistic for group effect is significant: †p < .10, *p ≤ .05, **p ≤.01.
Within the entire sample, correlation analyses of delta and alpha frequency bands with CBCL subscales, ADHD symptoms, and cognitive measures suggest that spectral power was strongly related to behavioral dysregulation, both within the CBCL/DP profile and extending also to other CBCL subscales (Table 3). Within the CBCL/DP profile, the centroparietal low delta/high alpha power profile was most strongly correlated with the Anxiety/Depression subscale (r’s range −.28 (delta) to .36 (alpha), followed by the Aggression subscale (r’s range −.21 (delta) to .31 (alpha). The EEG profile was not significantly associated with the CBCL attention problems scale, ADHD symptoms or cognitive measures, suggesting that centroparietal low delta/high alpha profile might be specific to behavioral and emotional dysregulation.
Table 3.
Pearson Correlations (N=114) Suggest the Electroencephalography (EEG) Profile is Specifically Associated With Behavioral Dysregulation
| central delta |
central alpha |
parietal delta |
parietal alpha | |
|---|---|---|---|---|
| CBCL subscales | ||||
| Anxiety/Depression | −0.28** | 0.36**** | −0.30** | 0.33*** |
| Attention Problems | −.18 | .16 | −.17 | .16 |
| Aggressive Behavior | −0.23* | 0.31** | −0.21* | 0.25* |
| Withdrawn | −0.24* | 0.32** | −0.23* | 0.29** |
| Somatic Complaints | −0.27** | 0.25* | −0.27** | .18 |
| Thought Problems | −0.23* | 0.24* | −0.24* | 0.23* |
| Delinquent Behavior | −.06 | .14 | −.07 | .12 |
| KSADS ADHD symptom counts | ||||
| Inattentive | .10 | −.09 | .15 | −.14 |
| Hyperactive-Impulsive | −.02 | −.03 | −.02 | −.06 |
| Wechsler scores | ||||
| Full Scale IQ | .11 | −.11 | .10 | −.12 |
| Digit span | .11 | .01 | .05 | .01 |
| Go/No-go task | ||||
| Omission Errors | −.12 | −.02 | −.12 | .10 |
| Commission Errors | −.03 | −.01 | −.08 | .11 |
| Reaction Time | −.11 | .12 | −.12 | .14 |
Note: Significant correlations are in bold. ADHD = attention-deficit/hyperactivity disorder; CBCL = Child Behavior Checklist; KSADS = Kiddie Schedule for Affective Disorders and Schizophrenia.
p<.05,
p<.01,
p<.001,
p<.0005.
DISCUSSION
The goal of this study was to examine and characterize behavioral, cognitive, and EEG functioning differences associated with the CBCL/DP, ADHD, and typically developing controls. Our results suggest the CBCL/DP+ group has a unique profile that differentiates it from the other 2. Behaviorally, youth with the CBCL/DP+ have higher rates of behavioral disturbance on CBCL scales not used in the dysregulation profile and exhibit psychiatric comorbidities 2 to 3 times more often than same-aged peers with ADHD alone. Cognitively, those with the CBCL/DP+ respond slowly and are more inattentive than ADHD/DP−, who showed a fast and impulsive response style. Finally, EEG data suggest that the CBCL/DP+ is associated with attenuated delta and elevated alpha band spectral power in both central and parietal regions, the latter of which has previously been associated with depression. The current work goes beyond previously described findings with the CBCL/DP by directly revealing patterns of underlying neural functioning and their associations with manifestations of emotional dysregulation, not ADHD or disruptive behaviors. This report adds to growing evidence that the CBCL/DP identifies a patient group with unique cognitive and biological attributes that are distinct from ADHD alone and from controls.
EEG findings failed to demonstrate the hypothesized differences between ADHD/DP− and control groups in the spectral power of theta and beta bands. This was unexpected and not consistent with previous studies. 22, 23, 31 Elevated theta band power, however, could be a nonspecific marker of cortical dysfunction common to other disorders such as epilepsy, bipolar disorder, and substance abuse.41 In addition, considerable EEG heterogeneity within ADHD has been noted, suggesting potential ADHD subgroups with differing neurophysiological substrates. Separate studies have identified an ADHD subgroup characterized by excess alpha band power.22, 42 Thus, it is possible that our selection of an ADHD sample with low CBCL/DP scores resulted in a group with less emotional/behavioral dysfunction and with a more normalized neurophysiologic substrate than typical of ADHD in general. Alternatively, emotional dysregulation might produce confounding effects on EEG characteristics in ADHD-affected youth, as suggested in previous work. 43 Future EEG studies of ADHD should consider the potential confounding of emotional dysregulation.
As hypothesized, the primary group differences between those with and without the CBCL/DP+ profile emerged in the alpha band, which is thought to arise from thalamocortical interactions30 and mood dysregulation.27 Functionally, elevated centroparietal alpha band power has also been associated with high behavioral inhibition,44 lower cortical arousal,45 lack of motor responsiveness, and longer reaction times.46 The specific EEG pattern of low delta and high alpha band power has been associated with melancholic temperament30 and early stage depression.28 These previous findings are consistent with the elevated rates of mood and anxiety disorders, as well as slower reaction time and high rate of omission errors exhibited by the CBCL/DP+ group. The current results suggest that the low delta/high alpha profile is associated with manifestations of emotional dysregulation, not ADHD or disruptive behaviors.
Procedures and findings from this study are consistent with recently proposed neuroscience-based approaches to research of mental disorders and psychiatric classification.47, 48 Specifically, our investigation utilized cognitive testing, brain imaging, and a dimensional measure of psychopathology to investigate differences in brain function that are less constrained by preconceived diagnostic classifications. An additionally surprising and novel finding from our data is the suggestion that the CBCL/DP is associated with a distinct cognitive endophenotype which diverges significantly from ADHD per se, despite the fact that both groups share the ADHD diagnosis. Our findings suggest that both the ADHD/DP- and CBCL/DP+ groups displayed significant inattention, but that the ADHD/DP− group demonstrated more difficulties with inhibiting motor responses relative to the CBCL/DP+ and control groups. Participants with the CBCL/DP+ showed greater omission errors than controls, but had significantly slower reaction times than those with ADHD alone, likely resulting from an imbalance in thalamocortical arousability. Taken together, these data suggest that the ADHD/DP− group had a fast and impulsive response style, whereas the CBCL/DP+ group was slow and had apparent difficulties sustaining attention and maintaining an appropriate level of cortical activation.
A natural extension of our approach would be to conduct additional investigations to confirm whether a refined EEG/cognitive profile stands as a true biosignature of the CBCL/DP, and whether or not the profile might also serve as an indicator of treatment outcome. Similarly, studies of EEG delta and alpha power differences in samples of children with mood and anxiety disorders, or risk for disorder, are needed to determine if our findings are unique to the CBCL/DP, or if these EEG differences overlap with emotional disorders not comorbid with ADHD or the externalizing features of the profile.
The utility of the CBCL/DP as a predictor of diagnostic category also requires further elaboration. The profile was initially proposed as a means to differentiate cases of ADHD from juvenile mania.37 This led to further work proposing that juvenile mania, in contrast to the adult form, was better characterized by chronic irritability than by episodic euphoria,49 and that features associated with this irritability were detected by the CBCL/DP. Other investigators proposed a differentiation between narrow phenotype bipolar disorder, which strictly adhered to DSM requirements for distinct episodes with associated hallmark features of grandiosity or elevated mood differing from baseline, and a broad phenotype, typified by chronic nonepisodic irritability that lacked the hallmark features of mania.50 Subsequent research based on this framework ultimately led to a redefinition of the broad bipolar phenotype as “severe mood dysregulation” (SMD).51 Research on SMD has demonstrated that patterns of adolescent irritability are stable and distinct, with long-term outcomes typified by diagnoses of depression and ADHD.52, 53 Youth with SMD also have deficiencies in face emotion labeling,54 increased family dysfunction, and patterns of amygdala hypoactivation also seen with depression.52 Increased recognition of SMD as a distinct biological syndrome has led to development of a newly proposed diagnostic category “disruptive mood dysregulation disorder” (DMDD) that is largely based on SMD criteria without the requirement for hyperarousal and with other minor clarifications in age-of-onset and exclusion criteria.55 Recently, some have suggested a relationship between the CBCL/DP and proposed DMDD category,6 although the predictive power of the profile for either clinically diagnosed SMD or DMDD has not been established. Nonetheless, confirmation of an EEG/cognitive biosignature for the CBCL/DP would provide a critical tool for understanding the biological underpinnings of these newly defined behavioral syndromes with a concomitant justification for inclusion of SMD/DMDD in the revised classification of psychiatric disorders.
The study has several limitations. Although well matched, the findings are based on a relatively small number of participants. It is critical to confirm these results in larger samples. Similarly, participants’ ages were relatively constrained and the potential effects of development on their EEG findings have not been examined. Study participants were ascertained through other studies of clinically affected and unaffected youth, and these studies both had additional inclusion and exclusion criteria that might influence outcomes described here. Finally, although several participants in the CBCL/DP+ group did not meet full threshold criteria for ADHD, the extent to which the CBCL/DP identifies individuals with no evidence of ADHD, even at a sub-threshold level, remains unstudied.
Both the study findings and its limitations provide a framework for future research. The CBCL/DP is a cross-disorder dimensional measure of psychopathology with proven biological correlates. Potential relationships between the CBCL/DP and psychiatric diagnosis should be investigated in a range of internalizing and externalizing disorders in order to determine the profile’s diagnostic specificity and predictive power. In particular, the utility of the profile in predicting SMD or DMDD requires clear delineation. The relationship of EEG findings to the CBCL/DP requires confirmation in larger samples that include broader age ranges and are not limited by strictly defined ADHD or other inclusion/exclusion criteria. Careful consideration should address whether the identified EEG profile represents a “state” or “trait” condition, along with the related question of whether these EEG findings suggest a risk for psychopathology that is independent of current clinical presentation. Finally, the potential role of the EEG profile as either a predictor of treatment response or mediator of clinical outcomes should be examined in an effort to move from a behavioral to brain-based approach in clinical decision making.
Acknowledgments
This study was supported by National Institute of Mental Health grants U01MH093582 (J.J.M.), R01MH92829 (S.K.L.), and P50MH77248 (J.T.M.).
Dr. McGough has received grant or research support from the National Institutes of Health (NIH), NeuroSigma Inc., Shionogi, Shire, and Supernus. He has served as a consultant to Alexza Pharmaceuticals, Akili Interactive, Inc., MedImmune, Sunovion, Targacept, and Theravance. He has provided expert testimony for Shire. Dr. McCracken has received grant or research support from NIH, Seaside Therapeutics, Roche, and Otsuka. He has served as a consultant to BioMarin and PharmaNet. Dr. Piacentini has received grant or research support from NIH, the Tourette Syndrome Association, the Furlotti Family Foundation, and Pfizer. He has served on the speakers’ bureau of the Tourette Syndrome Association and the International Obsessive Compulsive Disorder (OCD) Foundation. He has received book royalties from Guilford Press and Oxford University Press. He is a coauthor of the Child OCD Impact Scale–Revised (COIS-R) and the Child Anxiety Impact Scale (CAIS) assessment tools, none of which are commercially published and therefore no royalties have been received.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
Drs. Cowen and Loo, Mr. Cho, Mr. Castelo, and Ms. Sturm report no biomedical financial interests or potential conflicts of interest.
References
- 1.Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 profile. Burlington: Vermont, University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 2.Biederman J, Monuteaux MC, Kendrick E, Klein KL, Faraone SV. The CBCL as a screen for psychiatric comorbidity in paediatric patients with ADHD. Arch Dis Child. 2005;90:1010–1015. doi: 10.1136/adc.2004.056937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Althoff RR, Rettew DC, Ayer LA, Hudziak JJ. Cross-informant agreement of the Dysregulation Profile of the Child Behavior Checklist. Psychiatry Res. 2010;178:550–555. doi: 10.1016/j.psychres.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mick E, Biederman J, Pandina G, Faraone SV. A preliminary meta-analysis of the child behavior checklist in pediatric bipolar disorder. Biol Psychiatry. 2003;53:1021–1027. doi: 10.1016/s0006-3223(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 5.McGough JJ, Loo SK, McCracken JT, et al. CBCL pediatric bipolar disorder and ADHD: comorbidity and quantitative trait loci analysis. J Am Acad Child Adolesc Psychiatry. 2008;47:1151–1157. doi: 10.1097/CHI.0b013e3181825a68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Althoff RR, Ayer LA, Crehan ET, Rettew DC, Baer JR, Hudziak JJ. Temperamental profiles of dysregulated children. Child Psychiatry Hum Dev. 2012;43:511–522. doi: 10.1007/s10578-012-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Althoff RR, Verhulst FC, Rettew DC, Hudziak JJ, van der Ende J. Adult outcomes of childhood dysregulation: a 14-year longitudinal study. J Am Acad Child Adolesc Psychiatry. 2010;49:1105–1116. doi: 10.1016/j.jaac.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayer L, Althoff R, Ivanova M, et al. Child Behavior Checklist Juvenile Bipolar Disorder (CBCL-JBD) and CBCL Posttraumatic Stress Problems (CBCL-PTSP) scales are a measure of a single deregulatory syndrome. J Child Psychol Psychiatry. 2009;50:1291–1300. doi: 10.1111/j.1469-7610.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- 9.Holtmann M, Buchmann AR, Esser G, Schmidt MH, Banaschewski T, Laucht M. The Child Behavior Checklist-Dysregulation Profile predicts substance use suicidality, and functional impairment: a longitudinal analysis. J Child Psychol Psychiatry. 2011;52:139–147. doi: 10.1111/j.1469-7610.2010.02309.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Carlson GA, Meyer SE, et al. Correlates of the CBCL-dysregulation profile in preschool-aged children. J Child Psychol Psychiatry. 2012;53(9):918–926. doi: 10.1111/j.1469-7610.2012.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Althoff RR, Rettew DC, Faraone SV, Boomsa DI, Hudziak JJ. Latent class analysis shows strong heritability of the child behavior checklist-juvenile bipolar disorder phenotype. Biol Psychiatry. 2006;60:903–911. doi: 10.1016/j.biopsych.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Meyer SE, Carlson GA, Youngstrom E, et al. Long-term outcomes of youth who manifested the CBCL-Pediatric Bipolar Disorder phenotype during childhood and/or adolescence. J Affect Disord. 2009;113:227–235. doi: 10.1016/j.jad.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Biederman J, Petty CR, Day H, et al. Severity of the aggression/anxietydepression/attention child behavior checklist profile discriminates between different levels of deficits in emotional regulation in youth with attention-deficit/hyperactivity disorder. J Dev Beh Pediatr. 2012;33:236–243. doi: 10.1097/DBP.0b013e3182475267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jucksch V, Salbach-Andrae H, Lenz K, et al. Severe affective and behavioural dysregulation is associated with significant psychosocial adversity and impairment. J Child Psychol Psychiatry. 2011;52:686–695. doi: 10.1111/j.1469-7610.2010.02322.x. [DOI] [PubMed] [Google Scholar]
- 15.Mick E, McGough J, Loo S, et al. Genome-wide association study of the child behavior checklist dysregulation profile. J Am Acad Child Adolesc Psychiatry. 2011;50:807–817. doi: 10.1016/j.jaac.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doyle AE, Biederman J, Ferreira MA, Wong P, Smoller JW, Faraone SV. Suggestive linkage of the CBCL juvenile bipolar disorder phenotype to 1p21: 6p21 and 8q21. J Am Acad Child Adolesc Psychiatry. 2010;49:378–387. [PMC free article] [PubMed] [Google Scholar]
- 17.Zepf FD, Wöckel L, Poustka F, Holtmann M. Diminished 5-HT functioning in CBCL pediatric bipolar disorder profiled ADHD patients vs. normal ADHD: susceptibility to rapid tryptophan depletion influences reaction time performance. Human Psychopharmacol. 2008;23:291–299. doi: 10.1002/hup.934. [DOI] [PubMed] [Google Scholar]
- 18.Holtmann M, Duketis E, Goth K, Poustka L, Boelte S. Severe affective and behavioral dysregulation in youth is associated with increased serum TSH. J Affect Disord. 2010;121:184–188. doi: 10.1016/j.jad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Wozniak J, Gönenc A, Biederman J, et al. A magnetic resonance spectroscopy study of the anterior cingulate cortex in youth with emotional dysregulation. Isr J Psychiatry Relat Sci. 2012;49:62–69. [PMC free article] [PubMed] [Google Scholar]
- 20.Carter CS, Barch DM, Bullmore E, et al. Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia II: developing imaging biomarkers to enhance treatment development for schizophrenia and related disorders. Biol Psychiatry. 2011;70:7–12. doi: 10.1016/j.biopsych.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman R, Olincy A, Ross RG, et al. The genetics of sensory gating deficits in schizophrenia. Curr Psychatry Rep. 2003;5:155–161. doi: 10.1007/s11920-003-0032-2. [DOI] [PubMed] [Google Scholar]
- 22.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Children with attention-deficit/ hyperactivity disorder and comorbid oppositional defiant disorder: an EEG analysis. Psychiatry Res. 2002;111:181–190. doi: 10.1016/s0165-1781(02)00137-3. [DOI] [PubMed] [Google Scholar]
- 23.Snyder SM, Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. J Clin Neurophysiol. 2006;23:440–455. doi: 10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- 24.Feng X, Forbes E, Kovacs M, et al. Children’s depressive symptomatology in relation to EEG frontal asymmetry and maternal depression. J Abnorm Child Psychol. 2012;40:265–276. doi: 10.1007/s10802-011-9564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leuchter AF, Cook IA, Hunter AM, Cai C, Horvath S. Resting-state quantitative electroencephalographi reveals increased neurophysiologic connectivity in depression. PLoS One. 2012;7:e32508. doi: 10.1371/journal.pone.0032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychol Sci. 2005;16:805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- 27.Jackson D, Mueller C, Dolski I, et al. Now you feel it now you don’t: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol Sci. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- 28.Grin-Yatsenko V, Baas I, Ponomarev V, Kropotove J. EEG power spectra at early stages of depressive disorder. J Clin Neurophysiol. 2009;26:401–406. doi: 10.1097/WNP.0b013e3181c298fe. [DOI] [PubMed] [Google Scholar]
- 29.Segrave RA, Thomson RH, Cooper NR, Croft RJ, Sheppard DM, Fitzgerald PB. Emotive interference during cognitive processing in major depression: an investigation of lower alpha 1 activity. J Affect Disord. 2012;141:185–193. doi: 10.1016/j.jad.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Robinson DL. How brain arousal systems determine different temperament types and the major dimensions of personality. Personality and Individual Differences. 2001;31:1233–1259. [Google Scholar]
- 31.Loo SK, Hale ST, Hanada G, et al. Familial clustering and DRD4 effects on electronencephalgram measures in multiplex familie with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:368–377. [PMC free article] [PubMed] [Google Scholar]
- 32.Hollingshead AB. Two-Factor Index of Social Position. New Haven: Yale University Press; 1957. [Google Scholar]
- 33.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school aged children-present and Lifetime Version (K-SADS-PL) J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Loo SK, Rich EC, Ishii J, et al. Cognitive functioning in affected sibling pairs with ADHD: familial clustering and dopamine genes. J Child Psychol Psychiatry. 2008;49:950–957. doi: 10.1111/j.1469-7610.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- 35.Smalley SL, McGough JJ, Del’Homme M, et al. Familial clustering of symptoms and disruptive behaviors in multiplex families with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:1135–1143. doi: 10.1097/00004583-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 37.Faraone SV, Althoff RR, Hudziak JJ, Monuteaux M, Biederman J. The CBCL predicts DSM bipolar disorder in children: a receiver operating characteristic curve analysis. Bipolar Dis. 2005;7:518–524. doi: 10.1111/j.1399-5618.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler Intelligence Scale for Children. 4th Edition. San Antonio, TX: Pearson Education Inc.; 2003. [Google Scholar]
- 39.Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Pearson Education Inc.; 1999. [Google Scholar]
- 40.Conners C. CPTII: Conners' Continuous Performance Test. San Antonio, TX: Pearson Education Inc.; 2004. [Google Scholar]
- 41.Coutin-Churchman P, Anez Y, Uzcategui M, et al. Quantitative spectral analysis of EEG in psychiatry revisited: drawing signs out of numbers in a clinical setting. Clin Neurophysiol. 2003;114:2294–2306. doi: 10.1016/s1388-2457(03)00228-1. [DOI] [PubMed] [Google Scholar]
- 42.Chabot RJ, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biol Psychiatry. 1996;40:951–963. doi: 10.1016/0006-3223(95)00576-5. [DOI] [PubMed] [Google Scholar]
- 43.Loo SK, Makeig S. Clinical utility of EEG in attention-deficit/hyperactivity disorder: a research update. Neurotherapeutics. 2012;9:569–587. doi: 10.1007/s13311-012-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knyazev GG, Levin EA, Savostyanov AN. Impulsivity, anxiety, and individual differences in evoked and induced brain oscillations. Int J Psychophysiol. 2008;68:242–254. doi: 10.1016/j.ijpsycho.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- 46.Moore RA, Gale A, Morris PH, Forrester D. Alpha power and coherence primarily reflect neural activity related to stages of motor response during a continuous performance task. Int J Psychophysiol. 2008;69:79–89. doi: 10.1016/j.ijpsycho.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): towards a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 48.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions. Dialogues Clin Neurosci. 2012;14:29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wozniak J, Biederman J, Kwon A, et al. How cardinal are cardinal symptoms in pediatric bipolar disorder? An examination of clinical correlates. Biol Psychiatry. 2005;58:583–588. doi: 10.1016/j.biopsych.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 51.Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168:129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brotman MA, Rich BA, Guyer AE, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167:61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leibenluft E, Cohen P, Gorrindo T, Brook JS, Pine DS. Chronic versus episodic irritability in youth: a community-based, longitudinal study of clinical and diagnostic associations. J Child Adolesc Psychopharmacol. 2006;16:456–466. doi: 10.1089/cap.2006.16.456. [DOI] [PubMed] [Google Scholar]
- 54.Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJ, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev Psychopathol. 2008;20:529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DSM-5 Childhood and Adolescent Disorders Workgroup. [Accessed October 27, 2012];Justification for temper dysregulation disorder with dysphoria. http://www.dsm5.org.