Abstract
Objective
Children undergoing cardiac surgery with cardiopulmonary bypass (CPB) are susceptible to additional inflammatory and immunogenic insults from blood transfusions. We hypothesize that washing red blood cells (RBC) and platelets transfused to these patients will reduce post-operative transfusion-related immune modulation and inflammation.
Design
Prospective randomized controlled clinical trial.
Setting
University hospital pediatric cardiac intensive care unit.
Patients
Children from birth to 17 years old undergoing cardiac surgery with CPB.
Interventions
Children were randomized to an unwashed or washed RBC and platelet transfusion protocol for their surgery and postoperative care. All blood was leukoreduced, irradiated, and ABO identical. Plasma was obtained for laboratory analysis: pre-op, immediately, six and 12 hours after CPB. Primary outcome was the 12-hour post-CPB interleukin (IL)-6: IL-10 ratio. Secondary measures were IL levels, C-reactive protein (CRP), and clinical outcomes.
Measurements and main results
162 subjects were studied, 81 per group. 34 subjects (17 per group) did not receive any blood transfusions. Storage duration of blood products was similar between groups. Among transfused subjects, the 12-hour IL ratio was significantly lower in the washed group (3.8 v. 4.8; p=0.04) secondary to lower IL-6 levels (post-CPB: 65 v.100 pg/ml; p = 0.06; 6 hour: 89 v.152 pg/ml; p = 0.02; 12-hour: 84 v.122 pg/ml; p = 0.09). Post-operative CRP was lower in subjects receiving washed blood (38 v. 43 mg/L; p = 0.03). There was a numerical, but not statistically significant decrease in total blood product transfusions (203 v. 260) and mortality (2 v. 6 deaths) in the washed group compared to the unwashed group.
Conclusions
Washed blood transfusions in cardiac surgery reduced inflammatory biomarkers, number of transfusions, donor exposures, and were associated with a non-significant trend towards reduced mortality. A larger study powered to test for clinical outcomes is needed to determine whether these laboratory findings are clinically significant.
Keywords: congenital heart disease, cardiac surgery, cardiopulmonary bypass, transfusion, inflammation, C-reactive protein
Introduction
The heightened inflammatory state and altered hemostatic profile associated with cardiopulmonary bypass (CPB) (1) has been well described (2,3). Bioactive markers released in response to CPB have been implicated in many post-operative complications (4-9), contributing to organ dysfunction and adverse clinical outcomes (10-13). Surgical trauma, ischemic-reperfusion injury, activation of the coagulation/complement cascade (14,15), and blood transfusions (16) all contribute to post-operative systemic inflammation.
In adult cardiac surgery, leukoreduction of blood transfusions reduced the incidence of post-operative infection, multi-organ failure and mortality (17,18) but similar studies have not been performed in children.
In adults following CPB, increased numbers of transfusions are associated with adverse outcomes, including wound infections (19), nosocomial pneumonia (20), renal dysfunction (21), sepsis (22), and mortality (23,24). There are no similar transfusion dose-outcome data in the pediatric cardiac surgical literature; although recent studies indicate that a restrictive transfusion strategy is well tolerated (25), even in those with single ventricle physiology (26).
Blood transfusions alter humoral and cellular immunity in the recipient (27-32). Whether the cells themselves, the supernatant, or both, contribute to inflammation and poor clinical outcomes attributed to transfusion-related immunomodulation remains unknown (33,34). Bioactive substances and microparticles (35-44) in the supernatant of stored RBCs likely serve as a secondary inflammatory insult, compounding the diffuse inflammatory response to CPB, and contributing to postoperative organ dysfunction (45). Whether removal of these bioactive substances by post-storage RBC and platelet washing (46) can attenuate the recipient's inflammatory and immunogenic response, and improve post-operative clinical outcomes, remains to be studied. Washed transfusions have been associated with reduced cardiopulmonary complications in aggregate hospital data (47) and improved survival in acute leukemia in adults (48).
We hypothesize that washing blood cell products transfused into pediatric patients undergoing open heart surgery will reduce post-operative inflammation (as evidenced by a lower interleukin (IL)-6:IL-10 ratio) and may improve clinical outcomes. The IL-6:IL-10 ratio was chosen as primary outcome since elevated levels of both interleukins have been found to prospectively identify increased morbidity and mortality in critically ill children (49,50), with higher ratios significantly associated with injury severity scores (51), multi-organ failure (52,53), and death (54,55).
Our primary aim is to compare the 12-hour post-CPB interleukin (IL)-6 to IL-10 ratio in children status post open heart surgery randomized to a unwashed vs. washed RBC and platelet transfusion protocol. Secondary aims are to compare other laboratory measures of inflammation and immune response and clinical outcomes between transfusion groups.
Materials and Methods
Subjects
Children up to 18 years presenting to the University of Rochester Medical Center (URMC) for cardiac surgical repair/palliation with CPB were eligible. Exclusion criteria were: patent ductus arteriosus repair, if their parent/guardian was non-English speaking, if consent could not be obtained, or participation in another clinical transfusion trial. The protocol was approved by the URMC Research Subjects Review board and registered with ClinicalTrials.gov (NCT00693498).
Subjects were enrolled at their pre-anesthesia visit, with properly witnessed and documented informed consent. Once enrolled, subjects were divided into groups according to age and for the presence of cyanosis, and block randomization used to randomize subjects to the unwashed or washed transfusion strategy. The URMC blood bank was notified so that transfusions would be prepared accordingly.
Transfusion strategy
The transfusion strategy was initiated for the operating room and maintained until hospital discharge (including CPB prime). All blood products were pre-storage leukoreduced, irradiated and ABO identical, without restrictions on storage age.
Unwashed Group
All RBC and platelet transfusions were prepared according to standard protocol at the URMC for the duration of their hospital admission.
Washed Group
All RBC and platelet transfusions were post-storage washed for the duration of hospital admission.
The protocol could be temporarily suspended at the discretion of the attending physician should the time required to wash blood products (2 hrs for platelets; 30 minutes for RBCs at the URMC) interfere with patient care. The protocol was resumed promptly once the subject's condition no longer fulfilled the suspension criteria.
Surgical management
Peri-operative management including the initiation of anesthesia, CPB technique, and surgical repair was performed per current standard of care at URMC with the sole exception being the transfusion strategy. The cardiac surgeon (GMA, FG) was blinded to study assignment to prevent any potential bias on the surgical or CBP management. Obvious differences in packaging and labeling of washed blood products prevented blinding of the perfusionists and anesthesiologists.
The Terumo RX05 oxygenator (Terumo Cardiovascular Systems, Ann Arbor, MI) was used for subjects weighing ≤10kg, and the Terumo RX15 oxygenator for subjects >10kg. RBCs were used in CPB prime for infants weighing <10kg. Fresh frozen plasma (FFP), platelets and/or cryoprecipitate were never included in CPB prime, nor given routinely in the operating room. All patients were managed intra-operatively on CPB with unfractionated heparin adjusted according to activated clotting times and reversed with protamine. All patients received epsilon aminocaproic acid. Conventional ultrafiltration was employed for all study subjects as is current standard of care for all CPB cases performed at our center. All subjects undergoing deep hypothermic circulatory arrest (DHCA) and those children ≤ six months of age received steroids on CPB per our current standard of care.
Vasoactive medications (epinephrine, dopamine, and/or milrinone drips) were utilized to maintain hemodynamics during transition off CPB at the discretion of the cardiac surgical team. All patients remained intubated and sedated for transfer to the pediatric cardiac intensive care unit (PCICU). Surgical, CPB and transfusion details were collected, as well as any intra-operative complications (i.e., arrhythmias, acidosis, and/or poor ventricular function).
Post-operative management
Subjects were managed post-operatively per the current standard of care aside from their transfusion strategy. Subjects were weaned from mechanical ventilation and extubated as their cardiopulmonary status allowed. Crystalloid and/or 5% albumin were infused in 10-20mL/kg boluses for clinical findings of hypovolemia and poor cardiac output (i.e., tachycardia, poor pulses and perfusion, low urine output and/or hypotension) as needed to maintain hemodynamic stability. Vasoactive medications were adjusted to maintain hemodynamics and end-organ perfusion.
Transfusion of RBC, platelet and hemostatic products were based upon a standard PCICU protocol, and the protocol was adhered to throughout the trial. The RBC transfusion protocol takes into account: 1) the type of cardiac defect and repair, 2) the overall hemodynamic stability of the patient (as assessed by blood pressure, central venous pressure, oxygenation status, distal perfusion/toe temperature, urine output, serum lactate; and 3) the presence/absence of active bleeding/MT output, in addition to the hemoglobin, platelet or coagulation profile. RBC were transfused in 10mL/kg volumes at a rate at the discretion of the prescribing physician.
The platelet transfusion protocol takes into account: 1) the presence/absence of active bleeding/oozing, 2) the type of cardiac defect and repair; and 3) the presence/absence of artificial shunts/conduits, in addition to the platelet count. Platelets were transfused in a volume of 10mL/kg, with the rate of transfusion at the discretion of the prescribing physician. Coagulant components (FFP, cryoprecipitate and/or recombinant factors) were transfused according to the PCICU protocol, which was adhered to throughout the trial. Subjects received anticoagulation (i.e., aspirin, enoxaparin, warfarin) as appropriate for their type of cardiac defect, surgical palliation/repair, and post-operative medical management.
URMC Blood Bank blood component therapy procedures
All blood products were pre-storage leukoreduced with a PALL BPF High Efficiency Leukocyte removal filter that removes leukocytes and micro-aggregates from the packed RBC or platelet units. All platelets and RBC were irradiated using a CIS-US IBL 437 Blood Irradiator delivering 2500 centigray. The “age” (number of stored days) of RBC and platelet products prepared for each group was tracked. All blood products were ABO identical.
Washing procedures
A COBE (Caridian, Lakewood, CO) 2991 Blood Cell Processor separated and removed supernatant plasma proteins, preservative agents and micro-aggregates from the RBC or platelet unit by centrifugation (previously published method (56). After separation and concentration of the RBCs, the supernatant was expressed into a waste bag and two additional washing cycles were repeated by adding 0.9% saline solution.
Platelets underwent a two-wash procedure removing approximately 95% of original plasma proteins, isoagglutinins, antibodies, and blood group A&B substances (previously published method) (57). Platelet recovery is approximately 80-85% (58). After washing, platelet units were re-suspended in 0.9% saline.
IL-6/IL-10 ratio
2.0mL whole blood samples were collected in sodium citrate tubes, centrifuged immediately, and the plasma stored at -80° for later IL-6 and IL-10 cytokine quantification. Samples were obtained: 1) in the OR prior to initiation of CPB; 2) once off CPB after the protamine is completed (“post-CPB”); 3) 6 hours “post-CPB”; 4) 12 hours “post-CPB”. Measurements of IL-6 and IL-10 cytokine levels were determined by a Luminex beadlyte multiplex assay per manufacturer (Millipore Corporation, Billerica MA) instructions. Interleukin 6 and10 levels were quantified for each subject group and the ratio calculated at each time point.
High sensitivity C-reactive protein (CRP)
CRP was measured pre and post-CPB (as described for the interleukin levels) and on post-operative day (POD) 1-3. CRP testing was performed at the URMC Clinical Laboratories, analyzed by immunoturbidmetric assay (ADVIA 2400 Chemistry System, Bayer Healthcare, Tarrytown, NY).
Clinical outcome measures
Subjects were monitored daily for clinical complications including: sepsis (59), active infection, and thrombosis (based on clinical and/or radiographic data) (60).
Statistical analysis
All patients presenting for surgery with CPB require blood to be available for possible intra-operative transfusion. Therefore, subjects were randomized to their transfusion group pre-operatively, to provide adequate notification to the blood bank of their study assignment and allow for preparation of the blood cooler for surgery. Since the random assignment was not conducted at the time of transfusion, two samples are analyzed, an intention to treat sample and a treatment delivery sample (transfusion recipients).
Sample size calculations were conducted on the treatment delivery sample before initiation of the study. Since it could not be determined prospectively which subjects would not receive any blood products, enrollment was set to exceed that necessary for the sample size calculation to ensure adequate power for the primary aim in transfused subjects.
Previous studies suggest that the mean IL-6/IL-10 ratio rises to approximately 10 fold post-operatively, with a standard deviation conservatively estimated as 4. It was calculated that a sample size of 64 subjects per group would provide 80% power to detect a relatively small group difference of 2 units (one-half of a SD) of the mean IL-6:IL-10 ratio, using an unpaired t-test and a two-tailed significance level of 5%. In all subjects, descriptive statistics (e.g., mean, SD, median, quartiles) of the interleukin levels, their ratio, CRP levels, and clinical outcomes were calculated. As the laboratory outcome data (IL and CRP levels) were not normally distributed, median values were compared using non-parametric tests. For interleukin levels the Mann Whitney test for significance was performed. For comparison of CRP levels, the Wald Wolfowitz test was chosen to test for significance for its sensitivity to outliers after preliminary examination of the data. For clinical outcome data that was normally distributed, t-tests were performed to test for significance. For clinical outcome data that was not normally distributed, and when interleukin levels were compared, Mann Whitney tests were performed. We did not adjust for multiple comparisons as all secondary analyses are considered exploratory in nature
All subject data were analyzed at study completion in the manner of “intention-to-treat” to ensure proper statistical interpretation of the study results. Subgroup analysis was then performed for only those children who received RBC transfusions (N = 128) (treatment delivery sample) in order to compare the study intervention of blood cell modification via washing.
A two-way analysis of variance (ANOVA) was performed comparing IL-6 levels and survival between treatment groups. The statistical package for the social sciences (SPSS) version 17.0 (SPSS Inc., Chicago, IL) and Statview (SAS Institute, Cary, NC) were used for all statistical analyses. A p value < 0.05 was considered statistically significant.
Results
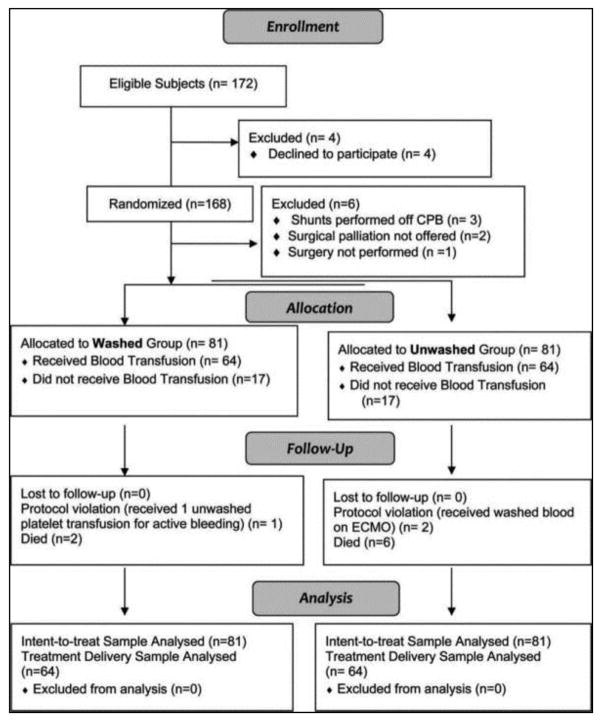
From October 2008 to September 2010, 172 children were eligible for study participation. 168 children and families gave informed consent and were randomized. Three subjects did not have surgery performed (palliation was not offered in 2, and central venous access could not be secured for the third), and three subjects had shunt procedures performed off CPB, and were therefore excluded. The remaining 162 subjects underwent surgery and completed study procedures; 81 were randomized to each group. No surviving subjects dropped out of the study, and none were lost to follow-up. Data from subjects that died were included in all analysis (CONSORT chart).
Subject characteristics
There were 62 females (38%) and 100 males (62%). Median age was 7 months (range: 2 days to 17 years) and median weight was 6.75 kg (2.2-106.8kg). Thirty-six (23%) were neonates (age <31 days), 39 (24.1%) had single ventricle physiology, 78 (48%) had cyanotic cardiac lesions, and 31 (19%) had diagnosed chromosomal abnormalities or syndromes. There were no significant differences in baseline characteristics between intent-to-treat or treatment delivery sample groups, and the CPB and surgical management were similar (Table 1).
Table 1.
Subject characteristics and intra-operative results.
| Variable | Washed Group | Unwashed Group | p-value |
|---|---|---|---|
| N=81 | N = 81 | ||
| Age (mo.) | 6 [3d-17yr] | 7 [2d-17yr] | 0.953 |
| Male sex | 63 (77.8%) | 64 (79%) | 1.0 |
| Weight (kg) | 6.8 [2.8-106] | 6.7 [2.2-66] | 0.456 |
| Neonate (≤30 days) | 16 (20%) | 20 (24.6%) | 0.451 |
| Genetic syndrome/comorbid condition | 16 (20%) | 15 (18.5%) | 0.842 |
| Cyanotic | 37 (45.7%) | 41 (50.6%) | 0.531 |
| Single Ventricle Physiology | 17 (21%) | 22 (27.2%) | 0.36 |
| RACHS-1 score | 3.1 ± 1.4 | 3.0 ± 1.4 | 0.736 |
| Surgical procedure: | |||
| Stage 1 palliation | 11 (13.5%) | 11 (13.5%) | 1.0 |
| Arterial switch operation | 7 (8.6%) | 3 (3.7%) | 0.35 |
| Truncus arteriosus & IAA repair | 1 (1.2%) | 1 (1.2%) | 1.0 |
| BDG | 4 (5%) | 4 (5%) | 1.0 |
| Fontan | 2 (2.5%) | 4 (5%) | 0.407 |
| Tetralogy of Fallot repair | 12 (14.8%) | 19 (23%) | 0.214 |
| Right Ventricle to PA conduit | 10 (12.3%) | 10 (12.3%) | 1.0 |
| Aortic root replacement or Ross | 3 (3.7%) | 3 (3.7%) | 0.651 |
| ASD, VSD or AVSD repair | 24 (29.6%) | 15 (18.5%) | 0.153 |
| Aortic arch recon. ± septal defect | 2 (2.5%) | 3 (3.7%) | 1.0 |
| Valve repair/replacement | 4 (4.9%) | 3 (3.7%) | 0.7 |
| Other* | 1 (1.2%) | 5 (6%) | 0.248 |
| Operating room duration (min) | 210 [100-540] | 240 [82-540] | 0.183 |
| CPB duration (min) | 95 [25-356] | 98 [28-290] | 0.855 |
| Aortic cross clamp | 66 (81.5%) | 63 (77.8%) | 0.698 |
| Aortic cross clamp duration (min) | 62 [6-234] | 61 [6-216] | 0.499 |
| DHCA | 11 (13.6%) | 11 (13.6%) | 0.799 |
| DHCA duration (cumulative min) | 14 [2-72] | 23.5 [2-42] | 0.372 |
| RBC in prime | 54 (66.7%) | 49 (60.5%) | 0.475 |
| Mean age RBC transfused (days) | 16.1 ± 7.3 | 17.6 ± 8.7 | 0.23 |
| CPB priming volume (mL) | 447 [292-1654] | 475 [60-1602] | 0.870 |
| Hemoconcentration volume (mL) | 600 [40-4750] | 600 [150-2500] | 0.957 |
| Additional RBCs in OR | 41 (50.6%) | 40 (49.4%) | 0.875 |
| Delayed chest closure | 8 (9.9%) | 7 (8.6%) | 0.806 |
| Duration chest open (days) | 4 ± 2.4 | 5.8 ± 5.8 | 0.709 |
Values expressed as: number (%); mean ± SD, or median [range]. Independent t-tests were performed for data that was normally distributed. Mann-Whitney tests were performed to compare data that was not normally distributed.
Other surgeries include: subAortic membrane resection, pulmonary artery unifocalization, coronary re-implantation and repair of partial or total anomalous pulmonary venous return.
CPB = cardiopulmonary bypass; RACHS = Risk adjusted congenital heart surgery; IAA = interrupted aortic arch; BDG = bidirectional glenn; PA = pulmonary artery; ASD = atrial septal defect; VSD = ventricular septal defect; AVSD = atrioventricular septal defect; DHCA = deep hypothermic circulatory arrest; OR = operating room; FFP = fresh frozen plasma.
Intent-to-Treat Sample (N = 162)
Interleukin (IL)-6 to IL-10 ratio
the median IL-6: IL-10 ratio was zero for both subject groups preoperatively and rose similarly at discontinuation of CPB (0.1 v. 0.2, p = 0.24 for washed and unwashed groups respectively). The median interleukin ratio rose at each post-operative time point, always lower in the washed group. This difference reached statistical significance 12 hours following CPB (6 hours: 3.2 v. 4.4, p = 0.255; 12 hours 4.4 v. 5.5, p = 0.014).
Interleukin 6
For both transfusion groups plasma IL-6 levels rose post-operatively with levels peaking six hours post-CPB. Median IL-6 levels were lower in the washed group at each time point, nearly significant immediately coming off CPB and significant 6 hours after CPB (post-op: 64 v. 94 pg/dl, p = 0.06; 6hr: 113 v. 157 pg/dl, p = 0.05; 12hr: 108 v. 129 pg/dl, p = 0.116).
Interleukin 10
Interleukin-10 levels rose in both transfusion groups immediately post CPB, with higher levels in the washed compared to unwashed group (517 v. 392 pg/dl, p = 0.922). IL-10 levels fell and were nearly identical between groups at the later post-operative time points (6 hours: 35 v 35 pg/dl, p=0.8; 12 hours: 25 v. 23 pg/dl, p = 0.96 for washed and unwashed groups respectively).
C-reactive protein (CRP)
For all subjects median CRP was zero preoperatively and rose significantly each post-operative day (POD) with numerically but not significantly higher levels in the unwashed group at each time point (POD #1: 40 v. 43mg/dl, p = 0.109; and POD #2: 65 v. 79mg/dl, p = 0.284).
Treatment Delivery Sample Subgroup Analysis (Transfused Subjects) (N = 128)
Interleukin (IL)-6 to IL-10 ratio
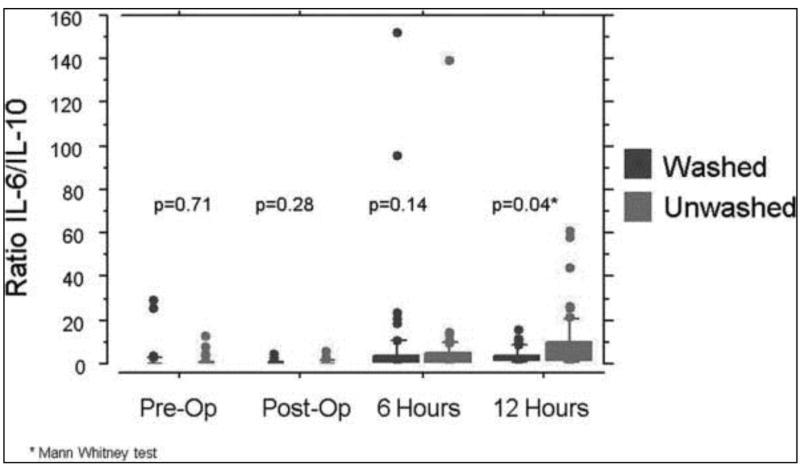
When data from only those subjects that received a blood transfusion were compared, the median IL-6: IL-10 ratio was zero for both subject groups preoperatively and rose slightly (0.11 and 0.2 for washed and unwashed groups respectively) at discontinuation of CPB. For both transfusion groups the median IL-6: IL-10 ratio climbed as time from CPB increased, with lower levels in the washed group and the largest between-group difference 12 hours after CPB: (6 hours: 2.4 v. 2.6, p = 0.14; 12 hours: 3.8 v. 4.8, p = 0.04 for washed and unwashed groups respectively) (Figure 1).
Figure 1.
Between group comparison of IL-6:IL-10 ratio in transfused subjects. Shown are box plots for the median (middle of the box) and 75th percentile (upper edge of each box) of each distribution, with outliers beyond the 95% confidence intervals shown as individual points. The lower end of the box (the 25th percentile) often falls below zero, and thus is not visible in some cases. For the pre-op and post-op values, the median is zero and is superimposed on the x axis and thus no box is shown. P values are shown for individual comparison of the distributions for the washed and unwashed transfusion recipients. Only at 12 hours post-op is there a striking difference between the washed and unwashed arms, with many more high outliers in the unwashed group.
Interleukin 6
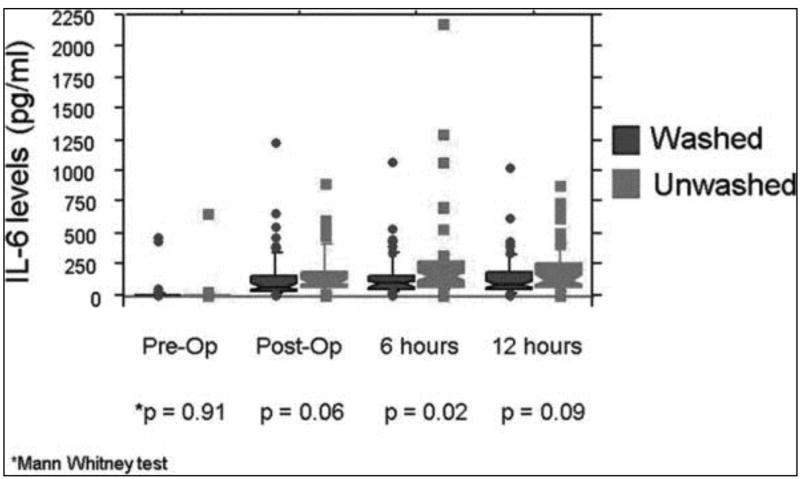
Median pre-operative IL-6 levels were zero in both transfusion groups, with IL-6 levels rising post-operatively, peaking six hours post-CPB. Median IL-6 levels remained lower in the washed than unwashed group at each time point, and were significantly lower six hours after coming off CPB (post-CPB: 65 v. 100 pg/dl, p = 0.06; 6 hours: 89 v. 152 pg/dl, p = 0.02; 12 hours: 84 v. 122 pg/dl, p = 0.09) (Figure 2).
Figure 2.
Between group comparison of IL-6 levels in transfused subjects. Shown are box plots for the median (middle of the box) and 75th percentile (upper edge of each box) of each distribution, with outliers beyond the 95% confidence intervals shown as individual points. For the pre-op values, the median is zero and superimposed on the x axis and thus no box is shown. The lower end of the box (the 25th percentile) often falls below zero, and thus is not visible in some cases. P values are shown for individual comparison of the distributions for the washed and unwashed transfusion recipients. Only at 6 hours post-op is there a striking difference between the washed and unwashed arms, with many more high outliers and a higher median level in the unwashed group.
Interleukin 10
Median pre-operative IL-10 levels were similar between groups (2 v. 3.1 pg/dl; p =0.17 in washed and unwashed groups respectively). Median IL-10 levels peaked immediately after discontinuation of CPB and were higher in the washed subjects (570 v. 492 pg/dl; p = 0.89). Post-operative median IL-10 were numerically but not statistically lower in washed subjects post-operatively (6 hours: 37 v. 57 pg/dl, p = 0.19; 12 hours: 22 v. 25 pg/dl, p = 0.33).
CRP
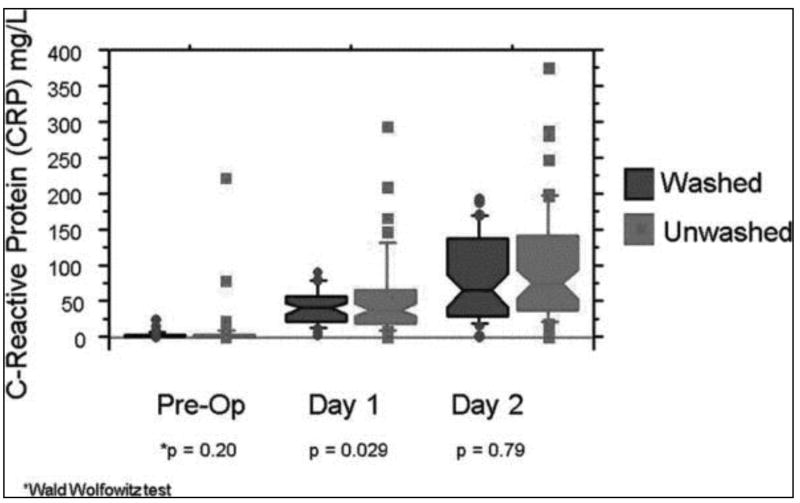
Median CRP levels were similar and less than one preoperatively for both subject groups (0.4 mg/dl for washed and 0.6mg/dl unwashed subjects). CRP rose postoperatively in all subjects, with lower levels in those subjects receiving washed versus unwashed blood, reaching significance the first post-operative day (POD #1: 38.4 v. 43.1 mg/dl, p = 0.03; POD#2: 58.6 v. 70 mg/dl, p = 0.29) (Figure 3).
Figure 3.
Between group comparison of C-reactive protein in transfused subjects. Shown are box plots for the median (middle of the box) and 75th percentile (upper edge of each box) of each distribution, with outliers beyond the 95% confidence intervals shown as individual points. The lower end of the box (the 25th percentile) often falls below zero, and thus is not visible in some cases. For the pre-op values, the median is zero and is superimposed on the x axis and thus no box is shown. P values are shown for individual comparison of the distributions for the washed and unwashed transfusion recipients. Only on day 1 post-op is there a striking difference between the washed and unwashed arms, with many more high outliers in the unwashed group.
Blood transfusions
Of the 162 subjects that completed study participation, 34 (21%) did not receive any blood products, 17 in each group. Total number of RBC, platelet, plasma and cryoprecipitate transfusions were numerically but not significantly lower in the washed group compared to the unwashed group (203 v. 260), with a mean of 2.48 ± 3.5 v. 3.22 ± 6.5 (p = 0.37) transfusions in the washed and unwashed groups respectively.
RBCs and donor exposures
Fewer RBC transfusions were given to subjects in the washed group compared to the unwashed group (191 v. 212), with a mean of 2.34 ± 3.1 transfusions per subject in the washed group, compared to 2.63 ± 4.0 in the unwashed group (p = 0.61). Correspondingly, the washed group had fewer RBC donor exposures than the unwashed group (154 v. 165), with a mean of 1.9 ± 2.1 RBC exposures per subject in the washed group, compared to 2.04 ± 2.5 in the unwashed group (p = 0.7).
Pro-hemostatic blood components
The washed group received fewer peri-operative platelet (4 v. 15), plasma (5 v. 15), and cryoprecipitate (2 v. 9) transfusions than the unwashed group, though these differences were not statistically significant (Table 2).
Table 2.
Clinical outcomes for Treatment delivery sample (Transfused subjects).
| Variable | Washed Group | Unwashed Group | p-value |
|---|---|---|---|
| N=64 | N = 64 | ||
| No. RBC transfusions | 2.97 ± 3.27 | 3.27 ± 4.3 | 0.662 |
| No. Platelet transfusions | 0.06 ± 0.244 | 0.36 ± 1.5 | 0.139 |
| No. FFP transfusions | 0.08 ± 0.324 | 0.25 ± 1.18 | 0.264 |
| No. Cryo transfusions | 0.03 ± 0.175 | 0.14 ± 0.639 | 0.189 |
| Mechanical ventilation (hrs) | 45 [4-1008] | 51.5 [3-1200] | 0.628 |
| Inotropic/pressors (hrs) | 72 [0-744] | 72 [0-1056] | 0.676 |
| CVC duration (days) | 5.25 ± 4 | 6.6 ± 8 | 0.227 |
| Mediastinal tube (days) | 8.75 ± 5.4 | 8.61 ± 5.0 | 0.883 |
| Antibiotics | 18 (28%) | 18 (28%) | 1.0 |
| Infection | 13 (20%) | 13 (20%) | 1.0 |
| Thrombosis | 7 (11%) | 8 (12.5%) | 0.759 |
| Arrhythmias | 14 (21.9%) | 16 (25%) | 0.677 |
| PCICU Admission lactate | 3.0 ± 2.3 | 2.94 ± 1.9 | 0.764 |
| Peak lactate | 4.0 ± 3.0 | 4.0 ± 2.9 | 0.965 |
| Volume [cryst + albumin] | 50 [0-980] | 48 [0-3195] | 0.882 |
| POD 0-2 (mL) | |||
| Highest WBC POD 0-2 | 17.9 ± 4.5 | 18.4 ± 6.6 | 0.613 |
| PCICU complication | 22 (34%) | 22 (34%) | 1.0 |
| PCICU length of stay (days) | 8.6 ± 10.8 | 8.9 ± 12.36 | 0.861 |
| Hospital length of stay (days) | 8 [3-78] | 8.5 [4-56] | 0.574 |
| ECMO | 0 | 2 | 0.156 |
| Death | 2 (3.1%) | 6 (9.3%) | 0.146 |
Values expressed as: number (%); mean ± SD, or median [range]. Independent t-tests were performed for normally distributed data. Mann Whitney tests were performed for data that was not normally distributed.
No. = number, RBC = red blood cell, FFP = fresh frozen plasma, Cryo = cryoprecipitate, PCICU = pediatric cardiac intensive care unit, POD = post-operative day, ECMO = extra-corporeal membrane oxygenation; CVC = central venous catheter.
Storage Age
The storage age of the RBCs transfused to each treatment group was similar (16.1 ± 7.3 and 17.6 ± 8.7 days; p = 0.23) for the unwashed and washed groups respectively. All platelets were transfused between 3-5 days following collection (the first 3 days were required for infectious disease testing). FFP was transfused within 24 hours, and cryoprecipitate within 6 hours of thawing.
Clinical Outcomes
Protocol violations occurred in three subjects (<2%), one in the washed group and two in the unwashed group. The washed group subject was a neonate with hypoplastic left heart syndrome (HLHS) following modified Norwood (Sano) palliation who received one unwashed platelet transfusion; (all other transfusions were according to protocol). The two unwashed subjects both required extracorporeal membrane oxygenation (ECMO) support, and per the current standard of care at URMC, all cellular blood products transfused on ECMO are washed to prevent hyperkalemia. The first subject had HLHS with Glenn shunt complicated by atrioventricular valve regurgitation. He underwent St. Jude valve replacement and was on ECMO for ten hours post-op, then returning to the unwashed strategy. The second subject had anomalous right and left coronary arteries (off the main pulmonary artery) and transitioned from CPB to ECMO. He received washed blood on ECMO and subsequently died when ECMO was discontinued on POD #4.
Although not powered to test for statistical differences, clinical outcomes were similar between subject groups (intent to treat). When clinical outcomes were compared between only those children who received transfusions, the results remained similar (Table 2).
Only one subject in the unwashed arm underwent re-exploration for bleeding (first ECMO subject described above). There were eight (4.9%) deaths overall (2 in washed group, 6 in unwashed group), with a higher mortality rate in the unwashed (7.4%) than the washed arm (2.5%) of the study, however this difference was not statistically significant (p=0.15). Five were neonates undergoing stage 1 palliation, two required ECMO (details above), and one was and infant with Trisomy 21 and an atrioventricular septal defect who succumbed from a pulmonary hypertensive crisis.
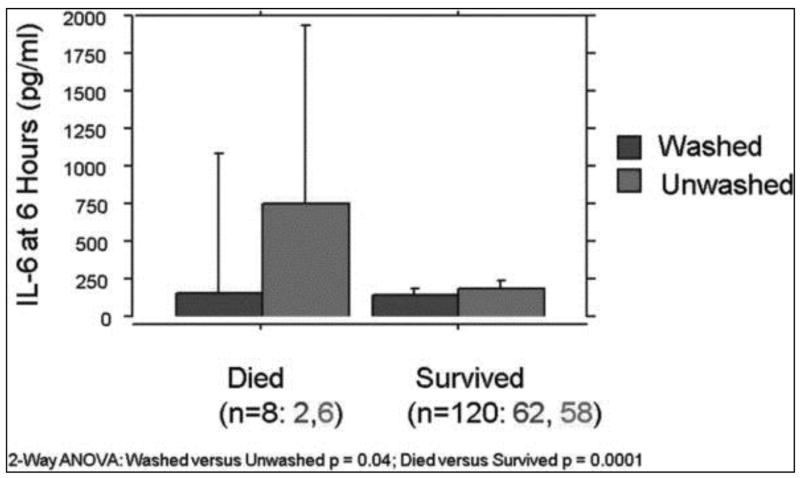
In the 120 subjects who survived, IL-6 levels were similar between transfusion groups. When the mean IL-6 levels were compared in subjects who later died, six hour post-op levels were significantly higher in those subjects who received unwashed transfusions (125 v. 750 pg/dl; p< 0.01) with higher levels of IL-6 associated with death (Figure 4).
Figure 4.
Association of IL-6 and survival in transfused subjects. Bar charts displaying the mean and 95% upper confidence interval for each group are shown. Patients in the unwashed group had higher mean levels of IL-6 at 6 hours as shown in Figure 2, this difference being primarily due to the very high 6 hour IL-6 levels in the six patients in the unwashed arm that died.
When subjects with clinically significant infections were studied, six hour IL-6 and POD #2 CRP levels were significantly higher in those subjects receiving unwashed transfusions (IL-6: 427 v. 176 pg/dl, p <0.01; CRP: 147 v. 91 mg/dl, p < 0.01) compared to washed group subjects.
Discussion
In this study, washing RBC and platelets transfused to children undergoing open heart surgery reduced post-operative inflammatory biomarkers. Subjects transfused with washed blood had significantly lower post-operative IL-6 levels and lower IL-6:IL-10 ratios than subjects receiving unwashed blood, consistent with a more favorable inflammatory profile. As previous work has demonstrated, higher IL-6 levels are associated with greater illness severity, longer length of hospital stay, sepsis and death (61), although not powered to assess for clinical outcomes, we believe our results may have clinical implications. This is supported by the fact that IL-6 levels are known to rise following CPB (62-64) and correlate with postoperative morbidity (65). Furthermore, in pediatric patients following CPB, postoperative IL-6 levels correlate with length of inotropic support, mechanical ventilation, and increased oxygen requirement (66), and patients undergoing the most complex surgeries have the highest levels of IL-6 (67).
In this series, IL-10 levels immediately off CPB were numerically but not significantly higher in subjects receiving washed blood compared to those receiving unwashed blood. These results are consistent with an improved inflammatory balance as IL-10, an anti-inflammatory cytokine (68), stimulates the compensatory anti-inflammatory response syndrome (69), with elevated levels correlating with adverse clinical outcomes including multiple organ dysfunction, sepsis (70-72) and mortality (73,74). Interleukin-10 levels are known to rise following pediatric cardiac surgery with CPB (75-77). In this study, IL-10 levels were lower 6 and 12 hours post-CPB in subjects receiving washed blood, likely due to lower IL-6 levels at those time points, requiring a less vigorous compensatory response.
Similar to our study, Bilgin et al 2010 studied the effect of leukocyte-depleted RBC transfusions on postoperative inflammatory mediators and postoperative complications in 346 adults undergoing cardiac valve surgery (78). Increased concentrations of IL-6 and IL-12 occurred in subjects receiving leukocyte-containing RBCs, and higher IL levels were measured in those subjects developing postoperative infections and multiple organ dysfunction. Multivariate analysis demonstrated an association between elevated IL-6 concentration and multiple organ dysfunction and hospital mortality. They conclude that leukocyte-containing RBCs adversely affect the postoperative proinflamatory response, and may affect postoperative complications.
Our results indicate that, in addition to leukoreduction, further modification of stored blood cells with the removal of residual leukocytes and bioactive substances by washing, decreases the inflammatory response in the recipient. Whether this may lead to improved clinical outcomes warrants further study. Additionally, the effect of RBC storage duration requires consideration. It has been hypothesized that “older” RBCs are more likely to be associated with adverse clinical outcomes than “fresh” blood (79). We believe that it is the bioactive mediators in the supernatant of older RBC that is likely responsible for the proinflammatory (and potential clinical) impact on the transfusion recipient. We propose that adverse effects from increased numbers of RBC and component therapy transfusions, and storage duration, may be ameliorated by washing. As neonates and infants undergoing cardiac surgery with CPB receive the largest numbers of RBC and platelet transfusions, we believe they are particularly susceptible to the inflammatory impact of those transfusions, and would benefit most from the washing of those cells.
This study is limited in that it was not powered to test for clinical outcomes. Biomarkers were chosen as primary outcome measures as they are continuous variables (improving power) strongly associated with adverse outcomes. Local patient numbers precluded powering the study for clinical outcomes which would be of greater relevance. It is possible that there is a significant genomic component to the measured biomarkers and children may behave very different clinically, with similar biochemical profiles. Fortunately, the two study groups had similar pre-operative interleukin levels and similar demographics including genetic and comorbid conditions. Larger studies following biomarkers over time, both pre and post-operatively, may help to better delineate the correlation between an individual's biomarker level and their clinical condition. The short term measurements of interleukin levels are a limitation of this study. Serial measurements of interleukin levels over a longer post-operative period would add important information regarding the impact of the transfusion strategy on both laboratory and clinical parameters.
A study with a more homogenous subject population undergoing similar surgeries would be of greater interest, as it would allow for more direct between-group comparisons. As neonates and young infants receive the largest numbers of RBC and platelet transfusions, it would be more rigorous to study only those subjects, and the results would be more powerful with less heterogeneous cardiac defects and surgical procedures. Local subject numbers precluded use of such a restricted study population and would require a multi-center trial. Additional subgroup analysis of subjects undergoing similar cardiac procedures and tests for associations between laboratory measures and clinical outcomes is needed.
Of note, none of the 34 patients who were randomized but did not receive transfusions died, emphasizing, in our view, that transfusion is both a marker for severity of illness/difficulty of surgery, as well as a potentially toxic therapy. Leukoreduction has been proven to reduce the toxicity of transfusion in cardiac surgery (18), and our results suggest that supernatant removal by washing may have the potential to further reduce the morbidity that follows transfusions in this setting. Preliminary data from a small randomized trial suggests that washed transfusions may improve clinical outcomes in a very different patient population, adults with acute leukemia (48).
Furthermore, at the time of study design reviewers and clinicians expressed concern that the time necessary to perform washing procedures would place subjects at risk from delays in receiving necessary blood transfusions (particularly platelets). There was only one subject where active mediastinal tube bleeding was thought to preclude waiting 2 hours for platelets to be washed, resulting in only one such protocol violation in all 164 subjects. The other two protocol violations in fact caused unwashed group subjects to receive washed products due to their increased safety profile. The extremely low rate of protocol violations in this study indicates that the technical challenge and time necessary for washing does not preclude use of washed products even in this critically ill post-operative population.
In this series very few subjects, <3%, required platelet, FFP and/or cryoprecipitate transfusions. This suggests that routine use of these products is unnecessary for achieving excellent results in terms of morbidity and mortality. Indeed, transfusion of these blood components probably carries significant risks. Our local CPB strategy does not include use off FFP in the prime, nor routine use of these pro-hemostatic products on/or coming off CPB, and very few of our patients required such products. Additionally, low numbers of RBC transfusions were utilized in this series when compared to many institutions. The low number of RBC transfusions and platelet transfusions may actually have hindered the detection of a beneficial clinical effect of washing. When larger numbers of RBC and platelet transfusions are given, washing potentially would have greater impact. A similar study performed in a center where larger numbers of RBC and platelet products are transfused, may have produced more striking clinical results.
Two subjects in the unwashed group (but no washed group subjects) required ECMO support, contributing to the larger numbers of total RBC and coagulant product transfusions in the unwashed group. A larger study with more patients on ECMO may demonstrate that the larger volume of blood product transfusions may overwhelm any potential benefit from blood cell modification via washing. However, the significant risk of electrolyte disturbances associated with large volume transfusions prompts washing of blood transfused on ECMO to be performed at many centers. Therefore the “sickest” patients may end up receiving washed blood and future studies may choose to exclude subjects requiring ECMO from data analysis. In the current study when the two ECMO subjects were removed from the analysis the difference between transfusion groups persisted (220 RBC and 14 coagulant product transfusions in the unwashed group v. 202 RBC and 12 coagulant product transfusions in the washed group).
Our results are particularly generalizable as virtually all eligible patients were enrolled over the 23 month period of the study. Data from the current study demonstrate that modification of RBC and platelet transfusions by washing mitigates post-transfusion inflammation and immunomodulation and non-significantly reduced the number of transfusions and donor exposures. Whether these laboratory findings are clinically relevant, and whether they are reproducible in other institutions, remains unknown. Whether the trend towards fewer transfusions in subjects receiving washed blood persists in larger studies would be of great interest, as would be testing for an association with post-operative complications. A large multi-center clinical trial powered for clinical outcomes is warranted. Focus on those subgroups undergoing similar cardiac procedures would add strength to such study. Expansion of clinical trials of washed transfusions to other critically ill patients is warranted by the results of this and previous studies.
Conclusions
This is the first investigation in any clinical setting demonstrating that removal of stored red cell supernatant prior to transfusion reduces inflammatory biomarkers in the period after transfusion. As previous studies have demonstrated an association of these laboratory signals with clinical outcomes, we postulate that washing blood cell products may decrease post-operative complications. Washing blood transfused to children undergoing cardiac surgery with CPB can be done safely, without increased risk of bleeding or adverse outcomes, despite the time required for this modification.
In this study use of washed products numerically but not significantly decreased the total number of RBC and pro-hemostatic component transfusions, and donor exposures. Adoption of a washed blood cell transfusion protocol should be considered in order to limit the potential risks of blood cell transfusions, particularly in high-risk infant and neonatal populations. That subjects randomized to receive washed transfusions required fewer RBC and component transfusions is of great interest as the risks associated with blood transfusions are increasingly recognized, with larger numbers of transfusions associated with worse clinical outcomes (80). These results should provide an impetus for larger studies of washed transfusions powered for clinical outcomes to confirm these results and determine their clinical significance.
CONSORT Flow Diagram.
Acknowledgments
We wish to thank the children and families who participated in this study. We also wish to thank Regina Cable, PNP, for her assistance with consent procedures. We thank the pediatric cardiac operating room staff and perfusionists for their assistance with study procedures. We thank Michael Anne for running the Luminex cytokine assays. We thank the PCICU attendings, fellows and staff for their assistance with laboratory data collection, and Eileen Taillie MG for her assistance with data entry and analysis.
Financial support: This study was funded in part by a Strong Children's Research Development award from the URMC-Department of Pediatrics (JMC), and from the National Institute of Environmental Health Sciences/National Institute of Health (ES01247) and the National Heart Lung and Blood Institute/National Institute of Health (HL100051, HL095467) (RP). These funding sources did not have any role in the study design, collection, analysis and/or in the decision to submit the manuscript for publication. There are no relationships with industry.
Footnotes
Conflict of interest statement: Dr. Blumberg has served as a consultant to and research grant recipient from manufacturers of leukoreduction filters (Pall Biomedical, Fenwall) and cell washing devices (Caridian). Caridian provided a small proportion of the cell washing sets for patients in the washed arm of the study. No other author has any financial or personal relationship with other people or organizations that could inappropriately influence his/her work.
References
- 1.Levy JH, Tanaka KA. Inflammatory Response to Cardiopulmonary Bypass. Ann Thorac Surg. 2003;75(2):S715–20. doi: 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- 2.Edmunds LH. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1998;66(5S):S12–6. doi: 10.1016/s0003-4975(98)00967-9. discussion S25-8. [DOI] [PubMed] [Google Scholar]
- 3.Gessler P, Pretre R, Hohl V, Rousson V, Fischer J, Dahinden C. CXC-chemokine stimulation of neutropils correlates with plasma levels of myeloperoxidase and lactoferrin and contributes to clinical outcome after pediatric cardiac surgery. Shock. 1994;22:513–520. doi: 10.1097/01.shk.0000145939.54838.51. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury D, Ojamaa K, Parnell VA, et al. A prospective randomized clinical study of thyroid hormone treatment after operations for complex congenital heart disease. J Thorac Cardiovasc Surg. 2001;12:1023–1025. doi: 10.1067/mtc.2001.116192. [DOI] [PubMed] [Google Scholar]
- 5.De Mendonca-Filho HT, Gomes RV, de Almeida Campos LA, et al. Circulating levels of macrophage migration inhibitory factor are associated with mild pulmonary dysfunction after cardiopulmonary bypass. Shock. 2004;22:533–537. doi: 10.1097/01.shk.0000142817.84070.df. [DOI] [PubMed] [Google Scholar]
- 6.Finkel MS, Oddis CV, Jacob TD, et al. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 7.Gando S, Nishihira J, Kemmotsu O, et al. An increase in macrophage migration inhibitory factor release in patients with cardiopulmonary bypasss surgery. Surg Today. 2000;30:689–694. doi: 10.1007/s005950050041. [DOI] [PubMed] [Google Scholar]
- 8.McMahon CK, Klein I, Ojamaa K. Interleukin-6 and thyroid hormone metabolism in pediatric cardiac surgery patients. Thyroid. 2003:301–304. doi: 10.1089/105072503321582123. [DOI] [PubMed] [Google Scholar]
- 9.Stayer SA, Diaz LK, East DL, et al. Changes in respiratory mechanics among infants undergoing heart surgery. Anesth Analg J. 2004;38:307–311. doi: 10.1213/01.ANE.0000096005.25218.74. [DOI] [PubMed] [Google Scholar]
- 10.Hirai S. Systemic inflammatory response syndrome after cardiac surgery under cardiopulmonary bypass. Ann Thorac Cardiovasc Surg. 2003;9:365–70. [PubMed] [Google Scholar]
- 11.Holmes JH, 4th, Connolly NC, Paull DL, et al. Magnitude of the inflammatory response to cardiopulmonary bypass and its relation to adverse clinical outcomes. Inflamm Res. 2002;51:579–586. doi: 10.1007/pl00012432. [DOI] [PubMed] [Google Scholar]
- 12.Madhok AB, Ojamaa K, Haridas V, Parnell VA, Pahwa S, Chowdhury D. Cytokine response in children undergoing surgery for congenital heart disease. Pediatr Cardiol. 2006;27:408–13. doi: 10.1007/s00246-006-0934-y. [DOI] [PubMed] [Google Scholar]
- 13.Allen ML, Hoschtitzky JA, Peters MJ, et al. Interleukin-10 and its role in clinical immunoparalysis following pediatric cardiac surgery. Crit Care Med. 2006;34:2658–65. doi: 10.1097/01.CCM.0000240243.28129.36. [DOI] [PubMed] [Google Scholar]
- 14.Hirai S. Systemic inflammatory response syndrome after cardiac surgery under cardiopulmonary bypass. Ann Thorac Cardiovasc Surg. 2003;9:365–370. [PubMed] [Google Scholar]
- 15.Varan B, Tokel K, Mercan S, Donmez A, Aslamaci S. Systemic inflammatory response related to cardiopulmonary bypass and its modification by methyl prednisolone; high dose versus low dose. Pediatr cardiol. 2002;23:437–441. doi: 10.1007/s00246-002-0118-3. [DOI] [PubMed] [Google Scholar]
- 16.Hamada Y, Kohtani T, Nakata T, et al. Blood transfusion under cardiopulmonary bypass is a possible inducer for inflammation. Kyobu Geka. 2001;54(10):835–8. [PubMed] [Google Scholar]
- 17.Bilgin YM, van de Watering LM, Eijsman L, et al. Double-blind, randomized controlled trial on the effect of leukocyte-depleted erythrocyte transfusions in cardiac valve surgery. Circulation. 2004;109(22):2755–60. doi: 10.1161/01.CIR.0000130162.11925.21. [DOI] [PubMed] [Google Scholar]
- 18.Van de Watering LM, Hermans J, Houbiers JG, et al. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation. 1998;97(6):562–8. doi: 10.1161/01.cir.97.6.562. [DOI] [PubMed] [Google Scholar]
- 19.Zacharias A, Habib RH. Factors predisposing to median sternotomy complications. Deep vs. superficial infection. Chest. 1996;110:1173–8. doi: 10.1378/chest.110.5.1173. [DOI] [PubMed] [Google Scholar]
- 20.Leal-Noval SR, Marques-Vacaro JA, Garcia-Curiel A, et al. Nosocomial pneumonia in patients undergoing heart surgery. Crit Care Med. 2000;28:935–40. doi: 10.1097/00003246-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Ranucci M, Pavesi M, Mazza E, et al. Risk factors for renal dysfunction after coronary surgery: the role of cardiopulmonary bypass technique. Perfusion. 1994;9:319–26. doi: 10.1177/026765919400900503. [DOI] [PubMed] [Google Scholar]
- 22.Michalopoulos A, Stavridis G, Geroulanos S. Severe sepsis in cardiac surgical patients. Eur J Surg. 1998;164:217–22. doi: 10.1080/110241598750004670. [DOI] [PubMed] [Google Scholar]
- 23.Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of Blood Transfusion on Long-Term Survival After Cardiac Operation. Ann Thorac Surg. 2002;74:1180–6. doi: 10.1016/s0003-4975(02)03766-9. [DOI] [PubMed] [Google Scholar]
- 24.Michalopoulos A, Tzelepis G, Dafni U, Geroulanos S. Determinants of hospital mortality after coronary artery bypass grafting. Chest. 1999;115:1598–603. doi: 10.1378/chest.115.6.1598. [DOI] [PubMed] [Google Scholar]
- 25.Willems A, Harrington K, Lacroix J, et al. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery: A subgroup analysis. Crit Care Med. 2010;38(2):649–56. doi: 10.1097/CCM.0b013e3181bc816c. [DOI] [PubMed] [Google Scholar]
- 26.Cholette JM, Rubenstein JS, Alfieris GM, Powers KS, Eaton M, Lerner NB. Children with single ventricle physiology do not benefit from higher hemoglobin levels post cavopulmonary connection: Results of a prospective, randomized controlled trial of a restrictive versus liberal red-cell transfusion strategy. Pediatr Crit Care Med. 2011;1(12):39–45. doi: 10.1097/PCC.0b013e3181e329db. [DOI] [PubMed] [Google Scholar]
- 27.Kao KJ. Mechanisms and new approaches for the allogenic blood transfusion-induced immunomodulatory effects. Transfus Med Rev. 2000;14:12–22. doi: 10.1016/s0887-7963(00)80112-1. [DOI] [PubMed] [Google Scholar]
- 28.Kirkley SA, Cowles J, Pellegrini VD, Jr, et al. Cytokine secretion after allogeneic or autologous blood transfusion. Lancet. 1995;345:527. doi: 10.1016/s0140-6736(95)90627-4. [DOI] [PubMed] [Google Scholar]
- 29.Kirkley SA, Cowles J, Pellegrini VD, Jr, et al. Blood transfusion and total joint replacement surgery: T helper 2 (Th2) cytokine secretion and clinical outcome. Tranfus Med. 1998;8:195–204. doi: 10.1046/j.1365-3148.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- 30.Blajchman MA, Bordin JO. Mechanisms of transfusion-associated immunosuppression. Curr Opin Hematol. 1994;1:457–461. [PubMed] [Google Scholar]
- 31.Jensen LS, Andersen AJ, Christiansen PM, et al. Postoperative infection and natural killer cell function following blood transfusion in patients undergoing elective colorectal surgery. Br J Surg. 1992;79:513–516. doi: 10.1002/bjs.1800790613. [DOI] [PubMed] [Google Scholar]
- 32.Brunson ME, Alexander JW. Mechanisms of transfusion-induced immunosuppression. Transfusion. 1990;30:651–658. doi: 10.1046/j.1537-2995.1990.30790385527.x. [DOI] [PubMed] [Google Scholar]
- 33.Blumberg N. Deleterious clinical effects of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion. 2005;45(s):33S–9S. doi: 10.1111/j.1537-2995.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 34.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: fact or fiction? Blood. 2001;97:1180–1195. doi: 10.1182/blood.v97.5.1180. [DOI] [PubMed] [Google Scholar]
- 35.Bordin JO, Heddle NM, Blajchman MA. Biologic effects of leukocytes present in transfused cellular blood products. Blood. 1994;84:1705–1721. [PubMed] [Google Scholar]
- 36.Frabetti F, Musiani D, Marini M, et al. White cell apoptosis in packed red cells. Transfusion. 1998;38:1082–9. doi: 10.1046/j.1537-2995.1998.38111299056320.x. [DOI] [PubMed] [Google Scholar]
- 37.Mincheff M. Changes in donor leukocytes during blood storage. Implications on post-transfusion immunomodulation and transfusion-associated GVHD. Vox Sang. 1998;74(S2):189–200. doi: 10.1111/j.1423-0410.1998.tb05420.x. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen HJ, Reimert CM, Pedersen AN, et al. Time-dependent, spontaneous release of white cell-and platelet-derived bioactive substances from stored human blood. Transfusion. 1996;36:960–965. doi: 10.1046/j.1537-2995.1996.36111297091738.x. [DOI] [PubMed] [Google Scholar]
- 39.Ghio M, Contini P, Mazzei C, et al. In vitro immunosuppressive activity of soluble HLA class I and Fas-ligand molecules: do they play a role in autologous blood transfusion? Transfusion. 2001;41:988–996. doi: 10.1046/j.1537-2995.2001.41080988.x. [DOI] [PubMed] [Google Scholar]
- 40.Kristiansson M, Soop M, Saraste L, Sundqvist KG. Cytokines in stored red blood cell concentrates: promoters of systemic inflammation and simulators of acute transfusion reactions? Acta Anaesthesiol Scand. 1996;40:496–501. doi: 10.1111/j.1399-6576.1996.tb04475.x. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen HJ, Reimert CM, Pedersen AM, et al. Time-dependent, spontaneous release of white cell and platelet derived bioactive substances from stored human blood. Tranfusion. 1996;36:960–965. doi: 10.1046/j.1537-2995.1996.36111297091738.x. [DOI] [PubMed] [Google Scholar]
- 42.Magee CC, Sayegh MH. Peptide-mediated immunosuppression. Curr Opin Immunol. 1997;9:669–675. doi: 10.1016/s0952-7915(97)80047-7. [DOI] [PubMed] [Google Scholar]
- 43.Silliman CC, Clay KL, Thurman GW, Hohnson CA, Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–694. [PMC free article] [PubMed] [Google Scholar]
- 44.Silliman CC, Johnson CA, Clay KL, Thurman GW, Ambruso Dr. Compounds biologically similar to platelet activating factor are present in stored blood components. Lipids. 1993;28:415–418. doi: 10.1007/BF02535939. [DOI] [PubMed] [Google Scholar]
- 45.Fransen E, Maessen J, Dentener M, Senden N, Burman W. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest. 1999;116:1233–1239. doi: 10.1378/chest.116.5.1233. [DOI] [PubMed] [Google Scholar]
- 46.Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370:415–26. doi: 10.1016/S0140-6736(07)61197-0. [DOI] [PubMed] [Google Scholar]
- 47.Blumberg N, Heal JM, Gettings KF, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50:2738–44. doi: 10.1111/j.1537-2995.2010.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blumberg N, Heal JM, Rowe JM. A randomized trial of washed red blood cell and platelet transfusions in adult acute leukemia. BMC Blood disorders. 2004;4:6. doi: 10.1186/1471-2326-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doughty LA, Kaplan SS, Carcill JA. Inflammatory cytokine and nitric oxide responses in pediatric sepsis and organ failure. Crit Care Med. 1996;24(7):1137–1143. doi: 10.1097/00003246-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Finnerty CC, Herndon DN, Chinkes DL, Jeschke MG. Serum Cytokine differences in severely burned children with and without sepsis. Shock. 2007;27(1):4–9. doi: 10.1097/01.shk.0000235138.20775.36. [DOI] [PubMed] [Google Scholar]
- 51.Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A. The ratio of interleukin-6 to interleukin-10 correlates with severity in patients with chest and abdominal trauma. Am J Emerg Med. 1999;17:548–551. doi: 10.1016/s0735-6757(99)90194-8. [DOI] [PubMed] [Google Scholar]
- 52.Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med. 1999;27(7):1262–1264. doi: 10.1097/00003246-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Loisa P, Rinne T, Laine S, Hurme M, Kaukinen S. Anti-inflammatory cytokine response and the development of multiple organ failure in severe sepsis. Acta Anaesthesiol Scan. 2003;47:319–325. doi: 10.1034/j.1399-6576.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- 54.Day NP, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TTH, Mai NTH, Phu NG, Sinh DX, White NJ, Ho M. The prognostic and pathophysiologic role of pro-and anti-inflammatory cytokines in severe malaria. JID. 1999;180:1288–97. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 55.Doughty LA, Kaplan SS, Carcill JA. Inflammatory cytokine and nitric oxide responses in pediatric sepsis and organ failure. Crit Care Med. 1996;24(7):1137–1143. doi: 10.1097/00003246-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 56.Kalmin ND, Brown DJ. Platelet washing with a blood cell processor. Transfusion. 1982;22:125–7. doi: 10.1046/j.1537-2995.1982.22282177118.x. [DOI] [PubMed] [Google Scholar]
- 57.Vesilind GW, Simpson MB, Shifman MA, Colman RE, Kao KJ. Evaluation of a centrifugal blood cell processor for washing platelet concentrates. Transfusion. 1988;28:46–51. doi: 10.1046/j.1537-2995.1988.28188127952.x. [DOI] [PubMed] [Google Scholar]
- 58.Vo TD, Cowles J, Heal JM, Blumberg N. Platelet washing to prevent recurrent febrile reactions to leucocyte-reduced transfusions. Transfusion medicine. 2001;11:45–7. doi: 10.1046/j.1365-3148.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 59.Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109:1033–7. doi: 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- 60.Monagle P, Chan A, Massicotte P, Chalmers E, Michelson AD. Antithrombotic Therapy in Children. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3):645S–687S. doi: 10.1378/chest.126.3_suppl.645S. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan JS, Kilpatrick L, Costarino AT, Jr, et al. Correlation of plasma cytokine elevations with mortality rate in children with sepsis. J Pediatr. 1992;120:510–515. doi: 10.1016/s0022-3476(05)82476-x. [DOI] [PubMed] [Google Scholar]
- 62.Schroeder VA, Pearl JM, Schwartz SM, Shanley TP, Manning PB, Nelson DP. Combined steroid treatment for congenital heart surgery improves oxygen delivery and reduces postbypass inflammatory mediator expression. Circulation. 2003;107:2823–2828. doi: 10.1161/01.CIR.0000070955.55636.25. [DOI] [PubMed] [Google Scholar]
- 63.Eggum R, Ueland T, Mollnes TE, Videm V, Aukrust P, Fiane AE, Lindberg HL. Effect of perfusion temperature on the inflammatory response during pediatric cardiac surgery. Ann Thorac Surg. 2008;85:611–7. doi: 10.1016/j.athoracsur.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 64.Gessler P, Pfenninger J, Pfammatter J, Carrel T, Baenziger O, Dahinden C. Plasma levels of interleukin-8 and expression of interleukin-8 receptors on circulating neutrophils and monocytes after cardiopulmonary bypass in children. J Thorac and Cardiovasc Surg. 2003;126(3):718–725. doi: 10.1016/s0022-5223(03)00685-8. [DOI] [PubMed] [Google Scholar]
- 65.Madhok AB, Ojamaa K, Haridas V, Parnell VA, Pahwa S, Chowdhury D. Cytokine response in children undergoing surgery for congenital heart disease. Pediatr Cardiol. 2006;27:408–13. doi: 10.1007/s00246-006-0934-y. [DOI] [PubMed] [Google Scholar]
- 66.Gessler P, Pfenninger J, Pfammatter J, Carrel T, Baenziger O, Dahinden C. Plasma levels of interleukin-8 and expression of interleukin-8 receptors on circulating neutrophils and monocytes after cardiopulmonary bypass in children. J Thorac and Cardiovasc Surg. 2003;126(3):718–725. doi: 10.1016/s0022-5223(03)00685-8. [DOI] [PubMed] [Google Scholar]
- 67.Madhok AB, Ojamaa K, Haridas V, Parnell VA, Pahwa S, Chowdhury D. Cytokine response in children undergoing surgery for congenital heart disease. Pediatr Cardiol. 2006;27:408–13. doi: 10.1007/s00246-006-0934-y. [DOI] [PubMed] [Google Scholar]
- 68.Hietbrink F, Koenderman L, Rijkers G, Leenen L. Trauma: the role of the innate immune system. World J Emerg Surg. 2006;1:15. doi: 10.1186/1749-7922-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mokart D, Capo C, Blache JL, et al. Early postoperative compensatory anti-inflammatory response syndrome is associated with septic complications after major surgical trauma in patients with cancer. Br J Surg. 2002;89:1450–1456. doi: 10.1046/j.1365-2168.2002.02218.x. [DOI] [PubMed] [Google Scholar]
- 70.Seekamp A, Jochum M, Ziegler M, van Griensven M, Martin M, Regel G. cytokines and adhesion moleculres in elective and accidental trauma-related ischemia/reperfusion. J Trauma. 1998;44:874–882. doi: 10.1097/00005373-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 71.Giannoudis PV, Smith RM, Perry SL, Windsor AJ, Dickson RA, Bellamy MC. Immediate IL-10 expression following major orthopaedic trauma: relationship to anti-inflammatory response and subsequent development of sepsis. Intensive Care Med. 2000;26:1076–1081. doi: 10.1007/s001340051320. [DOI] [PubMed] [Google Scholar]
- 72.Von Heymann C, Langenkamp J, Dubisz N, et al. Posttraumatic immune modulation in chronic alcoholics is associated with multiple organ dysfunction syndrome. J Trauma. 2002;52:95–103. doi: 10.1097/00005373-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 73.Hatherill M, Tibby S, Turner C, Tatnavel N, Murdock A. Procalcitonin and cytokine levels: Relationship to organ failure and mortality in pediatric septic shock. Crit Care Med. 2000;28(7):2591–2594. doi: 10.1097/00003246-200007000-00068. [DOI] [PubMed] [Google Scholar]
- 74.Harris MC, D'Angio CT, Gallagher PR, Kaufman D, Evans J, Kilpatrick L. Cytokine elaboration in critically ill infants with bacterial sepsis, necrotizing enterocolitis, or sepsis syndrome: correlation with clinical parameters of inflammation and mortality. J Pediatr. 2005;147:462–8. doi: 10.1016/j.jpeds.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 75.Schroeder VA, Pearl JM, Schwartz SM, Shanley TP, Manning PB, Nelson DP. Combined steroid treatment for congenital heart surgery improves oxygen delivery and reduces postbypass inflammatory mediator expression. Circulation. 2003;107:2823–2828. doi: 10.1161/01.CIR.0000070955.55636.25. [DOI] [PubMed] [Google Scholar]
- 76.Eggum R, Ueland T, Mollnes TE, Videm V, Aukrust P, Fiane AE, Lindberg HL. Effect of perfusion temperature on the inflammatory response during pediatric cardiac surgery. Ann Thorac Surg. 2008;85:611–7. doi: 10.1016/j.athoracsur.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 77.Madhok AB, Ojamaa K, Haridas V, Parnell VA, Pahwa S, Chowdhury D. Cytokine response in children undergoing surgery for congenital heart disease. Pediatr Cardiol. 2006;27:408–13. doi: 10.1007/s00246-006-0934-y. [DOI] [PubMed] [Google Scholar]
- 78.Bilgin YM, van de Watering LM, Versteegh MI, van Oers MH, Brand A. Effects of allogeneic leukocytes in blood transfusions during cardiac surgery on inflammatory mediators and postoperative complications. Crit Care Med. 2010;38(2):720–1. doi: 10.1097/CCM.0b013e3181c0de7b. [DOI] [PubMed] [Google Scholar]
- 79.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178(6):570–2. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 80.Murphy GJ, Reeves BC, Rogers CA, Rizvi SIA, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]