Abstract
Indoleamine 2,3-dioxygenase [indoleamine: oxygen 2,3-oxidoreductase (decyclizing)] activity in the supernatant fraction (30,000 X g, 30 min) of mouse lung homogenate increased approximately 120-fold after infection with PR8 influenza virus. Both specific and total enzyme activities started to increase linearly from the 5th day after infection, reached the highest level around the 11th day, and then gradually decreased to normal values in about 3 weeks. Other enzymes in the lung, such as certain lysosomal enzymes and monoamine oxidase, did not change significantly throughout the experiments. The time course of the increase in the enzyme activity was quite different from that of virus replication in the lung (a peak by the 3rd day and persistence until the 9th day) or that of serum antibody content (started to rise on the 9th day). Rather, it appeared to be closely related to the infiltrations of mononuclear and lymphocytic cells. When mice were exposed to a higher dose of virus and did not recuperate, the time course of the increase of the enzyme activity was essentially identical to that seen with a low concentration of virus. A maximum stimulation of the enzyme activity in the lung occurred on the 9th day after infection; the increase was approximately 100-fold. However, serum antibody content was slight and virus titer in the lung remained high.
Full text
PDF
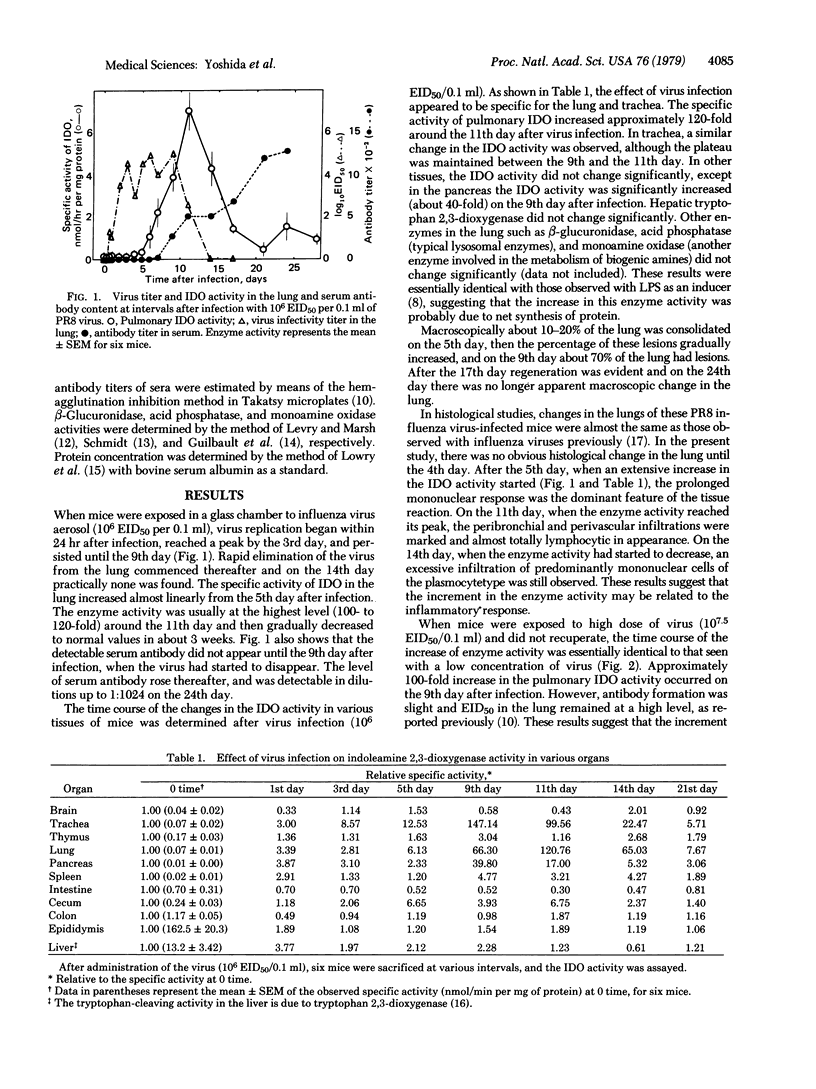
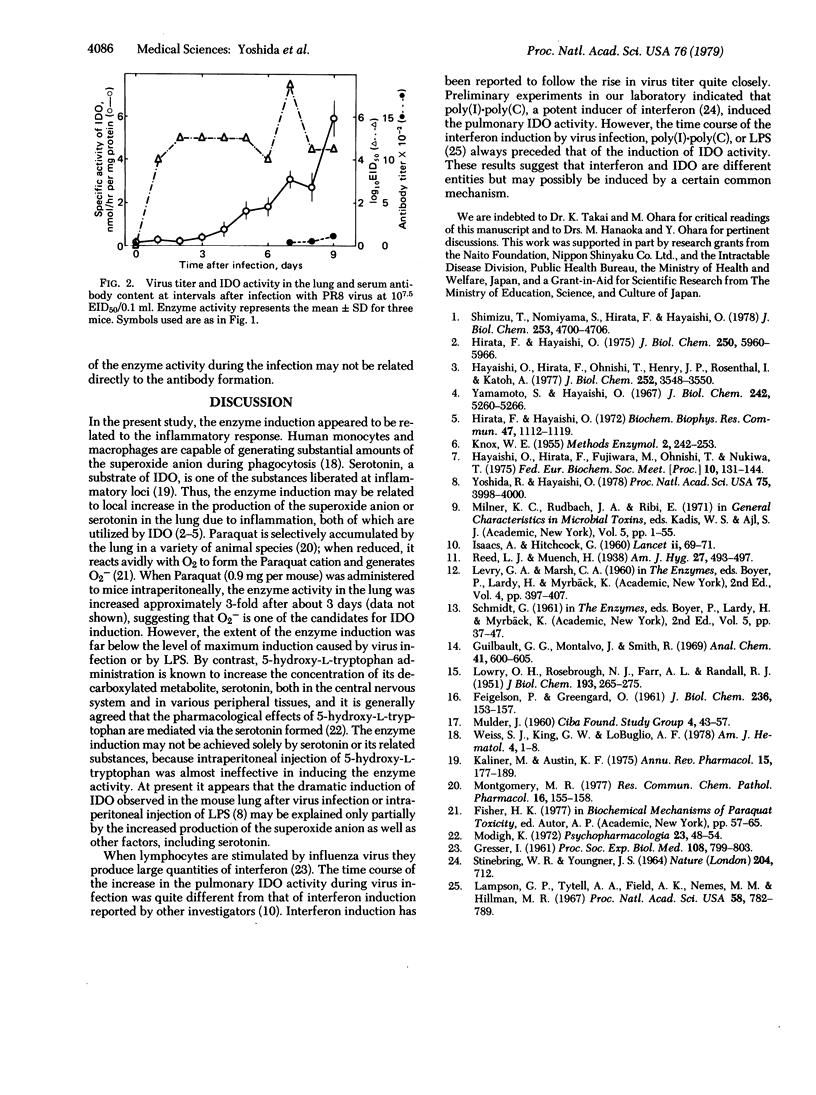
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FEIGELSON P., GREENGARD O. A microsomal iron-porphyrin activator of rat liver tryptophan pyrrolase. J Biol Chem. 1961 Jan;236:153–157. [PubMed] [Google Scholar]
- GRESSER I. Production of interferon by suspensions of human leucocytes. Proc Soc Exp Biol Med. 1961 Dec;108:799–803. doi: 10.3181/00379727-108-27072. [DOI] [PubMed] [Google Scholar]
- Hayaishi O., Hirata F., Ohnishi T., Henry J. P., Rosenthal I., Katoh A. Indoleamine 2,3-dioxygenase: incorporation of 18O2-- and 18O2 into the reaction products. J Biol Chem. 1977 May 25;252(10):3548–3550. [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. New degradative routes of 5-hydroxytryptophan and serotonin by intestinal tryptophan 2,3-dioxygenase. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1112–1119. doi: 10.1016/0006-291x(72)90949-7. [DOI] [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. Studies on indoleamine 2,3-dioxygenase. I. Superoxide anion as substrate. J Biol Chem. 1975 Aug 10;250(15):5960–5966. [PubMed] [Google Scholar]
- ISAACS A., HITCHCOCK G. Role of interferon in recovery from virus infections. Lancet. 1960 Jul 9;2(7141):69–71. doi: 10.1016/s0140-6736(60)91215-0. [DOI] [PubMed] [Google Scholar]
- Kaliner M., Austen K. F. Immunologic release of chemical mediators from human tissues. Annu Rev Pharmacol. 1975;15:177–189. doi: 10.1146/annurev.pa.15.040175.001141. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampson G. P., Tytell A. A., Field A. K., Nemes M. M., Hilleman M. R. Inducers of interferon and host resistance. I. Double-stranded RNA from extracts of Penicillium funiculosum. Proc Natl Acad Sci U S A. 1967 Aug;58(2):782–789. doi: 10.1073/pnas.58.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modigh K. Central and peripheral effects of 5-hydroxytryptophan on motor activity in mice. Psychopharmacologia. 1972;23(1):48–54. doi: 10.1007/BF00414413. [DOI] [PubMed] [Google Scholar]
- Montgomery M. R. Paraquat toxicity and pulmonary superoxide dismutase: an enzymic deficiency of lung microsomes. Res Commun Chem Pathol Pharmacol. 1977 Jan;16(1):155–158. [PubMed] [Google Scholar]
- STINEBRING W. R., YOUNGNER J. S. PATTERNS OF INTERFERON APPEARANCE IN MICE INFECTED WITH BACTERIA OR BACTERIAL ENDOTOXIN. Nature. 1964 Nov 14;204:712–712. doi: 10.1038/204712a0. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Nomiyama S., Hirata F., Hayaishi O. Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem. 1978 Jul 10;253(13):4700–4706. [PubMed] [Google Scholar]
- Weiss S. J., King G. W., LoBuglio A. F. Superoxide generation by human monocytes and macrophages. Am J Hematol. 1978;4(1):1–8. doi: 10.1002/ajh.2830040102. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Hayaishi O. Tryptophan pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme or enzymes. J Biol Chem. 1967 Nov 25;242(22):5260–5266. [PubMed] [Google Scholar]
- Yoshida R., Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3998–4000. doi: 10.1073/pnas.75.8.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]


