Abstract
Androgen receptor (AR) is a ligand-dependent transcription factor, which plays a significant role in prostate carcinogenesis. Blockade of AR and its ligand, androgen is the basis for the treatment of prostate cancer (PCa). Nevertheless, a modest increase in the critical levels of AR mRNA and corresponding protein is sufficient for the development of resistance to antiandrogen therapy. A strategy to further downregulate AR mRNA and protein expression in combination with antiandrogen therapy may prevent or delay the development of androgen-independent PCa. Recent studies show that microRNAs (miRNAs) perform tumor suppressor functions in various cancers. In this study, we demonstrate that the overexpression of miR 488* downregulates the transcriptional activity of AR and inhibits the endogenous AR protein production in both androgen-dependent and androgen-independent PCa cells. In addition, miR 488* blocks the proliferation and enhances the apoptosis of PCa cells. Our data indicate that miR 488* targets AR and is a potential modulator of AR mediated signaling. Our findings provide insight for utilizing miRNAs as novel therapeutics to target AR in PCa.
Keywords: androgen receptor, miRNA, prostate cancer
Introduction
Prostate cancer (PCa) is the most frequently diagnosed malignancy and the second leading cause of cancer-related death in American men.1 Both hereditary and environmental factors have been implicated in the development of PCa. A few PCa susceptibility genes, including HPC1, RNASEL, MSR1, CYP17, SRD5A2 and AR have been characterized.2 Although PCa is heterogeneous in its etiology and progression, AR gene has emerged as the most significant contributor to PCa development. The AR protein is a ligand-dependent transcription factor belonging to the nuclear hormone receptor superfamily.3 The physiological ligands for AR include the androgens, testosterone and dihydrotestosterone (DHT). On activation by the binding of androgens, AR undergoes rapid homodimerization and nuclear translocation, and binds to specific DNA sequences termed androgen-responsive elements (AREs) located in the promoter region of its target genes. After binding to promoters, AR recruits the coregulators together with the basal transcriptional machinery, and modulates the transcription of its target genes.4 This AR signaling axis is required for the growth and development of a normal prostate gland and it also plays a key role in all phases of PCa, from disease initiation to disease progression and the development of treatment resistance.4,5
PCa begins as an androgen-dependent disease, which is managed by a series of therapies that suppress AR signaling by androgen depletion and/or the administration of AR antagonists. However, the regression of tumor growth brought about by these therapies is only temporary and after a short remission period, prostate tumors regrow and become resistant to therapy. At this stage, the tumors are described as androgen-independent or hormone refractory. Accumulating evidence suggests that the progression of PCa to androgen-independent stage does not involve the loss of AR but instead, results from the restoration of AR signaling in PCa cells undergoing treatment.6 On their way to androgen-independence, PCa cells develop a multitude of mechanisms to activate AR in an androgen depleted environment. These mechanisms include AR gene amplification and mutations, overexpression of AR coactivators and ligand-independent activation of AR.7–9 Some prostate tumors have been reported to become truly androgen-independent by activating other cell survival and growth pathways that allow the tumor cells to survive in the absence of AR signaling.7,8 However, the majority of androgen-independent tumors retain dependence on AR signaling. This continued dependence on AR signaling pathway indicates the importance of AR for survival of PCa cells. Knockdown of AR expression in androgen-independent PCa cell lines has been found to inhibit cell proliferation, thus demonstrating the functional role of AR in the growth of androgen-independent PCa cells.10–15 Thus, AR has emerged as a promising therapeutic target for the treatment of both androgen-dependent and androgen-independent PCa.
At present, there is no curative treatment for androgen-independent PCa and it continues to have a fatal prognosis.16 There is a need to develop innovative strategies for targeting AR, which can produce more efficient and durable repression of AR activity in combination with the existing therapies, thereby preventing or delaying the onset of therapy-resistant disease. Several novel AR-inhibitory agents are currently being evaluated. These include more effective AR antagonists, inhibitors of enzymes required for androgen biosynthesis, inhibitors of 5α-reductase, an enzyme required for the conversion of testosterone to the more potent AR ligand, DHT, inhibitors of heat shock protein-90, which protects AR from degradation and inhibitors of histone deacetylases, which are required for AR-dependent transcription.9,16,17 Other strategies being explored for lowering AR expression include targeting AR mRNA expression by using ribozyme,11,18 antisense oligonucleotides,10,19–21 short hairpin RNA (shRNA)22 and small interfering RNAs (siRNAs).12,14,15,23,24 In this article, we have evaluated a microRNA (miRNA) based approach for the suppression of AR activity in PCa cells.
Regulatory miRNAs are small (18–25 nucleotides), endogenous, noncoding RNA molecules involved in the post-transcriptional modulation of gene expression. miRNAs function by binding to partially complementary target sites in 3’ untranslated regions (3’ UTR) of target mRNAs, resulting in translational repression or mRNA degradation.25 miRNAs play important roles in normal cellular processes such as, differentiation, proliferation and apoptosis, and they have also been implicated in cancer.26 Accumulating evidence suggests that miRNAs can contribute to carcinogenesis by acting as tumor suppressors or oncogenes.27 Aberrant expression of miRNAs has been reported in numerous cancers, including PCa.28 Some miRNAs including miR 221, miR 222, miR 125b, miR 126*, miR 146a, miR 330, miR 449a and miR 148a appear to play important roles in PCa by targeting the expression of growth regulatory genes.29–35 Surprisingly, despite the pivotal role of AR in the development and progression of PCa, there is no evidence of a link between AR expression and its potential regulation by miRNAs. Using computational and rational miRNA:mRNA base pairing analyses, we identified a potential target site for miR 488* in the 3’ UTR of AR mRNA. Here, we show the experimental validation of the predicted interaction between miR 488* and AR 3’ UTR. Our data show that miR 488* represses AR expression in PCa cells, leading to inhibition of cellular growth and an increase in apoptosis.
Material and Methods
Cell culture
Human PCa cell lines LNCaP, C4-2B and DU 145 were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine and antibiotics. CHO-K1 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 5% FBS, 2 mM L-glutamine, 1 mM L-proline, 10 mM 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) and antibiotics. All cell lines were maintained in a humidified 5% CO2 atmosphere at 37°C. LNCaP, DU 145 and CHO-K1 cells were obtained from ATCC (Manassas, VA). C4-2B cells were obtained from ViroMed Laboratories (Minnetonka, MN).
Western blotting
LNCaP and C4-2B cells were seeded in six-well plates one day before transfection. Cells were transfected with synthetic hsa-miR-488* mimic or negative control (NC) miRNA mimic (Dharmacon, Chicago, IL) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and harvested 48 hr post transfection for protein extraction. Five micrograms of protein was resolved on NuPAGE 4–12% Bis-Tris gels and electro-transferred to nitrocellulose membranes. Following antibodies were used: mouse monoclonal anti-androgen receptor (AR) antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-β-actin antibody (1:350, Santa Cruz Biotechnology) and horseradish peroxidase conjugated anti-mouse secondary antibody (1:10,000, GE Healthcare, Piscataway, NJ). Bands were detected using the ECL Plus Western blotting detection reagent (GE Healthcare). The signal intensities of bands were measured using the SCION IMAGE analysis software. The level of AR protein expression in each sample was determined by normalizing AR band intensity to β-actin band intensity.
Construction of reporter plasmids, transfections and luciferase assays
WT-3’ UTR (WT: wild type) reporter plasmid was constructed by cloning 77 base pairs (bp) fragment of AR 3’ UTR spanning the predicted target site for miR 488* downstream of firefly luciferase coding region in pMIR-REPORT vector (Ambion, Austin, TX). Site-directed mutagenesis of the putative target site for miR 488* in WT-3’ UTR construct was carried out to generate the MUT-3’ UTR construct. Nucleotide sequences of the constructs were confirmed by DNA sequencing. For luciferase assays, CHO-K1 cells (30,000 cells/well) were plated in 24-well plates one day before transfection. Cells were cotransfected using Lipofectamine 2000 (Invitrogen), with 100 ng of WT-3’ UTR or MUT-3’ UTR firefly luciferase reporter construct, 0.5 ng of renilla luciferase reporter plasmid (Promega, Madison, WI) and either miR 488* mimic (10 nM) or NC mimic (10 nM). Cell lysates were assayed for firefly and renilla luciferase activities 48 hr after transfection using the Dual-Luciferase Reporter Assay System (Promega) and Victor 3 Multilabel Counter 1420 (PerkinElmer). Renilla luciferase activity served as a control for transfection efficiency. Data are represented as ratio of firefly luciferase activity to renilla luciferase activity.
For vector-expressed miR 488*, pcDNA-pri-miR 488* was constructed by cloning 383 bp fragment containing pre-miR 488* and flanking region in pcDNA 3.1 (−) vector. CHO-K1 cells were cotransfected using polybrene (Sigma-Aldrich, St. Louis, MO), with 100 ng of WT-3’ UTR or MUT-3’ UTR firefly luciferase reporter construct, 0.5 ng of renilla luciferase reporter plasmid and either pcDNA-pri-miR 488* construct (2.5 µg) or empty pcDNA 3.1(−) plasmid (2.5 µg). Firefly and renilla luciferase activities were measured 48 hr post transfection as described above. Details of primers and cloning strategy are available by request to authors.
Quantitative real-time PCR analysis of miR 488* expression
CHO-K1 cells were transfected with different concentrations (1.5–2.5 µg) of pcDNA-pri-miR 488* construct using polybrene. Total RNA was extracted 48 hr after transfection using Trizol reagent (Invitrogen). First strand cDNA was synthesized from 10 ng of total RNA using primers specific for human mature miR 488* and rodent small nucleolar RNA 202 (snoRNA202). Reverse transcription and quantitative real-time (qRT)-PCR was carried out using the TaqMan MicroRNA Reverse Transcription kit and TaqMan MicroRNA Assays (Applied Biosystems, Foster City, CA) as described previously.36 SnoRNA202 expression was used as an invariant control. The relative expression of miR 488* was calculated as 2−ΔCt where ΔCt = Ct value of miR 488* in a sample – Ct value of snoRNA202 in that sample. Mean miR 488* expression ± standard error (SE) was calculated from three independent experiments.
Determination of prostate specific antigen mRNA expression by qRT-PCR
Total RNA was isolated from LNCaP cells transfected with miR 488* mimic (100 nM) or NC mimic (100 nM) and also treated with DHT (20 nM) or vehicle control (dimethyl sulfoxide; DMSO). One microgram of DNase treated RNA was reverse transcribed into cDNA and real-time PCR reactions were set up as described previously.36 The specificity of amplification was confirmed by melting curve analysis and also by running PCR products on 3% agarose gels. Prostate specific antigen (PSA) mRNA expression was normalized to GAPDH mRNA expression. Mean normalized PSA expression ± SE was calculated from three independent experiments.
Quantitation of PSA in cell culture supernatants
Amounts of secreted PSA protein in cell culture supernatants were determined by using the Quantikine human PSA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Serial dilutions of recombinant human PSA were used to plot the standard curve.
Cell viability assay
LNCaP, C4-2B and DU 145 cells were plated in six-well plates one day before transfection. The cells were transiently transfected with either miR 488* mimic (50 nM) or NC mimic (50 nM) using Lipofectamine 2000. Twenty-four hours after transfection, cells were seeded into 96-well plates at 5,000 cells/well. Cell viability was determined on 2nd, 4th and 5th day post transfection using CellTiter-Glo luminescent cell viability assay (Promega) according to the manufacturer’s protocol.
Detection of apoptosis
LNCaP cells were transiently transfected with miR 488* mimic (50 nM) or NC mimic (50 nM) or treated with a known AR antagonist, Bicalutamide (100 µM) and apoptosis was assayed 4 days post transfection. Apoptotic cells were detected by using the cell death detection ELISAPLUS kit (Roche Applied Science, Indianapolis, IN) and Annexin V-FITC apoptosis detection kit (BD Biosciences, San Diego, CA) according to the manufacturer’s protocols. Annexin V-FITC and propidium iodide binding was assessed by flow cytometry.
Statistical analyses
Statistical analyses were performed using SPSS software. Data were presented as mean ± SE from at least three independent experiments. Independent samples t-test was used to assess statistically significant differences. Statistical significance was accepted for p < 0.05.
Results
Computational prediction: AR is a potential target of miR 488*
To identify potential miRNAs which can regulate AR, we used PicTar,37 TargetScan,38 and miRanda.39 Only TargetScan identified a putative binding site for miR 488* in the 3’ UTR of human AR. Originally, miR 488* was identified from brain tissue small RNA libraries.40 miR 488* is encoded in the fifth intron of Astrotactin 1 (ASTN1) gene (Supporting Information Fig. 1). miR 488* and miR 488 are expressed from the 5’ and 3’ arms of the precursor stem-loop, respectively (Supporting Information Fig. 1). The predicted target site for miR 488* is encoded from nucleotides 4266 to 4289 in the 3’ UTR of AR mRNA (Supporting Information Fig. 2a). The minimum free energy (MFE) value of miR 488* to its target binding site was −27.3 kcal/mol with extended ten canonical Watson-Crick base-pairs suggesting a strong binding site (Supporting Information Fig. 2a). In addition, precursor (pre) miR 488* was almost 90% conserved among human, mouse, rat, cow and horse genomes (Supporting Information Fig. 2b). The mature miR 488* was found to be 95% conserved overall and 100% identical in the 10 nucleotide seed region (Supporting Information Fig. 2b). Furthermore, the target sequence of miR 488* in the human AR 3’ UTR was also found to be conserved in the AR 3’ UTR of other mammalian genomes including orangutan, rat, pig and cow (Supporting Information Fig. 2c). Based on the phylogenetic conservation of miR 488* and its cognate target site in the 3’ UTR of several mammalian AR, as well as the extended seed region binding with the target region and finally, the strong MFE binding value of the miRNA with its target site, we hypothesized that the AR 3’ UTR is a potential target of miR 488*.
miR 488* downregulates endogenous protein levels of AR
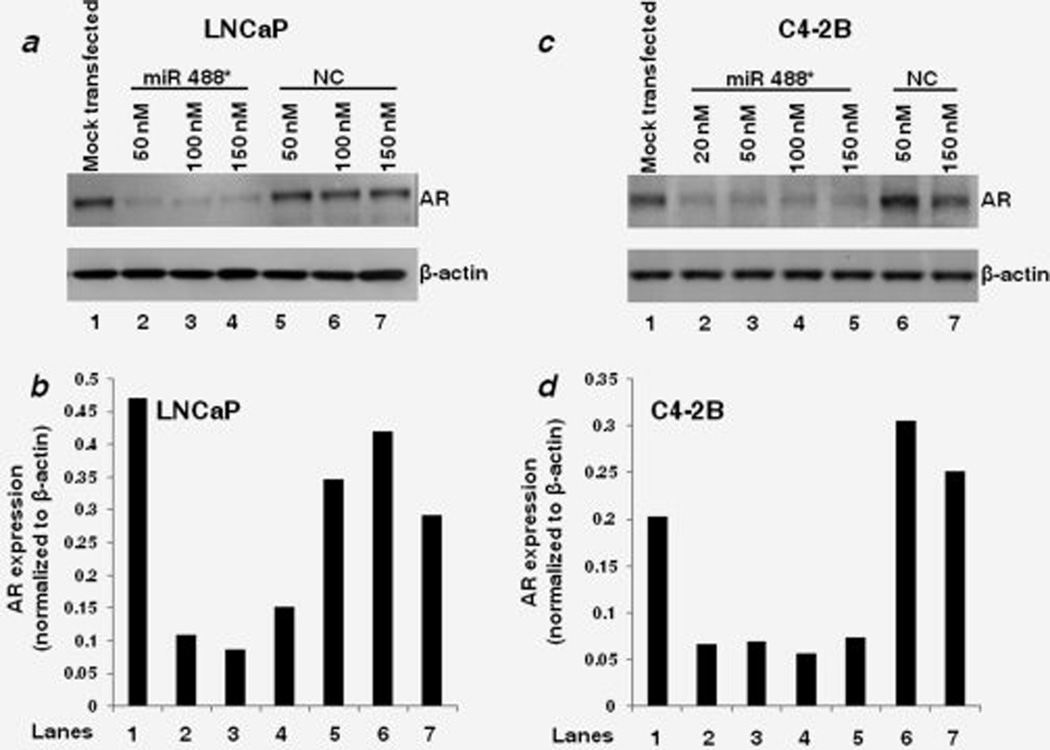
We first sought to determine if there was an inverse correlation between endogenous miR 488* expression and AR protein expression in PCa cell lines. However, we were unable to detect the endogenous expression of mature miR 488* in PCa cell lines (MDA PCa 2b, LNCaP, C4-2B, PC-3, DU 145) using qRT-PCR (data not shown). Hence, to experimentally test the potential of miR 488* to regulate the expression of AR, we overexpressed miR 488* in PCa cells. We transfected PCa cell lines (LNCaP and C4-2B) with miR 488* mimic or NC mimic and determined the AR protein expression by Western blotting. The LNCaP and C4-2B cell lines represent, respectively, the androgen-dependent and androgen-independent models of human PCa, which closely mimic the natural history of the disease. LNCaP cells express a mutant but functional AR and are responsive to DHT in terms of growth and expression of PSA.41 C4-2B is an androgen-independent subline of LNCaP obtained by passage and growth in castrated athymic mice.42 The transfection of increasing concentrations of miR 488* mimic in LNCaP cells markedly reduced the AR protein levels (Lanes 2–4) as compared to that in mock transfected (Lane 1) and NC mimic transfected cells (Lanes 5–7; Figs. 1a and 1b). Similar reduction in AR protein levels was observed in C4-2B cells transfected with miR 488* mimic (Figs. 1c and 1d), thus showing that miR 488* can effectively suppress AR protein expression in both androgen-dependent and androgen-independent cellular models of PCa. We also confirmed the expression of miR 488* in miR 488* mimic transfected LNCaP and C4-2B cells. Although the expression of miR 488* in mock transfected cells was undetectable, substantial expression of miR 488* was observed in miR 488* mimic transfected cells (Supporting Information Figs. 3a and 3b).
Figure 1.
miR 488* downregulates AR expression. Representative western blots showing the expression of AR and β-actin in LNCaP (a) and C4-2B (c) cells treated with indicated amounts of miR 488* mimic or NC mimic for 48 hr. Mock transfected cells represent cells treated with Lipofectamine 2000. β-actin expression was used as a loading control. (b and d) Quantitation of AR expression in the respective lanes of Western blots shown in (a) and (c). The signal intensities of bands were measured using the SCION IMAGE analysis software. The AR expression in each lane was determined by normalizing AR band intensity to β-actin band intensity.
miR 488* targets the 3’ UTR of AR mRNA
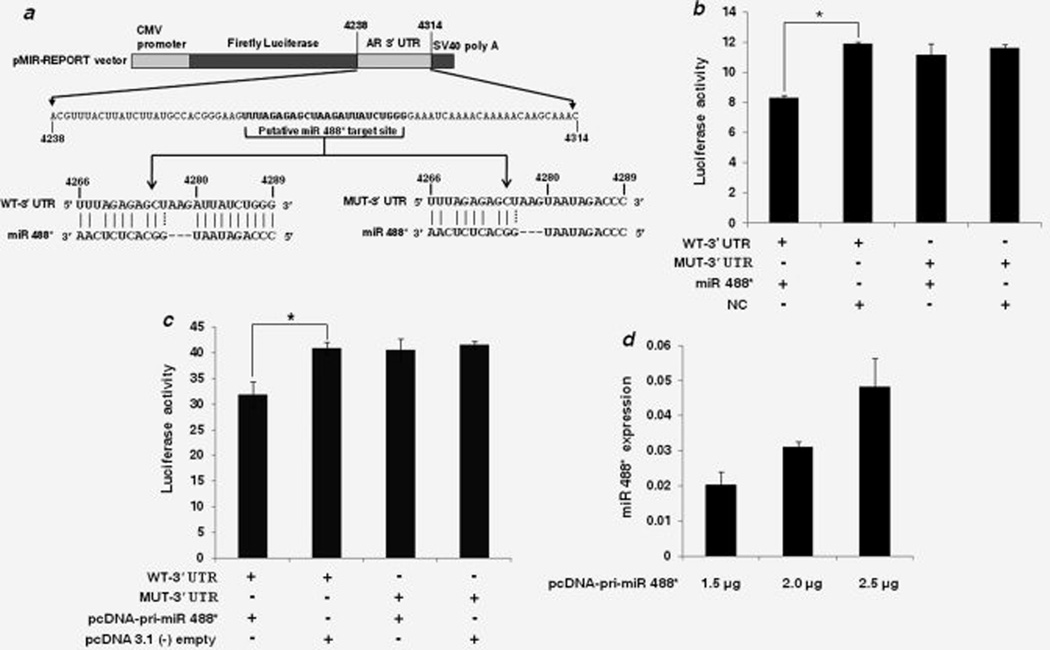
We next asked if the observed reduction in AR expression is due to the interaction of miR 488* with the predicted binding site in the 3’ UTR of AR mRNA. To address this, we cloned a segment of AR 3’ UTR containing the WT or mutated (MUT) miR 488* target site in a firefly luciferase reporter vector (Fig. 2a). In the MUT-3’ UTR, 10 nucleotides (4280–4289) of the target site were mutated to their complementary nucleotides to disrupt miR 488* binding in the seed match region (Fig. 2a). Each of these constructs was cotransfected with either miR 488* mimic or NC mimic in CHO-K1 cells and luciferase activity was measured after 48 hr. As shown in Fig. 2b, miR 488* reduced luciferase activity of the WT-3’ UTR construct by about 30% as compared to that with the NC mimic. However, in cells transfected with MUT-3’ UTR luciferase reporter, miR 488* was unable to suppress luciferase activity. Luciferase expression in these cells was similar to that seen in cells cotransfected with MUT-3’ UTR construct and NC mimic (Fig. 2b). Similar results were obtained when vector expressed miR 488* was used (Fig. 2c). The expression of mature miR 488* from the pcDNA-pri-miR 488* construct was verified using TaqMan miRNA assays. A dose-dependent increase in mature miR 488* expression was observed in CHO-K1 cells transfected with different amounts of pcDNA-pri-miR 488* construct (Fig. 2d). The highest amount (2.5 µg) of pcDNA-pri-miR 488* construct was selected for luciferase reporter assays. Although pcDNA-pri-miR 488* repressed luciferase activity of the WT-3’ UTR construct by about 22% as compared to that with the empty pcDNA 3.1 (−) control (Fig. 2c), it was unable to repress luciferase activity when the seed binding region of the putative target site was mutated (Fig. 2c). Taken together, these data show that the predicted target site in the AR 3’ UTR is an authentic, specific binding site for miR 488* and that AR is a direct target of miR 488*.
Figure 2.
AR is a direct target of miR 488*. (a) Schematic representation of firefly luciferase reporter construct containing 77 nucleotide sequence from AR 3’ UTR with either WT or mutant (MUT) miR 488* target site. In the MUT-3’ UTR construct, 10 nucleotides (4280–4289) in the seed matching region of the target site were mutated to their complementary nucleotides to disrupt miR 488* binding. (b) Luciferase reporter assay in CHO-K1 cells cotransfected with WT-3’ UTR or MUT-3’ UTR constructs and miR 488* mimic (10 nM) or NC mimic (10 nM) as indicated. (c) Luciferase reporter assay in CHO-K1 cells cotransfected with WT-3’ UTR or MUT-3’ UTR constructs and either pcDNA-pri-miR 488* construct (2.5 µg) or empty pcDNA 3.1(−) plasmid (2.5 µg) as indicated. (b and c) Luciferase activity is plotted as ratio of firefly to renilla luciferase activity. Each bar represents mean ± SE of three independent experiments. Asterisks indicate statistical significance as determined using the independent samples t-test (*p < 0.05). (d) miR 488* expression as determined by qRT-PCR. CHO-K1 cells were transfected with different amounts of pcDNA-pri-miR 488* construct as indicated and mature miR 488* expression was measured 48 hr post transfection. miR 488* expression is plotted as 2−ΔCt after normalization to snoRNA202 expression. Each bar represents mean ± SE of three independent experiments.
miR 488* mediated transcriptional downregulation of AR target gene, PSA
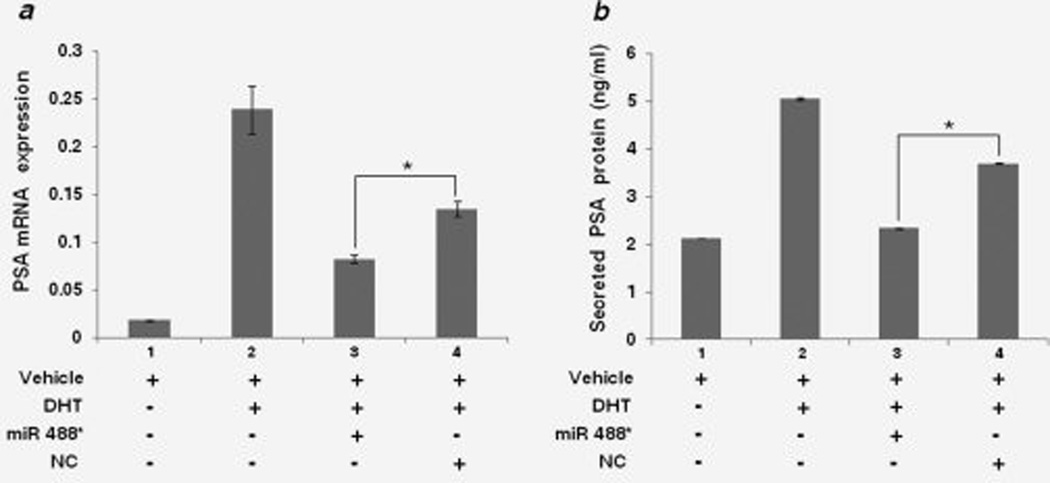
AR is a ligand-activated transcription factor, which activates the transcription of genes containing ARE in the promoter regions.4 Hence, we sought to determine the ability of miR 488* to repress the transcriptional activity of AR. For this, we assayed the androgen-dependent expression of PSA, a well characterized target gene of AR,43 in the presence of miR 488*. LNCaP cells were maintained in medium containing charcoal/dextran stripped FBS for two days before transfection with miR 488* mimic (100 nM) or NC mimic (100 nM). Forty-eight hours after transfection, DHT (20 nM) or vehicle control (DMSO) was added to the cells and 24 hr later, mRNA levels and secreted protein levels of PSA were measured. PSA mRNA expression was assayed using qRT-PCR. PSA mRNA levels increased approximately 12-fold in mock transfected cells treated with DHT as compared to mock transfected cells treated with vehicle (compare Bars 1 and 2 in Fig. 3a). This shows the androgen (DHT) and AR-dependent transcriptional upregulation of PSA expression. A similar increase, though of lesser magnitude, in DHT-dependent PSA mRNA expression was observed in cells transfected with NC mimic (Bar 4 in Fig. 3a). However, DHT mediated expression of PSA mRNA in cells transfected with miR 488* mimic (Bar 3 in Fig. 3a) was reduced by 38% as compared to the PSA mRNA levels in cells transfected with NC mimic and treated with DHT (Bar 4 in Fig. 3a), thus showing that miR 488* can inhibit the transcriptional activity of AR. Similar results were obtained with levels of secreted PSA in the same experiment. As seen in Fig. 3b, the amount of secreted PSA in miR 488* transfected cells treated with DHT (Bar 3) was decreased by 37% as compared to the secreted PSA levels in NC mimic transfected cells treated with DHT (Bar 4). Taken together, these data demonstrate the potential of miR 488* to disrupt the AR signaling axis by inhibiting the transcriptional activity of AR and downregulating the expression of AR target genes.
Figure 3.
miR 488* downregulates AR transcriptional activity. (a) qRT-PCR analysis of PSA mRNA expression in LNCaP cells transfected with miR 488* mimic (100 nM) or NC mimic (100 nM) in the presence of DHT (20 nM). Each bar represents PSA mRNA expression normalized to GAPDH mRNA expression. (b) Secreted PSA protein levels in LNCaP cells treated as in (a). Amounts of PSA in cell culture supernatants were determined using a quantitative sandwich enzyme immunoassay technique. (a and b) Cells treated with vehicle alone or DHT (bars 1 and 2) were also treated with Lipofectamine 2000 (mock transfected). Data are plotted as mean ± SE of three independent experiments. Asterisks indicate statistical significance as determined by independent samples t-test. *p < 0.01.
miR 488* inhibits the growth of PCa cells
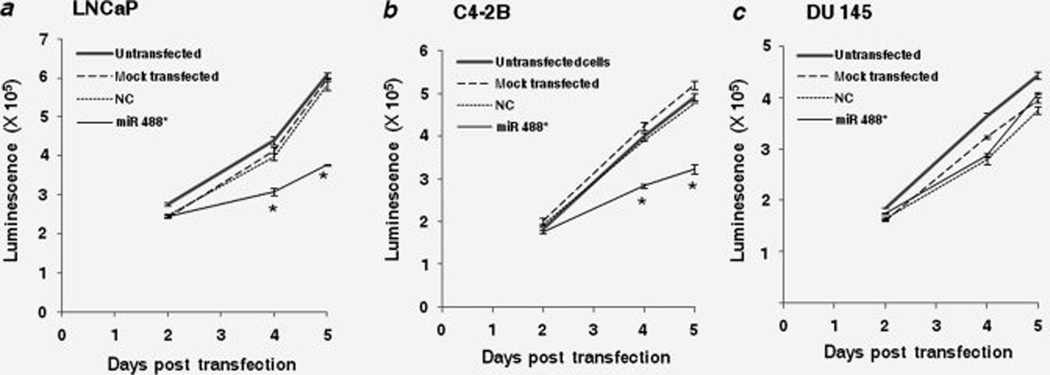
Because AR is required for the growth and proliferation of PCa cells, we investigated the potential of miR 488* to compromise the growth/viability of these cells by downregulating AR expression. Cells were transfected with either miR 488* mimic or NC mimic and numbers of viable cells were measured 2, 4 and 5 days post transfection by using the CellTiterGlo Luminescent Cell Viability Assay. The transfection of LNCaP cells with miR 488* mimic significantly inhibited cell growth as compared to that in NC mimic treated cells (Fig. 4a). Five days after transfection, the number of viable cells in the miR 488* mimic transfected pool was reduced by about 35% as compared to the viable cell number in the NC mimic transfected pool (Fig. 4a). Similar inhibition of cell proliferation was observed in C4-2B cells transfected with miR 488* mimic as compared to NC mimic treated cells (Fig. 4b). Five days after transfection, the viable cell count in the miR 488* mimic treated pool was reduced by 30% as compared to the viable cell count in the NC mimic transfected pool (Fig. 4b). These data demonstrate that miR 488* can substantially inhibit cell growth in both androgen-dependent and androgen-independent PCa cell lines.
Figure 4.
miR 488* inhibits the growth of PCa cells. Cell viability assay in LNCaP (a), C4-2B (b) and DU 145 (c) cells transfected with either miR 488* mimic (50 nM) or NC mimic (50 nM). Mock transfected cells represent cells treated with Lipofectamine 2000. CellTiter-Glo Luminescent Cell Viability Assay (Promega) was used to determine the number of viable cells. In this assay, the amount of ATP in viable cells is quantitated using a luciferase reaction and measurement of luminescence signal. Hence, luminescence signal serves as a measure of cell viability. Data are plotted as mean ± SE of three independent experiments. Independent samples t-test was used to assess statistically significant differences. Asterisks indicate a significant difference from NC mimic transfected cells. *p < 0.05.
To confirm if the observed effect of miR 488* on cell growth is specific to AR downregulation, we used the PCa cell line, DU 145, which does not express AR.41 As seen in Fig. 4c, miR 488* was unable to inhibit the growth of AR-negative DU 145 cells. The miR 488* mimic transfected cells showed a rate of growth similar to the NC mimic transfected cells (Fig. 4c). Hence, we conclude that miR 488* dependent inhibition of cell growth is mediated mainly by AR repression.
miR 488* enhances the apoptosis of PCa cells
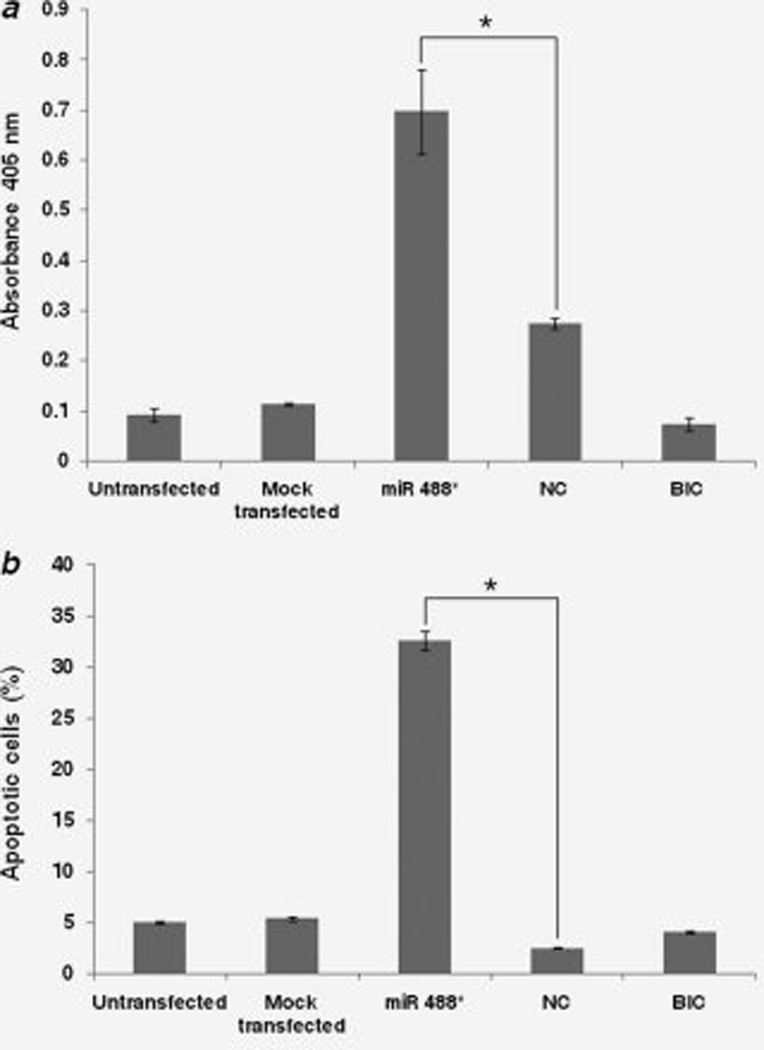
We next examined the potential of miR 488* to induce apoptosis in PCa cells. LNCaP cells were transfected with miR 488* mimic or NC mimic. Four days post transfection, apoptosis was assessed using cell death detection ELISAPLUS kit. This kit is based on a quantitative sandwich immunoassay technique and measures the amount of DNA degradation in a sample. In addition, percentage of apoptotic cells was determined by Annexin V/propidium iodide staining followed by flow cytometry. The transfection of miR 488* mimic in LNCaP cells significantly increased apoptosis as compared to the NC mimic treated cells (Figs. 5a and 5b). miR 488* mimic was more effective in inducing apoptosis than the antiandrogen, Bicalutamide (Figs. 5a and 5b). Interestingly, we failed to detect increased level of apoptosis in miR 488* mimic transfected C4-2B cells (data not shown).
Figure 5.
Assessment of apoptosis in LNCaP cells. LNCaP cells were transfected with miR 488* mimic (50 nM) or NC mimic (50 nM) or treated with Bicalutamide (BIC; 100 µM). Mock transfected cells were treated with Lipofectamine 2000. Apoptosis was measured 4 days post transfection using a quantitative sandwich immunoassay technique (a) and Annexin V/propidium iodide staining (b). (a) The immunoassay uses specific antibodies to detect the amount of mononucleosomes and oligonucleosomes that accumulate in the cytoplasm of the apoptotic cells because of degradation of DNA. The amount of antibodies bound to degraded DNA is determined by measuring absorbance at 405 nm. Absorbance (405 nm) is thus a measure of apoptosis. Data are plotted as mean ± SE of three independent experiments. Asterisks indicate statistical significance as determined by independent samples t-test (*p < 0.05). (b) Percentage of apoptotic LNCaP cells as assessed by Annexin V/propidium iodide staining and flow cytometry. Each bar represents the early apoptotic (Annexin V-FITC positive and propidium iodide negative) cells. *p < 0.05.
Discussion
AR expression is critical for both androgen-dependent and androgen-independent growth of PCa cells.6 Intense research has focused on deciphering the mechanism of AR regulation with the aim of designing better strategies for silencing AR and improving treatment options for PCa patients. In this study, we have identified miR 488* as a novel regulator of AR expression. Our computational analysis revealed a binding site for miR 488* in the 3’ UTR of AR. We used luciferase reporter assays to validate the predicted miR 488* target site. miR 488* significantly suppressed the activity of a luciferase reporter containing AR 3’ UTR (Figs. 2b and 2c). This repression of luciferase activity was reversed when the binding site for miR 488* was mutated (Figs. 2b and 2c), thus showing that AR is a direct target of miR 488*. Further, immunoblot analysis confirmed that miR 488* can effectively downregulate endogenous AR protein levels in PCa cells (Figs. 1a–1d). Ectopic expression of miR 488* in PCa cells resulted in (i) inhibition of AR expression, (ii) repression of functional activity of AR as evidenced by the decline of PSA expression, (iii) retardation of cellular growth and (iv) increase in apoptosis.
Both androgen-dependent (LNCaP) and androgen-independent (C4-2B) cellular models of PCa were used in the study. Transient transfection of miR 488* mimic resulted in robust suppression of AR protein expression in both cell types (Figs. 1a–1d). To confirm that the downregulation of AR expression by miR 488* also affects the transcriptional activity of AR, we assayed the expression of AR target gene, PSA. Transfection of miR 488* mimic in LNCaP cells reduced androgen-dependent expression of PSA at the mRNA and protein levels (Figs. 3a and 3b). We next tested the potential of miR 488* to affect the growth of PCa cells. Treatment with miR 488* mimic retarded the growth of LNCaP and C4-2B cells (Figs. 4a and 4b). miR 488* was unable to inhibit the growth of AR-negative DU 145 cells (Fig. 4c), thus suggesting that the observed miR 488* dependent inhibition of cell growth in LNCaP and C4-2B cells is primarily mediated by AR repression. An increase in apoptosis was observed in miR 488* mimic transfected LNCaP cells as compared to NC mimic treated cells (Figs. 5a and 5b). miR 488* was more effective in inducing apoptosis in LNCaP cells as compared to Bicalutamide, an antiandrogen routinely used for the treatment of PCa44 (Figs. 5a and 5b). However, we failed to detect significant apoptosis in miR 488* treated C4-2B cells (data not shown). It is possible that the androgen-independent C4-2B cells have evolved additional pathways for survival besides the AR-dependent survival pathway, thus making them resistant to miR 488* mediated apoptosis. The alternative survival pathways that bypass the AR-signaling cascade have been found to mainly include the upregulation of anti-apoptotic molecules.7,8,45 Our results are consistent with previous reports. Downregulation of AR expression by neutralizing antibody, ribozyme,11 antisense oligonucleotides10,19 and siRNAs12,14,15,23 has been shown to inhibit proliferation of both androgen-dependent and androgen-independent PCa cells. Inhibition of cell growth by AR-specific antisense oligonucleotides and siRNAs was accompanied by increase in apoptosis in androgen-dependent PCa cell lines.10,23,24 However, these studies did not examine the effect of AR silencing on the induction of apoptosis in androgen-independent PCa models. We are aware of a single report in which siRNA-directed silencing of AR in an androgen-independent PCa cell line, C4-2, was found to increase apoptosis as measured by a caspases assay.15 However, in the same study, siRNA-directed silencing of AR in another androgen-independent PCa cell line, 22RV1 inhibited cell proliferation but had no significant effect on apoptosis. These observations combined with ours indicate the need for more extensive analyses of the possible resistance of androgen-independent PCa cells to apoptosis induced by AR silencing.
miR 488* is encoded in intron 5 of ASTN1 gene (Supporting Information Fig. 1). The processing of precursor miR 488* stem-loop produces two mature miRNAs, namely miR 488* and miR 488 (Supporting Information Fig. 1). Although the expression of these miRNAs in normal human tissues and disease states has not been extensively studied, miR 488* and miR 488 have been reported to be predominantly expressed in human brain tissues.40,46 We failed to detect the endogenous expression of mature miR 488* in several PCa cell lines (data not shown). We are currently investigating the mechanism underlying the apparent repression of miR 488* expression in PCa cells. PCR analysis of genomic DNA revealed that the miR 488* locus is present in all the PCa cell lines tested (data not shown). Analysis of expression levels of primary and precursor forms of miR 488* is currently underway to determine if the processing of miR 488* is blocked in PCa cells. We are also exploring the possibility of epigenetic silencing of miR 488* expression. Since the reconstitution of miR 488* expression by synthetic miR 488* mimic molecules in LNCaP and C4-2B cells resulted in marked downregulation of AR expression and inhibition of cell growth, any strategy to upregulate the endogenous expression of miR 488* in PCa cells may have therapeutic implications. Further, since miR 488* regulates the expression of AR, a central molecule in PCa, it would be interesting to explore the potential roles of miR 488* and its host gene, ASTN1 in the development of PCa. ASTN1 is a neuronal adhesion molecule required for glial-guided neuronal movement in cortical regions of developing brain.47 Interestingly, ASTN1 gene (1q25.2) is located in a region of chromosome 1 that harbors the PCa susceptibility locus HPC1 (1q24-25),2 thus providing a basis for investigating ASTN1 with regard to PCa. Furthermore, the repression of miR 488* expression in PCa cells together with its growth inhibitory effects suggest the possibility of this miRNA to function as a tumor suppressor. The profiling of miR 488* expression in normal prostate tissue samples and tissue samples from different stages of PCa will provide useful insights regarding the potential role of miR 488* in the pathogenesis of PCa.
At present, the suppression of AR signaling in PCa is achieved by strategies that decrease circulating levels of androgens and by the administration of AR antagonists that compete with androgens for binding to AR.6 However, these therapeutic strategies become ineffective when PCa cells activate diverse molecular pathways to restore AR signaling during androgen-independent stage of PCa. To meet the need for better blockade of AR signaling, considerable research efforts are being focused on designing superior AR antagonists and testing other inhibitors of AR signaling axis such as, inhibitors of heat shock protein-90 and inhibitors of histone deacetylases.9 Strategies that directly inhibit AR expression by targeting AR mRNA may prove more effective than strategies that attempt to block the already expressed AR. However, the field of AR mRNA targeting is only beginning to emerge. AR mRNA specific ribozyme,11 antisense oligonucleotides10,19–21 and siRNAs12,14,15,23,24 have been tested as AR lowering strategies. In line with these strategies, our data support miR 488* mediated targeting of AR mRNA. Since large number of miRNAs are endogenously and ubiquitously expressed, the problems of toxicity and activation of innate immunity associated with siRNAs48 may not be an issue in case of miRNA-based therapy. Several recent studies have suggested the potential of miRNAs for PCa therapy; however, in these reports, miRNAs target molecules other than AR, the key regulatory molecule in PCa.31,33–35 Since PCa cells utilize multiple mechanisms to reactivate AR signaling in response to AR blocking therapies, there is a growing realization that no single therapeutic agent can eliminate PCa. A combinatorial approach that involves the simultaneous targeting of multiple pathways is now accepted as a rational approach to PCa therapy.16 Hence, the present emphasis is on the development of numerous therapeutic options, which can then be evaluated in a combinatorial therapy setting. It is in this light that miR 488* mediated repression of AR activity described in our study acquires significance. Further, miR 488* may be exploited as an effective tool to silence AR in studies aimed at gaining better understanding of AR signaling pathways. At present, no information is available about other genes targeted by miR 488* in any type of cancer. It would be interesting to investigate whether miR 488* targets a combination of genes, which are important in prostate carcinogenesis.
In conclusion, this study describes miRNA mediated regulation of AR expression. miR 488* negatively regulates AR expression by binding to the specific target site in 3’ UTR of AR mRNA. Further studies will confirm if miR 488* is involved in the transition of PCa to lethal androgen-independent stage. The data presented here may have implications for the control of specific form of PCa in which AR plays a dominant role.
Supplementary Material
Acknowledgements
The original concept was funded by CDMRP W81XWH-06-1-0191 to G.C.S. The authors are grateful to Dr. Crystal M. Weyman and Dr. Terri J. Harford (CSU) for their help with apoptosis assays. Research in GCS laboratory is supported by NSF, BCRP and PCRP of CDMRP/DoD and Board of Regents, Ohio grants.
Abbreviations
- AR
androgen receptor
- ARE
androgen-responsive elements
- ASTN1
astrotactin 1
- bp
base pairs
- CYP17
cytochrome P-450c17
- DHT
dihydrotestosterone
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HPC1
hereditary prostate cancer 1
- MFE
minimum free energy
- miRNA
microRNA
- MSR1
macrophage-scavenger receptor 1
- NC
negative control
- PCa
prostate cancer
- PSA
prostate specific antigen
- RNASEL
ribonuclease L
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- snoRNA
small nucleolar RNA
- SRD5A2
steroid-5-α-reductase type II
- UTR
untranslated region
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 3.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehm SM, Tindall DJ. Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol. 2007;21:2855–2863. doi: 10.1210/me.2007-0223. [DOI] [PubMed] [Google Scholar]
- 5.Buchanan G, Irvine RA, Coetzee GA, Tilley WD. Contribution of the androgen receptor to prostate cancer predisposition and progression. Cancer Metastasis Rev. 2001;20:207–223. doi: 10.1023/a:1015531326689. [DOI] [PubMed] [Google Scholar]
- 6.Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91:483–490. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- 7.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 8.Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1665–1671. doi: 10.1158/1078-0432.CCR-06-0067. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8:440–448. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eder IE, Culig Z, Ramoner R, Thurnher M, Putz T, Nessler-Menardi C, Tiefenthaler M, Bartsch G, Klocker H. Inhibition of LncaP prostate cancer cells by means of androgen receptor antisense oligonucleotides. Cancer Gene Ther. 2000;7:997–1007. doi: 10.1038/sj.cgt.7700202. [DOI] [PubMed] [Google Scholar]
- 11.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–1013. [PubMed] [Google Scholar]
- 12.Wright ME, Tsai MJ, Aebersold R. Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells. Mol Endocrinol. 2003;17:1726–1737. doi: 10.1210/me.2003-0031. [DOI] [PubMed] [Google Scholar]
- 13.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 14.Haag P, Bektic J, Bartsch G, Klocker H, Eder IE. Androgen receptor down regulation by small interference RNA induces cell growth inhibition in androgen sensitive as well as in androgen independent prostate cancer cells. J Steroid Biochem Mol Biol. 2005;96:251–258. doi: 10.1016/j.jsbmb.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Compagno D, Merle C, Morin A, Gilbert C, Mathieu JR, Bozec A, Mauduit C, Benahmed M, Cabon F. SIRNA-directed in vivo silencing of androgen receptor inhibits the growth of castration-resistant prostate carcinomas. PLoS One. 2007;2:e1006. doi: 10.1371/journal.pone.0001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15:4792–4798. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, et al. Development of a secondgeneration antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Song CS, Lavrovsky Y, Bi B, Vellanoweth R, Chatterjee B, Roy AK. Catalytic cleavage of the androgen receptor messenger RNA and functional inhibition of androgen receptor activity by a hammerhead ribozyme. Mol Endocrinol. 1998;12:1558–1566. doi: 10.1210/mend.12.10.0186. [DOI] [PubMed] [Google Scholar]
- 19.Eder IE, Hoffmann J, Rogatsch H, Schafer G, Zopf D, Bartsch G, Klocker H. Inhibition of LNCaP prostate tumor growth in vivo by an antisense oligonucleotide directed against the human androgen receptor. Cancer Gene Ther. 2002;9:117–125. doi: 10.1038/sj.cgt.7700416. [DOI] [PubMed] [Google Scholar]
- 20.Hamy F, Brondani V, Spoerri R, Rigo S, Stamm C, Klimkait T. Specific block of androgen receptor activity by antisense oligonucleotides. Prostate Cancer Prostatic Dis. 2003;6:27–33. doi: 10.1038/sj.pcan.4500603. [DOI] [PubMed] [Google Scholar]
- 21.Ko YJ, Devi GR, London CA, Kayas A, Reddy MT, Iversen PL, Bubley GJ, Balk SP. Androgen receptor down-regulation in prostate cancer with phosphorodiamidate morpholino antisense oligomers. J Urol. 2004;172:1140–1144. doi: 10.1097/01.ju.0000134698.87862.e6. [DOI] [PubMed] [Google Scholar]
- 22.Cheng H, Snoek R, Ghaidi F, Cox ME, Rennie PS. Short hairpin RNA knockdown of the androgen receptor attenuates ligandindependent activation and delays tumor progression. Cancer Res. 2006;66:10613–10620. doi: 10.1158/0008-5472.CAN-06-0028. [DOI] [PubMed] [Google Scholar]
- 23.Liao X, Tang S, Thrasher JB, Griebling TL, Li B. Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol Cancer Ther. 2005;4:505–515. doi: 10.1158/1535-7163.MCT-04-0313. [DOI] [PubMed] [Google Scholar]
- 24.Yang Q, Fung KM, Day WV, Kropp BP, Lin HK. Androgen receptor signaling is required for androgen-sensitive human prostate cancer cell proliferation and survival. Cancer Cell Int. 2005;5:8. doi: 10.1186/1475-2867-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 26.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2007;96(Suppl):R40–R44. [PubMed] [Google Scholar]
- 27.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Gandellini P, Folini M, Zaffaroni N. Towards the definition of prostate cancerrelated microRNAs: where are we now? Trends Mol Med. 2009;15:381–390. doi: 10.1016/j.molmed.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Galardi S, Mercatelli N, Giorda E, Massalini S, Frajese GV, Ciafre SA, Farace MG. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem. 2007;282:23716–23724. doi: 10.1074/jbc.M701805200. [DOI] [PubMed] [Google Scholar]
- 30.Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu M, Tepper CG, Evans CP, Kung HJ, DeVere White RW. An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci USA. 2007;104:19983–19988. doi: 10.1073/pnas.0706641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musiyenko A, Bitko V, Barik S. Ectopic expression of miR-126*, an intronic product of the vascular endothelial EGF-like 7 gene, regulates prostein translation and invasiveness of prostate cancer LNCaP cells. J Mol Med. 2008;86:313–322. doi: 10.1007/s00109-007-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin SL, Chiang A, Chang D, Ying SY. Loss of mir-146a function in hormonerefractory prostate cancer. RNA. 2008;14:417–424. doi: 10.1261/rna.874808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KH, Chen YL, Yeh SD, Hsiao M, Lin JT, Goan YG, Lu PJ. MicroRNA-330 acts as tumor suppressor and induces apoptosis of prostate cancer cells through E2F1- mediated suppression of Akt phosphorylation. Oncogene. 2009;28:3360–3370. doi: 10.1038/onc.2009.192. [DOI] [PubMed] [Google Scholar]
- 34.Noonan EJ, Place RF, Pookot D, Basak S, Whitson JM, Hirata H, Giardina C, Dahiya R. miR-449a targets HDAC-1 and induces growth arrest in prostate cancer. Oncogene. 2009;28:1714–1724. doi: 10.1038/onc.2009.19. [DOI] [PubMed] [Google Scholar]
- 35.Fujita Y, Kojima K, Ohhashi R, Hamada N, Nozawa Y, Kitamoto A, Sato A, Kondo S, Kojima T, Deguchi T, Ito M. MiR-148a attenuates paclitaxel-resistance of hormone-refractory, drug-resistant prostate cancer PC3 cells by regulating MSK1 expression. J Biol Chem. 2010 doi: 10.1074/jbc.M109.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sikand K, Slane SD, Shukla GC. Intrinsic expression of host genes and intronic miRNAs in prostate carcinoma cells. Cancer Cell Int. 2009;9:21. doi: 10.1186/1475-2867-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, Macmenamin P, da P I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 38.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 39.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 41.Navone NM, Logothetis CJ, von Eschenbach AC, Troncoso P. Model systems of prostate cancer: uses and limitations. Cancer Metastasis Rev. 1998;17:361–371. doi: 10.1023/a:1006165017279. [DOI] [PubMed] [Google Scholar]
- 42.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC, Chung LW. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. [PubMed] [Google Scholar]
- 43.Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol. 2003;21:383–391. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- 44.Lee EC, Zhan P, Schallhom R, Packman K, Tenniswood M. Antiandrogen-induced cell death in LNCaP human prostate cancer cells. Cell Death Differ. 2003;10:761–771. doi: 10.1038/sj.cdd.4401228. [DOI] [PubMed] [Google Scholar]
- 45.Cohen MB, Rokhlin OW. Mechanisms of prostate cancer cell survival after inhibition of AR expression. J Cell Biochem. 2009;106:363–371. doi: 10.1002/jcb.22022. [DOI] [PubMed] [Google Scholar]
- 46.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fink JM, Hirsch BA, Zheng C, Dietz G, Hatten ME, Ross ME. Astrotactin (ASTN), a gene for glial-guided neuronal migration, maps to human chromosome 1q25.2. Genomics. 1997;40:202–205. doi: 10.1006/geno.1996.4538. [DOI] [PubMed] [Google Scholar]
- 48.Robbins M, Judge A, Maclachlan I. siRNA and innate immunity. Oligonucleotides. 2009;19:89–102. doi: 10.1089/oli.2009.0180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.