Introduction and discussion
Recently, Dominique et al. reported that contamination of the dermoscopic lens by human papillomavirus (HPV) may be common [1]. Results of their study revealed that wart-specific HPV DNA is detected with a high frequency (43%) on a dermoscopic lens before examination and that the detection rate is increased to 82% after plantar wart examination. Furthermore, cleaning dermoscope lenses with an antiseptic cleansing wipe containing bactericidal and virucidal agents was not effective in removing HPV DNA, as 74% of samples were positive. Although actual infectivity of the viruses was not investigated, their data indicate that dermoscopy could be a possible source of nosocomial infection.
Dermoscopy has proven its diagnostic capabilities in pigmented and non-pigmented skin tumors [2]. It is also useful in the diagnosis of cutaneous infectious and inflammatory diseases [3,4]. As a dermatologist’s stethoscope, the use of dermoscopy is increasing since it is a remarkable screening tool in daily routine practice [2]. In this regard, the study which demonstrated that dermoscopy is a possible source of infectious disease transmission raises medical, ethical, and legal concerns with dermatologists. It increases the need for effective disinfection methods for the equipment. Proper sterilization or the use of preventive tools is crucial to prevent cross-contamination among patients.
Several previous studies have suggested methods to decrease possible nosocomial infection by dermoscopy.
Kelly SC and Purcell SM reported that alcohol-based gel inhibits bacterial colonization [5]. It can obviate the risk of nosocomial infection during dermoscopic examination. However, the risk of viral infection was not investigated in the study.
Zampino et al suggested that polyvinyl chloride (PVC) food wrap can be used to prevent possible transmission of infections from mucosal lesions [6]. The PVC film is placed on the dermoscopic lens with mineral oil on both surfaces, which acts as a barrier for viral contamination. However, this method has its limitations, such as low availability of PVC film in dermatologic clinics, cumbersomeness of the technique, and unpredicted transmission of infections, which could occur if the film is damaged.
Polarized noncontact dermoscopy (PND) may be ideal in evaluating possible infectious lesions because it does not require direct contact with the lesions [7]. Although direct contact can be theoretically avoided using PND, unintentional contact could happen while focusing and observing the lesion because the space between PND and skin is narrow.
Disposable dermoscopic lens covers can be used. Stauffer et al. used a disposable polyethylene lens cover that was self-made from standard household plastic wrap [8]. It can act as a mechanical barrier. However, the method has similar limitations as with PVC film described above.
We believe that a commercial disposable lens can be developed in the future, but cost would be factor.
Our suggestion
A glass microscope slide could be a possible practical tool to prevent cross-contamination among patients. As shown in Figures 1 and 2, a glass slide is placed on the front of the dermoscopic lens to evaluate viral wart-like lesions. This method completely inhibits contact of the lens with the skin. The used glass slide can then be discarded. Microscopic glass slides have several advantages as preventive covers because they are easily available at dermatologic clinics, as they are commonly used for KOH examination and diascopy, they are inexpensive, have perfect transparency, and are effective as solid mechanical barriers against bacterial and viral infections. The disadvantages of using this tool are that putting hard pressure on the slide glass can cause obliteration of dermoscopic vascular structures and breakage of the glass. Therefore, it should be noted that clinicians should put no or minimal pressure on the glass slide to preserve the vascular patterns of cutaneous lesions and to avoid unexpected injury to patients in breaking the slide.
Figure 1.

Microscope glass slide used as a cover for dermoscopic lens to lesions suspected to be plantar warts. [Copyright: ©2013 Mun et al.]
Figure 2.
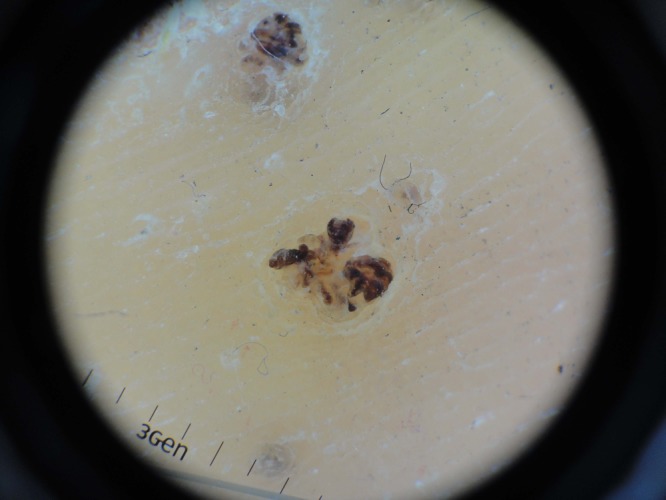
Thrombosed capillary loops are observed by dermoscopy. The transparent glass slide does not obscure observation of the lesion. As a mechanical barrier, it can prevent potential transmission of bacterial and viral infections. [Copyright: ©2013 Mun et al.]
Although several infection prevention measures have been suggested, further studies regarding actual risk of infection and preventive methods are required as dermoscopy is increasingly used. Herein, we suggest a glass slide as a simple, low cost, and effective tool that can be used to prevent cross-contamination among patients during dermoscopic examination.
References
- 1.Penso-Assathiany D, Gheit T, Pretet JL, et al. Presence and persistence of human papillomavirus types 1, 2, 3, 4, 27, and 57 on dermoscope before and after examination of plantar warts and after cleaning. J Am Acad Dermatol. 2013;68(1):185–6. doi: 10.1016/j.jaad.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Argenziano G, Ferrara G, Francione S, et al. Dermoscopy—the ultimate tool for melanoma diagnosis. Semin Cutan Med Surg. 2009;28(3):142–8. doi: 10.1016/j.sder.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Zalaudek I, Giacomel J, Cabo H, et al. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology. 2008;216(1):14–23. doi: 10.1159/000109353. [DOI] [PubMed] [Google Scholar]
- 4.Lallas A, Kyrgidis A, Tzellos TG, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br J Dermatol. 2012;166(6):1198–205. doi: 10.1111/j.1365-2133.2012.10868.x. [DOI] [PubMed] [Google Scholar]
- 5.Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32(4):552–5. doi: 10.1111/j.1524-4725.2006.32112.x. [DOI] [PubMed] [Google Scholar]
- 6.Zampino MR, Borghi A, Caselli E, et al. Virologic safety of polyvinyl chloride film in dermoscopic analysis of mucosal areas. Arch Dermatol. 2007;143(7):945–6. doi: 10.1001/archderm.143.7.945. [DOI] [PubMed] [Google Scholar]
- 7.Haliasos EC, Kerner M, Jaimes-Lopez N, et al. Dermoscopy for the pediatric dermatologist part I: dermoscopy of pediatric infectious and inflammatory skin lesions and hair disorders. Pediatr Dermatol. 2013;30(2):163–71. doi: 10.1111/pde.12097. [DOI] [PubMed] [Google Scholar]
- 8.Stauffer F, Kittler H, Forstinger C, Binder M. The dermatoscope: a potential source of nosocomial infection? Melanoma Res. 2001;11(2):153–6. doi: 10.1097/00008390-200104000-00010. [DOI] [PubMed] [Google Scholar]


