Abstract
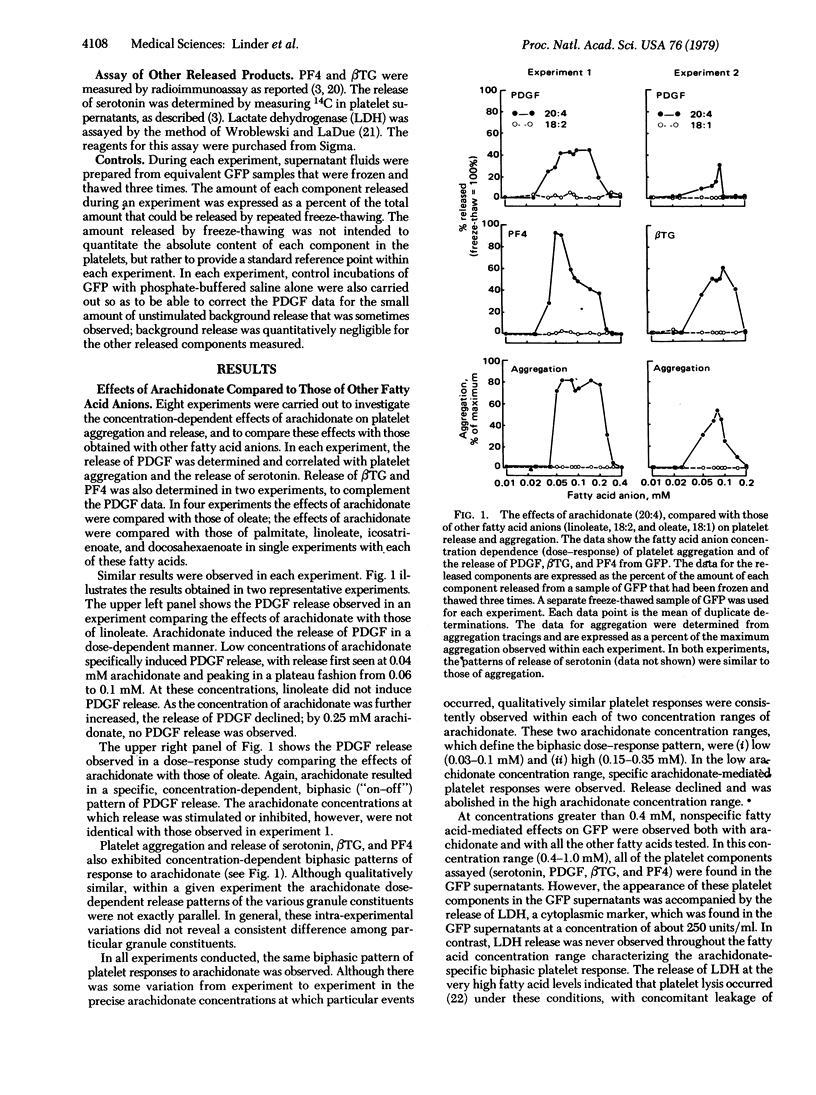
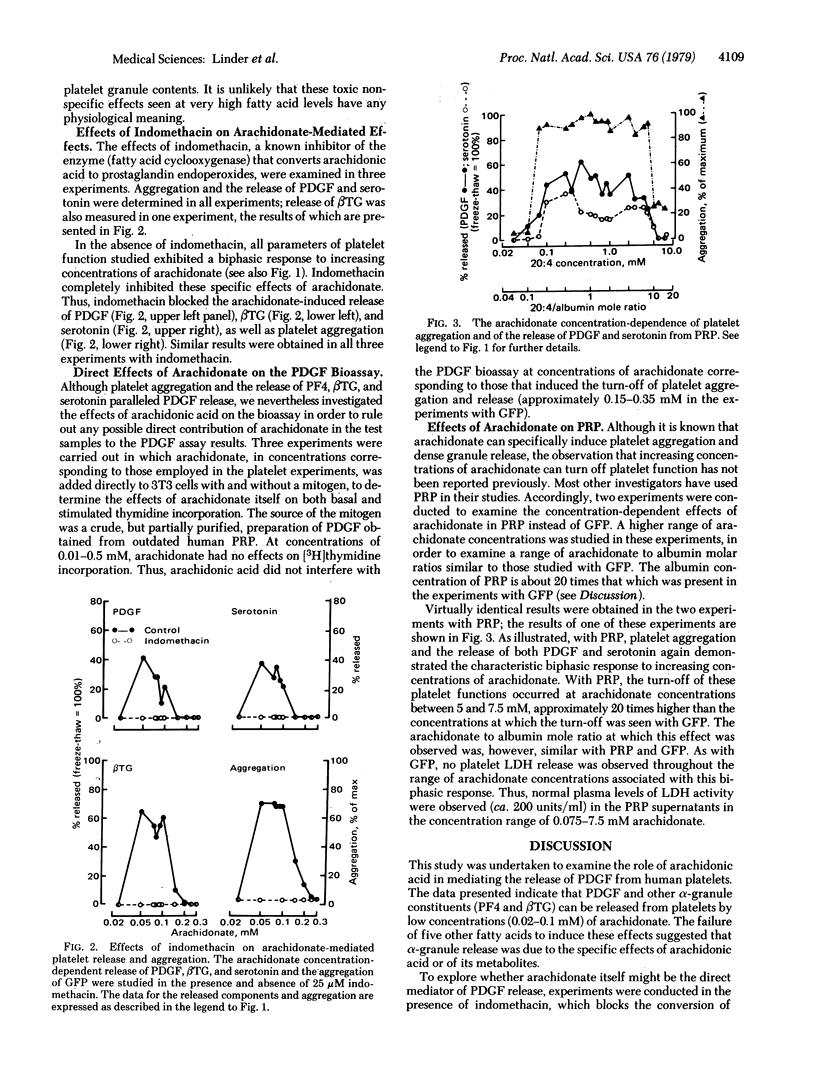
Platelet α-granules contain a factor that stimulates the proliferation of arterial smooth muscle cells and may play a role in atherogenesis. We have studied the role of arachidonic acid in mediating the release of the platelet-derived growth factor (PDGF) from human platelets. PDGF was assayed by stimulating of [3H]thymidine incorporation into DNA of mouse 3T3 cells. Platelet aggregation and the release of platelet factor 4,β-thromboglobulin, and serotonin were also studied. A biphasic response pattern was observed when gel-filtered platelets were incubated with arachidonate over the concentration range 0.01-0.4 mM. At low arachidonate levels (approximately 0.025-0.1 mM), specific concentration-dependent aggregation and release of PDGF and of the other components were observed. This effect was not seen with any of five other fatty acids tested and was suppressed by indomethacin (25 μM). At higher arachidonate concentrations (approximately 0.15-0.35 mM), a concentration-dependent turn-off of both aggregation and release occurred. At these concentrations the platelets remained functional, and no release of lactate dehydrogenase was observed. A similar biphasic pattern of arachidonate-induced aggregation and release was observed with platelet-rich plasma, over a similar range of arachidonate to albumin mole ratios. These studies demonstrate that PDGF and other α-granule constituents can be released from platelets specifically by arachidonate via an indomethacin-sensitive pathway, most probably involving the platelet cyclooxygenase and conversion of arachidonate to prostaglandin metabolities. The mechanisms responsible for the turn-off of the specific arachidonate-mediated responses at higher arachidonate concentrations remain to be defined.
Keywords: prostaglandin pathways, atherosclerosis, platelet aggregation, platelet factor 4, β-thromboglobulin
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Scher C. D. Radioimmunoassay of a human serum growth factor for Balb/c-3T3 cells: derivation from platelets. Proc Natl Acad Sci U S A. 1977 May;74(5):1973–1977. doi: 10.1073/pnas.74.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Selective release of archidonic acid from the phospholipids of human platelets in response to thrombin. J Clin Invest. 1977 Jul;60(1):1–6. doi: 10.1172/JCI108745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan R. W., Paxton J., Kuehl F. A., Jr Mechanism for irreversible self-deactivation of prostaglandin synthetase. J Biol Chem. 1976 Dec 10;251(23):7329–7335. [PubMed] [Google Scholar]
- Fitzpatrick F. A., Gorman R. R. Platelet rich plasma transforms exogenous prostaglandin endoperoxide H2 into thromboxane A2. Prostaglandins. 1977 Nov;14(5):881–889. doi: 10.1016/0090-6980(77)90304-5. [DOI] [PubMed] [Google Scholar]
- Friedman R. J., Stemerman M. B., Wenz B., Moore S., Gauldie J., Gent M., Tiell M. L., Spaet H. The effect of thrombocytopenia on experimental arteriosclerotic lesion formation in rabbits. Smooth muscle cell proliferation and re-endothelialization. J Clin Invest. 1977 Nov;60(5):1191–1201. doi: 10.1172/JCI108872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3824–3828. doi: 10.1073/pnas.71.10.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Wakabayashi T., Samuelsson B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc Natl Acad Sci U S A. 1974 Feb;71(2):345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S., Falardeau P. Resolution of prostaglandin endoperoxide synthase and thromboxane synthase of human platelets. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3691–3695. doi: 10.1073/pnas.74.9.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker L. A., Ross R., Slichter S. J., Scott C. R. Homocystine-induced arteriosclerosis. The role of endothelial cell injury and platelet response in its genesis. J Clin Invest. 1976 Sep;58(3):731–741. doi: 10.1172/JCI108520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Partial purification and characterization of platelet factors stimulating the multiplication of normal human glial cells. Exp Cell Res. 1977 Oct 15;109(2):429–437. doi: 10.1016/0014-4827(77)90023-4. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Day H. J., Setkowsky C. A. Secretory mechanisms. Behaviour of adenine nucleotides during the platelet release reaction induced by adenosine diphosphate and adrenaline. Biochem J. 1972 Aug;129(1):67–82. doi: 10.1042/bj1290067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Day H. J. The selectivity of the thrombin-induced platelet release reaction: subcellular localization of released and retained constituents. J Lab Clin Med. 1970 May;75(5):840–855. [PubMed] [Google Scholar]
- Kaplan K. L., Broekman M. J., Chernoff A., Lesznik G. R., Drillings M. Platelet alpha-granule proteins: studies on release and subcellular localization. Blood. 1979 Apr;53(4):604–618. [PubMed] [Google Scholar]
- Kaplan K. L., Nossel H. L., Drillings M., Lesznik G. Radioimmunoassay of platelet factor 4 and beta-thromboglobulin: development and application to studies of platelet release in relation to fibrinopeptide A generation. Br J Haematol. 1978 May;39(1):129–146. doi: 10.1111/j.1365-2141.1978.tb07135.x. [DOI] [PubMed] [Google Scholar]
- Kohler N., Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974 Aug;87(2):297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Cuatrecasas P. Rapid inactivation of cyclooxygenase activity after stimulation of intact platelets. Proc Natl Acad Sci U S A. 1979 Jan;76(1):121–125. doi: 10.1073/pnas.76.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmsten C., Hamberg M., Svensson J., Samuelsson B. Physiological role of an endoperoxide in human platelets: hemostatic defect due to platelet cyclo-oxygenase deficiency. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1446–1450. doi: 10.1073/pnas.72.4.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Friedman R. J., Singal D. P., Gauldie J., Blajchman M. A., Roberts R. S. Inhibition of injury induced thromboatherosclerotic lesions by anti-platelet serum in rabbits. Thromb Haemost. 1976 Feb 29;35(1):70–81. [PubMed] [Google Scholar]
- Nishizawa E. E., Miller W. L., Gorman R. R., Bundy G. L., Svensson J., Hamberg M. Prostaglandin d2 as a potential antithrombotic agent. Prostaglandins. 1975 Jan;9(1):109–121. doi: 10.1016/s0090-6980(75)80122-5. [DOI] [PubMed] [Google Scholar]
- Oelz O., Oelz R., Knapp H. R., Sweetman B. J., Oates J. A. Biosynthesis of prostaglandin D2. 1. Formation of prostaglandin D2 by human platelets. Prostaglandins. 1977 Feb;13(2):225–234. doi: 10.1016/0090-6980(77)90004-1. [DOI] [PubMed] [Google Scholar]
- Raz A., Minkes M. S., Needleman P. Endoperoxides and thromboxanes. Structural determinants for platelet aggregation and vasoconstriction. Biochim Biophys Acta. 1977 Aug 24;488(2):305–311. doi: 10.1016/0005-2760(77)90188-6. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Russell F. A., Deykin D. Mobilization of arachidonic acid in human platelets. Kinetics and Ca2+ dependency. Biochim Biophys Acta. 1977 Sep 28;488(3):370–380. doi: 10.1016/0005-2760(77)90196-5. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Rutherford R. B., Ross R. Platelet factors stimulate fibroblasts and smooth muscle cells quiescent in plasma serum to proliferate. J Cell Biol. 1976 Apr;69(1):196–203. doi: 10.1083/jcb.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L., Lands W. E. Oxygenation of polyunsaturated fatty acids during prostaglandin biosynthesis by sheep vesicular gland. Biochemistry. 1972 Aug 15;11(17):3276–3285. doi: 10.1021/bi00767a024. [DOI] [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J Lipid Res. 1975 May;16(3):165–179. [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Witte L. D., Kaplan K. L., Nossel H. L., Lages B. A., Weiss H. J., Goodman D. S. Studies of the release from human platelets of the growth factor for cultured human arterial smooth muscle cells. Circ Res. 1978 Mar;42(3):402–409. doi: 10.1161/01.res.42.3.402. [DOI] [PubMed] [Google Scholar]



