Abstract
Pharmacological levels of zinc oxide can promote growth and health of weaning piglets, but the underlying molecular mechanisms are yet not fully understood. The aim of this study was to determine changes in the global hepatic protein expression in response to dietary zinc oxide in weaned piglets. Nine half-sib piglets were allocated to three dietary zinc treatment groups (50, 150, 2500 mg/kg dry matter). After 14 d, pigs were euthanized and liver samples taken. The increase in hepatic zinc concentration following dietary supplementation of zinc was accompanied by up-regulation of metallothionein mRNA and protein expression. Global hepatic protein profiles were obtained by two-dimensional difference gel electrophoresis following matrix-assisted laser desorption ionization/time-of-flight mass spectrometry. A total of 15 proteins were differentially (P<0.05) expressed between groups receiving control (150 mg/kg) or pharmacological levels of zinc (2500 mg/kg) with 7 down- (e.g. arginase1, thiosulfate sulfurtransferase, HSP70) and 8 up-regulated (e.g. apolipoprotein AI, transferrin, C1-tetrahydrofolate synthase) proteins. Additionally, three proteins were differentially expressed with low zinc supply (50 mg/kg Zn) in comparison to the control diet. The identified proteins were mainly associated with functions related to cellular stress, transport, metabolism, and signal transduction. The differential regulation was evaluated at the mRNA level and a subset of three proteins of different functional groups was selected for confirmation by western blotting. The results of this proteomic study suggest that zinc affects important liver functions such as blood protein secretion, protein metabolism, detoxification and redox homeostasis, thus supporting the hypothesis of intermediary effects of pharmacological levels of zinc oxide fed to pigs.
Introduction
Zinc is an essential trace element that plays an important role in many metabolic processes. It acts as a co-factor in metalloenzymes and transcription factors and is involved in DNA replication and RNA transcription, signal transduction, apoptosis or oxidative stress response[1]. In addition, zinc is critical for growth and development as well as for proper immune function and is pivotal for animal and human health (reviewed by Chasapis et al. [2]). Zinc deficiency can result in gastrointestinal, renal and liver diseases; therefore supplementation of zinc has the potential to be a powerful therapeutic agent to prevent such disorders. In young children, for example, dietary supplementation with zinc has been reported to enhance growth and to prevent or treat gastrointestinal disorders [3]. Similar effects could be observed in animals. In pigs, feeding pharmacological (2000–4000 mg/kg) levels of dietary zinc as zinc oxide has been shown to improve performance [4]–[6], and reduce the incidence of diarrhea [7], [8]. The mechanisms are not yet entirely clear, however, possible modes of action have been attributed to the influence of zinc on the gut microbiota [9], [10], epithelial barrier function [11], [12] and/or systemic metabolic effects [13], [14].
Under normal dietary supply, zinc homeostasis is maintained within relatively narrow margins [15]. Zinc is stored in numerous organs with higher levels usually being found in bones, liver, kidney, pancreas, testis, skin, and the retina of the eye [1]. It has been shown that high levels of dietary zinc lead to increased zinc concentration and induction of metallothionein (MT) in various tissues including the liver [16]–[19]. The liver plays a central role in regulation of zinc homeostasis (reviewed by Stamoulis et al. [20]), which in turn is necessary for proper liver function. Due to its key function in the regulation of whole body metabolism of carbohydrates, lipids and proteins, the liver is in the focus of zinc-related health and nutrition research. Gene expression profiling in the liver of piglets revealed the regulation of several key genes when pharmacological zinc levels (2000 mg/kg) were fed [21]. These genes were associated with oxidative stress response and amino acid metabolism. However, whether similar effects can be determined at the protein level is yet unknown.
To our knowledge, this is the first study aiming to determine the influence of pharmacological dietary zinc supply on the global protein expression pattern in the liver of weaned piglets. We used a 2-dimensional differential gel electrophoresis approach (2D-DIGE), which has been previously demonstrated as a powerful tool in nutritional studies [8], [22]. Our hypothesis was that dietary zinc supplementation could modify hepatic protein expression of weaned piglets. Specifically, we identified potential targets in porcine liver that may have the potential to elucidate the cellular and molecular mechanisms of supplemental zinc.
Materials and Methods
Animals, feeding and sampling
All procedures involving animal handling and treatment were approved by the local state office of occupational health and technical safety ‘Landesamt für Gesundheit und Soziales, Berlin’ (LaGeSo Reg. No. G0179/09). A total of nine half-sib piglets after weaning at 25+/−1 days of life were used in this study. Experimental setup and samplings were described previously [23]. Briefly, after an adaptation period of 7 days, piglets (n = 3/group) were fed diets containing 50 mg zinc/kg [low zinc (LZn)], 150 mg zinc/kg [normal zinc (NZn)], or 2500 mg zinc/kg [high zinc (HZn)] (Table 1). The dietary zinc levels were adjusted by addition of analytical grade zinc oxide (Sigma, Taufkirchen, Germany) and confirmed by atomic absorption spectrometry (i.e. 50, 156, 2355 mg/kg DM). The animals remained on their respective diets for 14 days before sampling. Following euthanasia, liver samples were immediately snap-frozen in liquid N2 and stored at −80°C until further analysis.
Table 1. Ingredients and chemical composition of diets used in this study.
| Ingredients | g/kg | Chemical composition | g/kg |
| Dry matter | 885 | ||
| Optigrain®1 | 500 | ME(MJ/kg) | 13.5 |
| Barley | 100 | Crude ash | 70 |
| Wheat | 70 | Crude protein | 189 |
| Soybean meal | 190 | Crude fiber | 29 |
| Corn starch/zinc oxide2 | 10.0 | Ether extract | 39 |
| Whey powder | 60 | Starch | 386 |
| Mineral & Vitamin Premix3 | 10 | Lysine | 11.8 |
| Soy oil | 20 | Methionine | 4.1 |
| Monocalcium phosphate | 16 | Threonine | 7.1 |
| Limestone | 18 | Tryptophane | 2.3 |
| Salt | 2.5 | Calcium | 11.1 |
| Lysine HCl | 2.5 | Phosphorus | 7.7 |
| Methionine | 1.0 | Sodium | 2.7 |
| Magnesium | 1.8 | ||
| Zinc mg/kg4 | 50 | ||
| Iron mg/kg | 119 | ||
| Manganese mg/kg | 80 | ||
| Copper mg/kg | 18 |
Optigrain® (DEUKA, Deutsche Tiernahrung, Düsseldorf, Germany), consisting of hydrothermally processed wheat (50%), barley (25%) and corn (25%).
Corn starch in the basal diet was partially replaced in the diets containing 150, and 2500 mg/kg zinc with analytical grade zinc oxide (Sigma Aldrich, Deisenhofen, Germany) to adjust for the zinc level.
Mineral and Vitamin Premix (Spezialfutter Neuruppin Ltd., Neuruppin, Germany), containing per kg dry matter: 130 g Na (as sodium chloride), 55 g Mg (as magnesium oxide), 700,000 IU Vit A, 120,000 IU Vit D3, 8,000 mg Vit E, 300 mg Vit K3, 250 mg Vit B1, 250 mg Vit B2, 400 mg Vit B6, 2,000 µg Vit B12, 2,500 nicotinic acid, 100 mg folic acid, 25,000 µg biotin, 1,000 mg pantothenic acid, 80,000 mg choline chloride, 5,000 mg Fe (as iron-(II)-carbonate), 1,000 mg Cu (as copper-(II)- sulfate), 6,000 mg Mn (as manganese-(II)-oxide), 45 mg J (as calcium-iodate), 35 mg Se (as sodium-selenite).
Analyzed concentration of zinc in the basal diet without ZnO supplementation. The other diets contained 156, and 2355 mg/kg, respectively.
Liver zinc concentration
Zinc concentration in liver tissue was determined by atomic absorption spectrometry in an AAS vario 6 spectrometer (Analytik Jena, Germany) after hydrolysis of the incinerated tissue in concentrated hydrochloric acid as described by [23].
Protein extraction and 2-dimensional DIGE analysis
Protein extraction was performed by the addition of 1 ml lysis buffer (9 M urea, CHAPS 2%, biolyte pH 3–10 supplemented with 60 mM DTT, 5 µM PMSF) and protease inhibitor mixture (Calbiochem). Proteins were extracted using a FastPrep FP120 homogenizer (MP Biomedicals) with appropriate lysing matrix tubes followed by incubation for one hour on ice and subsequent centrifugation at 10 000×g, 4°C for 10 minutes. Protein lysates were further purified by a modified TCA-acetone precipitation method (2-D-Clean Up kit, GE Healthcare) and mixed with DIGE labeling buffer (8 M urea, 4% w/v CHAPS, 30 mM Tris, pH 8.5) and the concentration was determined by using a 2-D Quant Kit (GE Healthcare).
A pool consisting of equal amounts of liver tissue from each animal sample was prepared as the internal standard. This internal standard was always labeled with Cy2 and run together with individual liver samples on all gels. The use of this internal standard eliminated errors resulting from artifacts and enables accurate quantitative analysis [24]. Each sample (60 µg total protein) was labeled with 480 pmol appropriate CyDye following the manufacturer's protocol. Two DIGE analyses including 6 gels were performed. Each gel contained the internal standard, one sample obtained from NZn fed piglet and one sample obtained from either HZn or LZn fed animals, respectively. Samples were rehydrated, isoelectrically focused and equilibrated as described previously [22]. SDS-polyacrylamide gel electrophoresis (SDS–PAGE) for the second dimension was carried out in an ETTAN DALT six electrophoresis unit (GE Healthcare), first at 0.2 W per gel for 1 h and thereafter at 2 W per gel for further 18 h. Protein spots were visualized by using the Typhoon 9400 laser imager (GE Healthcare) choosing the appropriate wavelength for each CyDye (Cy2 = 520 nm; Cy3 = 580 nm; Cy5 = 670 nm) at a resolution of 100 µm, were cropped and imported into DeCyder V.7.0 software (GE Healthcare). During spot detection by a co-detection algorithm in the software, the estimated number of spots were set at 2500, and the exclude filter was set a slope >1.7 and area <200. The DeCyder differential in the gel analysis (DIA) module was used to process the images from a single gel and enables the pair-wise comparison of each sample to the pooled internal standard. The abundance of each protein spot was determined as a ratio to its corresponding spot present in the internal standard on the same gel. The DeCyder biological variation analysis (BVA) module was used to standardize the ratios across the gels accounting for differences compared with the internal standard.
Protein identification
Changes of protein expression in response to different Zn feeding detected by 2-DIGE analysis were matched with silver-stained protein patterns (400 µg protein), and the selected spots showing at least a 1.2-fold change in protein expression were excised from the gel. Protein spots were in-gel digested by trypsin (Promega, Germany) as described previously [25]. For protein-identification by matrix-assisted laser desorption/ionization-time-of-flight-mass spectrometry (MALDI-TOF-MS) an Ultraflex-II TOF/TOF instrument (Bruker Daltonics, Bremen, Germany) equipped with a Smart beam™ laser was used. The protein digests were measured in the reflector mode using an α-cyano-4-hydroxycinnamic acid (CHCA) as matrix. For the database search, listed contamination peaks from keratin and autoproteolytic products were excluded for peptide mass fingerprint database search with the Mascot server (www.matrixscience.com) in the NCBInr database and SwissProt. The search was restricted to mammalian sequences and one missed tryptic cleavage was considered. A mass accuracy of 50–100 ppm was used for the searches.
Immunoblotting
Liver samples were homogenized in DIGE buffer containing protease inhibitor cocktail (Merck Biosciences). Sample proteins (20 µg) and a pre-stained protein-weight marker (Bio-Rad) were resolved by SDS-PAGE in 12% polyacrylamide gels and transferred onto nitrocellulose membranes in Tris-glycine buffer with 20% (v/v) methanol. The membrane was saturated with 5% (w/v) non-fat milk powder (Roth) prepared in Tris-buffered saline containing 0.1% Tween20 (TBST) for 1 h at room temperature and then incubated with primary antibody overnight at 4°C. The primary antibodies employed were mouse monoclonal transferrin antibody (1∶5000, Santa Cruz Biotechnology, Inc.), rabbit polyclonal arginase antibody (1∶400, Santa Cruz Biotechnology, Inc.) and HSP70/72 mouse monoclonal antibody (1∶1000, Enzo Life Sciences). Western blot analysis of MT was performed as described previously [26] using mouse monoclonal MT-antibody (Clone E9, Dako, France) diluted 1∶150. The signals were detected by chemiluminescence with ECL Select (GE Healthcare) according to the manufacturer's instructions.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from tissue samples by using an InviTrap Spin Cell RNA Mini Kit (Stratec Molecular, Berlin, Germany) according to the manufacturer's instructions. Duplicates of each total RNA sample (1 µg) was treated with 3.75 µM random hexamers (GE Healthcare, Germany) and 200 U Moloney Murine Leukemia Virus reverse transcriptase (Fermentas, Germany) in a 60 µl reaction mixture in order to generate single-stranded cDNA[27]. Possible genomic DNA contamination was removed prior to reverse transcription by performing a DNase-treatment.
Real-time PCR in the presence of SYBR Green I was performed by using a Rotor-Gene 3000 (Corbett Research, Australia) as described previously [27]. Used primers are indicated in Table 2. The quantity of each specific mRNA was normalized with the program GeNorm [28] and the selected control genes 18S rRNA, GAPDH, SDHA, and RPLA13. Duplicates were performed of each cDNA sample. The normalized values were used for statistical assessment and for the generation of box and whisker plots. All PCR products were sequenced (GATC, Germany) and showed 100% homology to the known porcine sequences.
Table 2. Primer sequences used for real-time PCR analysis.
| Gene | Primer sequence | EMBL accession no. | Nucleotide range | Annealing |
| HSP70 | forward 5′-TTGACGCAGGTGTCTTTGAG-3′ | X68213 | 746–974 | 60°C |
| reverse 5′-AAGAGGGAGTCGATTTCCAG-3′ | ||||
| SERPINH1 | forward 5′-CCGTGGGTGTCACTATGATG-3′ | NM_001244132 | 887–1064 | 60°C |
| reverse 5′-AGCTGCTCTTTGGTCAGGAG-3′ | ||||
| APOA1 | forward 5′-CTCCTGGACAACTGGGACAG-3′ | X69477 | 246–532 | 60°C |
| reverse 5′-CTCAGCTTCTCCTGCAGCTC-3′ | ||||
| ARG1 | forward 5′-GGTGGAAGAAGGCCCTACAG-3′ | AY 039112 | 293–635 | 61°C |
| reverse 5′-GGTCGTGGTTGTCAGTGGAG-3′ | ||||
| EIF4A1 | forward 5′-GTTAAGCCGAGGGTTCAAGG-3′ | DQ351283 | 575–813 | 60°C |
| reverse 5′-ACAAGTCACACAGGGTGTCC-3′ | ||||
| AIFM1 | forward 5′-TCCTCCCTGAATACCTCAGC-3′ | XM_003135371 | 1251–1487 | 60°C |
| reverse 5′-GGAAGCCACCGAAATCAGAG-3′ | ||||
| MT1 | forward 5′-GTGAATCCGCGTTGCTCTCTGCT-3′ | NM_001001266 | 7–78 | 59°C |
| reverse 5′-CTGTGGGGCAGGAGCAGTTGG-3′ | ||||
| GAPDH | forward 5′-ATTCTACCCATGGCAAATTCC-3′ | AF017079 | 484–707 | 60°C |
| reverse 5′- AGGGGCAGAGATGATGACC-3′ | ||||
| SDHA | forward 5′- CAA ACT CGC TCC TGG ACC TC -3′ | DQ845177 | 869–1131 | 60°C |
| reverse 5′- CCG GAG GAT CTT CTC ACA GC -3′ | ||||
| RPLA13 | forward 5′-CCGTCTCAAGGTGTTCGATG-3′ | NM_001244068 | 328–527 | 60°C |
| reverse 5′-GGATCTTGGCCTTCTCCTTC-3′ | ||||
| 18S rRNA | forward 5′- AAT CGG TAG TAG CGA CGG -3′ | AY 265350 | 254–529 | 60°C |
| reverse 5′- AGA GGG ACA AGT GGC GTT C -3′ |
Statistics
Using Decyder Software proteins were defined as differentially regulated if the observed fold-change calculated as the ratio of the average standardized abundances corresponding to the groups of samples [treatment group (LZn), or (HZn) diet/control group (NZn) diet, respectively] was greater than 1.2 with P-values of less than 0.05 (Student's t-test).
For qRT-PCR the normalized mean obtained from the quadruplicates of each RNA sample were analyzed by the Kruskal-Wallis-H-test. In case of significance, the Mann-Whitney-U-test was used for group comparisons. Diagrams and statistical tests of mRNA expression were performed by using SPSS 20.0 (SPSS Inc., USA). The level of significance was set at P<0.05.
Results
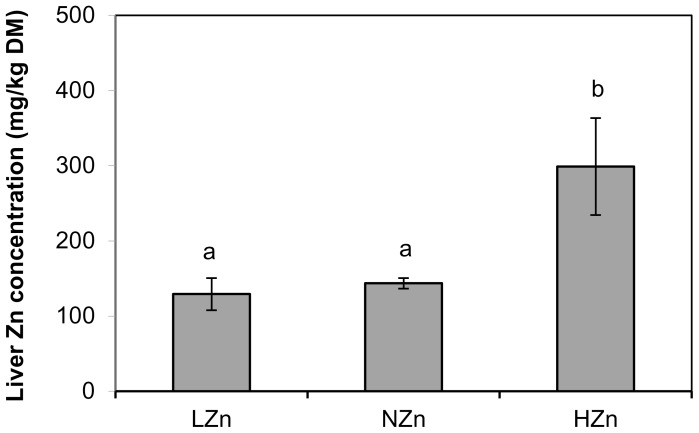
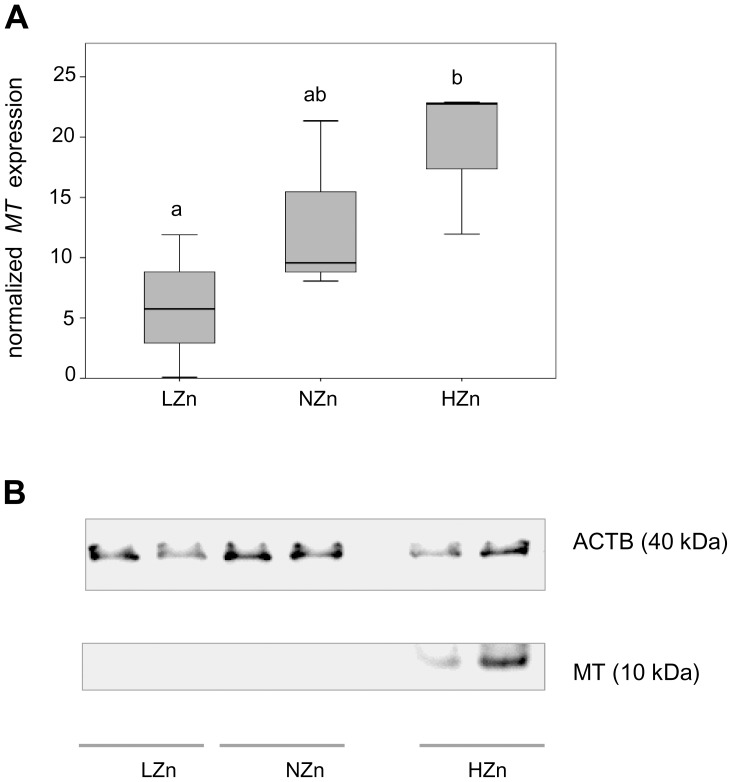
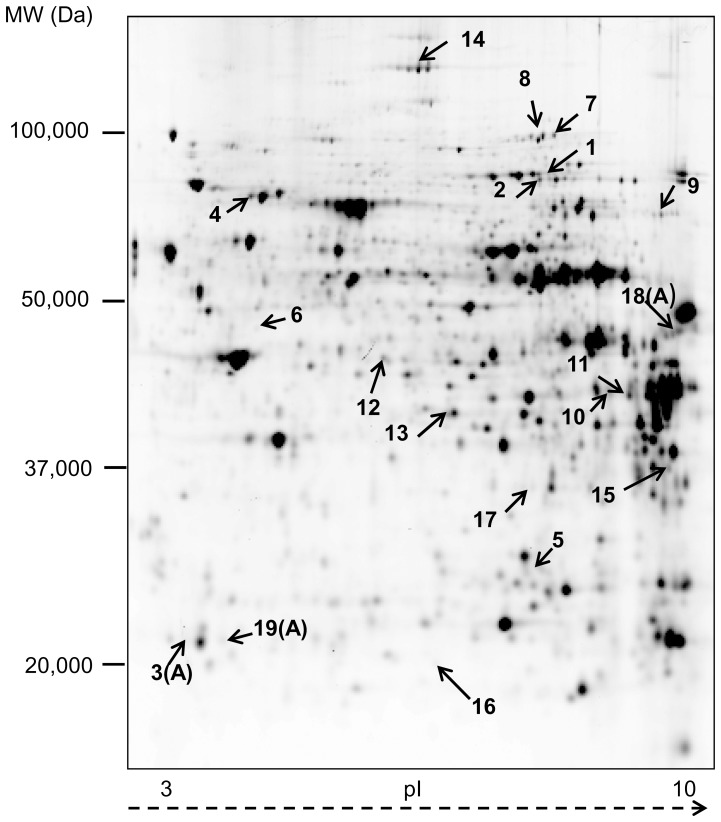
All piglets remained in a good health condition throughout the experimental period. As reported previously, performance (feed intake, average daily gain, feed conversion) did not differ between treatments [23]. Liver zinc concentration was higher (P<0.05) with the pharmacological dietary zinc supply (HZn group) but did not differ between NZn and LZn fed piglets (Fig. 1). Similarly, the mRNA level of hepatic MT (Fig. 2A) was significantly higher in HZn fed piglets as compared to the other groups. Induction of MT with pharmacological zinc supply was confirmed by western blot analysis (Fig. 2B). A total of ∼1,500 proteins were detected in hepatic tissue of piglets using the 2D-DIGE approach. Overall, DIGE analysis of the different gels revealed significant changes in expression of 19 proteins, which are assigned to a single representative 2D gel (Fig. 3). Particularly, 17 spots were differentially expressed (>1.2-fold; P<0.05) in the liver of piglets fed HZn diet in comparison with the corresponding spots from the liver of NZn fed piglets (spot 1–17). Among the differentially expressed proteins, 2 proteins were found in more than one spot (Table 3). Among the 15 identified proteins, 7 were up- and 8 down-regulated, respectively (Table 3). Only three proteins were differentially expressed (>1.2fold; P<0.05) between the LZn and NZn group (spot 3A, 18A, 19A). Important biochemical information of all identified proteins corresponding to the numbered spots is summarized in Tables 3 and 4. Transferrin (No. 1; 2) and C-1 tetrahydrofolate synthase (No. 7; 8) were identified in two different spots, respectively. Identified proteins were associated with transport processes (transferrin – TRFE, apolipoprotein A1 – APOA1), signal transduction (eukaryotic initiation factor 4A1 -EIF4A1, apoptosis inducing factor 1-AIFM1), stress response (heat-shock protein 70 – HSP70, endoplasmic reticulum resident protein –ERP 29, HSP47/SerpinH1 precursor-SERPINH1) and metabolic function (aldolase B – ALDOB, glyceral-3-phosphate-dehydrogenase – GAPDH, adenosine kinase – ADK, arginase 1 – ARG1, carbamoyl-phosphate synthase – CPSM, thiosulfate sulfurtransferase- THTR, malate- dehydrogenase – MDH, hypoxanthine-guanine phosphoribosyltransferase – HPRT, Catechol-o-methyltransferase – COMT, C1- tetrahydrofolate synthase – C1TC (Table 3 and 4). HZn supply down-regulated ARG1 and CPSM, while TRFE, APOA1, GAPDH, ALDOB, C1TC and ADK were up-regulated. Notably, compared to the control group (NZn fed piglets) one protein, identified as APOA1, was up-regulated in response to HZn supply (spot 3) and down-regulated in LZn fed piglets (spot 3A).
Figure 1. Zinc concentration in the liver of piglets fed LZn (50 mg/kg), NZn (150 mg/kg), or HZn (2500 mg/kg) levels of dietary zinc.
abdifferent superscripts indicate significant (P<0.05) differences.
Figure 2. Effects of dietary zinc supplementation on metallothionein expression.
Relative metallothionein mRNA levels (A) and protein expression (B) in the liver of piglets fed LZn (50 mg/kg), NZn (150 mg/kg), or HZn (2500 mg/kg) diet.
Figure 3. Effects of dietary zinc supplementation on proteomic proteomic profile of porcine liver.
Representative 2D gel containing liver proteins from a pool of all analyzed liver samples stained with Cy2. Marked spots represent differentially expressed proteins in hepatic tissue comparing HZn (spot 1–17) or LZn fed piglets (spot 3A, 18A, 19A) with those receiving NZn diet and correspond to the respective spot numbers found in Tables 3 and 4.
Table 3. List of identified proteins in hepatic tissue of piglets showing differential expression in response to various dietary zinc concentrations.
| Name | No. | Accession No. | Av. Ratio | P-value | MW | pI | score |
| Transferrin | 1 | gi 136192 | +1.81 | 0.028 | 78971 | 6.93 | 116 |
| 2 | +1.55 | 0.001 | 136 | ||||
| Apolipoprotein AI | 3 | gi 164359 | +1.45 | 0.028 | 30312 | 5.38 | 106 |
| Heat shock protein 70 | 4 | gi 178056524 | −1.29 | 0.021 | 70989 | 5.24 | 178 |
| Endoplasmic reticulum resident protein 29 | 5 | ERP29_BOVIN | +1.35 | 0.035 | 28845 | 5.63 | 56 |
| Eukaryotic initiation factor 4A1 | 6 | IF4AI_BOVIN | −1.21 | 0.024 | 46601 | 5.33 | 89 |
| C1-tetrahydrofolate synthase, | 7 | gi 311261216 | +1.40 | 0.026 | 101771 | 6.76 | 280 |
| 8 | +1.24 | 0.024 | 201 | ||||
| Apoptosis inducing factor 1 | 9 | gi 311276941 | −1.22 | 0.012 | 66420 | 9.31 | 28 |
| Fructose-biphosphate aldolase B-like | 10 | gi 350579435 | +1.20 | 0.007 | 27977 | 8.07 | 111 |
| Glyceraldehyde-3-phosphate dehydrogenase | 11 | G3P_PIG | +1.27 | 0.014 | 36041 | 8.51 | 98 |
| Adenosine kinase | 12 | ADK_mouse | +1.26 | 0.014 | 40466 | 5.84 | 60 |
| Arginase 1 | 13 | ARGI1_PIG | −1.27 | 0.026 | 35281 | 6.32 | 141 |
| Carbamoyl-phosphate synthase, mitochondrial | 14 | gi 74005321 | −1.27 | 0.005 | 165634 | 6.23 | 172 |
| Malate dehydrogenase | 15 | MDHM_PIG | −1.26 | 0.040 | 36029 | 8.93 | 118 |
| Hypoxanthine- guanine phosphoribosyl-transferase | 16 | HPRT_PIG | −1.54 | 0.047 | 24768 | 6.3 | 47 |
| Thiosulfate sulphurtransferase | 17 | gi 311255145 | −1.58 | 0.023 | 37861 | 8.58 | 132 |
| Apolipoprotein A1 | 3A | gi 164359 | −1.90 | 0.001 | 30307 | 5.48 | 70 |
| Serpin H1-precursor | 18A | gi 346421378 | +1.55 | 0.006 | 46648 | 8.91 | 115 |
| Catechol-o-methyltransferase | 19A | gi 305855180 | +1.28 | 0.047 | 30413 | 5.38 | 75 |
Table 4. Function of differentially expressed proteins.
| Protein name | No. | Predicted protein function |
| Transport function | ||
| Transferrin | 1/2 | Transport of iron, antioxidant |
| Apolipoprotein A1 | 3/3A | Transport of cholesterol, HDL-metabolism |
| Signal transduction | ||
| Eukaryotic initiation factor IF4A1 | 6 | Initiation of translation |
| Apoptosis inducing factor | 9 | Initiation of apoptosis |
| Stress response function | ||
| Heat shock protein 70 | 4 | Chaperone, protein folding |
| Endoplasmic reticulum resident protein 29 | 5 | Chaperone, processing and transport of proteins |
| Serpin H1 precursor | 18A | Chaperone, collagen binding protein |
| Metabolic function | ||
| Fructose-biphosphate aldolase | 10 | Glycolysis |
| Glyceraldehyde-3-phosphate dehydrogenase | 11 | Glycolysis |
| C-1 tetrahydrofolate synthase | 7/8 | C1-metabolism |
| Adenosine kinase | 12 | Phosphate transfer, methylation |
| Arginase 1 | 13 | Urea cycle |
| Carbamoylphosphate synthase | 14 | Urea cycle |
| Malate dehydrogenase | 15 | Tricarboxylic acid pathway |
| Hypoxanthine-guanine phosphoribosyl-transferase | 16 | Purine salvage pathway |
| Catechol-o-methyltransferase | 19A | Methylation of DNA |
| Thiosulfate sulfurtransferase | 17 | Sulfur metabolism, detoxification |
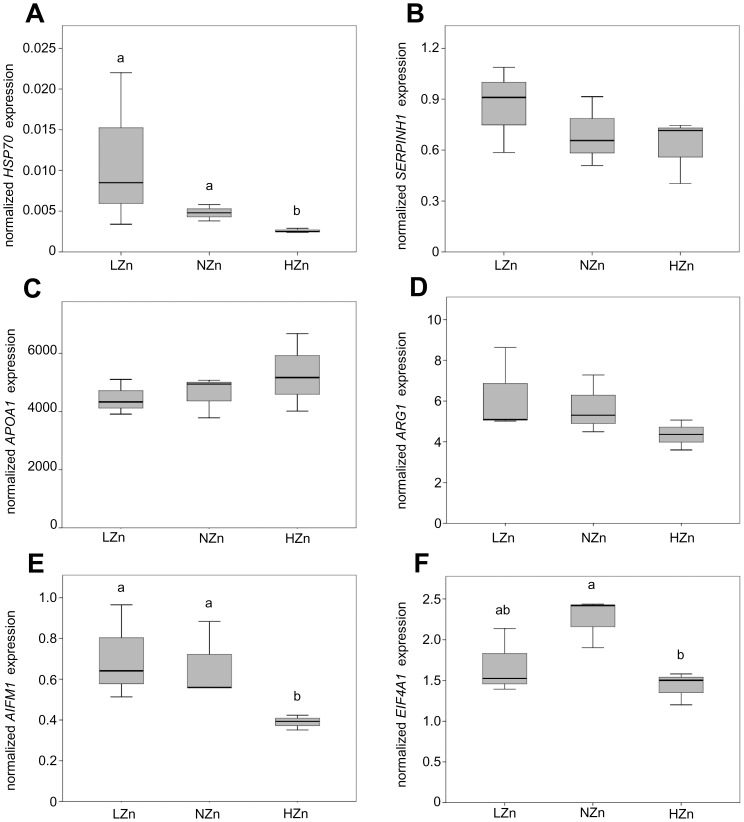
Differential expression of selected proteins (i.e. SERPINH1, HSP70, APOA1, ARG1, AIFM1, EIF4A1) was confirmed at the mRNA level (Fig. 4A–F). The mRNA level for HSP70, EIF4A1 and AIFM1 was decreased (P<0.05) with HZn supply compared with NZn fed animals. ARG1 showed the same tendency (P = 0.07). SERPINH1 was up-regulated and APOA1 was down-regulated in the LZn-feeding group, but failed to reach significance.
Figure 4. Effects of dietary zinc supplementation on relative mRNA level of differentially expressed liver proteins.
Relative mRNA level of HSP70 (A), SERPINH1 (B), APOA1 (C), ARG1 (D), AIFM1 (E), and EIF4A1 (F) in hepatic tissue of piglets fed LZn (50 mg/kg), NZn (150 mg/kg), or HZn (2500 mg/kg) levels of dietary zinc. Different superscripts indicate significant (P<0.05) differences.
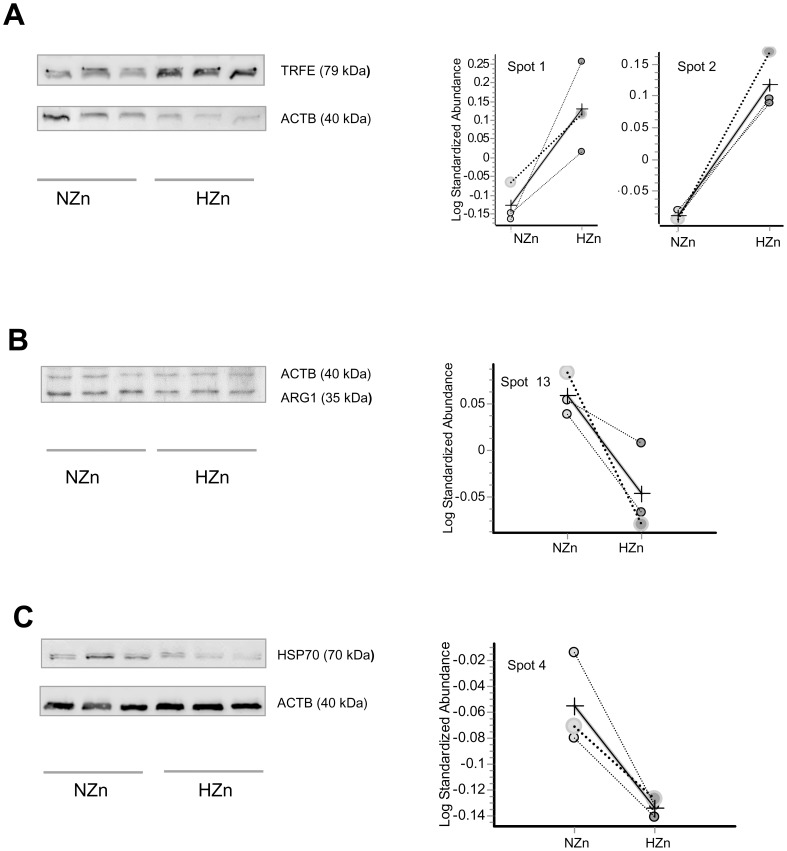
Western blotting was used to verify differential protein expression in hepatic tissue between piglets fed NZn and HZn diet (Fig. 5A–C). One representative protein out of three different functional groups (see Table 4), namely TRFE, ARG1 and Hsp70 was examined. On the right side of Fig.5 the protein abundances of these selected proteins (detected in each gel and calculated by the BVA module of the Decyder software) are indicated. Increased protein abundance in the liver of HZn-fed piglets was confirmed for TRFE (Fig. 5A), whereas reduced protein levels were confirmed for ARG1 (Fig. 5B) and HSP70 (Fig. 5C).
Figure 5. Validation of differential protein expression by Western blotting.
Western blot demonstration of differentially expressed proteins TRFE (A), ARG1(B), and HSP70(C) in the liver of piglets fed NZn (150 mg/kg), or HZn (2500 mg/kg) diets.
Discussion
The current study reveals that feeding pharmacological dietary zinc not only affects the hepatic zinc concentration in weaned piglets but, moreover, changes the global protein expression pattern. Zinc is one of the essential dietary elements for normal growth and development and innate immune function [1]. Feeding pigs with pharmacological levels of zinc to the diet of weanling piglets can enhance growth performance [17], [29], [30]. Proposed mechanisms for the enhanced growth of piglets involve MT as one candidate protein [17]–[19]. This was further underlined by our study demonstrating induced hepatic MT expression in response to HZn dietary zinc supply at transcript as well as protein level. MTs are a family of low molecular weight proteins (0.5–14 kDa) with high cysteine content that could regulate the availability of cellular zinc [31]. Thus, MT-level may be involved in triggering zinc signaling pathways, particularly in balancing redox homeostasis and metabolism. It has been demonstrated that the induction of MTs by zinc in the liver of mice or rats could play an important role in protection against oxidative stress [32], [33]. Although we did not observe significant differences in growth rate and feed intake in our approach, metabolic changes in the liver with HZn diets can be assumed. Interestingly, gene expression profiling in porcine hepatic tissue identified genes involved in reducing oxidative stress and amino acid metabolism [21]. Using a proteomic approach, we were able to even identify further targets of zinc in the liver providing a possible basis to unravel molecular mechanisms involved in the response HZn feeding. To our knowledge, the current study provides first evidence of a differential hepatic proteomic profile in pigs in response to pharmacological level of dietary zinc. The identified proteins are multifunctional and involved in important cellular processes.
Transport proteins
Two transport proteins were regulated in response to dietary zinc, namely APOA1 and TRFE. Particularly, the abundance of APOAI was lower in pigs fed LZn diet when compared to NZn-fed animals (P<0.05) and vice versa this protein was up-regulated in liver of HZn fed group. APOAI is mainly synthesized in the intestine and liver and is the major protein component of plasma high-density lipoprotein (HDL) particles. In growing rats, it has been demonstrated that Zn deficiency results in decreased serum HDL and plasma glucose, whereas cholesterol was increased [34], [35]. Accordingly, a down-regulation of hepatic APOAI mRNA abundance has been reported in rats and hamsters [36] and in Hep G2 cells [37], [38] under zinc-deficient conditions. On the other hand, Zn supplementation resulted in an increased cellular APOA1 mRNA abundance [37] and a concomitant increase of MT [39]. In accordance with these published results our data suggest that the Zn status regulates hepatic APOA1 expression in pigs.
High zinc level increased the expression of TRFE. TRFEs are iron-binding transport proteins that are involved in the transport of iron from absorption sites to those of storage and utilization (reviewed by Theil [40]). TRFE is not only an iron binding protein, but also binds zinc [41] and has functions in both, iron and zinc absorption and transport [42]. However, no difference in iron concentrations of the liver was detected in this study (data not shown). Thus, the up-regulation of TRFE might be a response to high hepatic zinc concentration. Other zinc binding proteins such as MT were also highly up-regulated in the liver. Additionally, due to its ability to chelate free iron, transferrin may have an important role as antioxidant [43].
Apoptosis
Our finding that feeding the HZn diet down-regulates the expression of AIFM1 in porcine liver is in line with other studies reporting zinc as a potent inhibitor of apoptosis [44]. Particularly, zinc supplementation has been shown to inhibit hepatic apoptosis in mice [45] and on the other side zinc deprivation induces apoptosis in several cell lines including HepG2 cells [46], [47].
Stress proteins
Increased expression of HSPs is considered as universal response to stress, which plays an important role in protecting cells from harmful environmental conditions as reviewed in Ref. [48], including pigs [49]. However, it is also known that these heat-shock proteins possess a more global role in cell metabolism, making it complicated to address their specific action. Heat shock proteins like HSP70 act as cellular chaperones and are involved in different cellular functions, such as, protein folding and assembly or reassembly. Particularly, HSP70 is known to inhibit the aggregation of nascent or miss-folded proteins to regulate protein degradation and to help in translocation of proteins between different cellular compartments [50], [51]. Previously our group has reported an up-regulation of HSP70 expression in porcine intestinal cells exposed to high concentrations of zinc [52]. This is consistent with data on mice, indicating enhanced HSP70 expression in the gastrointestinal tract in response to high dietary zinc [53]. Interestingly, here we found a down-regulation of liver HSP70, which could be confirmed by immunoblotting and was further underlined by decreased HSP70 mRNA. This is in accordance with the results of a gene expression study in hepatic tissue of newly weaned pigs fed with 2000 mg Zn/kg [21]. These authors speculated that zinc induced chaperone expression will be counteracted at higher zinc concentration by inhibiting their expression, previously discussed by other authors [54]. Recently published results indicate different heat shock response in different organs and tissues of pigs after transportation. [55]. Therefore it is possible that heat shock expression in different cell types and organs could be regulated through distinct mechanisms in a complex manner at both transcriptional and translational levels. However, the precise function of down-regulation of HSP70 in porcine liver in response to zinc needs further investigations. Interestingly, the ERP29, another putative chaperone, was found up-regulated in response to high zinc concentrations. ERP29 is known to self-associate into 51 kDa dimers (also seen in Fig. 3) and to lack redox enzyme properties, such as disulphide-editing, that are typical for other chaperones [56]. The functional role in the liver may be related to its important role in the early secretory pathway facilitating processing and transport of proteins [57]. The up-regulation of ERP29 under high dietary zinc possibly occurs through involvement of zinc regulatory transcription factors including Sp1 [58] that have been reported to be member of highly related zinc finger proteins [59]. It should be mentioned, that zinc deficiency is also associated with stress [35]. This suggestion can be underlined by the results of our study. As compared with NZn group, the LZn feeding of 50 mg Zn/kg affects the expression of the SERPINH1 (HSP47) precursor. The serpins are a family of serin proteinase inhibitors with pleiotropic functions extending from protease inhibition to hormone transport and regulation of chromatin organization [60]. Interestingly, SERPINH1 (Hsp47) is a stress inducible collagen binding protein acting as protein-specific “manager” assisting the synthesis, post-translational modification and transport of (pro)-collagen without protease inhibitory activity [60]. Its induction was reported by liver damage and markedly up-regulated during progression of liver fibrosis [61], [62].
Metabolism
The liver plays the major role in the metabolism of nutrients and other dietary substances [63]. Accordingly, 10 differentially expressed proteins involved in various metabolic pathways were identified (Tab. 3 and 4). Particularly, two enzymes of the glycolysis were found to be up-regulated by high dietary zinc concentrations, namely ALDOB and GAPDH. These enzymes have been recently characterized as zinc-binding proteins in human hepatocytes [64]. Some evidences indicate that high zinc concentrations stimulate glycolysis as shown on isolated rat hepatocytes and primary mouse hepatocytes [65], [66]. Furthermore this effect was markedly diminished in hepatocytes from MT-null (-/-) mice suggesting a close link between zinc induced increase of hepatic MT and increased glycolysis. Interestingly, our study reveals a down-regulation of the MDH by zinc. However, this inhibitory effect of zinc could be also demonstrated in chick-embryo hepatocytes [67] and may be underlined by studies on the isolated malic liver enzyme [68]. Furthermore, the expression of two enzymes of the urea-cycle was moderately decreased. This corresponds with an exponential decline in luminal ammonia concentration [23] and might suggest an adaptive depression of the urea cycle in the liver. The one-carbon metabolism is connected with other pathways like biosynthesis of nucleic acids, conversion of creatine and amino acids as well as in vitamin metabolism. Furthermore, zinc is also essential for synthesis of coenzymes that mediate biogenic-amine synthesis and metabolism [69]. Accordingly, in our study the expression of the C1TC was up-regulated by zinc. Notably, it has been reported that folate deficiency enhances perturbations in methionine metabolism and DNA damage while promoting alcoholic liver injury [70]. This in turn could be suppressed by zinc supply [71]. Another enzyme, the ADK playing an important role in the maintenance and balance of methylation reactions, was found to be up-regulated by high dietary zinc in the liver. The HPRT converts guanine to guanosine monophosphate, and hypoxanthine to inosine monophosphate. This enzyme plays a central role in the generation of purine nucleotides through the purine salvage pathway. In rats, which received a zinc-deficient diet, an enhanced activity of the HPRT was observed [72]. Accordingly, we found a down-regulation of this enzyme in response to high dietary zinc. The porcine THTR is found with increased activities in liver and kidney [73] and suggested to be involved in cyanide detoxification [73], [74]. However, this enzyme may be involved in other functions, including energy and sulfur metabolism; a function as thioredoxin oxidase and involvement in proliferation and progression of cell cycle are further discussed (reviewed by Cippolone et al. [75]). Rhodanese was found to be inhibited by zinc by more than 70%, implying an interaction of zinc with the sulfhydryl groups of the enzyme catalytic sites [76], [77]. Our study provides evidence that this enzyme is related to zinc status in liver but clearly requires further investigations to define its exact biological function.
In summary, high dietary zinc supplementation induced an increase in the levels of both hepatic zinc and MT expression, and altered the protein expression profiles. Using a 2D-DIGE proteomic approach, we were able to describe for the first time significant protein changes in the liver, despite of a limited piglet number. We provide initial insights on the complex regulatory network of zinc-induced protein expression pattern alterations in the liver. The identified proteins were involved in transport, stress response, metabolism, apoptosis and cellular signaling. Further work is needed to determine their functional relevance and to clarify whether changes in the level of the above mentioned proteins are due to a direct effect of zinc supplementation or a consequence of its interaction with other target(s).
Acknowledgments
We thank Ulla Scholz and Christoph Holder for technical assistance.
Funding Statement
This work was supported by the DFG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.Suttle N (2010) Mineral Nutrition of Livestock, 4th Edition Preface. Mineral Nutrition of Livestock, 4th Edition: Vii–Viii.
- 2. Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME (2012) Zinc and human health: an update. Arch Toxicol 86: 521–534. [DOI] [PubMed] [Google Scholar]
- 3. Krebs NF (2013) Update on Zinc Deficiency and Excess in Clinical Pediatric Practice. Annals of Nutrition and Metabolism 62: 19–29. [DOI] [PubMed] [Google Scholar]
- 4. Hahn JD, Baker DH (1993) Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J Anim Sci 71: 3020–3024. [DOI] [PubMed] [Google Scholar]
- 5. Case CL, Carlson MS (2002) Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J Anim Sci 80: 1917–1924. [DOI] [PubMed] [Google Scholar]
- 6. Hu C, Song J, You Z, Luan Z, Li W (2012) Zinc oxide-montmorillonite hybrid influences diarrhea, intestinal mucosal integrity, and digestive enzyme activity in weaned pigs. Biol Trace Elem Res 149: 190–196. [DOI] [PubMed] [Google Scholar]
- 7. Poulsen HD (1995) Zinc-Oxide for Weanling Piglets. Acta Agriculturae Scandinavica Section a-Animal Science 45: 159–167. [Google Scholar]
- 8. Wang X, Ou D, Yin J, Wu G, Wang J (2009) Proteomic analysis reveals altered expression of proteins related to glutathione metabolism and apoptosis in the small intestine of zinc oxide-supplemented piglets. Amino Acids 37: 209–218. [DOI] [PubMed] [Google Scholar]
- 9. Hojberg O, Canibe N, Poulsen HD, Hedemann MS, Jensen BB (2005) Influence of dietary zinc oxide and copper sulfate on the gastrointestinal ecosystem in newly weaned piglets. Appl Environ Microbiol 71: 2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vahjen W, Pieper R, Zentek J (2011) Increased dietary zinc oxide changes the bacterial core and enterobacterial composition in the ileum of piglets. J Anim Sci 89: 2430–2439. [DOI] [PubMed] [Google Scholar]
- 11. Zhang B, Guo Y (2009) Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr 102: 687–693. [DOI] [PubMed] [Google Scholar]
- 12.Hu C, Song J, Li Y, Luan Z, Zhu K (2013) Diosmectite-zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br J Nutr: 1–8. [DOI] [PubMed]
- 13. Li X, Yin J, Li D, Chen X, Zang J, et al. (2006) Dietary supplementation with zinc oxide increases Igf-I and Igf-I receptor gene expression in the small intestine of weanling piglets. J Nutr 136: 1786–1791. [DOI] [PubMed] [Google Scholar]
- 14. Yin J, Li X, Li D, Yue T, Fang Q, et al. (2009) Dietary supplementation with zinc oxide stimulates ghrelin secretion from the stomach of young pigs. J Nutr Biochem 20: 783–790. [DOI] [PubMed] [Google Scholar]
- 15. Lichten LA, Cousins RJ (2009) Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29: 153–176. [DOI] [PubMed] [Google Scholar]
- 16. Schell TC, Kornegay ET (1996) Zinc concentration in tissues and performance of weanling pigs fed pharmacological levels of zinc from ZnO, Zn-methionine, Zn-lysine, or ZnSO4. J Anim Sci 74: 1584–1593. [DOI] [PubMed] [Google Scholar]
- 17. Carlson MS, Hill GM, Link JE (1999) Early- and traditionally weaned nursery pigs benefit from phase-feeding pharmacological concentrations of zinc oxide: effect on metallothionein and mineral concentrations. J Anim Sci 77: 1199–1207. [DOI] [PubMed] [Google Scholar]
- 18. Martinez MM, Hill GM, Link JE, Raney NE, Tempelman RJ, et al. (2004) Pharmacological zinc and phytase supplementation enhance metallothionein mRNA abundance and protein concentration in newly weaned pigs. J Nutr 134: 538–544. [DOI] [PubMed] [Google Scholar]
- 19. Martinez MM, Link JE, Hill GM (2005) Dietary pharmacological or excess zinc and phytase effects on tissue mineral concentrations, metallothionein, and apparent mineral retention in the newly weaned pig. Biol Trace Elem Res 105: 97–115. [DOI] [PubMed] [Google Scholar]
- 20. Stamoulis I, Kouraklis G, Theocharis S (2007) Zinc and the liver: an active interaction. Dig Dis Sci 52: 1595–1612. [DOI] [PubMed] [Google Scholar]
- 21. Martinez-Montemayor MM, Hill GM, Raney NE, Rilington VD, Tempelman RJ, et al. (2008) Gene expression profiling in hepatic tissue of newly weaned pigs fed pharmacological zinc and phytase supplemented diets. BMC Genomics 9: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bondzio A, Gabler C, Badewien-Rentzsch B, Schulze P, Martens H, et al. (2011) Identification of differentially expressed proteins in ruminal epithelium in response to a concentrate-supplemented diet. Am J Physiol Gastrointest Liver Physiol 301: G260–268. [DOI] [PubMed] [Google Scholar]
- 23. Pieper R, Vahjen W, Neumann K, Van Kessel AG, Zentek J (2012) Dose-dependent effects of dietary zinc oxide on bacterial communities and metabolic profiles in the ileum of weaned pigs. J Anim Physiol Anim Nutr (Berl) 96: 825–833. [DOI] [PubMed] [Google Scholar]
- 24. Marouga R, David S, Hawkins E (2005) The development of the DIGE system: 2D fluorescence difference gel analysis technology. Anal Bioanal Chem 382: 669–678. [DOI] [PubMed] [Google Scholar]
- 25. Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858. [DOI] [PubMed] [Google Scholar]
- 26.Martin L, Lodemann U, Bondzio A, Gefeller EM, Vahjen W, et al.. (2013) A High Amount of Dietary Zinc Changes the Expression of Zinc Transporters and Metallothionein in Jejunal Epithelial Cells in Vitro and in Vivo but Does Not Prevent Zinc Accumulation in Jejunal Tissue of Piglets. J Nutr. [DOI] [PubMed]
- 27. Odau S, Gabler C, Holder C, Einspanier R (2006) Differential expression of cyclooxygenase 1 and cyclooxygenase 2 in the bovine oviduct. J Endocrinol 191: 263–274. [DOI] [PubMed] [Google Scholar]
- 28. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carlson D, Sehested J, Feng Z, Poulsen HD (2008) Serosal zinc attenuate serotonin and vasoactive intestinal peptide induced secretion in piglet small intestinal epithelium in vitro. Comp Biochem Physiol A Mol Integr Physiol 149: 51–58. [DOI] [PubMed] [Google Scholar]
- 30. Hill GM, Mahan DC, Carter SD, Cromwell GL, Ewan RC, et al. (2001) Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. J Anim Sci 79: 934–941. [DOI] [PubMed] [Google Scholar]
- 31. Cousins RJ, Liuzzi JP, Lichten LA (2006) Mammalian zinc transport, trafficking, and signals. J Biol Chem 281: 24085–24089. [DOI] [PubMed] [Google Scholar]
- 32. Wang X, Li H, Fan Z, Liu Y (2012) Effect of zinc supplementation on type 2 diabetes parameters and liver metallothionein expressions in Wistar rats. J Physiol Biochem 68: 563–572. [DOI] [PubMed] [Google Scholar]
- 33. Beattie JH, Gordon MJ, Reid MD, Rucklidge GJ, Kwon CS, et al. (2006) Hepatic responses to dietary stress in zinc- and metallothionein-deficient mice. Exp Biol Med (Maywood) 231: 1542–1547. [DOI] [PubMed] [Google Scholar]
- 34. El Hendy HA, Yousef MI, Abo El-Naga NI (2001) Effect of dietary zinc deficiency on hematological and biochemical parameters and concentrations of zinc, copper, and iron in growing rats. Toxicology 167: 163–170. [DOI] [PubMed] [Google Scholar]
- 35. Yousef MI, El-Hendy HA, El-Demerdash FM, Elagamy EI (2002) Dietary zinc deficiency induced-changes in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology 175: 223–234. [DOI] [PubMed] [Google Scholar]
- 36. Wu JY, Reaves SK, Wang YR, Wu Y, Lei PP, et al. (1998) Zinc deficiency decreases plasma level and hepatic mRNA abundance of apolipoprotein A-I in rats and hamsters. Am J Physiol 275: C1516–1525. [DOI] [PubMed] [Google Scholar]
- 37. Wu JY, Wu Y, Reaves SK, Wang YR, Lei PP, et al. (1999) Apolipoprotein A-I gene expression is regulated by cellular zinc status in hep G2 cells. Am J Physiol 277: C537–544. [DOI] [PubMed] [Google Scholar]
- 38. Cui L, Schoene NW, Zhu L, Fanzo JC, Alshatwi A, et al. (2002) Zinc depletion reduced Egr-1 and HNF-3beta expression and apolipoprotein A-I promoter activity in Hep G2 cells. Am J Physiol Cell Physiol 283: C623–630. [DOI] [PubMed] [Google Scholar]
- 39. Urani C, Melchioretto P, Gribaldo L (2010) Regulation of metallothioneins and ZnT-1 transporter expression in human hepatoma cells HepG2 exposed to zinc and cadmium. Toxicol In Vitro 24: 370–374. [DOI] [PubMed] [Google Scholar]
- 40. Theil EC (2004) Iron, ferritin, and nutrition. Annu Rev Nutr 24: 327–343. [DOI] [PubMed] [Google Scholar]
- 41. Quarles CD Jr, Brumaghim JL, Marcus RK (2010) Simultaneous multiple element detection by particle beam/hollow cathode-optical emission spectroscopy as a tool for metallomic studies: determinations of metal binding with apo-transferrin. Metallomics 2: 154–161. [DOI] [PubMed] [Google Scholar]
- 42. Evans GW (1976) Transferrin function in zinc absorption and transport. Proc Soc Exp Biol Med 151: 775–778. [DOI] [PubMed] [Google Scholar]
- 43. Gutteridge JM, Quinlan GJ (1993) Antioxidant protection against organic and inorganic oxygen radicals by normal human plasma: the important primary role for iron-binding and iron-oxidising proteins. Biochim Biophys Acta 1156: 144–150. [DOI] [PubMed] [Google Scholar]
- 44. Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, et al. (1997) Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J Biol Chem 272: 18530–18533. [DOI] [PubMed] [Google Scholar]
- 45. Zhou Z, Liu J, Song Z, McClain CJ, Kang YJ (2008) Zinc supplementation inhibits hepatic apoptosis in mice subjected to a long-term ethanol exposure. Exp Biol Med (Maywood) 233: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nakatani T, Tawaramoto M, Opare Kennedy D, Kojima A, Matsui-Yuasa I (2000) Apoptosis induced by chelation of intracellular zinc is associated with depletion of cellular reduced glutathione level in rat hepatocytes. Chem Biol Interact 125: 151–163. [DOI] [PubMed] [Google Scholar]
- 47. Cao J, Bobo JA, Liuzzi JP, Cousins RJ (2001) Effects of intracellular zinc depletion on metallothionein and ZIP2 transporter expression and apoptosis. J Leukoc Biol 70: 559–566. [PubMed] [Google Scholar]
- 48. Mukhopadhyay I, Nazir A, Saxena DK, Chowdhuri DK (2003) Heat shock response: hsp70 in environmental monitoring. J Biochem Mol Toxicol 17: 249–254. [DOI] [PubMed] [Google Scholar]
- 49. Zhong X, Li W, Huang X, Zhang L, Yimamu M, et al. (2012) Impairment of cellular immunity is associated with overexpression of heat shock protein 70 in neonatal pigs with intrauterine growth retardation. Cell Stress Chaperones 17: 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295: 1852–1858. [DOI] [PubMed] [Google Scholar]
- 51. Arya R, Mallik M, Lakhotia SC (2007) Heat shock genes - integrating cell survival and death. J Biosci 32: 595–610. [DOI] [PubMed] [Google Scholar]
- 52. Lodemann U, Einspanier R, Scharfen F, Martens H, Bondzio A (2013) Effects of zinc on epithelial barrier properties and viability in a human and a porcine intestinal cell culture model. Toxicol In Vitro 27: 834–843. [DOI] [PubMed] [Google Scholar]
- 53. Van Molle W, Van Roy M, Van Bogaert T, Dejager L, Van Lint P, et al. (2007) Protection of zinc against tumor necrosis factor induced lethal inflammation depends on heat shock protein 70 and allows safe antitumor therapy. Cancer Res 67: 7301–7307. [DOI] [PubMed] [Google Scholar]
- 54. Soti C, Csermely P (2003) Aging and molecular chaperones. Exp Gerontol 38: 1037–1040. [DOI] [PubMed] [Google Scholar]
- 55. Zhang M, Lv Y, Yue Z, Islam A, Rehana B, et al. (2011) Effects of transportation on expression of Hsp90, Hsp70, Hsp27 and alpha B-crystallin in the pig stomach. Veterinary Record 169: 312–U356. [DOI] [PubMed] [Google Scholar]
- 56. Mkrtchian S, Sandalova T (2006) ERp29, an unusual redox-inactive member of the thioredoxin family. Antioxid Redox Signal 8: 325–337. [DOI] [PubMed] [Google Scholar]
- 57. Zhang D, Richardson DR (2011) Endoplasmic reticulum protein 29 (ERp29): An emerging role in cancer. Int J Biochem Cell Biol 43: 33–36. [DOI] [PubMed] [Google Scholar]
- 58. Sargsyan E, Baryshev M, Szekely L, Sharipo A, Mkrtchian S (2002) Identification of ERp29, an endoplasmic reticulum lumenal protein, as a new member of the thyroglobulin folding complex. J Biol Chem 277: 17009–17015. [DOI] [PubMed] [Google Scholar]
- 59. Cook T, Gebelein B, Urrutia R (1999) Sp1 and its likes: biochemical and functional predictions for a growing family of zinc finger transcription factors. Ann N Y Acad Sci 880: 94–102. [DOI] [PubMed] [Google Scholar]
- 60. Ragg H (2007) The role of serpins in the surveillance of the secretory pathway. Cell Mol Life Sci 64: 2763–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Park KJ, Kim HY, Chang BJ, Lee HH (2004) Ameliorative effects of soy 11S protein on liver damage and hyperlipidemia in alcohol-fed rats. Biol Pharm Bull 27: 1636–1641. [DOI] [PubMed] [Google Scholar]
- 62. Nagata H, Ito M, Cai J, Edge AS, Platt JL, et al. (2003) Treatment of cirrhosis and liver failure in rats by hepatocyte xenotransplantation. Gastroenterology 124: 422–431. [DOI] [PubMed] [Google Scholar]
- 63. Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G (2006) Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 17: 571–588. [DOI] [PubMed] [Google Scholar]
- 64. She YM, Narindrasorasak S, Yang S, Spitale N, Roberts EA, et al. (2003) Identification of metal-binding proteins in human hepatoma lines by immobilized metal affinity chromatography and mass spectrometry. Mol Cell Proteomics 2: 1306–1318. [DOI] [PubMed] [Google Scholar]
- 65. Brand IA, Kleineke J (1996) Intracellular zinc movement and its effect on the carbohydrate metabolism of isolated rat hepatocytes. J Biol Chem 271: 1941–1949. [DOI] [PubMed] [Google Scholar]
- 66. Rofe AM, Philcox JC, Coyle P (2000) Activation of glycolysis by zinc is diminished in hepatocytes from metallothionein-null mice. Biol Trace Elem Res 75: 87–97. [DOI] [PubMed] [Google Scholar]
- 67. Zhu Y, Goodridge AG, Stapleton SR (1994) Zinc, Vanadate and Selenate Inhibit the Tri-Iodothyronine-Induced Expression of Fatty-Acid Synthase and Malic Enzyme in Chick-Embryo Hepatocytes in Culture. Biochemical Journal 303: 213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hung HC, Chang GG, Yang ZR, Tong L (2000) Slow binding of metal ions to pigeon liver malic enzyme: A general case. Biochemistry 39: 14095–14102. [DOI] [PubMed] [Google Scholar]
- 69. Sandstead HH, Frederickson CJ, Penland JG (2000) History of zinc as related to brain function. Journal of Nutrition 130: 496s–502s. [DOI] [PubMed] [Google Scholar]
- 70. Halsted CH, Villanueva JA, Devlin AM, Niemela O, Parkkila S, et al. (2002) Folate deficiency disturbs hepatic methionine metabolism and promotes liver injury in the ethanol-fed micropig. Proc Natl Acad Sci U S A 99: 10072–10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, et al. (2005) Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol 166: 1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cossack ZT, Prasad AS (1991) Activities of Purine Catabolism Related Enzymes in Zinc-Deficiency - Relationship to T-Lymphocyte Dysfunction and Hyperammonemia. International Journal for Vitamin and Nutrition Research 61: 51–56. [PubMed] [Google Scholar]
- 73. Aminlari M, Li A, Kunanithy V, Scaman CH (2002) Rhodanese distribution in porcine (Sus scrofa) tissues. Comp Biochem Physiol B Biochem Mol Biol 132: 309–313. [DOI] [PubMed] [Google Scholar]
- 74. Himwich WA, Saunders JP (1948) Enzymatic Conversion of Cyanide to Thiocyanate. American Journal of Physiology 153: 348–354. [DOI] [PubMed] [Google Scholar]
- 75. Cipollone R, Ascenzi P, Visca P (2007) Common themes and variations in the rhodanese superfamily. IUBMB Life 59: 51–59. [DOI] [PubMed] [Google Scholar]
- 76. Volini M, Van Sweringen B, Chen FS (1978) Effects of metal-ion binding on rhodanese activity. Arch Biochem Biophys 191: 205–215. [DOI] [PubMed] [Google Scholar]
- 77. Lee GD, Lee MH, Ihm J (1995) Role of d electrons in the zinc-blende semiconductors ZnS, ZnSe, and ZnTe. Phys Rev B Condens Matter 52: 1459–1462. [DOI] [PubMed] [Google Scholar]