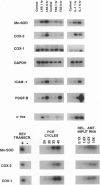
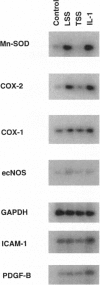
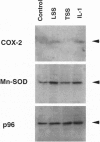
Abstract
Early atherosclerotic lesions develop in a topographical pattern that strongly suggests involvement of hemodynamic forces in their pathogenesis. We hypothesized that certain endothelial genes, which exhibit differential responsiveness to distinct fluid mechanical stimuli, may participate in the atherogenic process by modulating, on a local level within the arterial wall, the effects of systemic risk factors. A differential display strategy using cultured human endothelial cells has identified two genes, manganese superoxide dismutase and cyclooxygenase-2, that exhibit selective and sustained up-regulation by steady laminar shear stress (LSS). Turbulent shear stress, a nonlaminar fluid mechanical stimulus, does not induce these genes. The endothelial form of nitric oxide synthase also demonstrates a similar LSS-selective pattern of induction. Thus, three genes with potential atheroprotective (antioxidant, antithrombotic, and antiadhesive) activities manifest a differential response to distinct fluid mechanical stimuli, providing a possible mechanistic link between endothelial gene expression and early events in atherogenesis. The activities of these and other LSS-responsive genes may have important implications for the pathogenesis and prevention of atherosclerosis.
Full text
PDF

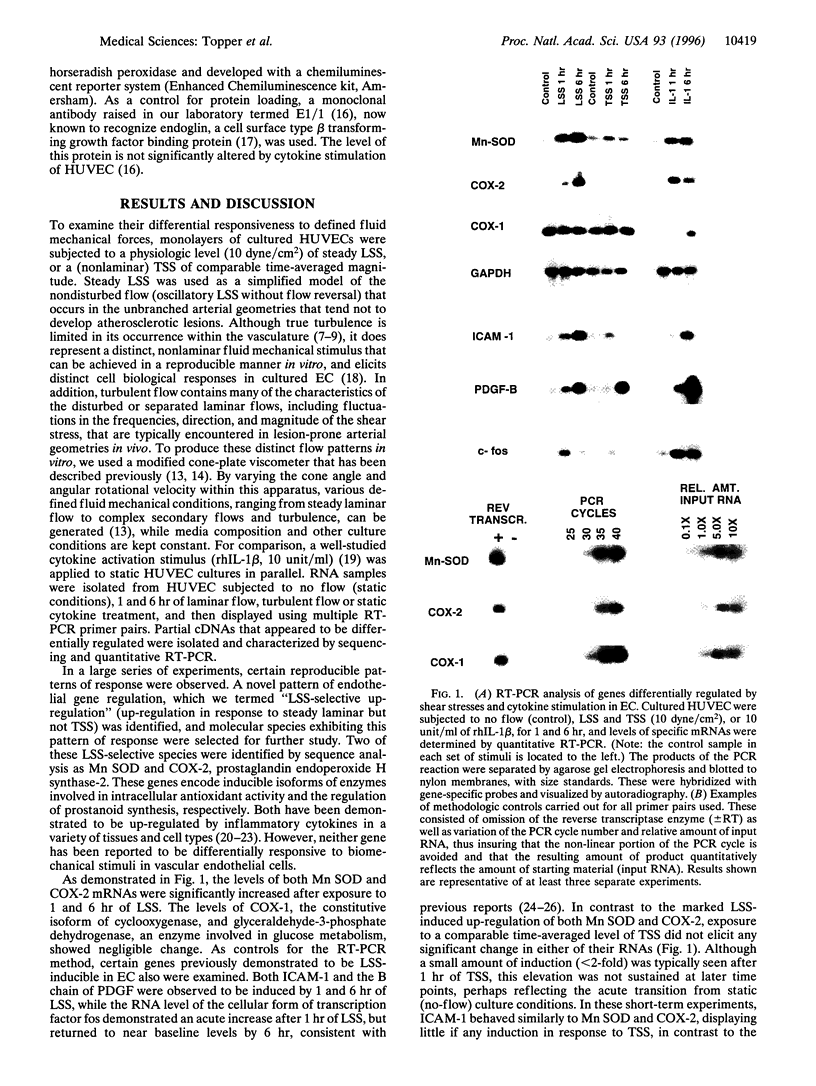


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. W. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995 Feb;25(2):155–161. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- Ando J., Tsuboi H., Korenaga R., Takada Y., Toyama-Sorimachi N., Miyasaka M., Kamiya A. Shear stress inhibits adhesion of cultured mouse endothelial cells to lymphocytes by downregulating VCAM-1 expression. Am J Physiol. 1994 Sep;267(3 Pt 1):C679–C687. doi: 10.1152/ajpcell.1994.267.3.C679. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bussolari S. R., Dewey C. F., Jr, Gimbrone M. A., Jr Apparatus for subjecting living cells to fluid shear stress. Rev Sci Instrum. 1982 Dec;53(12):1851–1854. doi: 10.1063/1.1136909. [DOI] [PubMed] [Google Scholar]
- Caro C. G., Fitz-Gerald J. M., Schroter R. C. Arterial wall shear and distribution of early atheroma in man. Nature. 1969 Sep 13;223(5211):1159–1160. doi: 10.1038/2231159a0. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Bellón T., Calés C., Vera S., Bernabeu C., Massagué J., Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992 Sep 25;267(27):19027–19030. [PubMed] [Google Scholar]
- Collins T. Endothelial nuclear factor-kappa B and the initiation of the atherosclerotic lesion. Lab Invest. 1993 May;68(5):499–508. [PubMed] [Google Scholar]
- Cornhill J. F., Roach M. R. A quantitative study of the localization of atherosclerotic lesions in the rabbit aorta. Atherosclerosis. 1976 May-Jun;23(3):489–501. doi: 10.1016/0021-9150(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Davies P. F. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995 Jul;75(3):519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. F., Remuzzi A., Gordon E. J., Dewey C. F., Jr, Gimbrone M. A., Jr Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caterina R., Libby P., Peng H. B., Thannickal V. J., Rajavashisth T. B., Gimbrone M. A., Jr, Shin W. S., Liao J. K. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995 Jul;96(1):60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusting G. J. Nitric oxide in cardiovascular disorders. J Vasc Res. 1995 May-Jun;32(3):143–161. doi: 10.1159/000159089. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cybulsky M. I., Kume N., Collins T., Resnick N. Vascular endothelium. An integrator of pathophysiological stimuli in atherogenesis. Ann N Y Acad Sci. 1995 Jan 17;748:122–132. [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H. J., Li N. Q., Frangos J. A. Shear stress increases endothelial platelet-derived growth factor mRNA levels. Am J Physiol. 1991 Feb;260(2 Pt 2):H642–H646. doi: 10.1152/ajpheart.1991.260.2.H642. [DOI] [PubMed] [Google Scholar]
- Hsieh H. J., Li N. Q., Frangos J. A. Shear-induced platelet-derived growth factor gene expression in human endothelial cells is mediated by protein kinase C. J Cell Physiol. 1992 Mar;150(3):552–558. doi: 10.1002/jcp.1041500316. [DOI] [PubMed] [Google Scholar]
- Inoue N., Ramasamy S., Fukai T., Nerem R. M., Harrison D. G. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ Res. 1996 Jul;79(1):32–37. doi: 10.1161/01.res.79.1.32. [DOI] [PubMed] [Google Scholar]
- Jones D. A., Carlton D. P., McIntyre T. M., Zimmerman G. A., Prescott S. M. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993 Apr 25;268(12):9049–9054. [PubMed] [Google Scholar]
- Karino T., Motomiya M. Flow visualization in isolated transparent natural blood vessels. Biorheology. 1983;20(2):119–127. doi: 10.3233/bir-1983-20202. [DOI] [PubMed] [Google Scholar]
- Khachigian L. M., Resnick N., Gimbrone M. A., Jr, Collins T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest. 1995 Aug;96(2):1169–1175. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku D. N., Giddens D. P., Zarins C. K., Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985 May-Jun;5(3):293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- Kume N., Cybulsky M. I., Gimbrone M. A., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992 Sep;90(3):1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume N., Gimbrone M. A., Jr Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human endothelial cells. J Clin Invest. 1994 Feb;93(2):907–911. doi: 10.1172/JCI117047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Cybulsky M. I., Gimbrone M. A., Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993 Feb;13(2):197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Lindau-Shepard B., Shaffer J. B., Del Vecchio P. J. Overexpression of manganous superoxide dismutase (MnSOD) in pulmonary endothelial cells confers resistance to hyperoxia. J Cell Physiol. 1994 Nov;161(2):237–242. doi: 10.1002/jcp.1041610207. [DOI] [PubMed] [Google Scholar]
- Marui N., Offermann M. K., Swerlick R., Kunsch C., Rosen C. A., Ahmad M., Alexander R. W., Medford R. M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993 Oct;92(4):1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morham S. G., Langenbach R., Loftin C. D., Tiano H. F., Vouloumanos N., Jennette J. C., Mahler J. F., Kluckman K. D., Ledford A., Lee C. A. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995 Nov 3;83(3):473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Nagel T., Resnick N., Atkinson W. J., Dewey C. F., Jr, Gimbrone M. A., Jr Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest. 1994 Aug;94(2):885–891. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y., Plump A. S., Raines E. W., Breslow J. L., Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994 Jan;14(1):133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Nishida K., Harrison D. G., Navas J. P., Fisher A. A., Dockery S. P., Uematsu M., Nerem R. M., Alexander R. W., Murphy T. J. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992 Nov;90(5):2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermann M. K., Medford R. M. Antioxidants and atherosclerosis: a molecular perspective. Heart Dis Stroke. 1994 Jan-Feb;3(1):52–57. [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Pomerantz K. B., Hajjar D. P. Eicosanoids in regulation of arterial smooth muscle cell phenotype, proliferative capacity, and cholesterol metabolism. Arteriosclerosis. 1989 Jul-Aug;9(4):413–429. doi: 10.1161/01.atv.9.4.413. [DOI] [PubMed] [Google Scholar]
- Pritchard K. A., Jr, O'Banion M. K., Miano J. M., Vlasic N., Bhatia U. G., Young D. A., Stemerman M. B. Induction of cyclooxygenase-2 in rat vascular smooth muscle cells in vitro and in vivo. J Biol Chem. 1994 Mar 18;269(11):8504–8509. [PubMed] [Google Scholar]
- Resnick N., Collins T., Atkinson W., Bonthron D. T., Dewey C. F., Jr, Gimbrone M. A., Jr Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick N., Gimbrone M. A., Jr Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. 1995 Jul;9(10):874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- Ristimäki A., Garfinkel S., Wessendorf J., Maciag T., Hla T. Induction of cyclooxygenase-2 by interleukin-1 alpha. Evidence for post-transcriptional regulation. J Biol Chem. 1994 Apr 22;269(16):11769–11775. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Shyy J. Y., Lin M. C., Han J., Lu Y., Petrime M., Chien S. The cis-acting phorbol ester "12-O-tetradecanoylphorbol 13-acetate"-responsive element is involved in shear stress-induced monocyte chemotactic protein 1 gene expression. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):8069–8073. doi: 10.1073/pnas.92.17.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Tatsumi H., Satoh S., Senda T., Nakata T., Fujii J., Taniguchi N. Manganese-superoxide dismutase in endothelial cells: localization and mechanism of induction. Am J Physiol. 1993 Oct;265(4 Pt 2):H1173–H1178. doi: 10.1152/ajpheart.1993.265.4.H1173. [DOI] [PubMed] [Google Scholar]
- Tsujii M., DuBois R. N. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995 Nov 3;83(3):493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- Vane J. R., Mitchell J. A., Appleton I., Tomlinson A., Bishop-Bailey D., Croxtall J., Willoughby D. A. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennmalm A. Endothelial nitric oxide and cardiovascular disease. J Intern Med. 1994 Apr;235(4):317–327. doi: 10.1111/j.1365-2796.1994.tb01081.x. [DOI] [PubMed] [Google Scholar]
- Wispé J. R., Warner B. B., Clark J. C., Dey C. R., Neuman J., Glasser S. W., Crapo J. D., Chang L. Y., Whitsett J. A. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem. 1992 Nov 25;267(33):23937–23941. [PubMed] [Google Scholar]
- Zarins C. K., Giddens D. P., Bharadvaj B. K., Sottiurai V. S., Mabon R. F., Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983 Oct;53(4):502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- Zembowicz A., Jones S. L., Wu K. K. Induction of cyclooxygenase-2 in human umbilical vein endothelial cells by lysophosphatidylcholine. J Clin Invest. 1995 Sep;96(3):1688–1692. doi: 10.1172/JCI118211. [DOI] [PMC free article] [PubMed] [Google Scholar]