Abstract
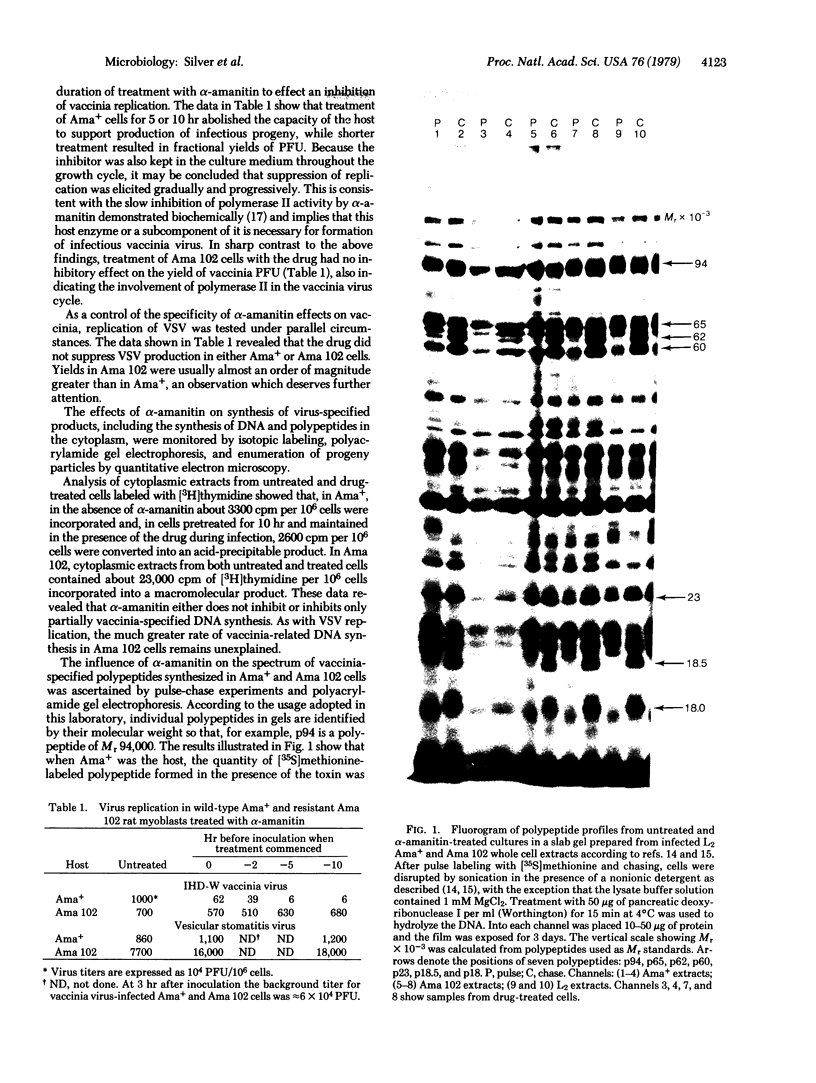
The participation of host RNA polymerase II in the vaccinia life cycle was examined by comparing efficiency of multiplication after treating the Ama+ sensitive and Ama 102 drug resistant lines with alpha-amanitin. In the latter, resistance is due to a mutation in RNA polymerase II. The toxin profoundly reduces synthesis of virus-specified polypeptides and morphopoeisis in Ama+ but not in Ama 102 rat myoblasts without appreciably altering vaccinia DNA replication in either cell type. This implicates RNA polymerase II in the expression of late virus functions. Circumstantial evidence from a model system indicates that gamma irradiation of the host prior to infection might disrupt transcription into functional mRNA from the nucleus. Irradiation does not, however, alter the capability of the host to support vaccinia multiplication fully. Therefore, ongoing host nuclear transcription may not be required by this virus. The above results are consistent with the ability of cytoplasts to produce small quantities of mature progeny. Our studies lead us to hypothesize that RNA polymerase II or a subunit of the host enzyme may participate directly in late transcription of the vaccinia genome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati P., Blasi F., Di Porzio U., Riccio A., Traboni C. Hamster alpha-amanitine-resistant RNA polymerase II able to transcribe polyoma virus genome in somatic cell hybrids. Proc Natl Acad Sci U S A. 1975 Feb;72(2):753–757. doi: 10.1073/pnas.72.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodner O. G., Wieland T. Identification of the amatoxin-binding subunit of RNA polymerase B by affinity labeling experiments. Subunit B3-the true amatoxin receptor protein of multiple RNA polymerase B. Biochemistry. 1976 Aug 10;15(16):3480–3484. doi: 10.1021/bi00661a013. [DOI] [PubMed] [Google Scholar]
- Costanzo F., Fiume L., La Placa M., Mannini-Palenzona A., Novello F., Stirpe F. Ribonucleic acid polymerase induced by vaccinia virus: lack of inhibition by rifampicin and alpha-amanitin. J Virol. 1970 Feb;5(2):266–269. doi: 10.1128/jvi.5.2.266-269.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Milovanovitch V., Pogo B. G., Weintraub S. B., Huima T., Wilton S., McFadden G. Biogenesis of vaccinia: isolation of conditional lethal mutants and electron microscopic characterization of their phenotypically expressed defects. Virology. 1978 Feb;84(2):403–428. doi: 10.1016/0042-6822(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Guarino L. A., Kates J. R. Vaccinia virus replication. I. Requirement for the host-cell nucleus. J Virol. 1979 Feb;29(2):705–715. doi: 10.1128/jvi.29.2.705-715.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Lynn D. L., Kates J. R. Vaccinia virus replication requires active participation of the host cell transcriptional apparatus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1887–1890. doi: 10.1073/pnas.76.4.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y., Dales S. Biogenesis of poxviruses: interrelationship between hemagglutinin production and polykaryocytosis. Virology. 1971 Dec;46(3):533–543. doi: 10.1016/0042-6822(71)90057-2. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus polypeptides in cells resistant to alpha-amanitin: evidence for the involvement of cellular RNA polymerase II in virus replication. J Virol. 1977 Sep;23(3):816–819. doi: 10.1128/jvi.23.3.816-819.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Isolation and partial characterization of the poly(A) polymerases from HeLa cells infected with vaccinia virus. J Biol Chem. 1977 Oct 10;252(19):6939–6947. [PubMed] [Google Scholar]
- Pennington T. H., Follett E. A. Vaccinia virus replication in enucleate BSC-1 cells: particle production and synthesis of viral DNA and proteins. J Virol. 1974 Feb;13(2):488–493. doi: 10.1128/jvi.13.2.488-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Biogenesis of vaccinia: separation of early stages from maturation by means of hydroxyurea. Virology. 1971 Jan;43(1):144–151. doi: 10.1016/0042-6822(71)90232-7. [DOI] [PubMed] [Google Scholar]
- Somers D. G., Pearson M. L., Ingles C. J. Isolation and characterization of an alpha-amanitin-resistant rat myoblast mutant cell line possessing alpha-amanitin-resistant RNA polymerase II. J Biol Chem. 1975 Jul 10;250(13):4825–4831. [PubMed] [Google Scholar]
- Somers D. G., Pearson M. L., Ingles C. J. Regulation of RNA polymerase II activity in a mutant rat myoblast cell line resistant to alpha-amanitin. Nature. 1975 Jan 31;253(5490):372–374. doi: 10.1038/253372a0. [DOI] [PubMed] [Google Scholar]
- Stern W., Dales S. Biogenesis of vaccinia: relationship of the envelope to virus assembly. Virology. 1976 Nov;75(1):242–255. doi: 10.1016/0042-6822(76)90023-4. [DOI] [PubMed] [Google Scholar]
- Stern W., Pogo B. G., Dales S. Biogenesis of poxviruses: analysis of the morphogenetic sequence using a conditional lethal mutant defective in envelope self-assembly. Proc Natl Acad Sci U S A. 1977 May;74(5):2162–2166. doi: 10.1073/pnas.74.5.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniolo D., Meiss H. K., Basilico C. A temperature-sensitive mutation affecting 28S ribosomal RNA production in mammalian cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1273–1277. doi: 10.1073/pnas.70.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]