Abstract
The occurrence of hypersensitivity reactions including rare but life-threatening Stevens-Johnson syndrome (SJS) and drug-induced hypersensitivity syndrome (HSS) limits the use of the anticonvulsant carbamazepine (CBZ). HLA-B*15:02 and HLA-A*31:01 have been identified as predictive genetic markers for CBZ hypersensitivity in Asian and European patients. To replicate these genetic associations in pediatric patients from North America with a diverse ethnic background, we investigated HLA-A*31:01 and HLA-B*15:02 in 42 children with CBZ hypersensitivity, and 91 CBZ-tolerant children from across Canada. HLA-A*31:01 was significantly associated with CBZ-HSS (odds ratio (OR): 26.4, p=0.0025) and maculopapular exanthems (OR: 8.6, p=0.0037), but not with CBZ-SJS. Conversely, HLA-B*15:02 was associated with CBZ-SJS (OR: 38.6, p=0.002), but not HSS and maculopapular exanthems. This study is the first to demonstrate the association of HLA-A*31:01 with CBZ hypersensitivity in children, providing important replication of this association and highlighting the importance of HLA-A*31:01 as a predictive biomarker across various ancestries.
Keywords: HLA-A*31:01, HLA-B*15:02, HLA, carbamazepine, drug hypersensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis, rash, adverse drug reaction, pharmacogenetics
Introduction
Carbamazepine (CBZ) is one of the most frequently used anticonvulsants in adults and children (1, 2) for the treatment of epilepsy, trigeminal neuralgia, and bipolar disorder. In particular, CBZ and its derivative oxcarbazepine (OXC) are the drugs of choice in the treatment of complex partial seizures in children (3, 4).
CBZ-induced hypersensitivity reactions of varying clinical presentation and severity (5–8) are common and occur in approximately 3–10% of patients (9–11) with similar frequencies reported for adults and children (12, 13). The majority of hypersensitivity reactions are relatively mild skin rashes that often require the discontinuation of CBZ for symptoms to resolve (9–11). However, CBZ also causes severe and life-threatening hypersensitivity reactions, which include the Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) spectrum (14–16), and drug-induced hypersensitivity syndrome (HSS) (17). SJS/TEN is characterized by a blistering rash and hemorrhagic erosions of mucous membranes, with TEN being the more severe form with more extensive skin detachment (18). HSS is characterized by a skin eruption, fever, and involvement of at least one internal organ, most frequently the liver (17, 18). Even though rare, the morbidity and mortality associated with these dramatic hypersensitivity reactions is substantial (long term complications in 45% and mortality of 2% in children with SJS/TEN; mortality of up to 10% for SJS and HSS, and up to 50% for TEN in adults) (19–22).
A genetic basis of CBZ-induced hypersensitivity reactions has previously been investigated in primarily adult patients. In these studies, strong associations of two genetic variants in the human leukocyte antigen (HLA) region, HLA-B*15:02 and HLA-A*31:01, with CBZ hypersensitivity were identified. Patients carrying HLA-B*15:02 were shown to be at strongly increased risk of CBZ-induced SJS/TEN (23–25). This discovery resulted in drug label changes for CBZ, and a recent prospective study demonstrated the clinical potential of this pharmacogenetic marker to reduce the occurrence of CBZ-induced SJS/TEN (26). However, HLA-B*15:02 is observed primarily in certain Asian populations and only very rarely in patients outside of Asia (27, 28). Correspondingly, a higher incidence of CBZ-induced SJS/TEN in countries where HLA-B*15:02 is common has been suggested (29). More recently, HLA-A*31:01 was reported to be associated with various CBZ-induced hypersensitivity reactions, including HSS, SJS/TEN and skin-specific maculopapular exanthems (MPE) in European (30) and Asian (24, 31, 32) patients.
HLA-B*15:02 and HLA-A*31:01 have been clearly established as risk factors for CBZ hypersensitivity in adult patients from Asia and Europe. However, even though some of the studies on HLA-B*15:02 included pediatric patients (26, 33), to our knowledge, these genetic associations have not been replicated in an entirely pediatric patient cohort. As children often metabolize drugs differently than adults (34), dedicated pediatric pharmacogenomic studies are of value to confirm these associations (35), even though no pediatric-specific effects so far have been reported for HLA-dependent adverse drug reactions. Furthermore, no studies have investigated these genetic risk variants in North American patients with diverse and admixed ancestries. In particular, the more recently reported association of HLA-A*31:01 requires further replication both in patients of European decent, and in patients with non-European or non-Asian ancestries. This study aimed to address these gaps by investigating HLA-B*15:02 and HLA-A*31:01, two important pharmacogenetic markers with consistent evidence from adult-based studies, in Canadian children with diverse ancestries.
Results
Patient Characteristics
A total of 42 children experiencing CBZ-induced hypersensitivity reactions (CBZ cases) and 91 pediatric CBZ-tolerant control patients (CBZ controls) were genotyped. CBZ cases included nine children with SJS/TEN, six patients with HSS, 26 children with MPE, and one patient with acute generalized exanthematous pustulosis (AGEP) (Table 1). Median age at the start of CBZ treatment was higher in CBZ cases compared to CBZ controls (P=0.006, Table 1), whereas no differences were observed for mean age during treatment and age at the end of treatment or follow up. With a total daily dose of 750 mg, the median CBZ dose was higher in CBZ controls compared to CBZ cases (P <0.001; Table 1). A larger proportion of CBZ cases were of non-European origin (60% vs 38%; P=0.03; Table 1). All patients received CBZ for the treatment of a seizure disorder.
Table 1.
Patient characteristics.
| CBZ Cases (n = 42) | CBZ Controls (n = 91) | P-valuea | OXC cases (n = 5) | |
|---|---|---|---|---|
| Age, years | ||||
| Therapy start, median (range) | 9.9 (0.64–16.9) | 7.4 (0.62–18.7) | 0.006 | 6.6 (3.4–15.3) |
| Therapy mean, median (range) | 9.6 (0.92–21.2) | 0.52 | ||
| Therapy end, median (range) | 10.8 (1.22–23.8) | 0.21 | ||
| Sex, n (%) | 0.85 | |||
| female | 19 (45) | 43 (47) | 4 (80) | |
| male | 23 (55) | 48 (53) | 1 (20) | |
| CBZ duration, days | n = 38b | n = 88b | <0.001 | |
| median (range) | 14 (4–55) | 728 (58–6801) | 14 (10–22) | |
| CBZ dose, mg/day | n = 35b | n = 88b | <0.001 | |
| median (range) | 400 (80–1000) | 750 (100–2000) | 600 (525–900) | |
| Ancestry, n (%)c | 0.03 | |||
| Europe | 17 (40) | 56 (62) | 3 (60) | |
| Asia | 6 (14) | 6 (7) | - | |
| Africa | 1 (2) | 1 (1) | - | |
| Aboriginald | 2 (5) | 3 (3) | - | |
| Latin America/Caribbean | 4 (10) | 1 (1) | - | |
| mixed | 10 (24) | 14 (15) | - | |
| unknown | 2 (5) | 10 (11) | 2 (40) | |
| Hypersensitivity reaction | ||||
| HSS | 6 | - | - | |
| SJS/TEN | 9 | - | 2 | |
| MPE | 26 | - | 3 | |
| AGEP | 1 | - | - | |
| Time to onset of reaction, days | n = 37b | |||
| median (range) | 13 (1–48) | - | 13 (10–22) | |
| HSS | 16.5 (10–48) | - | - | |
| SJS/TEN | 14 (10–24) | - | 16 (10–22) | |
| MPEe | 11 (1–28) | - | 13 (11–14) | |
| AGEP | 45 | - | - | |
AGEP, Acute Generalized Exanthematous Pustulosis; CBZ, carbamazepine; HSS, drug-induced hypersensitivity syndrome; MPE, maculopapular exanthem; SJS/TEN, Stevens-Johnson syndrome/toxic epidermal necrolysis; OXC, oxcarbazepine
Test between CBZ cases and CBZ controls
n = number known; only indicated if not known for all patients
Country of origin; patients were classified as European if the country of origin of all four grandparents was European or Canada. Mixed origin was defined as ≥1 grandparent having a different origin from the other grandparents. Origin was classified as unknown if country of origin was not known for ≥1 grandparent.
Aboriginal Canadian (First Nations, Inuit, Métis)
Exact time to onset of reaction was not known for 5 patients.
Median time to onset of hypersensitivity reactions in CBZ cases was 13 days (range: 1–48 days; Table 1). In all but one patient, CBZ was the only possible causative drug with the appropriate temporal relationship between therapy initiation and onset of symptoms. One patient was taking CBZ and phenytoin, but developed similar symptoms upon re-challenge with CBZ only. Of the nine children with CBZ-SJS/TEN, eight were classified as SJS, and one as SJS/TEN overlap. Four of the children with CBZ-SJS/TEN were of European origin, two were of Southeast Asian origin, and one patient each reported First Nations, Sri Lankan, and Caribbean ancestry, respectively. All SJS/TEN patients had involvement of skin and oral mucosa, with eight patients also experiencing conjunctivitis. Genital mucosa was affected in two patients. Fever was frequent in children with CBZ-SJS/TEN (7 of 9 patients). Elevation of liver enzymes was observed in two CBZ-SJS/TEN patients. All patients with CBZ-HSS experienced a body-wide skin eruption, fever, and elevation of liver enzymes. Eosinophilia was observed in two patients with CBZ-HSS, with one patient also experiencing lymphadenopathy. In one patient with CBZ-SJS, serology suggested an acute Mycoplasma pneumoniae infection. However, widespread skin lesions were observed in this patient, which are less common in Mycoplasma-induced compared to drug-induced reactions (36). In the other patients, no clear infectious etiology, which could have contributed to the clinical manifestation and severity of hypersensitivity reactions, was identified, even though full screens for infections or virus reactivation were not routinely performed. The presence of mucous membrane erosions in all children with CBZ-SJS allowed for the exclusion of staphylococcal scalded skin syndrome as a possible differential diagnosis in these patients (14, 37).
Of the five patients with OXC-induced hypersensitivity reactions, included for an exploratory analysis of potential genetic associations with this structurally similar drug, two were classified as SJS, and three as MPE. In all hypersensitivity cases, symptoms resolved after the suspected causative drug (CBZ or OXC, respectively) was discontinued.
Genotyping results
Overall, 20 (15%) children treated with CBZ carried the T allele of rs1061235, a single nucleotide polymorphism (SNP) previously shown to be in complete linkage disequilibrium with HLA-A*31:01 in Europeans (30). However, only 12 (9%) of these patients also carried HLA-A*31:01 as determined by direct sequence-based typing. Of the remaining patients, five carried HLA-A*33:03, and three HLA-A*33:01 (Supplementary Table 1). HLA-A*31:01 was not observed in any of the children not carrying rs1061235T.
Only four carriers of HLA-B*15:02 were observed. This was expected, given the low frequency of this variant in non-Asian populations, and the small number of patients with Asian origin in our study (9% of all patients). Genotyping for HLA-B*15:02 failed for four CBZ-tolerant controls due to low DNA quality.
HSS and MPE
Three of six patients (50%) with CBZ-HSS carried HLA-A*31:01, whereas only three (3.3%) of the 91 CBZ-tolerant controls carried the allele, resulting in a very strong association with CBZ-HSS (OR: 26.4; P=0.0025; Table 2). A significant association was also observed with CBZ-induced MPE, with six of 26 (23.1%) CBZ-MPE cases carrying HLA-A*31:01 (OR: 8.6; P=0.0037; Table 2). HLA-A*31:01 was thus very strongly associated with CBZ-HSS or MPE in a combined analysis of the two reactions (OR: 11.2; P=2.6×10−4; Table 2), with nine (28.1%) of the cases carrying HLA-A*31:01.
Table 2.
Association of HLA-A*31:01, proxy SNP rs1061235 and HLA-B*15:02 with CBZ hypersensitivity.
| Total N | HLA-A*31:01 | rs1061235T | HLA-B*15:02 | Combinedd | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N positive | OR (95% CI) | P-valuea | N positive | OR (95% CI) | P-valuea | N positive | OR (95% CI) | P-valuea | OR (95% CI) | P-valuea | ||
| CBZ-HSS | 6 | 3 (50) | 26.36 (2.53–307.89) | 0.0025 | 4 (66.7) | 25.97 (3.07–340.87) | 8.2×10−4 | - | 4.44 (0.16–120.08)b | 1.000 | 19.05 (1.95–197.65) | 0.0048 |
| CBZ-MPE | 26 | 6 (23.1) | 8.57 (1.67–57.50) | 0.0037 | 9 (34.6) | 7.32 (2.03–28.65) | 7.4×10−4 | - | 1.09 (0.04–27.51)b | 1.000 | 6.09 (1.31–32.25) | 0.0095 |
| CBZ-SJS/TEN | 9 | - | 1.33 (0.06–27.76)b | 1.000 | - | 0.69 (0.04–13.27)b | 1.000 | 3 (33.3) | 38.65 (2.68–2239.5) | 0.0022 | 9.87 (1.18–75.50) | 0.017 |
| CBZ-AGEP | 1 | - | - | - | 1 (100) | - | - | - | - | - | - | - |
| All CBZ cases | 42 | 9 (21.4) | 7.85 (1.82–47.80) | 0.0016 | 14 (33.3) | 6.96 (2.25–24.32) | 1.5×10−4 | 3 (7.1) | 6.51 (0.50–350.5) | 0.101 | 8.14 (2.25–37.34) | 2.6×10−4 |
| HSS/MPE | 32 | 9 (28.1) | 11.18 (2.53–69.27) | 2.6×10−4 | 13 (40.6) | 9.45 (2.91–34.50) | 2.6×10−5 | - | 0.86 (0.03–21.66)b | 1.000 | 7.94 (2.00–38.55) | 8.9×10−5 |
| CBZ-tolerant | 91/87c | 3 (3.3) | - | - | 6 (6.6) | - | - | 1 (1.1) | - | - | - | - |
AGEP, Acute Generalized Exanthematous Pustulosis; CBZ, carbamazepine; CI, confidence interval; HSS, drug-induced hypersensitivity syndrome; MPE, maculopapular exanthem; SJS/TEN, Stevens-Johnson syndrome/toxic epidermal necrolysis; OR, odds ratio
Calculated using Fisher’s exact test
Calculated by adding 0.5 to each value in a 2×2 contingency table and using normal approximation.
HLA-B*15:02 genotyping failed for four CBZ-tolerant patients
Combined analysis of HLA-A*31:01 and HLA-B*15:02
Significant P-values are indicated in bold.
A highly significant association with CBZ-HSS and MPE was also observed for the HLA-A*31:01 proxy SNP rs1061235 with 14 (33%) CBZ hypersensitivity cases and only six (6.6%) control patients carrying the T allele (combined OR: 9.45; P=2.6×10−5; Table 2). Interestingly, rs1061235 was also overrepresented in hypersensitivity cases after excluding HLA-A*31:01-positive children (OR: 5.83; P=0.033; Supplementary Table 1), resulting in very similar effect sizes of the associations with HSS and MPE for HLA-A*31:01 and rs1061235.
None of the patients with CBZ-HSS or MPE carried HLA-B*15:02 (Table 2). Therefore, in agreement with previous studies (24, 31), no significant association of this risk variant with CBZ-HSS or MPE was observed.
SJS/TEN
In contrast to the strong associations with HSS and MPE, no HLA-A*31:01 or rs1061235T carriers were detected among the nine patients with CBZ-induced SJS/TEN, and no significant association with CBZ-SJS/TEN was observed (OR: 1.33; P=1.00; Table 2).
On the other hand, three of the nine patients with CBZ-SJS/TEN were carriers of HLA-B*15:02, resulting in a significant association of HLA-B*15:02 with CBZ-SJS/TEN (OR: 38.6; P=0.002; Table 2). All three HLA-B*15:02-positive patients with CBZ-SJS/TEN were of Asian origin and included the patient with concurrent Mycoplasma infection. In this patient, even though Mycoplasma infection can also be associated with oral and ocular inflammation and blistering (36), the genetic result was thus consistent with a CBZ-induced reaction.
AGEP
Interestingly, the single patient with CBZ-induced AGEP tested positive for the proxy SNP rs1061235, but did not carry HLA-A*31:01 (Supplementary Table 1). Rs1061235T has also been reported in one adult patient with CBZ-AGEP of European descent (30). Whereas direct typing of HLA-A*31:01 was not performed in this patient, a perfect concordance between rs1061235 and HLA-A*31:01 was reported for other European patients in this study, suggesting that the patient also carried HLA-A*31:01. Therefore, our finding may be in disagreement with the previously suggested association of HLA-A*31:01 with AGEP.
Combined analyses
When considering all CBZ hypersensitivity reactions combined, a highly significant association of HLA-A*31:01 was observed (OR: 7.85; P=0.0016; Table 2), even though the effect size was reduced compared to an analysis only including HSS and MPE cases. On the other hand, HLA-B*15:02 was not significantly overrepresented in the CBZ cases overall. In a combined analysis of both risk variants, a strong association was observed with all CBZ-induced hypersensitivity reactions (OR: 8.14; P=2.6×10−4; Table 2). Overall, 12 of 42 (28.6%) CBZ cases carried HLA-A*31:01 or HLA-B*15:02, whereas only four (4.6%) CBZ-tolerant patients were positive for either risk variant. Using an estimated incidence of CBZ-induced hypersensitivity reactions (pre-test probability) of 5–10%, the positive post-test probability of a combined genetic test for both risk variants, based on the positive likelihood ratio of 6.21 observed here, was estimated as 25–41%, with a negative post-test probability of 3.8–7.7%.
HLA-A*31:01 in European patients
Given the differences in origin between CBZ cases and controls, we performed a subgroup analysis for HLA-A*31:01 in patients with three or more grandparents of European origin. Twenty CBZ cases and 65 controls were included in this analysis. The frequency of HLA-A*31:01 in European CBZ-tolerant children (3.1%; Table 3) was similar to the frequency reported previously in a European study (3.9%). Similar results were observed for the association of HLA-A*31:01 with CBZ hypersensitivity as for the full cohort, with 20% of CBZ cases carrying HLA-A*31:01 (OR: 7.62; P=0.025; Table 3). As in the full cohort, a stronger association was observed when only considering patients with CBZ-HSS or MPE (OR: 10.05; P=0.013; Table 3).
Table 3.
Subgroup analysis of HLA-A*31:01 in European patients only.
| Total N | N positive | OR (95% CI) | P-valuea | |
|---|---|---|---|---|
| CBZ-HSS | 2 | 1 (50.0) | 25.92 (0.27–2437.8) | 0.088 |
| CBZ-MPE | 14 | 3 (21.4) | 8.23 (0.84–109.23) | 0.037 |
| CBZ-SJS/TEN | 4 | - | 2.82 (0.12–68.10)b | 1.000 |
| CBZ-AGEP | - | - | - | - |
| All CBZ cases | 20 | 4 (20.0) | 7.62 (0.99–91.40) | 0.025 |
| HSS/MPE | 16 | 4 (25.0) | 10.05 (1.28–122.88) | 0.013 |
| CBZ-tolerant | 65 | 2 (3.1) | - | - |
AGEP, Acute Generalized Exanthematous Pustulosis; CBZ, carbamazepine; CI, confidence interval; HSS, drug-induced hypersensitivity syndrome; MPE, maculopapular exanthem; SJS/TEN, Stevens-Johnson syndrome/toxic epidermal necrolysis; OR, odds ratio
Calculated using Fisher’s exact test
Calculated by adding 0.5 to each value in a 2×2 contingency table and using normal approximation.
Significant P-values are indicated in bold.
OXC hypersensitivity cases
In the five children with OXC-induced hypersensitivity, one of the two patients with OXC-SJS was positive for rs1061235T, but not HLA-A*31:01. None of the three children with OXC-MPE carried HLA-A*31:01 or rs1061235T. Even though the number of cases is too small to draw any conclusions, we thus did not observe any evidence indicating a strong association of HLA-A*31:01 with OXC hypersensitivity. Similarly, no carriers of HLA-B*15:02 were observed among the patients with OXC hypersensitivity. However, this was not unexpected, as none of the patients with OXC hypersensitivity reported Asian ancestry.
Origin of risk variant carriers
All four HLA-B*15:02-positive children reported Asian countries of origin. Eleven of the sixteen grandparents of these children were of Chinese origin, and one grandparent originated from the Philippines, both populations where HLA-B*15:02 is common (38). Interestingly, one HLA-B*15:02-positive child with CBZ-SJS/TEN was of Sri Lankan origin, where, to our knowledge, no HLA-B*15:02-positive cases have been reported so far.
In contrast to HLA-B*15:02, children carrying HLA-A*31:01 or rs1061235T had a variety of origins (Figure 1). Due to the high proportion of European ancestry in the overall cohort, European origin was also common in HLA-A*31:01 carriers. However, significant proportions of patients also had Aboriginal and Latin American origins (Figure 1A). Interestingly, a majority of patients with West or South Asian origin who carried rs1061235T did not carry HLA-A*31:01, suggesting that rs1061235 alone is not an optimal proxy SNP in some populations.
Figure 1.
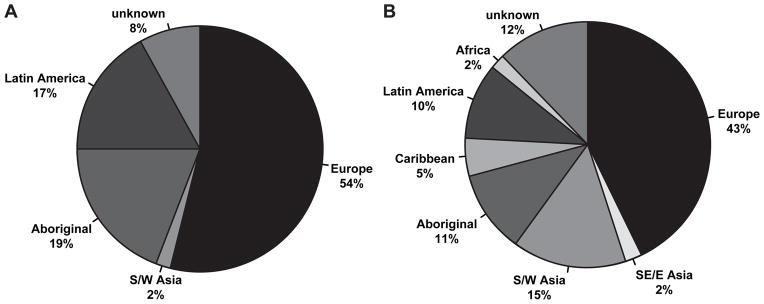
Origin of HLA-A*31:01 risk variant and proxy SNP rs1061235T carriers. Self-reported origins of HLA-A*31:01 positive patients’ grandparents (A; n = 12 patients) and of rs1061235T carriers’ grandparents (B; n = 21 patients) are shown. SE/E Asia: South East or East Asia; S/W Asia: South or Western Asia; Aboriginal: Aboriginal Canadian, including First Nations, Inuit, Métis.
Discussion
Hypersensitivity reactions are a significant problem in the treatment of children with CBZ. Here, we replicated important associations of two genetic risk factors, HLA-B*15:02 and HLA-A*31:01, in pediatric patients. Overall, our findings were in agreement with previous studies, suggesting similar genetic associations in children and adults and an increased risk of CBZ hypersensitivity in children carrying these risk variants. As the first study in ethnically diverse North American patients, we replicated the association of HLA-A*31:01 with CBZ hypersensitivity and demonstrated the relevance of this risk variant across a broader range of ancestries, as well as provided further replication in European patients.
Even though the number of HSS cases included in our study was small, our observation of a stronger association of HLA-A*31:01 with HSS compared to MPE is in agreement with most previous findings (30, 31), again suggesting a similar association in children and adults. Overall, the frequency of HLA-A*31:01 in children with CBZ-HSS or MPE was similar to the frequencies reported previously in European patients (30), but lower than frequencies reported in Japanese and Korean populations (31, 32). This is in agreement with the large proportion of patients in our study reporting European origins, and suggests that also the overall frequency of HLA-A*31:01 in our multiethnic patient cohort was similar. Of importance, we showed that the proxy SNP rs1061235 is not an optimal surrogate marker for HLA-A*31:01 in ancestrally diverse populations. Our observation of rs1061235T being overrepresented among CBZ hypersensitivity cases also in patients not carrying HLA-A*31:01 suggests that further investigation of this proxy SNP, particularly in multiethnic populations, may still be of interest.
We did not detect an association of HLA-A*31:01 with CBZ-SJS/TEN, which is in contrast to two previous studies that reported a stronger association with SJS/TEN compared to CBZ-HSS or MPE with odds ratios of 26 and 34 (30, 31). The number of SJS cases in our study was limited, particularly when taking into consideration that three SJS cases carried HLA-B*15:02, reducing our power to detect an association. However, besides the two studies reporting a strong association (30, 31), evidence from other adult-based studies regarding the association of HLA-A*31:01 with CBZ-SJS/TEN is also conflicting. For example, no significant association was observed in another study in Japanese patients (39) and in a Korean study (32). Random sampling effects due to the small numbers of cases and differences in the clinical characterization of SJS/TEN may partly explain these discrepant findings. Nevertheless, assuming an effect size as reported previously (OR ≥26), the probability of, by chance, observing no risk variant carriers among six CBZ-SJS cases in our study was <2.3%. Random sampling error alone is thus unlikely to explain the absence of HLA-A*31:01 among SJS cases. Therefore, whereas no conclusions can be drawn regarding an association of HLA-A*31:01 with CBZ-SJS/TEN or lack thereof, our findings combined with others suggest that the association of HLA-A*31:01 may not be as strong as initially reported. Further studies investigating HLA-A*31:01 in the context of CBZ-SJS/TEN are thus needed in both pediatric and adult patients.
In contrast, the observed association of HLA-B*15:02 with CBZ-SJS/TEN is in full agreement with previous findings (23–25, 40–42) with HLA-B*15:02 being observed in all children with CBZ-SJS/TEN who reported Asian origins. Of particular interest, we report here for the first time an HLA-B*15:02-positive patient with Sri Lankan origin, suggesting the presence and clinical relevance of HLA-B*15:02 in Sri Lanka. Data on the population frequency of HLA-B*15:02 in Sri Lanka is, to our knowledge, not available. However, HLA-B*15:02 has been associated with CBZ-SJS/TEN in patients from India (43). Due to the geographical proximity, the presence of HLA-B*15:02 in patients from Sri Lanka would not be surprising.
Similarly, we report here for the first time the presence of HLA-A*31:01 in CBZ hypersensitivity cases with Aboriginal and Latin American origin, demonstrating the relevance of this risk variant across a broader range of ancestries. This finding is in agreement with high population frequencies reported for HLA-A*31:01 in many ethnic groups (38). In particular, high HLA-A*31:01 population frequencies (up to 48% carrier frequency) have been reported in Aboriginal Americans. This is in agreement with our observation that Aboriginal Canadian ancestry was common (19%) in HLA-A*31:01 carriers and suggests a high relevance of HLA-A*31:01 for Aboriginal American patients receiving CBZ.
A larger proportion of patients were of European origin in the CBZ-tolerant group compared to the hypersensitivity cases in our study. As the frequencies of HLA alleles differ between populations, this difference in ancestry between cases and controls could potentially affect our results. In particular, the overrepresentation of patients carrying rs1061235T but not HLA-A*31:01 may be partly attributed to the different frequencies of non-European origins between cases and controls. Many of these patients carried HLA-A*33:03 and were of Asian origin, where HLA-A*33:03 is more common compared to European populations. On the other hand, given the consistency of our findings between the overall cohort and a subgroup analysis in European patients, and the overall agreement of our findings with other studies, any effect of ancestral differences between cases and controls is unlikely to affect the overall conclusions of this study regarding the associations of HLA-A*31:01 and HLA-B*15:02 with CBZ hypersensitivity. Nevertheless, future studies in pediatric patients should be conducted that include CBZ-tolerant children with non-European ancestries.
Finally, even though HLA-B*15:02 and HLA-A*31:01 are important risk factors in the context of CBZ hypersensitivity, not all patients carrying these variants developed a hypersensitivity reaction. In fact, based on the combined evaluation of both risk markers in our population of ancestrally-diverse Canadian children and an estimated frequency of hypersensitivity reactions of 5%, approximately 3 out of 4 of patients carrying a risk variant are expected to tolerate CBZ. Similarly, HLA-A*31:01 or HLA-B*15:02 were not present in all CBZ hypersensitivity cases. The two risk variants therefore appear to be neither sufficient nor necessary to trigger CBZ hypersensitivity. Further studies are needed to identify additional risk factors in order to improve the prediction of hypersensitivity reactions and avoid unnecessary withholding of CBZ from patients in whom it would have been safe. Promising findings have recently been reported in the context of HLA-B*15:02, where certain T-cell receptor clonotypes were found to be expressed in combination with HLA-B*15:02 to elicit an in vitro reaction to CBZ (44).
Nevertheless, in spite of the relatively low positive predictive power of these genetic tests, the severity of SJS/TEN and HSS combined with the availability of equally effective alternative therapies for many indications justifies genetic testing for HLA-B*15:02 and HLA-A*31:01 to identify children and adults at increased risk for CBZ hypersensitivity. Even though HLA-B*15:02 is rare in some populations, carriers of the risk variant can still occur (32). Furthermore, the frequency of HLA-B*15:02 is unknown for many populations. As the safest approach, we therefore suggest predictive testing for both variants in all patients, irrespective of their ancestry. In particular, a combined test for both markers is likely to be beneficial in ethnically diverse populations, where the full genetic ancestry of patients may not be known and many patients are of mixed origins.
In conclusion, these findings demonstrate that HLA-A*31:01 and HLA-B*15:02 are predictive of CBZ-induced hypersensitivity reactions in children and that HLA-A*31:01 is a relevant marker in patients of various ancestries. Even though further investigation is needed to elucidate the magnitude of the association of HLA-A*31:01 with CBZ-SJS/TEN and AGEP, these pharmacogenetic markers have great potential to reduce the occurrence of severe and life-threatening hypersensitivity reactions and improve the safety of CBZ therapy.
Patients and Methods
Patients
All study participants were recruited through the Canadian Pharmacogenomics Network for Drug Safety (CPNDS) (45) in pediatric hospitals across Canada. Blood or saliva samples were obtained for genetic analyses. Clinical data was obtained through detailed review of medical records, performed blind to genotype data by a clinical pharmacologist, a dermatologist and an adverse drug reaction surveillance clinician. Self-reported ancestry was obtained from all patients as country of origin of patients, parents and grandparents. No genetic analyses were conducted to confirm reported ancestries; however, a previous investigation of 524 CPNDS samples revealed a good concordance between self-reported and genetic ancestry (46).
SJS/TEN (including SJS, SJS/TEN overlap, and TEN), HSS, and AGEP were defined according to the criteria suggested by The Phenotype Standardization Project for immune-mediated drug-induced skin injury (18). Patients with SJS/TEN or HSS were further characterized using an in-house scoring tool (available from the authors by request) to assess the likelihood of diagnosis, differentiating between possible, probable, and highly likely cases, based on the available clinical data (e.g. presented symptoms, temporal relationship between drug and reaction onset, likelihood of other etiologies), and incorporating elements from the ALDEN algorithm (47) to assess drug causality. All SJS/TEN and HSS cases included in this study were classified as probable or highly likely.
MPE were defined as any cutaneous reaction occurring within the first eight weeks of CBZ treatment and resulting in CBZ discontinuation, with or without fever but without other systemic symptoms. One patient developed a rash, fever and conjunctival injection after initiation of CBZ and phenytoin, and developed a rash with fever upon rechallenge with CBZ only. This patient was classified as MPE. One patient developed mucosal lesions and bilateral conjunctivitis, but no skin lesions. As not all diagnostic criteria for SJS were fulfilled, the patient was afebrile and no clear infectious etiology was identified, this patient was classified as possible erythema multiforme and grouped with MPE for analyses.
CBZ-tolerant controls were defined as patients taking CBZ for at least eight weeks without any adverse reaction. Patients tolerating CBZ who experienced a cutaneous adverse reaction to other drugs (n = 13) were excluded from the control population.
Five patients with OXC-induced hypersensitivity reactions were genotyped for an exploratory evaluation of indications for an association of the same HLA variants with hypersensitivity reactions to this structurally similar drug. Characterization of OXC-induced hypersensitivity reactions was performed using the same criteria as for CBZ. No OXC-tolerant controls were included as the number of available control patients (n = 13) was too small to perform any meaningful statistical analyses.
Written informed consent or assent was obtained from all study participants or their parents or legal guardians. This study was approved by the ethics committees of all participating universities and hospitals.
Genotyping
Genomic DNA was extracted using the QIAamp or the QIAsymphony DNA purification systems (Qiagen, Toronto, Canada). Genotyping was performed using the PG1502 DNA Detection Kit (Pharmigene, Inc., Taipei, Taiwan) for direct typing of HLA-B*15:02. For HLA-A*31:01 a Custom Taqman SNP Genotyping Assay (Applied Biosystems, Foster City, CA) was used for rs1061235, a proxy SNP showing an absolute correlation (R2 = 1) with HLA-A*31:01 in Europeans (48). Genotyping was performed according to standard protocols on a 7500 Fast Real-Time PCR System (Applied Biosystems). In addition, direct sequence-based typing for HLA-A*31:01 was also performed as described previously (49), using primers I1-240 and I3-249 for allele-specific amplification of exons 2 and 3 for HLA-A*31 and *33.
Statistical analysis
All statistical analyses were conducted using the statistical software R (50). Associations of genetic variants with CBZ hypersensitivity were assessed using Fisher’s exact test and a dominant genetic model. For evaluation of clinical variables in patients with and without CBZ hypersensitivity, the Wilcoxon-Mann-Whitney test was used for continuous variables, and Fisher’s exact test for categorical variables. Two-sided P-values <0.05 were considered statistically significant.
Supplementary Material
Study Highlights.
What is the current knowledge on this topic?
Up to 10% of patients initiating carbamazepine therapy experience hypersensitivity reactions including life-threatening Stevens-Johnson syndrome and hypersensitivity syndrome. HLA-B*15:02 is a well-known genetic marker for carbamazepine-induced Stevens-Johnson syndrome in Asian patients. HLA-A*31:01 was recently identified as a risk factor for carbamazepine hypersensitivity in adult patients from Europe and Asia.
What question this study addressed
The association of HLA-A*31:01 with carbamazepine hypersensitivity required further replication, particularly in children and patients of other ancestries. To address this gap, we investigated HLA-A*31:01 together with HLA-B*15:02 in ancestrally-diverse children with carbamazepine hypersensitivity from across Canada.
What this study adds to our knowledge
HLA-A*31:01 was associated with carbamazepine-induced hypersensitivity syndrome and maculopapular exanthems also in children with diverse ancestries. Only HLA-B*15:02, but not HLA-A*31:01, was associated with carbamazepine-induced Stevens-Johnson syndrome.
How this might change clinical pharmacology and therapeutics
These results demonstrate the clinical relevance of predictive HLA genotyping in children to identify patients at risk for carbamazepine hypersensitivity, and the importance of HLA-A*31:01 as a risk factor across ancestries.
Acknowledgments
We especially thank all study children and their families for their participation in the Canadian Pharmacogenomics Network for Drug Safety (CPNDS) and this specific study. We would also like to acknowledge all members of the CPNDS active adverse drug reaction surveillance consortium, who were instrumental in identifying and enrolling study participants and in the detailed clinical characterization of hypersensitivity reactions:
Vancouver, British Columbia: British Columbia Children’s Hospital, Child and Family Research Institute, Pharmaceutical Outcomes Programme and Centre for Molecular Medicine and Therapeutics: Bruce Carleton, Michael Hayden, Colin Ross, Stuart MacLeod, Anne Smith, Claudette Hildebrand, Lucila Castro-Pastrana, Reza Ghannadan, Ursula Amstutz, Catherine Carter, Michelle Higginson, Linhua Zhang, Nasim Massah, Fudan Miao, Adrienne Borrie;
Calgary, Alberta: Alberta Children’s Hospital: Cheri Niissen-Jordan, David Johnson, Linda Verbeek, Rick Kaczowka, Andrea Hurton, Patti Stevenson;
London, Ontario: London Health Sciences Centre: Michael Rieder, Becky Malkin;
Montréal, Quebec: Hôpital Sainte-Justine: Jean-Francois Bussières, Denis Lebel, Pierre Barret, Aurélie Closon, Eve Courbon;
Ottawa, Ontario: Children’s Hospital of Eastern Ontario: Régis Vaillancourt, Pat Elliott-Miller, Elaine Wong, Herpreet Mankoo, Brenda Wilson, Lauren O’Connor; Health Canada: Maurica Maher;
Toronto, Ontario: Hospital for Sick Children: Gideon Koren, Shinya Ito, Paul Nathan, Mark Greenberg, Miho Inoue, Facundo Garcia Bournissen, Toshihiro Tanaka, Sachi Sakaguchi, Hisaki Fujii, Mina Ogawa, Ryoko Ingram, Taro Kamiya, Smita Karande; Sunnybrook Health Sciences Centre: Neil Shear;
Winnipeg, Manitoba: Winnipeg Children’s Hospital: Kevin Hall, Nick Honcharik, Shanna Chan, Michelle Staub.
This study was funded by the Canadian Institutes of Health Research and the Canada Foundation for Innovation. Smaller amounts of support were received from Genome Canada, the Canadian Dermatology Foundation, the University of British Columbia and the Child and Family Research Institute.
Footnotes
Conflict of Interest/Disclosure
MJR holds the CIHR-GSK Chair in Pediatric Clinical Pharmacology at the University of Western Ontario; NHS has been a paid consultant for Novartis; the other authors declared no financial relationships or conflicts of interest relevant to this article.
Author Contributions
Carleton, Amstutz, Ross, Castro-Pastrana, Rieder, Shear, and Hayden wrote the manuscript
Carleton, Amstutz, Ross, and Hayden designed the research
Carleton, Amstutz, Castro-Pastrana, Rieder, and Shear performed research
Amstutz analyzed data
References
- 1.Landmark CJ, Fossmark H, Larsson PG, Rytter E, Johannessen SI. Prescription patterns of antiepileptic drugs in patients with epilepsy in a nation-wide population. Epilepsy Res. 2011;95:51–9. doi: 10.1016/j.eplepsyres.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 2.van de Vrie-Hoekstra NW, de Vries TW, van den Berg PB, Brouwer OF, de Jong-van den Berg LT. Antiepileptic drug utilization in children from 1997–2005--a study from the Netherlands. Eur J Clin Pharmacol. 2008;64:1013–20. doi: 10.1007/s00228-008-0480-z. [DOI] [PubMed] [Google Scholar]
- 3.Wheless JW, Clarke DF, Arzimanoglou A, Carpenter D. Treatment of pediatric epilepsy: European expert opinion, 2007. Epileptic Disord. 2007;9:353–412. doi: 10.1684/epd.2007.0144. [DOI] [PubMed] [Google Scholar]
- 4.Wheless JW, Clarke DF, Carpenter D. Treatment of pediatric epilepsy: expert opinion, 2005. J Child Neurol. 2005;20(Suppl 1):S1–56. doi: 10.1177/088307380502000101. quiz S9–60. [DOI] [PubMed] [Google Scholar]
- 5.Segal AR, Doherty KM, Leggott J, Zlotoff B. Cutaneous reactions to drugs in children. Pediatrics. 2007;120:e1082–96. doi: 10.1542/peds.2005-2321. [DOI] [PubMed] [Google Scholar]
- 6.Castro-Pastrana LI, Ghannadan R, Rieder MJ, Dahlke E, Hayden M, Carleton B. Cutaneous adverse drug reactions in children: an analysis of reports from the Canadian Pharmacogenomics Network for Drug Safety (CPNDS) J Popul Ther Clin Pharmacol. 2011;18:e106–20. [PubMed] [Google Scholar]
- 7.Pellock JM. Carbamazepine side effects in children and adults. Epilepsia. 1987;28 (Suppl 3):S64–70. doi: 10.1111/j.1528-1157.1987.tb05780.x. [DOI] [PubMed] [Google Scholar]
- 8.Roujeau JC. Clinical heterogeneity of drug hypersensitivity. Toxicology. 2005;209:123–9. doi: 10.1016/j.tox.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Chang DK, Shear NH. Cutaneous reactions to anticonvulsants. Semin Neurol. 1992;12:329–37. doi: 10.1055/s-2008-1041189. [DOI] [PubMed] [Google Scholar]
- 10.Marson AG, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000–15. doi: 10.1016/S0140-6736(07)60460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arif H, et al. Comparison and predictors of rash associated with 15 antiepileptic drugs. Neurology. 2007;68:1701–9. doi: 10.1212/01.wnl.0000261917.83337.db. [DOI] [PubMed] [Google Scholar]
- 12.Kramlinger KG, Phillips KA, Post RM. Rash complicating carbamazepine treatment. J Clin Psychopharmacol. 1994;14:408–13. [PubMed] [Google Scholar]
- 13.Konishi T, Naganuma Y, Hongo K, Murakami M, Yamatani M, Okada T. Carbamazepine-induced skin rash in children with epilepsy. Eur J Pediatr. 1993;152:605–8. doi: 10.1007/BF01954091. [DOI] [PubMed] [Google Scholar]
- 14.Roujeau JC, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333:1600–7. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 15.Wolkenstein PE, Roujeau JC, Revuz J. Drug-induced toxic epidermal necrolysis. Clin Dermatol. 1998;16:399–408. doi: 10.1016/s0738-081x(98)00011-x. [DOI] [PubMed] [Google Scholar]
- 16.Roujeau JC, Guillaume JC, Fabre JP, Penso D, Flechet ML, Girre JP. Toxic epidermal necrolysis (Lyell syndrome). Incidence and drug etiology in France, 1981–1985. Arch Dermatol. 1990;126:37–42. doi: 10.1001/archderm.126.1.37. [DOI] [PubMed] [Google Scholar]
- 17.Schlienger RG, Shear NH. Antiepileptic drug hypersensitivity syndrome. 1998;39 (Suppl 7):S3–7. doi: 10.1111/j.1528-1157.1998.tb01678.x. [DOI] [PubMed] [Google Scholar]
- 18.Pirmohamed M, et al. Phenotype standardization for immune-mediated drug-induced skin injury. Clin Pharmacol Ther. 2011;89:896–901. doi: 10.1038/clpt.2011.79. [DOI] [PubMed] [Google Scholar]
- 19.Mockenhaupt M. The current understanding of Stevens-Johnson syndrome and toxic epidermal necrolysis. Expert Rev Clin Immunol. 2011;7:803–13. doi: 10.1586/eci.11.66. quiz 14–5. [DOI] [PubMed] [Google Scholar]
- 20.Wolf R, Orion E, Marcos B, Matz H. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171–81. doi: 10.1016/j.clindermatol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331:1272–85. doi: 10.1056/NEJM199411103311906. [DOI] [PubMed] [Google Scholar]
- 22.Finkelstein Y, et al. Recurrence and outcomes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Pediatrics. 2011;128:723–8. doi: 10.1542/peds.2010-3322. [DOI] [PubMed] [Google Scholar]
- 23.Chung WH, et al. A marker for Stevens-Johnson syndrome. Nature. 2004;428:486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- 24.Hung SL, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genom. 2006;16:297–306. doi: 10.1097/01.fpc.0000199500.46842.4a. [DOI] [PubMed] [Google Scholar]
- 25.Tassaneeyakul W, et al. Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia. 2010;51:926–30. doi: 10.1111/j.1528-1167.2010.02533.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen P, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364:1126–33. doi: 10.1056/NEJMoa1009717. [DOI] [PubMed] [Google Scholar]
- 27.Lonjou C, et al. A marker for Stevens-Johnson syndrome ...: ethnicity matters. Pharmacogenomics J. 2006;6:265–8. doi: 10.1038/sj.tpj.6500356. [DOI] [PubMed] [Google Scholar]
- 28.Kaniwa N, et al. HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia. 2010;51:2461–5. doi: 10.1111/j.1528-1167.2010.02766.x. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Food and Drug Administration. [accessed Dec 12, 2012];Drug Safety Information for Healthcare Professionals: Dangerous or Even Fatal Skin Reactions - Carbamazepine (marketed as Carbatrol, Equetro, Tegretol, and generics) 2007 Available at http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124718.htm.
- 30.McCormack M, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364:1134–43. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozeki T, et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011;20:1034–41. doi: 10.1093/hmg/ddq537. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011;97:190–7. doi: 10.1016/j.eplepsyres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Then SM, Rani ZZ, Raymond AA, Ratnaningrum S, Jamal R. Frequency of the HLA-B*1502 allele contributing to carbamazepine-induced hypersensitivity reactions in a cohort of Malaysian epilepsy patients. Asian Pac J Allergy Immunol. 2011;29:290–3. [PubMed] [Google Scholar]
- 34.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–67. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 35.Becker ML, Leeder JS. Identifying genomic and developmental causes of adverse drug reactions in children. Pharmacogenomics. 2010;11:1591–602. doi: 10.2217/pgs.10.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer Sauteur PM, Goetschel P, Lautenschlager S. Mycoplasma pneumoniae and mucositis - part of the Stevens-Johnson syndrome spectrum. J Dtsch Dermatol Ges. 2012;10:740–5. doi: 10.1111/j.1610-0387.2012.07951.x. [DOI] [PubMed] [Google Scholar]
- 37.Levi N, et al. Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatrics. 2009;123:e297–304. doi: 10.1542/peds.2008-1923. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR. Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res. 2011;39:D913–9. doi: 10.1093/nar/gkq1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikeda H, et al. HLA class I markers in Japanese patients with carbamazepine-induced cutaneous adverse reactions. Epilepsia. 2010;51:297–300. doi: 10.1111/j.1528-1167.2009.02269.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, et al. Association between HLA-B*1502 allele and carbamazepine-induced severe cutaneous adverse reactions in Han people of southern China mainland. Seizure-Eur J Epilep. 2011;20:446–8. doi: 10.1016/j.seizure.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Wu XT, et al. Association between carbamazepine-induced cutaneous adverse drug reactions and the HLA-B*1502 allele among patients in central China. Epilepsy Behav. 2010;19:405–8. doi: 10.1016/j.yebeh.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, et al. Strong association between HLA-B*1502 and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in mainland Han Chinese patients. Eur J Clin Pharmacol. 2011;67:885–7. doi: 10.1007/s00228-011-1009-4. [DOI] [PubMed] [Google Scholar]
- 43.Mehta TY, et al. Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J Dermatol Ve. 2009;75:579–82. doi: 10.4103/0378-6323.57718. [DOI] [PubMed] [Google Scholar]
- 44.Ko TM, et al. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol. 2011;128:1266–76e11. doi: 10.1016/j.jaci.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Carleton BC, et al. Adverse drug reaction active surveillance: developing a national network in Canada’s children’s hospitals. Pharmacoepidemiol Drug Saf. 2009;18:713–21. doi: 10.1002/pds.1772. [DOI] [PubMed] [Google Scholar]
- 46.Visscher H, et al. Application of principal component analysis to pharmacogenomic studies in Canada. Pharmacogenomics J. 2009;9:362–72. doi: 10.1038/tpj.2009.36. [DOI] [PubMed] [Google Scholar]
- 47.Sassolas B, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010;88:60–8. doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- 48.de Bakker PI, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat Genet. 2006;38:1166–72. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotsch K, Wehling J, Kohler S, Blasczyk R. Sequencing of HLA class I genes based on the conserved diversity of the noncoding regions: sequencing-based typing of the HLA-A gene. Tissue Antigens. 1997;50:178–91. doi: 10.1111/j.1399-0039.1997.tb02857.x. [DOI] [PubMed] [Google Scholar]
- 50.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


