Abstract
Although signs of empathy have now been well documented in non-human primates, only during the past few years have systematic observations suggested that a primal form of empathy exists in rodents. Thus, the study of empathy in animals has started in earnest. Here we review recent studies indicating that rodents are able to share states of fear, and highlight how affective neuroscience approaches to the study of primary-process emotional systems can help to delineate how primal empathy is constituted in mammalian brains. Cross-species evolutionary approaches to understanding the neural circuitry of emotional ‘contagion’ or ‘resonance’ between nearby animals, together with the underlying neurochemistries, may help to clarify the origins of human empathy.
Keywords: affective neuroscience, rodent, social behavior, emotion, contagion, reciprocity.anterior cingulate cortex
Introduction
Empathy reflects the capacity of one animal to experience the emotional feelings of another, a process with many cognitive refinements in humans. Thus, investigators commonly distinguish between emotional and cognitive forms of empathy (see below) [1,2]. Studies of empathy make up a relatively new subdiscipline in neuroscience, with human brain imaging providing many correlates of relevant, higher psychological functions [3–5]. Neuroscience research on empathy in other animals has lagged far behind, but simplified animal behavior models based on emotional contagion, the presumed foundations of empathy, have been developed (Figure 1) [6]. Our goal here is to summarize such novel empirical approaches for studying empathy in laboratory rats and mice, and to highlight an integrated neuro-evolutionary strategy for understanding human empathy.
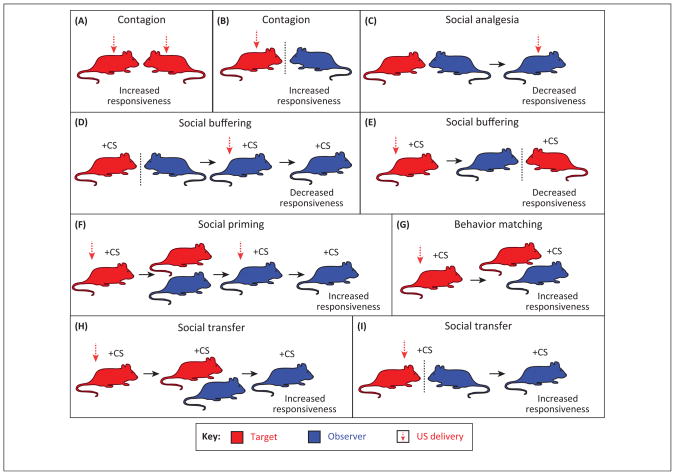
Figure 1.
Experimental approaches for assessing primal empathy in laboratory rodents. Nearly all of the studies that tap into some aspect of empathy processing in rodents utilize a painful sensory experience that can result in fear. As outlined in this cartoon depiction, the general approach entails presenting a noxious stimulus (often a shock) to a ‘target’, while an ‘observer’ witnesses this experience. Measures of behavioral responsiveness in an observer occur during the actual experience, or subsequently when a conditioned stimulus (CS) is presented, or when the target expresses a conditioned response (CR) to the CS. For example, (A) mice increase responsiveness to a painful unconditioned stimulus (US) when a familiar conspecific is concurrently exposed [62]. (B) Rodents increase freezing or express correlated freezing responses when observing a conspecific receiving a US [38,41,61]. (C) Prior social interaction coupled with a subeffective dose of morphine produces thermal analgesia [92], whereas observing a social partner in pain also produces subsequent thermal analgesia [62]. (D) Observing a non-fearful conspecific within a context reduces subsequent acquisition of contextual fear [63]. (E) The presence of a non-fearful social partner reduces retrieval of a fearful memory [67]. (F) Social interaction with a fear-conditioned conspecific increases subsequent acquisition of fear [66]. (G) Experienced rats respond to a CR of fear in others with increased freezing behavior [41]. (H) The CR of a partner rat is also sufficient to engender subsequent freezing in an observer [68]. (I) Mice acquire learned fear and avoidance responses after observing others being conditioned [39,41,69]. Not illustrated above are studies that have explored the role of social factors in fear-extinction (e.g., [64,65]).
Before proceeding, we consider the meteoric rise of neuro-empathy studies during the past few decades. The study of empathy was sparse in the biologically-oriented sciences of the 20th century until E.O. Wilson’s Sociobiology (1975), where constructs such as kin selection and reciprocal altruism were seen as major evolutionary explanations for individuals behaving unselfishly, even ‘altruistically’, toward others, provided that such behaviors supported the survival of one’s own genes [7]. Indeed, in Descent of Man, Darwin suggested that ‘We are thus impelled to relieve the sufferings of another, in order that our own painful feelings may at the same time be relieved’ and ‘those communities which included the greatest number of the most sympathetic members would flourish best, and rear the greatest number of offspring’ ([8], p. 88). Thus, inspired by writings of philosophers such as John Stuart Mill and Adam Smith, together with American social psychologists such as William McDougall [9] and Russian evolutionist Pyotr Kropotkin [10], a prosocial perspective emerged in late 20th century suggesting that individuals might be constitutionally more cooperative and emotionally interdependent than previously considered.
By the late 1990s human brain imaging offered robust approaches for identifying brain regions aroused during emotional states, encouraging systematic neuropsychological studies of empathy [11,12] that have now yielded diverse affective, cognitive, and social neuroscience perspectives [1,13–15]. Concurrently, primatologists recognized signs of empathic sensitivities [16,17] and now neuroscientists, inspired by classic early behavioral studies [18–20], are fashioning reliable simplified models to study the evolutionary roots of empathy (Box 1 and Figure 1)
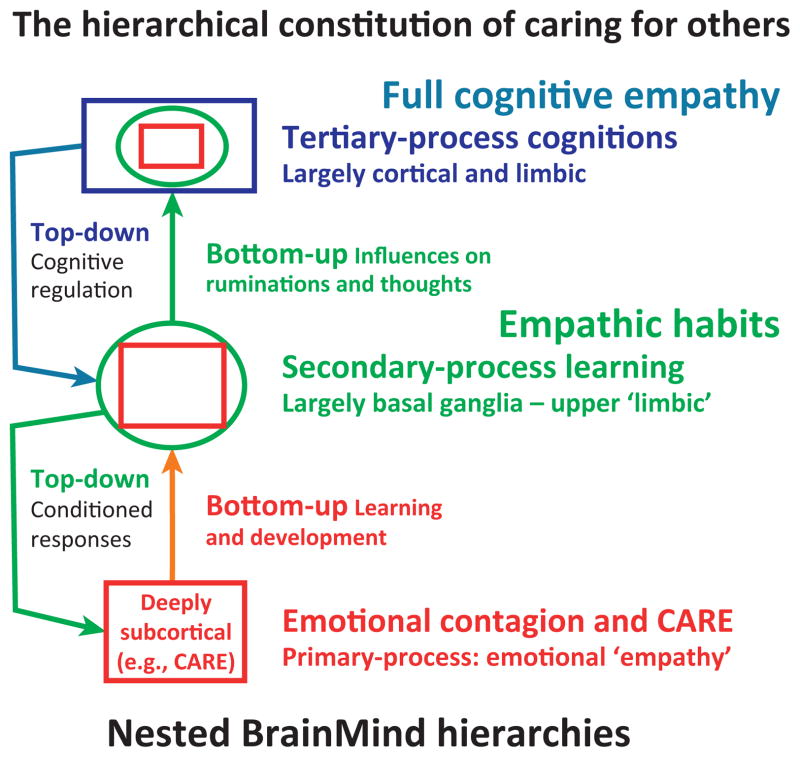
Box 1. Levels of empathic control and the nested hierarchies of the brain.
Tertiary processes: the highest brain functions, represented most richly in the expansions of the neocortex. These are much easier to study in humans because they are reflected in our cognitive consciousness which can be semantically described in humans. Its capacities are permitted by lower levels of brain organization, which are essential for consciousness [32,48] (Figure I).
Secondary processes: this intermediary level of brain organization mediates learning and memory, and is well studied in both animals and humans. The actual mechanisms of learning and memory have been largely clarified through animal research.
Primary processes: these deeply subcortical functions, homologous presumably in all mammals, constitute the primary affective processes – which include sensory affects (e.g., taste, touch, and pain), bodily homeostatic affects (e.g., hunger and thirst) and emotional affects (see Box 2), which are most important for understanding empathy. These brain functions are most clearly analyzed and understood through cross-species mammalian research, which is largely inaccessible to routine human experimentation. This foundational level is of critical importance for understanding the higher brain functions [23,32,48].
Figure I.
Nested hierarchies of control within the brain. A summary of circular, bottom-up and top-down causation by which lower BrainMind functions (e.g., primal emotional systems) are integrated, through bottom-up control, with higher-order MindBrain functions that then provide top-down regulatory control. Primary processes are shown as red squares, secondary process learning as green circles, and tertiary processes as blue rectangles (adapted from [33]).
The vagaries of ‘empathic’ terminologies
The term ‘empathy’ continues to have a diverse as well as nebulous usage, with ‘sympathy’ and ‘compassion’ being perennial colloquialisms used to describe related phenomena. One must remember that the term is a recent contribution to the vernacular, emerging in the early 20th century from the Greek empatheia (from em− ‘in’ + pathos ‘feeling’) and translated into the German Einfühlung, namely ‘feeling into’, especially when humans aesthetically appreciate the beauty of art. The English version of the term was coined in 1909 by Titchener [21] who was interested in describing the structure of the mind, and was further developed by Lipps [22] to recognize that humans have an intrinsic ability to recognize and appreciate the emotions of others through their bodily gestures and facial expressions.
Considering the variety of definitions and the relatively new intellectual coinage of the concept, all investigators must be careful to specify how they utilize the term. Obviously, the use of words such as ‘understand’, ‘recognize’, and ‘imagine’ can cause considerable problems for cross-species research because those words typically imply a critical role for higher cognitive functions which are difficult to study in animals. In this paper we use the term ‘primal empathy’ to refer to processes such as emotional contagion and emotional resonance in which there is a convergence of inferred affective states between individuals. This type of ‘affect-matching’ is monitored via shared emotional behavioral states which can be used as validated proxies of affective experiential states [23], but which do not require the additional ability to reflect cognitively upon one’s own states nor upon those of others. Such an approach suggests that primal empathy is a shared neurobehavioral, and we argue a shared neuroaffective, process rather than a unique emotional state per se. However, in humans and perhaps particular other mammals (cetaceans? higher primates?), primal empathy may interact with higher cognitive functions, allowing feelings such as compassion or sympathy to emerge (Box 1). Thereby, cross-species approaches to the neural origins of primal empathy may help to clarify how higher (more cognitive) forms of empathy are elaborated in humans.
Evolutionary affective foundations of empathy: levels of analysis in the brain and mind
Clearly a detailed, constitutive understanding of the mechanisms of empathy must come from cross-species neuroscience. Given the many excellent reviews covering correlative human brain imaging of empathy [3,11,24–27], we focus here on the primal emotional foundations of empathy in mammalian brains. The ‘primary-process’ emotional systems of the brain, which generate affective feelings (Box 2), are more accessible in animal models than in humans [23,28]. The interaction of primal affective states with ‘secondary-process’ learning and memory processes may eventually illuminate the higher ‘tertiary-process’ empathic abilities that are best studied in humans (Box 1).
Box 2. The primary-process emotional networks of mammalian brains and empathy.
Neural analysis with DBS supports the existence of seven basic highly interactive emotional systems in mammalian brains (see [23,42,48] for more detailed descriptions of these systems). Their names are capitalized to emphasize that specific neural networks exist in the brain. Each system has abundant descending and ascending components that work together to coordinate various instinctual emotional behaviors and associated autonomic changes, as well as the raw affective states (as evaluated by the rewarding and punishing properties of these systems). We highlight here the key brain regions and neuropeptides/neurotransmitters that help to mediate these emotions.
-
The SEEKING/desire system
This general-purpose appetitive motivational system allows all other emotional systems to operate effectively. It unconditionally allows animals to find all kinds of resources they need for survival, and eagerly anticipates forthcoming resources when conditioned.
Key anatomies: ventral tegmental area (VTA), medial forebrain bundle (MFB), nucleus accumbens (NAcc), medial prefrontal cortex (mPFC).
Key neurochemistries: dopamine, neurotensin, orexin.
-
The RAGE/anger system
RAGE is aroused by frustration and attempts to curtail the freedom of action of an animal. The RAGE system invigorates aggressive behaviors when animals are irritated or restrained and also helps animals to defend themselves by arousing FEAR in opponents.
Key anatomies: dorsal periaqueductal grey (dPAG), ventral MFB, medial amygdala, PFC.
Key neurochemistries: substance P, neuropeptide Y (NPY).
-
The FEAR/anxiety system
FEAR helps animals to reduce the likelihood of being inflicted with pain and the possibility of destruction by predators.
Key anatomies: ventral and dorsal PAG, ventral MFB, lateral and central amygdala, PFC.
Key neurochemistries: corticotrophin releasing factor (CRF); NPY.
-
The LUST/sexual system
Male and female sexual urges are mediated by several distinct brain neuropeptide circuits, whose activities are regulated by their respective gonadal steroids. The role of this circuitry in empathy is unclear although, because empathy is typically higher in females than males, testosterone may reduce and estrogen increase empathic tendencies.
Key anatomies: ventral and dorsal PAG, ventral MFB, lateral and central amygdala, PFC.
Key neurochemistries: estrogen facilitates oxytocin action, testosterone-facilitates vasopressin action.
-
The CARE/maternal nurturance system
Brain evolution has provided safeguards to assure that parents (usually the mother) take care of offspring. This system may provide pre-eminent control over primary-process empathy through the ministration of maternal devotions.
Key anatomies: ventral PAG, MFB, medial hypothalamus and preoptic area, corticomedial amygdala, mid-cingulate.
Key neurochemistries: oxytocin, vasopressin.
-
The PANIC/GRIEF system
Young mammals exhibit separation-distress calls resembling panic attacks when isolated; reunion with caretakers promotes social bonding. In adults this system promotes sadness and depression. It may be a major system that evokes empathy.
Key anatomies: dorsal PAG, dorsomedial thalamus, bed nucleus of the stria terminalis, and anterior cingulate.
Key neurochemistries: CRF, opioids, oxytocin, prolactin.
-
The PLAY/physical social-engagement system
Young animals have strong urges for rough-and-tumble, physical play. Physical play is infectious, and animals learn about the affective values of social interactions, which may provide fundamental learning experiences for higher forms of empathy.
Key anatomies: VTA, parafasicular thalamus, mPFC.
Key neurochemistries: endocannabinoids, endogenous opioids, and probably many other neuropeptides (as with all of the systems).
Such a multi-tiered, cross-species approach to understanding the brain and mind [29] helps to underscore the evolutionary complexities of empathy [4,30,31]. An unparalleled advantage of animal models is the ability to focus on the unconditional primary-process sensory and emotional systems that underlie empathic tendencies (Box 2), which may clarify how some cognitive forms of empathy (e.g., compassion and sympathy) emerge via social learning.
The anatomical trajectories of these subcortical emotion-generating systems originate in ancient medial regions of the upper brainstem that are conserved across mammalian species [23]. Arousal of these systems is subjectively experienced, evinced by ‘rewarding’ and ‘punishing’ effects, thereby facilitating learning and memory (secondary processes), as well as thinking, ruminating, and other higher mental abilities (tertiary processes). All mammalian brains are equipped with at least seven systems that mediate the unconditioned autonomic and behavioral displays of emotions; these same brain systems also engender the raw affective feelings of emotions (see Box 2 for descriptions of SEEKING, RAGE, FEAR, LUST, CARE, PANIC, and PLAY systems). Insofar as ‘empathy’ depends upon shared feelings, as Lipps first conjectured [22], cross-species affective neuroscience provides a framework for understanding empathy by concurrently delineating the behavioral-instinctive and subjective-experiential aspects of core emotional arousals, namely the primal brain reward and punishment systems that may be foundational for higher mental life [32].
Animal brain research allows us to envisage empathy as a bottom-up, emotional and developmental process of the brain [33] more clearly than top-down perspectives that are typically adopted in human research. New strategies are needed to help us to resolve the degree to which empathy is fundamentally an affective-emotional or cognitive process of the brain [34] and, if the former, how it connects to a variety of cognitive capacities. The bottom-up view taken here allows us to focus on primary-process ‘emotional contagion’ or ‘emotional resonance’ issues in animal models, working toward tertiary-process levels best addressed in humans [35,36]. The secondary-process, learning and memory level is well addressed in both, with animal research providing insight into neural mechanisms and human work into the neural correlates together with phenomenological and semantic complexities addressed by diverse aesthetic and cultural studies [12].
Beyond terminological and conceptual conundrums
A cross-species analysis readily synergizes with the original approach of Lipps, whereby empathy was characterized by how ‘the perception of an emotional gesture in another directly activates the same emotion in the perceiver, without any intervening labeling, associative or cognitive perspective-taking processes’ ([22], p. 2). As succinctly put by Hoffman [14], empathy is the process through which an individual generates an ‘affective response more appropriate to someone else’s situation than to one’s own.’ This complements the well-known, top-down ‘perception-to-action’ view [16] with a bottom-up ‘action-to-perception’ perspective because primal emotional systems are fundamentally brain action systems which concurrently help to engender emotional feelings [36,37] that may be conjointly experienced among animals in close proximity.
For instance, by considering the shared primal emotional roots of empathy, the infectiousness of FEAR has now been modeled in animals [38–41]. But, one might ask, how can we know other animals have emotional feelings [36,37]? This has been achieved by identifying the inherited primary-process emotional-action systems, shared by all mammals, using deep brain stimulations (DBS), and by concurrently evaluating the rewarding and punishing effects of such evoked neural states [23,28,42]. Given the underlying neuroanatomical and neurochemical homologies, and considering that the neocortex cannot sustain consciousness on its own [32], class-similarities in the evoked rewarding and punishing affective states are highly likely [43–45].
Here our concern is largely with the primary-process emotional level of analysis which, in the case of human investigations, has been best fleshed out with the experience of pain.
Investigators of human empathy have revealed that our empathy for the pain of others is mediated by brain regions aroused by our own experiences of pain [4,46,47]. This interoceptive/homeostatic, sensory affect appears to have been reutilized in the evolution of basic emotional systems, such as PANIC, which elaborates separation-distress and social bonding [23,48,49]. Parenthetically, such emotional attunements may promote language development [50] and psychotherapeutic practices [51], especially when deployed with affectively positive memory-reconsolidation procedures [48,52]. Empathic resonances may run deeply into the evolved neural networks that mediate affective consciousness and memory formation [53], and which mediate ‘laws of affect’ that control learning and socialization [36,37,48]. Indeed, the nested hierarchical organization of brain functions (Box 1) has implications for many facets of empathy that remain to be explored (Box 3): perhaps the higher-order empathic functions cannot be understood without understanding the foundational levels.
Box 3. Outstanding questions.
Are there correlated neural responses in primary emotional systems (Box 2) during emotional contagion, which would suggest affective resonance, as was recently demonstrated for mothers and fathers who observed their offspring during solitary play [81]?
To what extent is affective and cognitive empathy more dependent on the functional integrity of the primary-process emotional circuits as compared to higher brain modulatory regions such as the mPFC and anterior insula? How are these systems coordinated, and what does each contribute to experiential states of empathy?
How are changes in empathic tendencies in psychiatric disorders related to personality dimensions, as well as their disorders?
Drugs of abuse co-opt reward (i.e., SEEKING) pathways in the brain. However, some have proposed that particular drugs can serve as empathogens [93]. To what extent might compulsive consumption of specific drugs be attributed to an attempt to achieve ‘empathic states of mind’?
A better characterization of the roles of positive and negative emotions needs to be mapped out for empathy. Perhaps natural PLAYfulness is among the most empathic states of brain and mind – it is not possible for young animals to indulge in intense rough-and-tumble activities without their underlying brain systems being dynamically coordinated. Is PLAY infectious?
Conceptual problems are still widespread in the extended field. For instance, there are many related concepts such as altruism, cooperation, and fairness. Do they have different underlying mechanisms? Are they genetically and/or neurally correlated phenomena?
Do mirror neurons participate in the affective aspects of empathy, above and beyond their role in motor mimicry? To what extent is primary-process empathy dependent on emotional system resonances among nearby animals.
Do highly empathic individuals resonate comparably with both the positive and negative emotional states of others?
Top-down cognitive regulation of empathy
With maturation, guided by the affective qualities of childhood [54], humans develop the thought-related cognitive functions that vary considerably among individuals, providing diverse top-down control over social relationships [33,55]. Obviously, much having to do with empathy among humans is cognitive and elaborated by higher brain functions (e.g., sense of fairness, sympathetic perspective-taking, and compassion). However, we need to consider how those higher functions developmentally arise from the primal affective social-emotional circuits of our brains [35] that are shared homologously by mammals. To anticipate our major conclusions, particular primary-process, subcortical, emotional-affective functions of the brain, which can instigate primal forms of shared feelings (e.g., often labeled ‘emotional contagion’, ‘affect sharing’, or ‘reflexive empathy’), are critically important for the genesis of learning and memory – secondary processes that provide essential information for higher mental processes. Cognitive empathy (at times better described as compassion and sympathy) is often permitted by individualized memories, creativity, thinking, cognitive and emotional intelligence, social perspective taking, executive control of behavior, and the thoughtful ‘awareness’ that promotes conscious decision making – processes yielding the highest variants of empathy, with no empirically known, and perhaps unknowable, counterparts in other animals.
Thus, across brain levels, expressions of empathy are constituted by psychological processes of varying complexity, ranging from simple fellow-feelings as in the infectiousness or emotional limbic resonances of primary-process emotions, whether of primal FEAR or PLAYfulness, to the higher-order convictions about the ethically/morally right and wrong ways to behave toward each other, especially in the midst of socially challenging situations [56]. Empathy at the primary-process level is largely constituted of spontaneous affect sharing which is the psychological manifestation of primal emotional contagion as discussed elsewhere [57].
A cross-species affective neuroscience approach allows such processes to be studied empirically at the primary-process level, especially with electrical and neurochemical recording of emotional network activities in nearby animals. As described in the next section, such studies are possible with recent animal models for emotional resonance or reflexive empathy, already studied systematically by several laboratories [6].
Primary-process empathy
In its most basic form, empathy may be an inherent property of primal emotional systems, reflecting the fact that there is perceptually induced resonance of the same affective states in nearby animals. This may take its most poignant form in the capacity of mothers to intrinsically understand the emotional feelings of their infants. For instance, PANIC networks engender separation calls to signal psychological distress (probably a form of psychic pain evolving from pre-existing systems that mediated the affective qualities of physical pain) [23,47,58,59]. The auditory systems of the mothers may be evolutionarily primed to understand the distress of infants, whose cries reach the mothers’ separation distress-mediating PANIC systems. In this way each mother’s affective feelings can resonate with those of her child. Indeed, infants may also have such empathic capacities; it has long been known that in a large nursery, when one baby begins to cry, many others join the chorus [60]. But little empathy modeling has been done on this important social system in animals. Instead, because FEAR is the easiest to study, most recent empirical work has focused on that system.
Both rats [38,40,61] and mice [41] express increased freezing behaviors when distress is induced in social partners, highlighting the emotional contagion of FEAR. Mice also express infectious pain-related behaviors so as to closely match the pain states of social partners [62]. Within such experimental contexts, rats that witness social distress appear to be responding to the negatively valenced 22 kHz vocalizations of their partners [40,61], whereas mice seem to be more sensitive to the visual aspects of social distress [41,62,63] (however, also see [39]).
Social interactions also can prime rodents for subsequent learning. In mice, prior experiences with non-fearful conspecifics inhibit the acquisition of conditioned freezing [63], whereas experiences with fearful conspecifics strengthen conditioned freezing [64]. In addition, social experiences with frightened partners can both retard [65] and enhance [66] subsequent acquisition of fearful memories in mice and rats, respectively. Moreover, for rats, concurrent testing with fearful [40] or non-fearful [67] social partners respectively can increase and decrease fear. Other studies illuminate the acquired aspects of empathy – vicarious fear was promoted by familiarity both with emotional experiences [38,40] and social partners [41,62]. Taken together, these studies demonstrate that fear in rodents is broadly infectious upon the real-time, primary-process expression of behavior and upon subsequent learning abilities.
Other such studies indicate how fearful experiences in demonstrators can simply be transferred to observers. For instance, fear in rats can be transferred to others simply by observing a demonstrator that expresses a conditioned fear response [40,68]. Moreover, mice that witness others in cued [39] or contextual [41,69] fear learning paradigms express what may be primal FEAR. The mouse studies are particularly intriguing (e.g., [41]), suggesting that affective pain networks, including those of anterior cingulate cortex (ACC), lateral amygdala, and medial thalamus, are important neural substrates for processing the fear of others, including right-lateralized control within the ACC [70]. However, whether this reflects FEAR or PANIC circuitry remains unclear because DBS of these brain regions evoke separation-distress in species such as guinea pigs [71]. Furthermore, individuals from a gregarious strain of mice (C57BL/6J) were found to exhibit heart rate deceleration –a physiological correlate of empathic concern in humans [15] – when they witnessed distress in others [39]. In rats, social interaction with a previously distressed cage mate results in c-Fos induction within several regions of the amygdala, with the most specific activations being seen in the central amygdala of observer rats [72], a region long recognized as a key node in FEAR circuitry.
A provocative recent study [73] suggested that rats not only perceive and respond to the distress of their social partners, but also they go out of their way to alleviate their distress. Rats that witnessed constrained partners emitting a modest number of distress vocalizations gradually learned to free them from this situation. Although several control experiments were performed in this study [73], it still remains unclear whether the rats were working to alleviate the partner’s distress (the authors’ preferred interpretation), or rather merely to obtain social stimulation (visually or perhaps via other sensory modalities). It is noteworthy that similar findings have been observed in previous studies (e.g., [19]).
As noted earlier, the most solid case for primal empathy in humans (e.g., emotional contagion) is the strong tendency of babies in nurseries to cry together [60], suggesting infants are emotionally engaging with the affects of others [74]. Because it takes human infants many months to develop cortical inhibitory processes, which are presumably essential for regulated cognitive activities [75], such emotional resonances may be as close as we can get to primary-process empathic processes in the human species. In highly empathic adults this affective resonance continues for a lifetime, as reflected by the concern aroused in adults who hear babies cry [76]. Whether the well-studied facial imitations of human infants [77], now also observed in other primate infants [78], indicate the actions of cortical mirror neurons or more primal subcortical affective resonances presently remains unresolved.
Secondary-process modulation and parsing of primal empathy
It is not clear that secondary learning and memory processes contribute anything unique to the spectrum of primal empathic responses beyond parsing those responses in terms of space, time, and intensity. This level of brain processing appears to be completely unconscious [32,48], with well-established circuits for emotional learning (especially fear-conditioning) operating without the addition of anything new in terms of subjective emotional experiences. Learning can both intensify and moderate emotional feelings. For example, in the preceding coverage of the inter-animal infectiousness of fear, animals that had previously experienced the employed fearful situations exhibited significantly more contagious fear than inexperienced animals.
Once the fundamental substrates of affective mentality and the capacity to relate these feelings to world events through learning were solidified through evolution, these foundational processes presumably allowed higher neocortical brain regions to think about the world. Perhaps these fundamental substrates for social feelings – both the psychic pain of social loss, and the relief and comfort from reunion – provided critical substrates for the learning of higher forms of social empathy, to which we turn now.
Tertiary processes: the social emotions writ large
These highest levels of psychological processing are best studied in humans. There is a vast literature on human empathy, with insufficient space here to add much of significance. However, this is where research on the neurochemistries of social bonding – first opioids and subsequently oxytocin – both of which reduce separation-induced PANIC robustly (reviewed in [23,48]), may engender differential predictions about empathy – opioids may reduce empathy by dampening experienced social pain whereas the prosocial effects of oxytocin, without analgesia, may promote empathic responding. Indeed, oxytocin promotes feelings of social warmth, further highlighting its importance of maternal feelings in parent–infant bonding [79,80].
Brain emotional systems of mothers and fathers watching infant interactions exhibit heightened synchronies to their respective plasma oxytocin and vasopressin levels, suggestive of improved social attunements between the parents [81]. In addition, fathers given intranasal oxytocin exhibited greater playful social engagements with their infants, exhibiting increased social gaze, exploration, and social reciprocity, resulting in elevations of oxytocin levels in their offspring [82]. Oxytocin elevations in infant rats also increase their attraction to maternal cues [83].
Among future inquiries (Box 3), oxytocin facilitation of social synchronies among animals deserves more attention. Indeed, newborn chicks given oxytocin into their ventricular systems exhibit robust elevations of three simple behaviors – head shaking, yawning, and wing flapping – when tested alone [84]. When tested in groups, individual rates of head shaking and yawning remained unchanged, but wing flapping was dramatically facilitated, perhaps reflecting elevated social sensitivity and confidence [85].
The social foundations of empathy
Modern neuroscience is beginning to provide tools to clarify how the evolutionary sources of empathy are deeply grounded in fundamental brain processes – not only in our capacity to experience physical pains but also in the emotional networks that make us socially interdependent creatures. Although empathy has different connotations for different people, most agree that it is deeply emotional and pro-social – whereby one responds to the bodily needs, pains, and psychological losses of others through a mental appreciation of what other humans are experiencing. Such pro-social dimensions extend empathy considerably beyond Lipps’ original conception, whereby empathy at its foundation is essentially the spontaneous sharing (or fusion) of the same emotional experiences. His definition works well in basic animal research where the neurology of all fundamental emotional networks can be studied in detail (Box 2). Perhaps the ‘social emotions’ – LUST, CARE, PANIC/GRIEF, and PLAY – exhibit more robust emotional attunements or empathic resonances than the others (e.g., FEAR, RAGE, and SEEKING). However, this remains to be evaluated (Box 3).
Maternal CARE behaviors allow parents to soothe their distressed infants, perhaps via the neurophysiological coordination of primary-process PANIC and CARE systems –key sources of social attachments [23,48] – which may be foundational for the developmental emergence of the most socially sensitive, tertiary-process forms of empathy. Although the LUST and PLAY systems may exhibit abundant primary-process social resonance, they (together with RAGE and FEAR) may not intrinsically promote those mental states that most investigators would envisage as empathy. Perhaps it is through our mammalian capacity to experience not only the psychic pain of social loss (PANIC), but the balm of social concern, writ large in maternal CARE urges, that our fundamental social urges for LUST and PLAY become tamed to manifest social sensitivities that are widely considered to lie at the core of modern conceptions of empathy. In any event, the dictates of PANIC/GRIEF, CARE, and PLAY, together with our SEEKING of social warmth (solace) when in distress, may provide affective grounding from which the maturation of uniquely human capacities for empathy emerge through developmental landscapes currently being studied [35,54]. Ultimately, empathy involves emotional synchronies among individuals, suggesting a reduction in egocentric self-related processing, whose neural underpinnings are finally being elucidated [86,87].
In ongoing cycles of cultural-historical progressions, some have seen periodic signs of increasing empathy in human affairs, albeit with periods of intensified self-serving greed [88]. Indeed, the fuller manifestation of empathy in humans (e.g., primary-process emotional contagion integrated with tertiary-process theory of mind) may have promoted the emergence of human rights movements within the past two centuries of human cultural evolution, as exemplified by democratization, emancipation of slaves, together with women’s and gay rights movements. Indeed, perhaps our lateralized neocortical specializations have differential capacities for empathy, with the left hemisphere being more capable of promoting self-serving social dominance and arrogance, whereas the right is more prosocially attuned toward altruism and empathic views of social life [89]. Thus, the emergence of modern conceptions of fairness may still need to compete with our natural tendencies toward racially and kin-biased social favoritism [90–92]. Clearly we have much left to learn about the ways of empathy. What we can be most confident of is that most neural knowledge about how empathy is constituted remains to be discovered (Box 3).
Acknowledgments
J.B.P. was supported by a National Institutes of Health (NIH) training grant (F32 MH096475) during the writing of this paper.
References
- 1.Davis MH. Empathy: A Social Psychological Approach. Brown and Benchmark; 1994. [Google Scholar]
- 2.de Vignemont F, Singer T. The empathic brain: how, when and why? Trends Cogn Sci. 2006;10:435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Bernhardt BC, Singer T. The neural basis of empathy. Annu Rev Neurosci. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- 4.Decety J, et al. A neurobehavioral evolutionary perspective on the mechanisms underlying empathy. Prog Neurobiol. 2012;98:38–48. doi: 10.1016/j.pneurobio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Singer T, Lamm C. The social neuroscience of empathy. Ann N Y Acad Sci. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- 6.Panksepp JB, Lahvis GP. Rodent empathy and affective neuroscience. Neurosci Biobehav Rev. 2011;35:1864–1875. doi: 10.1016/j.neubiorev.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panksepp J. Altruism, neurobiology. In: Adelman G, editor. Neuroscience Year; Supplement 1 to the The Encyclopedia of Neuroscience. Birkhäuser; 1989/1999. pp. 7–8. [Google Scholar]
- 8.Darwin C. The Descent of Man and Selection in Relation to Sex. A.L. Fowler; 1888. [Google Scholar]
- 9.Preston SD, Hofelich AJ. The many faces of empathy: parsing empathic phenomena through a proximate, dynamic-systems view of representing the other in the self. Emot Rev. 2011;4:24–33. [Google Scholar]
- 10.Dugatkin LA. The Prince of Evolution: Peter Kropotkin’s Adventures in Science and Politics. University of Chicago Press; 2011. [Google Scholar]
- 11.Decety J, Ickes W, editors. The Social Neuroscience of Empathy. MIT Press; 2009. [Google Scholar]
- 12.Coplan A, Goldie P, editors. Empathy: Philosophical and Psychological Perspectives. Oxford University Press; 2011. [Google Scholar]
- 13.Eisenberger NI, et al. Prosocial development. In: Eisenberger NI, editor. Handbook of Child Psychology. Vol. 3. John Wiley and Sons; 2006. pp. 646–718. [Google Scholar]
- 14.Hoffman ML. The contribution of empathy to justice and moral judgment. In: Eisenberg N, Strayer J, editors. Empathy and Its Development. Cambridge; 1987. pp. 47–80. [Google Scholar]
- 15.Zahn-Waxler C, et al. Psychophysiological correlates of empathy and prosocial behaviors in preschool children with behavior problems. Dev Psychopathol. 1995;7:27–48. [Google Scholar]
- 16.Preston SD, de Waal FBM. Empathy: its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–71. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- 17.de Waal F. Primates and Philosophers: How Morality Evolved. Princeton University Press; 2006. [Google Scholar]
- 18.Church RM. Emotional reactions of rats to the pain of others. J Comp Physiol Psychol. 1959;52:132–134. doi: 10.1037/h0043531. [DOI] [PubMed] [Google Scholar]
- 19.Rice GE, Gainer P. Altruism in the albino rat. J Comp Physiol Psychol. 1962;55:123–125. doi: 10.1037/h0042276. [DOI] [PubMed] [Google Scholar]
- 20.Riess D. Vicarious conditioned acceleration: successful observational learning of an aversive Pavlovian stimulus contingency. J Exp Anal Behav. 1972;18:181–186. doi: 10.1901/jeab.1972.18-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titchener EB. Lectures on the Experimental Psychology of Thought-Processes. Macmillan; 1909. [Google Scholar]
- 22.Lipps T. Einfühlung, innere Nachahmung, und Organepfindungen. Arch Psychol. 1903;1:185–204. [Google Scholar]
- 23.Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotions. Oxford University Press; 1998/2005. [Google Scholar]
- 24.Keysers C, Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Curr Opin Neurobiol. 2009;19:666–671. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Decety J. The neuroevolution of empathy. Ann N Y Acad Sci. 2011;1231:35–45. doi: 10.1111/j.1749-6632.2011.06027.x. [DOI] [PubMed] [Google Scholar]
- 26.Decety J, Svetlova M. Putting together phylogenetic and ontogenetic perspectives on empathy. Dev Cogn Neurosci. 2012;1:1–24. doi: 10.1016/j.dcn.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci. 2012;15:681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- 28.Panksepp J. How Primary-process emotional systems guide child development: ancestral regulators of human happiness, thriving, and suffering. In: Narvaez D, et al., editors. Evolution, Early Experience and Human Development: From Research to Practice and Policy. Oxford University Press; 2013. pp. 74–94. [Google Scholar]
- 29.Northoff G, et al. The ‘resting-state hypothesis’ of major depressive disorder-A translational subcortical-cortical framework for a system disorder. Neurosci Biobehav Rev. 2011;35:1929–1945. doi: 10.1016/j.neubiorev.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Panksepp J. Empathy and the laws of affect. Science. 2011;334:1358–1359. doi: 10.1126/science.1216480. [DOI] [PubMed] [Google Scholar]
- 31.de Waal FBM. The antiquity of empathy. Science. 2012;336:874–876. doi: 10.1126/science.1220999. [DOI] [PubMed] [Google Scholar]
- 32.Solms M, Panksepp J. The ‘Id’ knows more than the ‘Ego’ admits: neuropsychoanalytic and primal consciousness perspectives on the interface between affective and cognitive neuroscience. Brain Sci. 2012;2:147–175. doi: 10.3390/brainsci2020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panksepp J. Cross-species affective neuroscience decoding of the primal affective experiences of humans and related animals. PLoS ONE. 2011;6:e21236. doi: 10.1371/journal.pone.0021236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legerstee M, et al., editors. The Infant Mind: Origins of the Social Brain. Guildford Press; 2013. [Google Scholar]
- 35.Baumeister RF, Mesicampo EJ. Conscious thought is for facilitating social and cultural interactions: how mental simulations serve the animal–culture interface. Psychol Rev. 2010;117:945–971. doi: 10.1037/a0019393. [DOI] [PubMed] [Google Scholar]
- 36.Watt DW. Toward a neuroscience of empathy: Integrating affective and cognitive perspectives. Neuropsychoanalysis. 2007;9:119–172. [Google Scholar]
- 37.Panksepp J. The basic emotional circuits of mammalian brains: do animals have affective lives? Neurosci Biobehav Rev. 2011;35:1791–1804. doi: 10.1016/j.neubiorev.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Atsak P, et al. Experience modulates vicarious freezing in rats: a model for empathy. PLoS ONE. 2011;6:e21855. doi: 10.1371/journal.pone.0021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Q, et al. Empathy is moderated by genetic background in mice. PLoS ONE. 2009;4:e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim EJ, et al. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS ONE. 2010;5:e15077. doi: 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeon D, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panksepp J. Affective consciousness: core emotional feelings in animals and humans. Conscious Cogn. 2005;14:30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Coenen VA, et al. Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): diffusion tensor imaging of two major subcortical pathways that may promote a dynamic balance of opposite affects relevant for understanding depression. Neuropsychiatry Clin Neurosci. 2012;24:223–236. doi: 10.1176/appi.neuropsych.11080180. [DOI] [PubMed] [Google Scholar]
- 44.Panksepp J. Mood changes. In: Vinken PJ, et al., editors. Handbook of Clinical Neurology. Elsevier; 1985. pp. 271–285. (Revised Series) [Google Scholar]
- 45.Heath RG. Exploring the Mind–Body Relationship. Moran Printing; 1996. [Google Scholar]
- 46.Lamm C, et al. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- 48.Panksepp J, Biven L. Archaeology of Mind: The Neuroevolutionary Origins of Human Emotions. Norton; 2012. [Google Scholar]
- 49.Reite M, Field T. Psychobiology of Attachment and Separation. Academic press; 1985. [Google Scholar]
- 50.Trevarthen C, Delafield-Butt J. Biology of shared experience and language development: regulations for the inter-subjective life of narratives. In: Legerstee M, et al., editors. The Infant Mind: Origins of the Social Brain. Guildford Press; 2013. pp. 167–199. [Google Scholar]
- 51.Farrow TFD, Woodruff PWR, editors. Empathy in Mental Illness. Cambridge University Press; 2007. [Google Scholar]
- 52.Ecker B, et al. Unlocking the Emotional Brain: Eliminating Symptoms at Their Roots Using Memory Reconsolidation. Routledge; 2012. [Google Scholar]
- 53.Panksepp J. Affective consciousness. In: Velmans M, Schneider S, editors. The Blackwell Companion to Consciousness. Blackwell Publishing; 2007. pp. 114–129. [Google Scholar]
- 54.Narvaez D, et al., editors. Human Nature, Early Experience and the Environment of Evolutionary Adaptedness. Oxford University Press; 2012. [Google Scholar]
- 55.Gallese V. The roots of empathy: the shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology. 2003;36:171–180. doi: 10.1159/000072786. [DOI] [PubMed] [Google Scholar]
- 56.Casebeer WD. Moral cognition and its neural constituents. Nat Rev Neurosci. 2003;4:841–847. doi: 10.1038/nrn1223. [DOI] [PubMed] [Google Scholar]
- 57.Rapson RL, et al. Emotional Contagion. Cambridge University Press; 1993. [Google Scholar]
- 58.MacDonald G, Jensen-Campbell LA, editors. Social Pain: Neuropsychological and Health Implications of Loss and Exclusion. American Psychological Association; 2011. [Google Scholar]
- 59.Panksepp J. Brain opioids: a neurochemical substrate for narcotic and social dependence. In: Cooper S, editor. Progress in Theory in Psychopharmacology. Academic Press; 1981. pp. 149–175. [Google Scholar]
- 60.Hoffman ML. Developmental synthesis of affect and cognition and its implications of altruistic motivation. Dev Psychol. 1975;23:97–104. [Google Scholar]
- 61.Wöhr M, Schwarting RKW. Ultrasonic calling during fear conditioning in the rat: no evidence for an audience effect. Anim Behav. 2008;76:749–760. [Google Scholar]
- 62.Langford DJ, et al. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 63.Guzmán YF, et al. Social modeling of conditioned fear in mice by non-fearful conspecifics. Behav Brain Res. 2009;201:73–78. doi: 10.1016/j.bbr.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nowak A, et al. Social modulation in the extinction of aversive memories. Behav Brain Res. 2013;238:200–205. doi: 10.1016/j.bbr.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 65.Bredy TW, Barad M. Social modulation of associative fear learning by pheromone communication. Learn Mem. 2009;16:12–18. doi: 10.1101/lm.1226009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Knapska E, et al. Social modulation of learning in rats. Learn Mem. 2009;17:35–42. doi: 10.1101/lm.1670910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiyokawa Y, et al. Main olfactory system mediates social buffering of conditioned fear responses in male rats. Eur J Neurosci. 2009;29:777–785. doi: 10.1111/j.1460-9568.2009.06618.x. [DOI] [PubMed] [Google Scholar]
- 68.Bruchey AK, et al. Fear conditioning by-proxy: social transmission of fear during memory retreival. Behav Brain Res. 2010;214:80–84. doi: 10.1016/j.bbr.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kavaliers M, et al. Recognition and avoidance of the odors of parasitized conspecifics and predators: differential genomic correlates. Neurosci Biobehav Rev. 2005;29:1347–1359. doi: 10.1016/j.neubiorev.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 70.Kim S, et al. Lateralization of observational fear learning at the cortical but not thalamic level in mice. Proc Natl Acad Sci USA. 2012;109:15497–15501. doi: 10.1073/pnas.1213903109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panksepp J, et al. Neural and neurochemical control of the separation distress call. In: Newman JD, editor. The Physiological Control of Mammalian Vocalizations. Plenum; 1988. pp. 263–300. [Google Scholar]
- 72.Knapska E, et al. Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proc Natl Acad Sci USA. 2006;103:3858–3862. doi: 10.1073/pnas.0511302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bartal BAI, et al. Empathy and pro-social behavior in rats. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trevarthen C. Action and emotion in development of the human self, its sociability and cultural intelligence: why infants have feelings like ours. In: Nadel J, Muir D, editors. Emotional Development. Oxford University Press; 2005. pp. 61–91. [Google Scholar]
- 75.Cuevas K, et al. Measures of frontal functioning and the emergence of inhibitory control processes at 10 months of age. Early Educ Dev. 2012;23:59–73. doi: 10.1016/j.dcn.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swain JE, et al. Brain basis of early parent infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meltzoff AN, Moore MK. Infant inter-subjectivity: broadening the dialogue to include imitation, identity and intention. In: Braten S, editor. Intersubjective Communication and Emotion in Early Ontogeny. Cambridge University Press; 1998. pp. 47–62. [Google Scholar]
- 78.Paukner A, et al. Delayed imitation of lipsmacking gestures by infant rhesus macaques (Macaca mulatta) PLoS ONE. 2011;6:e28848. doi: 10.1371/journal.pone.0028848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feldman R. Oxytocin and social affiliation in humans. Horm Behav. 2012;61:380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Nelson EE, Panksepp J. Brain substrates of infant–mother attachment; contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- 81.Atzil Z, et al. Synchrony and specificity in the maternal and the paternal brain: Relations to oxytocin and vasopressin. J Am Acad Child Adolesc Psychiatry. 2012;51:798–811. doi: 10.1016/j.jaac.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 82.Weisman O, et al. Oxytocin administration to parent enhances infant physiological and behavioral readiness for social engagement. Biol Psychiatry. 2012;72:982–989. doi: 10.1016/j.biopsych.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 83.Nelson E, Panksepp J. Oxytocin and infant–mother bonding in rats. Behav Neurosci. 1996;110:583–592. doi: 10.1037//0735-7044.110.3.583. [DOI] [PubMed] [Google Scholar]
- 84.Panksepp J. Oxytocin effects on emotional processes: separation distress, social bonding, and relationships to psychiatric disorders. Ann N Y Acad Sci. 1992;652:243–252. doi: 10.1111/j.1749-6632.1992.tb34359.x. [DOI] [PubMed] [Google Scholar]
- 85.Panksepp J. Primary process affects and brain oxytocin. Biol Psychiatry. 2009;65:725–727. doi: 10.1016/j.biopsych.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 86.Northoff G, et al. Differential parametric modulation of self-relatedness and emotions in different brain regions. Hum Brain Mapp. 2009;30:369–382. doi: 10.1002/hbm.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Northoff G, Panksepp J. The trans-species concept of self and the subcortical–cortical midline system. Trends Cogn Sci. 2008;12:259–264. doi: 10.1016/j.tics.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 88.Rifkin J. The Empathic Civilization: The Race to Global Consciousness in a World of Crisis. Tarcher/Penguin; 2009. [Google Scholar]
- 89.McGilchrist I. The Master and His Emissary: The Divided Brain and the Making of the Western World. Yale University Press; 2009. [Google Scholar]
- 90.Asma S. Beyond Fairness. University of Chicago Press; 2012. [Google Scholar]
- 91.Azevedo RT, et al. Their pain is not our pain: brain and autonomic correlates of empathic resonance with the pain of same and different race individuals. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22133. http://dx.doi.org/10.1002/hbm.22133. [DOI] [PMC free article] [PubMed]
- 92.D’Amato FR. Kin interaction enhances morphine analgesia in male mice. Behav Pharmacol. 1998;9:369–373. [PubMed] [Google Scholar]
- 93.Valea D, Hautefeuille M. Empathogenic agents: their use, abuse, mechanism of action and addiction potential. In: Farrow TFD, Woodruff PWR, editors. Empathy in Mental Illness. Cambridge University Press; 2007. pp. 199–216. [Google Scholar]