Abstract
In schizophrenia, neurocognition, social cognition and functional outcome are all inter-related, with social cognition mediating the impact that impaired neurocognition has on functional outcome. Less clear is the nature of the relationship between neurocognition, social cognition and functional outcome in individuals at clinical high risk (CHR) for psychosis. 137 CHR participants completed a neurocognitive test battery, a battery of social cognition tasks and the Social Functioning Scale. Confirmatory factor analysis showed that all social cognition tasks were reliable and valid measures of the latent variable. The path from neurocognition to functioning was statistically significant (standardized coefficient β = 0.22, p <0.01). The path from social cognition to functioning was also statistically significant (β= 0.27, p<0.05). In the mediation model the bootstrapping estimate revealed a nonsignificant indirect effect that was the association of social cognition with neurocognition and with functional outcome (β =0.20, 95% CI =−0.07 to 0.52, p=0.11). However, social cognition was significantly associated with neurocognition (β = 0.80, p < 0.001) and the path from neurocognition to functioning was no longer significant as soon as the mediator (social cognition) was entered into the mediation model (β = 0.02, p = 0.92). All of the model fit indices were very good. Unlike what has been observed with psychotic patients, social cognition does not seem to mediate the pathway from neurocognition to functional outcome when assessed with a measure of social attainment in individuals at CHR for psychosis.
Keywords: schizophrenia, clinical high risk, neurocognition, social cognition, functional outcome, mediation
1. Introduction
It is well established that individuals with schizophrenia at all stages of the illness evidence deficits in neurocognition, social cognition, and functional outcome (Keefe and Harvey, 2012; Green et al., 2012). In particular, poor functional outcome tends to persist even when symptoms are in remission (Tandon et al., 2010). Thus, to ultimately achieve recovery, it is necessary to understand its key determinants and to direct rehabilitation efforts to factors that may contribute to improved functioning.
Many studies have highlighted links between neurocognition, social cognition and functional outcome at both the first episode of psychosis as well as for individuals who are experiencing a more chronic course of illness (e.g. Allott et al., 2011; Fett et al., 2011). Using sophisticated modelling techniques, several of these studies have demonstrated a mediational role for social cognition (e.g. Addington et al., 2006b; Addington et al., 2010; Schmidt et al., 2011). Furthermore, in these models social cognition is more proximal to outcome than is neurocognition, that is, the average amount of variance in outcome explained by social cognition is usually greater than the variance explained by neurocognition (Fett et al., 2011). Understanding this relationship may be important for planning targeted interventions. In fact growing attention is being given to the development of new treatments specifically focused on cognitive (e.g. Wykes et al., 2011) or social cognitive training (e.g. Horan et al., 2008) with encouraging preliminary results.
Recent progress in risk identification methodology has made it possible to identify individuals who are putatively prodromal for psychosis, that is at clinical high risk (CHR) of developing psychosis (McGlashan et al., 2010). It has been consistently reported that similar or less severe deficits in neurocognition, social cognition and functional outcome are observed in CHR individuals when compared to individuals at either their first episode or those who have a more chronic course of illness (Addington and Barbato, 2012; Thompson et al., 2011; Addington et al., 2008; Fusar-Poli et al., 2012b; Green et al., 2012). Although deficits in neurocognition (Fusar-Poli et al., 2012b) may play a role in predicting transition to psychosis in individuals at CHR, only one study to date has demonstrated that a combination of neurocognitive tasks and social cognition assessed by a theory of mind task was related to conversion (Kim et al., 2011). However, the associations amongst social cognition, neurocognition and functional outcome have never been assessed in CHR individuals. Examining if social cognition does mediate between neurocognition and functional outcome at this stage of risk may add to our understanding of the development of psychosis. In terms of prevention, CHR individuals represent a unique opportunity to intervene early and possibly delay or prevent illness progression. Although only about one third of individuals at CHR will develop psychosis, the remaining two thirds will most likely continue to have poor functional outcome (Addington et al., 2011), and thus may benefit from more effective treatment intervention as well. Therefore, an improved understanding of the role of these early deficits in cognition and social cognition could be essential to intervening with respect to functional outcome.
Thus, based on the fact that a) a mediation role for social cognition has been observed at both the first episode and later stages of psychosis; b) deficits in social cognition, neurocognition and functional outcome are relatively stable across phases of the illness including both the acute and remission phase; and c) CHR individuals as a group experience similar deficits in neurocognition, social cognition and functional outcome, we predict that social cognition will play a mediation role in the CHR population. The specific aim of this study is, using Structural Equation Modeling (SEM), to verify if social cognition has a mediating role between neurocognition and functioning. To our knowledge this is the first study that attempted to verify this model in CHR individuals.
2. Method
2.1. Sample
The sample consisted of 137 (81 males, 56 females) individuals at CHR of psychosis. All of the participants were part of a multi-site NIMH funded study called “Enhancing the Prospective Prediction of Psychosis” (PREDICT). This was a 2-year longitudinal study conducted at the University of Toronto, the University of North Carolina (UNC), and Yale University to determine predictors of conversion in individuals at CHR of developing psychosis; 57 were recruited at Toronto, 55 at UNC and 25 at Yale. All participants met the Criteria of Prodromal Syndromes (COPS) based on the Structured Interview for Prodromal Syndromes (SIPS) (McGlashan et al., 2010). One-hundred and thirty-six CHR participants met attenuated positive symptom syndrome (APSS) criteria, which includes the emergence or worsening of a non-psychotic level disturbance in thought content, thought process or perceptual abnormality over the past year, and one participant met criteria for genetic risk and deterioration (GRD), which required either a first degree relative with a psychotic disorder or the subject having schizotypal personality disorder (SPD) plus at least a 30% drop in functioning on the General Assessment of Functioning (GAF) scale in the past 12 months. Participants were excluded if they met criteria for any current or lifetime axis I psychotic disorder, prior history of treatment with an antipsychotic, IQ< than 70, or past or current history of a clinically significant central nervous system disorder that may confound or contribute to clinical high risk symptoms. Participants were excluded if they were using antipsychotics at baseline. Furthermore, antipsychotics were not used at any later points in this study.
2.2. Measures
Criteria for a prodromal syndrome were determined using the Structured Interview for Prodromal Syndromes (SIPS) (McGlashan et al., 2010). Symptoms were assessed with the Scale of Prodromal Symptoms (SOPS), which consists of 19 items in 4 symptom domains: positive, negative, general, and disorganized.
2.2.1. Neurocognition
Neurocognitive tests were chosen on the basis of their demonstrated reliability, ability to discriminate patients with schizophrenia from healthy participants, lack of ceiling and floor effects in a CHR population, and appropriateness for individuals as young as 14 years of age. When available, age appropriate test versions were used, and raw scores were converted to age matched standard scores where appropriate. Our sample included participants younger than 14 (12–13). Therefore, we re-analyzed the data without the 12–13 year old participants and the results did not change. The neurocognitive tests battery included: Controlled Oral Word Association Test (COWAT), Category Instances, Trail Making Test A and B, Stroop Test (Color Naming and Color-Word), Finger Oscillation Test, Wisconsin Card Sorting Test (WCST), Ray Auditory Verbal Learning Test (RAVLT), Computerized Test of Visuospatial Working Memory, N-back task, Letter Number Sequencing Test, Continuous Performance Test – Identical Pairs (CPT-IP), and Digit Span Distractibility. These tests covered the neurocognitive domains of verbal fluency, processing speed, motor function, executive function, verbal memory, verbal and spatial working memory, and attention. This study was designed prior to the MATRICS battery but our battery is very similar with the CPT-IP and the TMT A being common to both.
2.2.2. Social cognition
Measures of social cognition included: the Facial Emotion Identification Test (FEIT), the Facial Emotion Discrimination Test (FEDT) (Kerr and Neale, 1993), and Affective prosody (AP) (Edwards et al., 2001), to assess affect processing, and the “Reading the Mind in the Eyes” task (Baron-Cohen et al., 2001) to assess theory of mind.
2.2.3. Functional outcome
Functional outcome was assessed using the Social Functioning Scale (SFS), a self-report questionnaire that has excellent psychometric properties (Birchwood et al., 1990). The SFS has a total score and 7 sub-scores: Withdrawal/social engagement, Interpersonal communication, Independence-performance, Independence-competence, Recreation, Prosocial, and Employment/Occupation.
2.3. Procedures
All three sites involved in this longitudinal study recruited CHR individuals. Raters were experienced research clinicians who demonstrated adequate reliability at routine reliability checks. Gold standard post-training agreement on the critical threshold for determining initial eligibility and subsequent conversion status based on the SIPS was excellent (kappa= 0.90). The PI or clinical psychiatrist or psychologist at each site conducted a comprehensive clinical assessment to determine if entry criteria were met. J. Addington chaired weekly conference calls to review criteria for all individuals admitted to the study to ensure consensus that all participant met COPS criteria. The study protocols and informed consents were reviewed and approved by the ethical review boards of all three study sites. All of the data was collected at the baseline assessment.
2.4. Data analysis
To perform the mediator analysis, a neurocognitive factor was obtained from all neurocognitive measures by using principle component factor analysis, which was deemed appropriate for the data (Bartlett test p < 0.001, Kaiser–Meyer–Olkin index= 0.83). This analysis generated 6 factors with eigenvalues greater than 1. However, the tests all loaded on one factor with most of the variance being accounted for by the first factor (36.5%), therefore only this factor was used in the mediation model. We computed two-tailed Pearson product–moment correlations to examine relationships among neurocognition, social cognition and functional outcome. We used Structural Equation Modeling (SEM) with maximum likelihood estimation of the AMOS 20.0 package to estimate and test mediation effects among the three constructs of neurocognition, social cognition and functional outcome at the baseline assessment. SEM consists of a combination of confirmatory factor analyses and multiple regressions to determine the relations among latent constructs. Prior to evaluating the mediation hypothesis, we checked raw data for normality and outliers, and replaced missing values by regression imputation. We employed confirmatory factor analysis to ensure that the latent variable social cognition was assessed with sufficient reliability and validity. To evaluate potential mediation effects we used 1) a basic model postulating a direct relationship between neurocognition and functional outcome and 2) a mediation model evaluating the strength of the indirect relationship while controlling for the direct effect of neurocognition on functional outcome. The indirect effects in our mediation model are the associations of social cognition with neurocognition as well as functional outcome. We used the bootstrap method to calculate the indirect effect as simulation research shows that bootstrapping the indirect effect tends to have the highest power and the best type I error control. To assess the degree to which the data fitted the structural equation model we used the chi-square test (χ2), the comparative fit index (CFI), and the root mean-squared error of approximation (RMSEA). A non-significant χ2, a CFI> 0.9 and an RMSEA< 0.08 indicate a good-fitting model (Schermelleh-Engel et al., 2003). Although larger samples are always preferable, a minimum sample of 100 has been recommended for SEM (e.g. Kline, 1998), therefore our sample size (n=137) was adequate.
3. Results
Sample characteristics are shown in Table 1. The mean age of our sample was 19.96 (age range 12–31). The majority of the participants were white (79%), single (94.2%), and had completed high school (58.4%). The correlation matrix for the variables employed in the SEM analyses is shown in Table 2. The three constructs were all associated thus meeting the requirements for mediation. There was no association between any of these constructs and attenuated positive symptoms.
Table 1.
Sample characteristics
| Measure | Value |
|---|---|
| Age – Mean (SD) | 19.96 (4.67) |
| Race – % | |
| White | 79% |
| Black | 7.3% |
| Asian | 7.3% |
| Hawaiian | 0.7% |
| Mixed | 5.1% |
| Marital status – % | |
| Married | 5.1% |
| Separated | 0.7% |
| Never Married | 94.2% |
| Education – % | |
| Did not complete High School | 41.6% |
| Completed High School | 10.2% |
| College or University Degree | 43.8% |
| Post graduate Degree | 4.4% |
| Symptoms – Mean (SD) | |
| SOPS Positive | 10.88 (3.04) |
| SOPS Negative | 8.04 (5.78) |
| Social cognition– Mean (SD) | |
| Affective Prosody | 44.31 (7.00) |
| Eyes Test | 25.16 (4.94) |
| FEDT | 25.67 (1.98) |
| FEIT | 12.74 (2.40) |
| Social Functioning Scale– Mean (SD) | 127.50 (19.14) |
SOPS: Structured Interview for Prodromal Symptoms; FEDT: Facial Emotion Discrimination Test; FEIT: Facial Emotion Identification Test;
Table 2.
Pearson product-moment correlations among measures of neurocognition, social cognition and functional outcome
| Cog. Factor |
Affective Prosody |
Eyes Test | FEIT | FEDT | |
|---|---|---|---|---|---|
| SFS | 0.19* | 0.12 | 0.18* | 0.25** | 0.08 |
| Cog. Factor | - | 0.49** | 0.63** | 0.42** | 0.24** |
| Affective Prosody | - | 0.48** | 0.36** | 0.15 | |
| Eyes Test | - | 0.41** | 0.26** | ||
| FEIT | - | 0.21* | |||
FEDT: Facial Emotion Discrimination Test; FEIT: Facial Emotion Identification Test; SFS: Social Functioning Scale.
p<0.05,
p<0.01
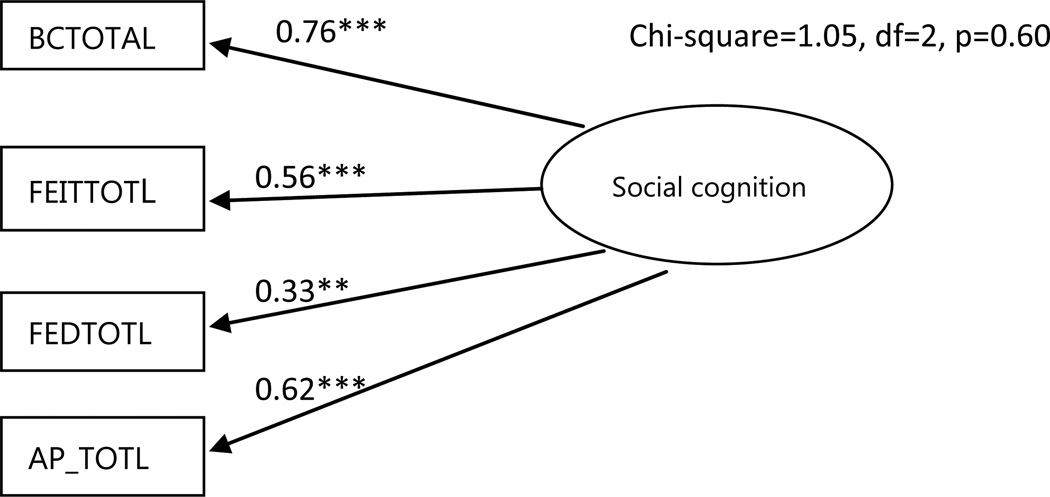
The confirmatory factor analysis showed that all measures of social cognition made significant contributions to their latent variable (loading range 0.33–0.76, p< 0.01; Figure 1).
Figure 1.
Confirmatory factor analysis model.
**p<0.01; ***p<0.001. Circles represent unobserved latent variables. Rectangles represent observed measured variables. Values are standardized path coefficients. BCTOTAL: Reading the Mind in the Eyes Test, FEITTOTL: Facial Emotion Identification Test, FEDTOTL: Facial Emotion Discrimination Test, AP_TOTL: Affective prosody Test.
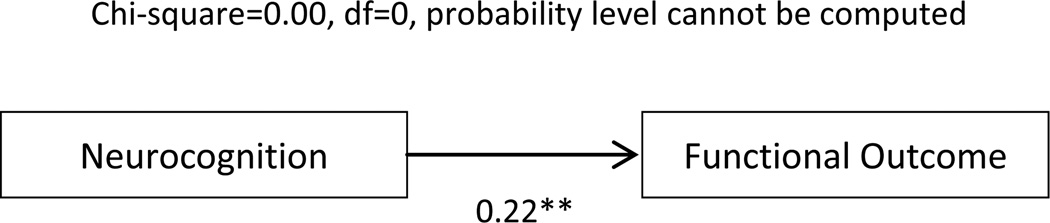
The basic model (Figure 2) depicts the direct relationship between neurocognition and functioning. This path was statistically significant (standardized coefficient β = 0.22, p < 0.01). The path from social cognition to functioning was also statistically significant (β= 0.27, p<0.05). The model explained 4.6% of the variance in functional outcome. This model had no degrees of freedom. Probability level could not be computed, CFI =1.0, RMSEA =0.20 (90% CI 0.08–0.36).
Figure 2.
Basic Model
** p<0.01. Rectangles represent observed measured variables.
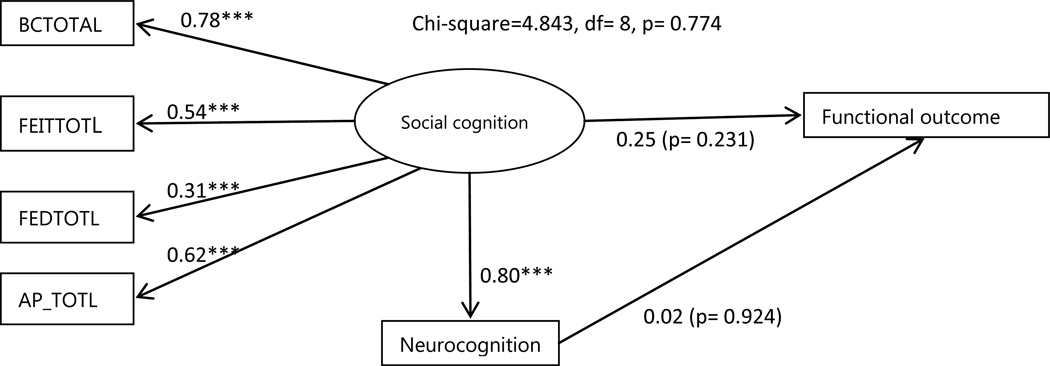
The mediation model intends to evaluate the strength of the indirect relationship while controlling for the direct effect of neurocognition on functional outcome (see Figure. 3). The direct path from neurocognition to functioning was no longer significant as soon as the mediator was entered into the model (β = 0.02, p = 0.92). Instead, social cognition was significantly associated with neurocognition (β = 0.80, p < 0.001). The impact of social cognition on functioning (β=0.25) was greater than the direct impact of neurocognition (β=0.02) but the regression weight for social cognition in the prediction of functional outcome was not significantly different from zero at the 0.05 level (two-tailed) (p=0.23).The model explained 7% of the variance in functional outcome. All of the model fit indices were very good (χ2 = 4.84, df = 8, p =0.77, CFI =1.0, RMSEA = 0.0). The bootstrapping estimate revealed the indirect effect (β =0.20, 95% CI =−0.07 to 0.52, p=0.11). The path coefficient for the indirect effect (β=0.20) represents the change in functional outcome for every unit change in neurocognition that is mediated through social cognition. Bootstrap approximation with 1000 iterations yielded a percentile-based confidence interval CI =−0.06 to 0.52. As zero is between the lower and upper bound, we cannot conclude that the indirect effect is significantly different from zero.
Figure 3.
Mediation Model
*** p<0.001 Circles represent unobserved latent variables. Rectangles represent observed measured variables. Values are standardized path coefficients. BCTOTAL: Reading the Mind in the Eyes Test, FEITTOTL: Facial Emotion Identification Test, FEDTOTL: Facial Emotion Discrimination Test, AP_TOTL: Affective prosody Test.
4. Discussion
The aim of this study was to understand the pathway from neurocognition to functional outcome in individuals at CHR for psychosis. Since it has been demonstrated that in individuals with schizophrenia social cognition mediated this pathway (Schmidt et al., 2011), we used SEM to test the hypothesis that in the CHR population the relationship between neurocognition and functioning is mediated by social cognitive abilities. All three constructs were associated, justifying including them in the model. Although the results of SEM showed that the data fitted very well with the model, after controlling for mediation effects, neurocognition and functioning were no longer significantly related. Furthermore, the significant association between social cognition and functioning also disappeared, although the association between neurocognition and social cognition strengthened. Therefore, contrary to what has been observed with individuals with schizophrenia, social cognition did not mediate between neurocognition and functioning in this CHR sample.
It has to be noted that in the CHR population, as a group, neurocognition and social cognition are only slightly impaired, with individuals at CHR performing at an intermediate level between individuals with a full blown psychotic illness and healthy controls (Addington and Barbato, 2012; Thompson et al., 2011). Therefore, it is possible that since these impairments are attenuated, the relationship among the three constructs is weaker than that observed in those with a full-blown psychotic illness who may have more severe deficits. This may be a function of the fact that in samples of those at CHR probably less than 30% will go on to develop psychosis (Fusar-Poli et al., 2012a). With a few exceptions, the role of social cognition as a mediator has not been examined in non-psychotic samples. In one study examining neurocognition, social cognition and functioning in schizophrenia, it was observed that in the healthy control group, there was no mediation effect for facial affect recognition (Addington et al., 2006b). However in a second study (Addington et al., 2006a) it was suggested that social perception was a potential mediator between cognition and social problem solving in both patients and controls. It is possible that the mediation model is more relevant to explain pathways to functioning in psychotic patients and may be a function of the specificity of the social functioning being assessed. In assessing functional outcome, a distinction has been made between competence-based measures and attainment-based measures. Competence measures assess functional capacity and are typically performance based simulations or role-plays of activities required for daily living whereas attainment-based measures focus on what an individual has accomplished and are assessed by either obtaining an individual’s subjective sense of their functioning or a more objective measure of their functioning. Generally, functional capacity is more likely to be affected by factors such as neurocognition (Horan et al., 2013). This may be the reason why in the two studies with healthy controls mediation was only observed with a competence-based measure of outcome. Interestingly, once social cognition was included in the mediation model, the strength of the relationship between neurocognition and social cognition increased significantly suggesting that even in this early stage of psychosis the two concepts are significantly associated.
There are several limitations to this study. First we only had one measure of functional outcome, the SFS, which is an attainment-based measure of functional outcome. This may have increased the variance in functional outcome and possibly led to a reduced path coefficient between social cognition and functional outcome. Secondly, we did assess two domains of social cognition; however, we had three measures of affect recognition, but only one measure of theory of mind. Thirdly, our measures of social cognition and the SFS were developed for adult populations and therefore may not be completely appropriate for such a young sample. More recently other scales have been designed to assess social and role functioning in this population (Cornblatt et al., 2007). The strength of this study is that we used a comprehensive and well validated cognitive battery and that the sample was antipsychotic free.
In conclusion, unlike what has been observed with psychotic patients, social cognition does not seem to mediate the pathway from neurocognition to functional outcome when assessed with a measure of social attainment in individuals at CHR for psychosis. Future studies may need to use a variety of approaches to assess functional outcome and to consider other interacting and intervening variables as the pathway through which neurocognition impacts functional outcome is likely highly complex.
Acknowledgements
This work was supported by National Institute of Mental Health grants to J. Addington (grant number U01MH06634), to D. Perkins (grant number U01MH0660694), and to S. Woods (grant number U01MH066160).
Role of funding source
The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
There are no conflicts of interest for any of the authors with respect to the data in this paper or for the study.
Contributors
Drs Addington, Perkins and Woods were responsible for the design of the whole study and the supervision of all aspects of data collection. Drs Penn and Keefe contributed to the design and data collection with respect to the social cognition data and the neurocognition data respectively. Ms Liu and Dr Barbato were responsible for data analysis. Dr Barbato took the lead on writing the manuscript with help in writing from Dr Addington and Dr Penn. All authors contributed to and approved the final manuscript.
Reference List
- Addington J, Cornblatt B, Cadenhead K, Cannon TD, McGlashan TH, Perkins DO. At clinical high risk for psychosis: outcome for nonconverters. American Journal of Psychiatry. 2011;13:800–805. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Girard TA, Christensen BK, Addington D. Social cognition mediates illness-related and cognitive influences on social function in patients with schizophrenia-spectrum disorders. Journal of Psychiatry and Neuroscience. 2010;35:49–54. doi: 10.1503/jpn.080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Barbato M. The role of cognitive functioning in the outcome of those at clinical high risk for developing psychosis. Epidemiology and Psychiatric Sciences. 2012;21:335–342. doi: 10.1017/S204579601200042X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Penn D, Woods SW, Addington D, Perkins DO. Social functioning in individuals at clinical high risk for psychosis. Schizophr Res. 2008;99:119–124. doi: 10.1016/j.schres.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Saeedi H, Addington D. Influence of social perception and social knowledge on cognitive and social functioning in early psychosis. The British Journal of Psychiatry. 2006a;189:373–378. doi: 10.1192/bjp.bp.105.021022. [DOI] [PubMed] [Google Scholar]
- Addington J, Saeedi H, Addington D. Facial affect recognition: A mediator between cognitive and social functioning in psychosis? Schizophr Res. 2006b;85:142–150. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Allott K, Liu P, Proffitt TM, Killackey E. Cognition at illness onset as a predictor of later functional outcome in early psychosis: Systematic review and methodological critique. Schizophr Res. 2011;125:221–235. doi: 10.1016/j.schres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The "reading the mind in the eyes" test revised version: A study with normal adults and adults with Asperger Syndrome or high-functioning autism. J Child Psychol Psychiatr. 2001;42:241–251. [PubMed] [Google Scholar]
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale: The Development and Validation of a New Scale Adjustment for use in Family Intervention Programmes with Schizophrenic Patients. British Journal of Psychiatry. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, Cannon TD. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33:688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J, Pattison PE, Jackson HJ, Wales RJ. Facial affect and affective prosody recognition in first episode schizophrenia. Schizophrenia Research. 2001;48:235. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MM-G, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, Barale F, Caverzasi E, McGuire P. Predicting psychosis: Meta-analysis of transition outcomes in individuals at high clinical risk. Archives of General Psychiatry. 2012a;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, Stieglitz R, Vita A, McGuire P, Borgwardt S. Cognitive functioning in prodromal psychosis: A meta-analysis. Archives of General Psychiatry. 2012b;69:562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Green MF, Bearden CE, Cannon TD, Fiske AP, Hellemann GS, Horan WP, Kee K, Kern RS, Lee J, Sergi MJ, Subotnik KL, Sugar CA, Ventura J, Yee CM, Nuechterlein KH. Social Cognition in Schizophrenia, Part 1: Performance Across Phase of Illness. Schizophrenia Bulletin. 2012;38:854–864. doi: 10.1093/schbul/sbq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kern RS, Green MF, Penn DL. Social Cognition Training for Individuals with Schizophrenia: Emerging Evidence. American Journal of Psychiatric Rehabilitation. 2008;11:205–252. [Google Scholar]
- Horan WP, Lee J, Green MF. Social Cognition and Functional Outcome in Schizophrenia. In: Roberts D, Penn D, editors. Social Cognition in Schizophrenia. Oxford University Press; 2013. [Google Scholar]
- Keefe R, Harvey P. Cognitive Impairment in Schizophrenia. In: Geyer MA, Gross G, editors. Novel Antischizophrenia Treatments. Berlin Heidelberg: Springer; 2012. pp. 11–37. [Google Scholar]
- Kerr SL, Neale JM. Emotion perception in schizophrenia: specific deficit or further evidence of generalized poor performance? J Abnorm Psychol. 1993;102:312–318. doi: 10.1037//0021-843x.102.2.312. [DOI] [PubMed] [Google Scholar]
- Kim HS, Shin NY, Jang JH, Kim E, Shim G, Park HY, Hong KS, Kwon JS. Social cognition and neurocognition as predictors of conversion to psychosis in individuals at ultra-high risk. Schizophr Res. 2011;130:170–175. doi: 10.1016/j.schres.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York (NY): The Guilford Press; 1998. [Google Scholar]
- McGlashan TH, Walsh BC, Woods SW. The Psychosis Risk Syndrome: Handbook for Diagnosis and Follow-up. New York: Oxford University Press; 2010. [Google Scholar]
- Schermelleh-Engel K, Moosbrugger H, Mueller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. Methods of Psychological Research. 2003;8:23–74. [Google Scholar]
- Schmidt SJ, Mueller DR, Roder V. Social cognition as a mediator variable between neurocognition and functional outcome in schizophrenia: empirical review and new results by structural equation modeling. Schizophrenia Bulletin. 2011;37(Suppl.2):S41–S54. doi: 10.1093/schbul/sbr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, "Just the Facts" 5. Treatment and prevention Past, present, and future. Schizophrenia Research. 2010;122:1–23. doi: 10.1016/j.schres.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Thompson AD, Bartholomeusz C, Yung AR. Social cognition deficits and the ultra high risk for psychosis population: a review of literature. Early Intervention in Psychiatry. 2011;5:192–202. doi: 10.1111/j.1751-7893.2011.00275.x. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]