Abstract
Background
Colorectal cancer (CRC) is a leading cause of cancer death worldwide. Epidemiological risk factors for CRC included alcohol intake, which is mainly metabolized to acetaldehyde by alcohol dehydrogenase and further oxidized to acetate by aldehyde dehydrogenase; consequently, the role of genes in the alcohol metabolism pathways is of particular interest. The aim of this study is to analyze the association between SNPs in ADH1B and ALDH2 genes and CRC risk, and also the main effect of alcohol consumption on CRC risk in the study population.
Methodology/Principal Findings
SNPs from ADH1B and ALDH2 genes, included in alcohol metabolism pathway, were genotyped in 1694 CRC cases and 1851 matched controls from the Molecular Epidemiology of Colorectal Cancer study. Information on clinicopathological characteristics, lifestyle and dietary habits were also obtained. Logistic regression and association analysis were conducted. A positive association between alcohol consumption and CRC risk was observed in male participants from the Molecular Epidemiology of Colorectal Cancer study (MECC) study (OR = 1.47; 95%CI = 1.18-1.81). Moreover, the SNPs rs1229984 in ADH1B gene was found to be associated with CRC risk: under the recessive model, the OR was 1.75 for A/A genotype (95%CI = 1.21-2.52; p-value = 0.0025). A path analysis based on structural equation modeling showed a direct effect of ADH1B gene polymorphisms on colorectal carcinogenesis and also an indirect effect mediated through alcohol consumption.
Conclusions/Significance
Genetic polymorphisms in the alcohol metabolism pathways have a potential role in colorectal carcinogenesis, probably due to the differences in the ethanol metabolism and acetaldehyde oxidation of these enzyme variants.
Introduction
Colorectal cancer (CRC) is a leading cause of death worldwide, with over one million new cases and half a million deaths around the world every year [1], [2]. Risk factors for CRC include advanced age, medical history of benign adenomatous polyps and inflammatory bowel diseases, family history of CRC, low intake of vegetables and fruits and high intake of dietary fat (particularly animal fat) and processed meat [3], [4], [5], [6]. Chronic consumption of non-steroidal anti-inflammatory drugs, hormone replacement therapy and statins are protective [7], [8]. The role of other lifestyle factors such as tobacco smoking [9], [10] or alcohol consumption [11], [12], [13], [14], [15], [16] remains inconclusive. Alcohol consumption has been reported to be associated with modest increased risks of CRC in some studies [17], but cancer risk may differ by tumor molecular subtype and anatomical site.
Although the mechanism by which alcohol influences CRC risk also remains not well understood [18], different hypothesis have been suggested: a carcinogenic effect of chemicals other than ethanol present in alcoholic beverages such as nitrosamines, a solvent action than facilitates absorption of other carcinogens, an inhibition of methylation caused by ethanol, or a carcinogenic and genotoxic role for acetaldehyde, the major metabolite of ethanol [19], [20]. There is sufficient evidence for the carcinogenicity of ethanol in experimental animals, and it is also considered carcinogenic to humans. Especially, there is evidence on the fact that drinking of alcoholic beverages is causally related to cancers of the oral cavity, pharynx, larynx and liver (IARC Group1); However, there is controversial evidence on the effect of alcohol consumption on gastric or colorectal cancer [21]. Moreover, there is increasing evidence that acetaldehyde, a cytotoxic, mutagenic, and carcinogenic metabolite of ethanol, is responsible for tumor enhancing effects leading to aberrant cell proliferation. Acetaldehyde can also bind to DNA, leading to the formation of stable DNA adducts and the generation of reactive oxygen species (ROS) that may cause replication errors and mutations in oncogenes and tumor suppressor genes [19], [22], [23], [24], [25]. Moreover, some studies have reported epigenetic alterations by alcohol metabolites including selective acetylation, methylation, and phosphorylation of histones that regulate gene expression during disease pathogenesis [26], [27], [28].
The amount of acetaldehyde present in various tissues following ethanol ingestion may not only depend on the amount of ethanol consumed but also on specific alleles of genes coding for ethanol-metabolizing enzymes. Ethanol is metabolized to acetaldehyde mainly by alcohol dehydrogenase (ADH) enzyme, and is further oxidized to acetate by acetaldehyde dehydrogenase (ALDH) (Supporting file Figure S1). Single nucleotide polymorphisms (SNPs) of ADH1B and ALDH2, the genes that encodes the key enzymes in alcohol metabolism, might cause variations in the amount of production and/or oxidation of acetaldehyde between individuals [18], [19], [29]. Therefore, polymorphisms in genes involved in alcohol metabolism pathways might influence CRC susceptibility.
The aim of the present study is to assess the association between SNPs in alcohol metabolism genes, in particular in ADH1B and ALDH2 genes, and CRC, and the main effect of alcohol consumption on CRC risk in the study population. The analyses have been performed within a large population-based case-control study conducted in Israel, the Molecular Epidemiology of Colorectal Cancer study (MECC) study.
Methods
Patients
The MECC study has already been described in detail [8], [30]. In brief, it is a population based case-control study of incident, pathologically confirmed, invasive CRC diagnosed between May 31, 1998 and March 31, 2004 and who lived in a geographically-defined area of Northern Israel.
Controls with no prior history of CRC were identified from the same source population using the Clalit Health Services (CHS) database, covering ∼70% of the older population. Controls were matched to cases by year of birth, gender, primary clinic location, and Jewish or Arab ethnicity. Participants provided written informed consent at the time of enrollment. The Institutional Review Boards at the Carmel Medical Center, the University of Michigan, and the University of Southern California approved all procedures. The study population included 2100 matched pairs. For this analysis, 1780 cases and 1864 controls with available DNA for genetic analysis will be used. The demographic characteristics of the subjects not included do not differ from those analyzed (data not shown).
Life-style information
Participants were interviewed face-to-face by trained monitors with a comprehensive epidemiological questionnaire that assessed demographic information, personal and family history of cancer, reproductive history and medical history, medication use, health habits and a food-frequency questionnaire.
Patient’s weight was recorded by self-report, as estimated one year before diagnosis for cases and at the time of interview for controls. Body mass index (BMI) was estimated one year before the diagnosis from self-reported weight and height.
Comprehensive dietary habits were obtained with the use of a validated food-frequency questionnaire modified for the Israeli diet. Alcohol frequency was registered as the alcohol consumption for the past five years before CRC diagnosis; beer, wine and other hard liquor were recorded separately (the question was exactly “have you ever consumed beer and/or wine and/or other hard liquor at least once a weak for at least one year?”). Alcohol consumption was converted to a categorical variable that includes three levels of consumption: non-drinkers, occasional drinkers (participants who only drink wine for the blessing) and drinkers. The prevalence of alcohol consumption in the study population is low, like in other Jewish population [31], [32]. Total fat and vegetable intakes were estimated from the questionnaire using local food composition tables. Similar estimates provided total energy consumption.
Physical activity was recorded for the longest occupation and also recreational physical exercise. These were combined into an ordinal index ranging from sedentary to intense physical activity.
SNP selection and genotyping
SNPs in two candidate genes related to key alcohol metabolism pathways, specifically rs1229884 in ADH1B gene and rs886205 in ALDH2, were selected for analysis within a larger exploratory project including other hypothesis. The SNPs were selected as maximally informative htSNPs when the regions were analyzed with HaploTagger. SNPs were genotyped using Illumina BeadStation and BeadExpress platforms. Data were analyzed using unsupervised Illumina BeadStation SNP calling automated routines, and the distribution of genotypes was compared to those expected from Hardy-Weinberg equilibrium. Genotypes were also confirmed with a second platform in at least 1% of samples.
Statistical Methods
Unconditional logistic regression was used to assess the association between genotypes and CRC risk. All models included age and gender to account for the matching design and avoid excluding incomplete pairs due to DNA unavailability or missing values in other variables. The total number of cases and controls with both, lifestyle information and genetic data available for this analysis were 1694 and 1851, respectively. Baseline characteristics did not differ to the complete dataset. Analyses regarding alcohol have been restricted to men due to the low prevalence of alcohol consumption among the female population.
To estimate the magnitude of the associations, multivariate-adjusted odds ratios (OR) and their corresponding 95% confidence intervals (CI) were calculated for each genotype compared to those homozygous for the common allele. Also a log-additive model was fitted (trend test). Genetic analysis were performed with the SNPstats web software [33] and SNPassoc library from R package [34]. A panel of 300 anonymous SNPs useful for population stratification analysis was analyzed in these subjects in relation to other larger genotyping project. No relevant population structure was identified that could not be explained by reported ethnicity. Although the potential confounding of this variable was rejected after a sensitivity analysis (data not shown) it was also included in the models as a potential confounder.
A global 5% significance level was desired for the analyses of SNPs. All reported p-values are two-tailed, and adjusted for age, sex, ethnicity, energy intake and physical activity. Other variables were assessed as potential confounders (i.e., BMI, vegetable consumption, fat intake, red meat consumption) but were not included in the modes since they did not significantly alter the estimates (results not shown).
To simultaneously study the associations between SNPs, potential confounding variables and CRC we also fitted structural equation models (SEM) to assess the direct effect of genetic polymorphisms on CRC (unexplained by candidate variables), and also their indirect effect, mediated through lifestyle related factors, by including all variables in the same model to assess overall associations. These analyses were performed using the structural equation modeling library from R package. SEM was used to test the conceptual model since, in contrast to traditional analytical procedures as linear regression analysis, SEM allows distinguishing between direct and indirect effects and provides information on the degree of fit for the entire model. In SEM the covariance structure that follows from the proposed model is fitted to the observed covariance. The maximum likelihood estimate method yields estimates of the regression coefficients in the model, standard errors and an overall goodness-of-fit test [35].
Results
The study population included 3545 participants, 1694 cases and 1851 controls, with available data regarding epidemiological interviews (including alcohol consumption) and genetic analysis. A description of the subjects’ characteristics is in Table 1. Cases had larger reported weight and BMI one year before the diagnosis than controls. Cases also reported more family history of CRC and lower physical activity and vegetable intake than controls, and were more often Ashkenazi than Sephardi.
Table 1. Baseline characteristics of cases and controls from the study population.
| Clinical/Baseline characteristics | All participants | Men only | ||||
| Controls | Cases | p-value | Controls | Cases | p-value | |
| N (%) | 1864 (51) | 1780 (49) | 931 (51) | 894 (49) | ||
| Age - mean (SD) | 70.6 (11.7) | 70.2 (11.7) | 0.34 | 71.3 (11.3) | 70.9 (11.3) | 0.40 |
| Sex | 0.87 | |||||
| Male - N (%) | 931 (50) | 894 (50) | ||||
| Female - N (%) | 933 (50) | 886 (50) | ||||
| BMI - mean (SD) | 26.9 (4.6) | 27.3 (4.6) | 0.027 | 26.4 (3.7) | 27.1 (4.1) | 0.00058 |
| History of colorectal cancer in first-degree relative | 0.0018 | 0.12 | ||||
| No - N (%) | 1722 (93) | 1571 (90) | 866 (93) | 806 (91) | ||
| Yes - N (%) | 132 (7) | 175 (10) | 61 (7) | 75 (9) | ||
| Physical activity | <0.0001 | 0.0011 | ||||
| Sedentary | 1105 (62) | 1231 (69) | 516 (56) | 571 (64) | ||
| Intermediate | 447 (25) | 311 (18) | 226 (24) | 173 (19) | ||
| Active | 241 (13) | 238 (13) | 189 (20) | 150 (17) | ||
| Ethnicity | <0.0001 | 0.0009 | ||||
| Ashkenazi | 1176 (63) | 1249 (70) | 595 (64) | 633 (71) | ||
| Sephardi | 445 (24) | 297 (17) | 219 (23) | 149 (17) | ||
| Other | 241 (13) | 234 (13) | 117 (13) | 112 (12) | ||
| Religion | 0.33 | 0.94 | ||||
| Jew | 1651 (89) | 1576 (88.54) | 828 (88.94) | 796 (89.04) | ||
| Christian | 91 (5) | 102 (5.73) | 49 (5.26) | 49 (5.48) | ||
| Moslem | 122 (6) | 102 (5.73) | 54 (5.8) | 49 (5.48) | ||
| Vegetable intake (g/day) - mean (SD) | 7.9 (3.7) | 7.5 (4.5) | 0.0061 | 8.1 (3.7) | 7.6 (4.1) | 0.0026 |
| Regular aspirin intake | ||||||
| No - N (%) | 1170 (63) | 1192 (70) | <0.0001 | 524 (56) | 558 (64) | 0.0001 |
| Yes - N (%) | 692 (37) | 531 (30) | 406 (44) | 317 (36) | ||
| Regular statin intake | ||||||
| No - N (%) | 1623 (88) | 1630 (94) | <0.0001 | 795 (86) | 819 (93) | <0.0001 |
| Yes - N (%) | 228 (12) | 101 (9) | 128 (14) | 59 (7) | ||
| Energy intake (Kcal/day) - mean (SD) | 1856 (1114) | 1889 (1587) | 0.48 | 1996 ( 1249) | 2058 (1878) | 0.41 |
| Total fat intake (g/day) - mean (SD) | 66.1 (44.9) | 69.3 (67.5) | 0.0968 | 71.3 (50.4) | 76.6 (81.8) | 0.1016 |
| Smoking | ||||||
| Never smoker- N (%) | 1035 (56) | 993 (57) | 0.38 | 327 (35) | 323 (37) | 0.53 |
| Ever smoker - N (%) | 826 (44) | 747 (43) | 603 (65) | 560 (63) | ||
| Alcohol consumption, past five years | ||||||
| No or occasionally- N (%) | 1587 (85) | 1405 (81) | 0.0002 | 693 (75) | 599 (68) | 0.0013 |
| Yes - N (%) | 272 (15) | 336 (19) | 235 (25) | 284 (32) | ||
Though energy intake was similar for cases and controls, total fat intake was larger for cases than controls. Long term regular use of aspirin and statins were also inversely associated with CRC risk. Smoking habits were similar in cases and controls, while higher proportion of cases reported alcohol consumption for the five years before diagnosis than controls (p<0.001).
A description of subjects’ characteristics by categories of alcohol consumption is in Table 2. Only 17% of participants reported alcohol consumption (other than wine for the blessing) for the past five years. No differences were observed regarding reported BMI, ethnicity, long term use of aspirin and statins and family history of CRC. Alcohol drinkers were mainly men, Christian religion, and had higher vegetables, fat and energy intakes. A large difference of alcohol consumption prevalence was observed between men and women. Since the prevalence of alcohol drinking in women was very low (<5%), women were excluded from the association analysis. Baseline characteristics of men’s population included in the study is shown in Table 1, and also by alcohol consumption in Table 2.
Table 2. Distribution of participant characteristics by categories of alcohol consumption.
| Clinical/Baseline characteristics | Alcohol consumption (all participants) | Alcohol consumption (men only) | ||||
| No or Occasional | Yes | p-value | No or Occasional | Yes | p-value | |
| N (%) | 2992 (83) | 608 (17) | 1292 (71) | 519 (29) | ||
| Age - mean (SD) | 70.5 (11.7) | 69.4 (11.3) | 0.028 | 71.59 (11.37) | 69.87 (11.10) | 0.0032 |
| Sex | <0.0001 | |||||
| Male - N (%) | 1292 (43) | 519 (85) | ||||
| Female - N (%) | 1700 (57) | 89 (15) | ||||
| BMI - mean (SD) | 27.1 (4.8) | 26.7 (3.7) | 0.016 | 26.7 (4.1) | 26.7 (3.5) | 0.82 |
| History of colorectal cancer in first-degree relative | 0.96 | 0.45 | ||||
| No - N (%) | 2707 (91) | 552 (91) | 1186 (93) | 473 (92) | ||
| Yes - N (%) | 253 (9) | 52 (9) | 93 (7) | 43 (8) | ||
| Physical activity | <0.0001 | <0.0001 | ||||
| Sedentary | 1990 (66) | 307 (51) | 815 (63) | 261 (50) | ||
| Intermediate | 593 (20) | 141 (23) | 274 (21) | 124 (24) | ||
| Active | 409 (14) | 160 (26) | 203 (16) | 134 (26) | ||
| Ethnicity | 0.25 | 0.14 | ||||
| Ashkenazi | 1977 (66) | 413 (68) | 868 (67) | 351 (68) | ||
| Sephardi | 627 (21) | 110 (18) | 272 (21) | 93 (18) | ||
| Other | 388 (13) | 85 (14) | 152 (12) | 75 (14) | ||
| Religion | <0.0001 | <0.0001 | ||||
| Jew | 2647 (88) | 538 (89) | 1155 (89) | 457 (88) | ||
| Christian | 135 (5) | 57 (9) | 48 (4) | 49 (9) | ||
| Moslem | 210 (7) | 13 (2) | 89 (7) | 13 (3) | ||
| Vegetable intake (g/day) - mean (SD) | 7.5 (4.1) | 8.5 (4.1) | <0.0001 | 7.6 (3.9) | 8.4 (4.0) | 0.0003 |
| Regular aspirin intake | ||||||
| No - N (%) | 1977 (66.2) | 378 (62.3) | 0.17 | 763 (59.3) | 315 (60.8) | 0.12 |
| Yes - N (%) | 994 (33.3) | 225 (37.1) | 522 (40.6) | 199 (38.4) | ||
| Regular statin intake | ||||||
| No - N (%) | 2700 (91) | 540 (90) | 0.25 | 1147 (90) | 459 (89) | 0.66 |
| Yes - N (%) | 265 (9) | 63 (10) | 130 (10) | 56 (11) | ||
| Energy intake (Kcal/day) - mean (SD) | 1811 (931) | 2178 (1022) | <0.0001 | 1957 (1042) | 2200 (1031) | 0.0059 |
| Total fat intake (g/day) - mean (SD) | 65.1 (53.5) | 80.9 (70.1) | <0.0001 | 70.8 (65.1) | 81.8 (72.7) | 0.0030 |
| Smoking | ||||||
| Never smoker- N (%) | 1853 (62) | 170 (28) | <0.0001 | 528 (41) | 120 (23) | <0.0001 |
| Ever smoker - N (%) | 1132 (38) | 438 (72) | 762 (59) | 399 (77) | ||
| Participant status | ||||||
| Control- N (%) | 1587 (53) | 272 (45) | 0.0002 | 693 (54) | 235 (45) | 0.0013 |
| Case - N (%) | 1405 (47) | 336 (55) | 599 (46) | 284 (55) | ||
Alcohol consumption and CRC risk
Although the prevalence of alcohol consumption in the study population was low, and only 29% of men reported alcohol consumption (other than wine for the blessing) for five years before CRC diagnostic, the prevalence of alcohol consumption was higher in male cases than in male controls (p<0.01) (Table 1). The odds ratio (OR) adjusted for age, ethnicity, religion, energy intake and physical activity was 1.47 (95%CI = 1.18-1.81; p = 0.0004).
Alcohol metabolism SNPs and CRC risk
SNPs in 2 candidate genes related to key alcohol metabolism pathways, specifically rs1229884 in ADH1B gene and rs886205 in ALDH2, were genotyped in CRC cases and controls and selected for the present analysis. A detailed description of the allele and genotype frequencies for the studied SNPs is shown in Supporting Information file Table S1. Details of the association between ADH1B and ALDH2 polymorphisms and CRC risk are shown in Table 3. Except for those indicated, ORs were adjusted for age, ethnicity, religion energy intake and physical activity, and the association analysis was restricted to men. The model of inheritance presented in tables was selected on the basis of the lowest value of Akaike's information criterion.
Table 3. Association between polymorphisms in alcohol metabolism genes and colorectal cancer risk (men only).
| SNP | Controls | Cases | OR1 | 95% CI | p-value |
| N (%) | N (%) | ||||
| ADH1B rs1229984 | 927 (51.9) | 860 (48.1) | 0.0096 | ||
| G/G | 513 (55.3) | 457 (53.1) | 1.00 | ||
| G/A | 360 (38.8) | 324 (37.7) | 1.04 | (0.85–1.26) | |
| A/A | 54 (5.8) | 79 (9.2) | 1.77 | (1.22–2.58) | |
| Recessive (A/A) | 54 (5.8) | 79 (9.2) | 1.75 | (1.21–2.52) | 0.0025 |
| ALDH2 rs886205 | 916 (51.8) | 854 (48.2) | 0.61 | ||
| T/T | 658 (71.8) | 637 (74.6) | 1.00 | ||
| T/C | 228 (24.9) | 189 (22.1) | 0.91 | (0.73–1.15) | |
| C/C | 30 (3.3) | 28 (3.3) | 1.16 | (0.67–1.99) | |
| Dominant (T/C-C/C) | 258 (28.2) | 217 (25.4) | 0.94 | (0.76–1.17) | 0.57 |
OR adjusted for age, ethnicity, religion, energy intake and physical activity.
An association was observed between rs1229984 polymorphism in ADH1B and CRC risk. The A allele of this SNP was associated with an increased risk of CRC. Under the recessive model, the multi-adjusted OR was 1.75 for A/A genotype (95%CI = 1.21-2.52, p-value = 0.0025).
No association between rs886205 polymorphism in ALDH2 and CRC risk was observed. Under the dominant model, the OR adjusted for potential confounders was 0.94 for T/C and C/C genotypes (95%CI = 0.76-1.17, p-value = 0.57). No significant interaction between ADH1B and ALDH2 polymorphisms was observed (p = 0.89; data not shown).
Alcohol metabolism SNPs and alcohol consumption
The association between ADH1B and ALDH2 polymorphisms and alcohol consumption in men controls is shown in Table 4. The recessive model for polymorphism rs1229984 in ADH1B showed a non-significant association with alcohol consumption (OR = 1.79, 95%CI = 0.97-3.28, p = 0.068). However, polymorphism rs886205 in ALDH2 was not associated to alcohol consumption (OR = 1.06, 95%CI = 0.75-1.50, p = 0.75). No significant interactions were observed between ADH1B polymorphisms and alcohol consumption or between ALDH2 polymorphisms and alcohol consumption (p = 0.73 and p = 0.81, respectively; data not shown).
Table 4. Association between polymorphisms in alcohol metabolism genes and alcohol consumption (men only).
| SNP | Alcohol consumption | OR1 | 95% CI | p-value | |
| No or Occasional | Yes | ||||
| N (%) | N (%) | ||||
| ADH1B rs1229984 | 690 (74.7) | 234 (25.3) | 0.10 | ||
| G/G | 378 (54.8) | 133 (56.8) | 1.00 | ||
| G/A | 276 (40.0) | 83 (35.5) | 0.83 | (0.60–1.15) | |
| A/A | 36 (5.2) | 18 (7.7) | 1.66 | (0.89–3.08) | |
| Recessive (A/A) | 36 (5.2) | 18 (7.7) | 1.79 | (0.97–3.28) | 0.068 |
| ALDH2 rs886205 | 682 (74.7) | 231 (25.3) | 0.69 | ||
| T/T | 493 (72.3) | 164 (71.0) | 1.00 | ||
| T/C | 166 (24.3) | 62 (26.8) | 1.10 | (0.77–1.57) | |
| C/C | 23 (3.4) | 5 (2.2) | 0.72 | (0.25–2.02) | |
| Dominant (T/C-C/C) | 189 (27.7) | 67 (29.0) | 1.06 | (0.75–1.50) | 0.75 |
OR adjusted for age, ethnicity, religion, energy intake and physical activity.
Path analysis of SNPs, alcohol consumption, physical activity, energy intake, ethnicity and CRC
The main aim of this analysis was to perform, beyond the association study, a combined analysis of all players in alcohol metabolism. Path analysis using structural equation models (SEM) provide an interesting tool for this, since a simultaneous estimate of associations can be done for an a priori proposed model. We built the model from the observed associations in the multiple univariate analyses previously described and tried to fit SEM to identify strong association resistant to adjustment for indirect effects.
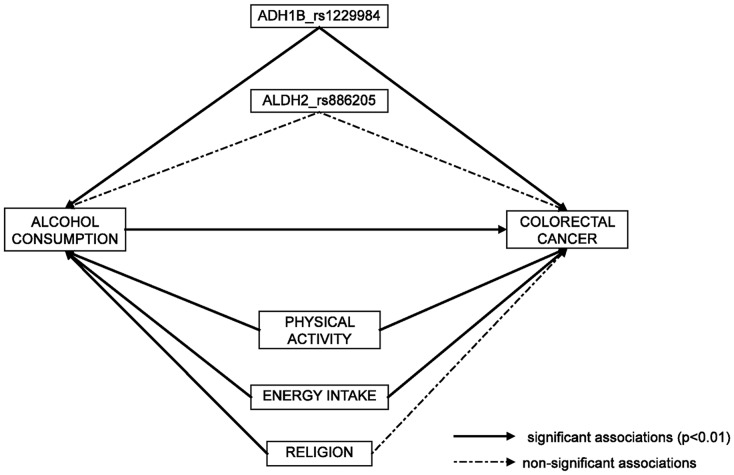
Figure 1 shows the proposed model reduced to relevant associations. The continuous arrows show the associations that remain significant in the SEM. Discontinuous arrows show associations that were no longer significant and could be considered as indirect effects. This analysis confirms the direct effect of ADH1B on CRC and also an indirect effect of such polymorphisms through alcohol consumption. Behavioral variables, like energy intake and physical exercise, also remain related among each other and associated to CRC.
Figure 1. Polymorphisms in alcohol metabolism genes ADH1B and ALDH2, alcohol consumption and colorectal cancer: path analysis using Structural Equation Modeling (SEM).
Continuous arrows indicate significant associations (p-value < 0.01) and discontinuous arrows indicate non-significant associations.
Discussion
We have examined the association between alcohol consumption, polymorphisms in alcohol metabolism genes and the risk of CRC. An increased risk of CRC was observed in alcohol drinkers. Polymorphism rs1229984 in ADH1B has been found to be directly associated with CRC risk and it also shows an indirect effect, mediated through alcohol consumption, even when energy intake, physical activity and religion are included in the model as potential confounders. No association between ALDH2 polymorphism rs886205 and CRC risk was observed, neither by the indirect effect of such polymorphisms on alcohol consumption.
In humans, the major enzymes involved in the alcohol metabolizing pathways are alcohol dehydrogenase-1B (ADH1B) and aldehyde dehydrogenase-2 (ALDH2). ADH1B, that is expressed in the liver and present in gastrointestinal tract, metabolize ethanol to acetaldehyde that is further oxidized to acetate by ALDH2 [19]. The activity of these enzymes varies between individuals, so genetic polymorphisms in ADH1B and ALDH2 genes could be responsible for differences in the expression and activity of the enzymes as well as the subsequent metabolites generated by the enzymes. Polymorphisms in genes responsible for these pathways can affect the amount of acetaldehyde and reactive oxygen species generated during the metabolic process, altering the effects of alcohol, and potentially leading to carcinogenesis [36], [37].
It has been reported that after alcohol absorption, the concentration of alcohol in the colon is higher than in the blood. In animal experiments, acetaldehyde concentration in the mucosa of large intestine could exceed the concentration that is considered to be mutagenic. Moreover, when aldehyde dehydrogenase activity was inhibited, tumorigenesis was observed in the colon of rats. In addition, bacteria in the large intestine can also metabolize alcohol into acetaldehyde, but do not metabolize acetaldehyde to acetic acid for detoxification, so this cytotoxic metabolite could accumulate and reach high concentrations in the colon [19], [38], [39], [40].
Previous studies have reported associations between polymorphisms of alcohol metabolizing enzymes and CRC risk, mainly in oriental population, but results are inconclusive since associations reported are not always replicated by other studies [36], [37], [41], [42], [43], [44], [45]. The most frequently reported loci are ADH1B Arg47His (rs1229984), because the activity of ADH1B decreased by 40-fold in ADH1B His/His individuals, and ALDH2 Glu487Lys (rs671), which affects the Km of this enzyme for alcohol with loss of the enzyme activity in individuals with the ALDH2 Lys/Lys phenotype. However, most of these studies were focused on the functional loci reported in other diseases; only the study of Yang et al, 2009 [36] reported a tagSNP-based association study to investigate novel loci that have not been reported before, including the ones analyzed in our study, rs1229984 SNP of ADH1B gene and rs886205 of ALDH2 gene. In this hospital-based case-control study of 440 CRC patients and 800 cancer-free controls in Southwestern Chinese population, none of these 2 SNPs were significantly associated with CRC risk. Recently, Ferrari et al. [44] evaluated the impact of rs1229984 polymorphisms and CRC risk in a nested case-control study (1269 cases and 2107 matched controls) within the European Prospective Investigation into Cancer and Nutrition study (EPIC) and they founded that the SNP was associated with a reduction in alcohol consumption but not with CRC risk overall in Western-European Populations.
Our results showed direct effect of ADH1B gene polymorphisms on colorectal carcinogenesis and also a weak indirect effect of such polymorphisms mediated through alcohol consumption, even including energy intake and physical activity in the models as potential confounders. Since polymorphisms in ADH1B gene contributed in the increased risk of CRC, the role of this enzyme in colorectal carcinogenesis could be due to its efficiency in ethanol metabolization and acetaldehyde detoxification and to the amount of acetaldehyde that is consequently accumulated leading to colorectal carcinogenesis.
This study is one of the largest conducted so far in which polymorphisms in alcohol metabolism genes have been studied in the global context of energy intake and physical activity in relation to CRC. The population-based case-control design, though retrospective, has revealed useful to identify a series of associations that allow a global picture of the key players. Some of the reported associations had been previously reported in studies of specific factors, which reinforces the strength of the proposed model. The path analysis using structural equations models, though not novel, is a good tool to provide a comprehensive analysis of all the players in the putative causal model. This analysis has identified direct and mediated effects of alcohol-related factors and CRC risk. The study also has some limitations. Most important ones are that alcohol intake was assessed only by self-report questionnaire, which may be liable to reporting biases. The population under study is characterized by a low prevalence of alcohol consumption, mostly related to religious traditions among Jewish and Arabs. This has impacted in a reduced sample size in the alcohol-exposed categories. Also, the polymorphisms studied have been reduced to one SNP per gene, which may not be enough to capture all the genetic variability.
Conclusion
Our results suggest a role of polymorphism rs1229984 in ADH1B in colorectal carcinogenesis but have failed to show such association for polymorphism rs886205 in ALDH2. Although our findings require validation in other independent population, further characterization of ADH1B and ALDH2 polymorphisms may provide new insights into their contribution to incidence and progression of CRC.
Supporting Information
Role of ADH1B and ALDH2 in ethanol metabolism.
(TIF)
Allele and genotype frequencies for the studied SNPs.
(DOCX)
Funding Statement
This work was supported by a grant from the National Cancer Institute [N01-CN43308 (SML), NCI R01-CA81488 (SBG)], University of Michigan's Cancer Center Support Grant [5 P30 CA46592]. Also the Catalan Institute of Oncology and the Private Foundation of the Biomedical Research Institute of Bellvitge (IDIBELL), the Instituto de Salud Carlos III [grants PI08-1635, PI08-1359, PS09-1037], CIBERESP CB06/02/2005 and the “Acción Transversal del Cancer”, the Catalan Government DURSI [grant 2009SGR1489], and the AECC (Spanish Association Against Cancer) Scientific Foundation. USC Norris Cancer Center Support Grant [NCI P30 CA014089 (SBG). No authors have financial conflict of interest with this manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, et al. (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European journal of cancer 49: 1374–1403. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA: a cancer journal for clinicians 63: 11–30. [DOI] [PubMed] [Google Scholar]
- 3.Potter JD (2008) Colorectal cancer. in Adami HO, Hunter D, Trichopoulos D.Textbook of cancer epidemiology. Oxford University Press, New York..
- 4. Weitz J, Koch M, Debus J, Hohler T, Galle PR, et al. (2005) Colorectal cancer. Lancet 365: 153–165. [DOI] [PubMed] [Google Scholar]
- 5. van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, Jenab M, Boshuizen HC, et al. (2009) Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 89: 1441–1452. [DOI] [PubMed] [Google Scholar]
- 6. Gerber M (2009) Background review paper on total fat, fatty acid intake and cancers. Ann Nutr Metab 55: 140–161. [DOI] [PubMed] [Google Scholar]
- 7. Clevers H (2006) Colon cancer—understanding how NSAIDs work. N Engl J Med 354: 761–763. [DOI] [PubMed] [Google Scholar]
- 8. Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, et al. (2005) Statins and the risk of colorectal cancer. N Engl J Med 352: 2184–2192. [DOI] [PubMed] [Google Scholar]
- 9. Liang PS, Chen TY, Giovannucci E (2009) Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 124: 2406–2415. [DOI] [PubMed] [Google Scholar]
- 10. Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, et al. (2008) Smoking and colorectal cancer: a meta-analysis. JAMA 300: 2765–2778. [DOI] [PubMed] [Google Scholar]
- 11. Park JY, Dahm CC, Keogh RH, Mitrou PN, Cairns BJ, et al. (2010) Alcohol intake and risk of colorectal cancer: results from the UK Dietary Cohort Consortium. Br J Cancer 103: 747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park JY, Mitrou PN, Dahm CC, Luben RN, Wareham NJ, et al. (2009) Baseline alcohol consumption, type of alcoholic beverage and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition-Norfolk study. Cancer Epidemiol 33: 347–354. [DOI] [PubMed] [Google Scholar]
- 13. Poynter JN, Haile RW, Siegmund KD, Campbell PT, Figueiredo JC, et al. (2009) Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status. Cancer Epidemiol Biomarkers Prev 18: 2745–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bongaerts BW, van den Brandt PA, Goldbohm RA, de Goeij AF, Weijenberg MP (2008) Alcohol consumption, type of alcoholic beverage and risk of colorectal cancer at specific subsites. Int J Cancer 123: 2411–2417. [DOI] [PubMed] [Google Scholar]
- 15. Moskal A, Norat T, Ferrari P, Riboli E (2007) Alcohol intake and colorectal cancer risk: a dose-response meta-analysis of published cohort studies. Int J Cancer 120: 664–671. [DOI] [PubMed] [Google Scholar]
- 16. Bagnardi V, Blangiardo M, La Vecchia C, Corrao G (2001) Alcohol consumption and the risk of cancer: a meta-analysis. Alcohol Res Health 25: 263–270. [PMC free article] [PubMed] [Google Scholar]
- 17.(AICR) WCRFWAIoCR (2007) Food, nutrition, physical activity and the prevention of cancer: a global perspective. World Cancer Research Fund global network's diet and cancer report website. http://www.dietandcancerreport.org/. Accessed October 2013).
- 18. Poschl G, Seitz HK (2004) Alcohol and cancer. Alcohol Alcohol 39: 155–165. [DOI] [PubMed] [Google Scholar]
- 19. Seitz HK, Stickel F (2007) Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer 7: 599–612. [DOI] [PubMed] [Google Scholar]
- 20. Boffetta P, Hashibe M (2006) Alcohol and cancer. Lancet Oncol 7: 149–156. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization IAfRoCI (2006) Monographs on the evaluation of carcinogenic risks to humans. International Agency for Cancer Research on Cancer website. http://monographs.iarc.fr/ENG/Preamble/CurrentPreamble.pdf. Accessed October 2013.
- 22. Seitz HK, Matsuzaki S, Yokoyama A, Homann N, Vakevainen S, et al. (2001) Alcohol and cancer. Alcohol Clin Exp Res 25: 137S–143S. [DOI] [PubMed] [Google Scholar]
- 23. Simanowski UA, Homann N, Knuhl M, Arce L, Waldherr R, et al. (2001) Increased rectal cell proliferation following alcohol abuse. Gut 49: 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salaspuro MP (2003) Alcohol consumption and cancer of the gastrointestinal tract. Best Pract Res Clin Gastroenterol 17: 679–694. [DOI] [PubMed] [Google Scholar]
- 25. Bongaerts BW, de Goeij AF, van den Brandt PA, Weijenberg MP (2006) Alcohol and the risk of colon and rectal cancer with mutations in the K-ras gene. Alcohol 38: 147–154. [DOI] [PubMed] [Google Scholar]
- 26. Mandrekar P (2011) Epigenetic regulation in alcoholic liver disease. World journal of gastroenterology : WJG 17: 2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moghe A, Joshi-Barve S, Ghare S, Gobejishvili L, Kirpich I, et al. (2011) Histone modifications and alcohol-induced liver disease: are altered nutrients the missing link? World journal of gastroenterology : WJG 17: 2465–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shukla SD, Velazquez J, French SW, Lu SC, Ticku MK, et al. (2008) Emerging role of epigenetics in the actions of alcohol. Alcoholism, clinical and experimental research 32: 1525–1534. [DOI] [PubMed] [Google Scholar]
- 29. Seitz HK, Meier P (2007) The role of acetaldehyde in upper digestive tract cancer in alcoholics. Transl Res 149: 293–297. [DOI] [PubMed] [Google Scholar]
- 30. Lipkin SM, Chao EC, Moreno V, Rozek LS, Rennert H, et al. (2010) Genetic variation in 3-hydroxy-3-methylglutaryl CoA reductase modifies the chemopreventive activity of statins for colorectal cancer. Cancer Prevention Research 3: 597–603. [DOI] [PubMed] [Google Scholar]
- 31. Hasin D, Aharonovich E, Liu X, Mamman Z, Matseoane K, et al. (2002) Alcohol and ADH2 in Israel: Ashkenazis, Sephardics, and recent Russian immigrants. Am J Psychiatry 159: 1432–1434. [DOI] [PubMed] [Google Scholar]
- 32. Neumark YD, Friedlander Y, Durst R, Leitersdorf E, Jaffe D, et al. (2004) Alcohol dehydrogenase polymorphisms influence alcohol-elimination rates in a male Jewish population. Alcohol Clin Exp Res 28: 10–14. [DOI] [PubMed] [Google Scholar]
- 33. Solé X, Guinó E, Valls J, Iniesta R, Moreno V (2006) SNPStats: a web tool for the analysis of association studies. Bioinformatics 22: 1928–1929. [DOI] [PubMed] [Google Scholar]
- 34. Gonzalez JR, Armengol L, Sole X, Guino E, Mercader JM, et al. (2007) SNPassoc: an R package to perform whole genome association studies. Bioinformatics 23: 644–5. [DOI] [PubMed] [Google Scholar]
- 35. Joreskog KG (1996) Modeling development: using covariance structure models in longitudinal research. Eur Child Adolesc Psychiatry 5 Suppl 18–10. [DOI] [PubMed] [Google Scholar]
- 36. Yang H, Zhou Y, Zhou Z, Liu J, Yuan X, et al. (2009) A novel polymorphism rs1329149 of CYP2E1 and a known polymorphism rs671 of ALDH2 of alcohol metabolizing enzymes are associated with colorectal cancer in a southwestern Chinese population. Cancer Epidemiol Biomarkers Prev 18: 2522–2527. [DOI] [PubMed] [Google Scholar]
- 37. Gao CM, Takezaki T, Wu JZ, Zhang XM, Cao HX, et al. (2008) Polymorphisms of alcohol dehydrogenase 2 and aldehyde dehydrogenase 2 and colorectal cancer risk in Chinese males. World J Gastroenterol 14: 5078–5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seitz HK, Homann N (2007) The role of acetaldehyde in alcohol-associated cancer of the gastrointestinal tract. Novartis Found Symp 285: 110-119; discussion 119–114, 198–119. [DOI] [PubMed]
- 39. Jokelainen K, Siitonen A, Jousimies-Somer H, Nosova T, Heine R, et al. (1996) In vitro alcohol dehydrogenase-mediated acetaldehyde production by aerobic bacteria representing the normal colonic flora in man. Alcohol Clin Exp Res 20: 967–972. [DOI] [PubMed] [Google Scholar]
- 40. Seitz HK, Simanowski UA, Garzon FT, Rideout JM, Peters TJ, et al. (1990) Possible role of acetaldehyde in ethanol-related rectal cocarcinogenesis in the rat. Gastroenterology 98: 406–413. [DOI] [PubMed] [Google Scholar]
- 41.Seitz HK, Becker P (2007) Alcohol metabolism and cancer risk. Alcohol Res Health 30: 38–41, 44–37. [PMC free article] [PubMed]
- 42. Yin G, Kono S, Toyomura K, Moore MA, Nagano J, et al. (2007) Alcohol dehydrogenase and aldehyde dehydrogenase polymorphisms and colorectal cancer: the Fukuoka Colorectal Cancer Study. Cancer Sci 98: 1248–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matsuo K, Wakai K, Hirose K, Ito H, Saito T, et al. (2006) A gene-gene interaction between ALDH2 Glu487Lys and ADH2 His47Arg polymorphisms regarding the risk of colorectal cancer in Japan. Carcinogenesis 27: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 44. Ferrari P, McKay JD, Jenab M, Brennan P, Canzian F, et al. (2012) Alcohol dehydrogenase and aldehyde dehydrogenase gene polymorphisms, alcohol intake and the risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition study. Eur J Clin Nutr 66: 1303–1308. [DOI] [PubMed] [Google Scholar]
- 45. Seitz HK, Stickel F (2010) Acetaldehyde as an underestimated risk factor for cancer development: role of genetics in ethanol metabolism. Genes Nutr 5: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Role of ADH1B and ALDH2 in ethanol metabolism.
(TIF)
Allele and genotype frequencies for the studied SNPs.
(DOCX)