Abstract
Objective
To determine whether nonobese adolescents with polycystic ovary syndrome (PCOS) have higher levels of retinol-binding protein 4 (RBP4) and ectopic fat than controls and whether RBP4 and ectopic fat correlate with comorbidities of metabolic disease.
Design
Cross-sectional case-control study.
Setting
Pediatric clinical research center based in a quaternary care medical center.
Patient(s)
Twenty-four nonobese adolescents between the ages of 13 and 21 years, 13 with PCOS and 11 controls.
Intervention(s)
Measurement of RBP4, insulin resistance, lipids, and body composition.
Main Outcome Measure(s)
Retinol-binding protein 4, reproductive and adrenal hormones, insulin resistance, intrahepatic and intramyocellular lipid levels, and visceral adipose tissue.
Result(s)
Adolescents with PCOS had higher intrahepatic lipid content and a statistical trend for higher RBP4 compared with controls. Retinol-binding protein 4 correlated with body fat, triglycerides, insulin resistance, and androgens but not intrahepatic lipid content; however, when adjusted for body fat, the correlation between RBP4 and triglycerides weakened to a statistical trend and was no longer statistically significant for the other measures.
Conclusion(s)
This small preliminary study of nonobese adolescent girls suggests that RBP4 may be involved in the dyslipidemia associated with PCOS and that there may be an independent relationship between RBP4 and triglycerides but not between RBP4 and insulin resistance. Although intrahepatic lipid content was higher in PCOS, it did not correlate with RBP4, triglycerides, or insulin resistance.
Keywords: Polycystic ovary syndrome, retinol-binding protein 4, intrahepatic lipid, triglycerides, insulin resistance
Polycystic ovary syndrome (PCOS) is a common disorder that affects 1 of every 15 women worldwide; its diagnostic traits include hyperandrogenism, chronic anovulation, and polycystic ovaries (1). PCOS appears to be a form of the metabolic syndrome in women because of its large degree of overlap in symptomatology. Adolescents and women with PCOS are at increased risk for infertility, central obesity, insulin resistance, type 2 diabetes mellitus, dyslipidemia, hypertension, and cardiovascular disease (2).
Retinol-binding protein 4 (RBP4) is an adipokine secreted primarily by the liver and, to a lesser extent, adipose tissue. Retinol-binding protein 4 may be a useful marker that signals the presence of early insulin resistance and may, in fact, be involved in the pathogenesis of insulin resistance. An elevation in the level of circulating RBP4 indicates possible impairment in whole body insulin sensitivity and is associated with GLUT4 dysregulation (3). Retinol-binding protein 4 may also be a sign of hepatic insulin resistance and may herald the onset of the metabolic syndrome (4).
Mouse studies suggest that RBP4 may play a causative role in insulin resistance (5, 6). Clinical studies have yielded conflicting results regarding the relationship of RBP4 and insulin resistance. Several studies do demonstrate a relationship between RBP4 and insulin resistance (7, 8), whereas others show no apparent relationship (9). In addition, several studies have shown correlation between RBP4 and serum triglyceride levels (10–12).
Several pediatric studies, including one from our group, have examined RBP4 in children and adolescents (10–16); in addition, there are a number of studies that look at RBP4 in women with PCOS (17–22). In both pediatric and adult studies, RBP4 consistently correlated with body mass index (BMI), body fat, and triglycerides and inconsistently with insulin resistance. Among the adult PCOS studies, there is inconsistency in whether RBP4 levels are higher in patients with PCOS compared with controls. There are no studies of RBP4 in nonobese adolescents with PCOS.
Ectopic fat deposition in the muscle (intramyocellular lipid) (23), liver (intrahepatic lipid) (24), and omentum (visceral adipose tissue) (25) have all been purported to be involved in the pathogenesis of insulin resistance. Indeed, PCOS has been shown to be associated with hepatic steatosis (26, 27), and women with PCOS with nonalcoholic fatty liver disease had evidence of insulin resistance (26, 27). One study of healthy adults has noted that the level of intrahepatic lipid correlated with that of RBP4 (8). There are no studies, to our knowledge, that examine ectopic fat deposition in nonobese adolescents with PCOS.
The goal of this pilot study was to determine whether RBP4 may function as a marker for current metabolic disease in nonobese adolescents with PCOS. Our primary hypotheses were that nonobese adolescents with PCOS would have higher RBP4 and ectopic fat deposition than BMI z score and age-comparable control subjects. Our secondary hypotheses were that RBP4 and ectopic fat would correlate with insulin resistance and triglyceride levels.
MATERIALS AND METHODS
Subjects
The study group comprised 24 nonobese (BMI < 95th percentile) adolescents (13 with PCOS, 11 controls) (Table 1) (28). Subjects were recruited from the pediatric endocrinology and adolescent clinics of Children’s Hospital of New York, Columbia University Medical Center, and from posted flyers. Informed consent was obtained from subjects 18 years old and older and from a parent or guardian for subjects under age 18. Assent was obtained also from subjects under age 18. The protocol was approved by the Institutional Review Boards of Columbia University Medical Center and St. Luke’s-Roosevelt Hospital Center, New York, New York.
TABLE 1.
Clinical characteristics and hormonal profile of the study population (mean ± SD).
| Characteristic | PCOS (n = 13) | Control (n = 11) | P value |
|---|---|---|---|
| Age (years) | 16.4 ± 2.1 | 18.8 ± 2.36 | .02 |
| Weight (kg) | 56.6 ± 9.3 | 57.9 ± 8.3 | NS |
| Height (cm) | 158.9 ± 8.0 | 160.5 ± 8.5 | NS |
| BMI (kg/m2) | 22.4 ± 2.7 | 22.4 ± 2.5 | NS |
| BMI z score | 0.41 ± 0.82 | 0.33 ± 0.62 | NS |
| Age at menarche (years) | 12.3 ± 1.4 | 11.6 ± 1.0 | NS |
| Years since menarche | 3.6 ± 2.2 | 6.6 ± 2.3 | .003 |
| Ferriman-Gallwey score | 21.8 ± 6.5 | 8.4 ± 4.8 | < .0001 |
| RBP4 (mg/L) | 24.1 ± 3.6 | 21.8 ± 3.7 | .087a |
| E2 | 28.5 ± 33.5 | 27.5 ± 34.2 | NSa |
| T (ng/dL) | 37.7 ± 18.9 | 26.6 ± 9.8 | .11a |
| Free T (pg/mL) | 5.8 ± 4.2 | 2.7 ± 0.9 | .01a |
| SHBG (nmol/L) | 33.8 ± 23.7 | 45.4 ± 11.7 | .09a |
| DHEAS (µg/dL) | 178.7 ± 91.5 | 123.2 ± 55.3 | .01a |
| Androstenedione (ng/dL) | 194.7 ± 98.0 | 111.6 ± 38.1 | .004a |
| Free androgen index | 1.7 ± 1.3 | 0.6 ± 0.2 | .005a |
Adjusted for menarcheal age.
Sopher. Young nonobese PCOS: RBP4 & ectopic fat. Fertil Steril 2012.
The diagnosis of PCOS was made using the National Institutes of Health criteria (29). The inclusion criteria for PCOS were [1] oligomenorrhea or amenorrhea; [2] clinical or laboratory evidence of hyperandrogenemia; [3] no evidence of 21-hydroxylase deficiency, as documented by basal 17-hydroxyprogesterone levels <200 ng/dL, or of other hormonal, adrenal or gonadal disorders by hormonal analysis (30); [4] BMI less than the 95th percentile for age; and [5] menarche at least 1.5 years before enrollment. The inclusion criteria for controls were [1] eumenorrhea; [2] absence of significant hirsutism or acne; [3] no evidence of hormonal, adrenal, or gonadal disorder by hormonal analysis; [4] BMI less than the 95th percentile for age; and [5] menarche at least 1.5 years before enrollment. Exclusion criteria for all subjects included [1] gestational history complicated by maternal diabetes, multiple gestation, preterm delivery, or small for gestational age; [2] chronic medical conditions; or [3] gluco-corticoid or other hormonal therapy.
Pubertal and Clinical Assessment
Pubertal and clinical assessment of subjects was performed on the day of testing at the Pediatric Outpatient Unit of the Clinical Research Resource affiliated with the Irving Institute for Clinical and Translational Research at Columbia University Medical Center. Height, weight, heart rate, and blood pressure were measured; BMI and BMI z scores were calculated using reference data (31). Degree of hirsutism was assessed by use of the Ferriman-Gallwey hirsutism scale (32).
Procedures
Blood samples were drawn between 8 am and 9 am after an overnight fast for RBP4, glucose (G0), insulin (I0), DHEAS, androstenedione, total T, free T, LH, FSH, hemoglobin A1c, lipids, and thyroid function tests. Glucose and insulin levels were measured at 30, 60, 90, and 120 minutes after a 75 g oral glucose tolerance load (using Glutol; Paddock Laboratories, Minneapolis, MN). Retinol-binding protein 4 was collected in a serum separator tube.
Measures of Insulin Sensitivity and Insulin Secretion
Fasting glucose-insulin ratio (33), homeostasis model assessment (HOMA) (34), insulin sensitivity measure (35), whole-body insulin sensitivity index (36), insulin area under the curve (IAUC), and glucose area under the curve (GAUC) (37) were calculated as measures of insulin resistance.
Assays
Retinol-binding protein 4 was measured by ELISA (R&D Systems Quantikine, Minneapolis, MN). The RBP4 assay has a sensitivity of 0.053–0.628 ng/mL, an intra-assay coefficient of variation of 5.7%–8.1% and an inter-assay coefficient of variation of 5.8%–8.6%. The following were measured by Esoterix Laboratories (Calabasas Hills, CA): insulin by chemiluminometric assay; DHEAS by RIA after hydrolysis; E2, androstenedione, and total T by high-performance liquid chromatography with tandem mass spectrometry; free T by equilibrium dialysis, sex hormone–binding globulin by immunoradiometric assay, FSH and LH by electrochemiluminescent assay, hemoglobin A1c by affinity chromatography, and c-peptide by immunochemiluminescent assay. In the Core Laboratory at Columbia University Medical Center, plasma glucose was measured by the glucose hexokinase method and serum total cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides were measured by using Hitachi analyzer; low-density lipoprotein (LDL) cholesterol was calculated.
Body Composition
Truncal subcutaneous adipose tissue and visceral adipose tissue were measured using contiguous-slice magnetic resonance imaging of the thorax, abdomen, and pelvis (GE 6x Horizon 1.5 T). Intramyocellular lipid and intrahepatic lipid contents were measured using single-voxel 1H nuclear magnetic resonance spectroscopy (GE 6x Horizon 1.5 T) of the right tibialis anterior muscle and the liver, respectively. Trunk fat and percentage body fat were determined using whole-body dual-energy x-ray absorptiometry (GE Lunar Prodigy).
Statistical Analysis
Comparison of groups for continuous variables were achieved by Student’s t test for age, height, weight, BMI z-score, age at menarche and menarcheal age (years since menarche). Menarcheal age–adjusted analysis of covariance (ANCOVA) was used to compare RBP4, lipids, total T, free T, DHEAS, androstenedione, E2, Ferriman-Gallwey score, visceral adipose tissue, subcutaneous adipose tissue, intramyocellular lipid content, intrahepatic lipid content, trunk fat and percentage body fat, and total body fat mass–adjusted ANCOVA was used for blood pressure, lipids, oral glucose tolerance test, and derived insulin resistance measures. Results for continuous variables are presented as mean ± SD.
Pearson correlations were performed on the entire group (n = 24) and assessed the association between RBP4 and the following parameters: BMI z-score, systolic and diastolic blood pressure, lipids, total T, free T, DHEAS, androstenedione, E2, c-peptide, insulin and glucose measures from the oral glucose tolerance test, derived insulin resistance measures, visceral adipose tissue, subcutaneous adipose tissue, intramyocellular lipid content, intrahepatic lipid content, total body fat mass, trunk fat and percentage body fat. Pearson correlations were then adjusted for total body fat mass. P values less than .05 were considered to represent statistical significance for all analyses.
RESULTS
Clinical Characteristics
Height, weight, BMI, BMI z score, and age at menarche were similar in the PCOS and control groups (Table 1). Chronologic age and menarcheal age were statistically significantly higher in the control group (P<.030).
Hormonal Profile of the Study Population
In a menarcheal age adjusted analysis, there was a statistical trend for higher RBP4 in the PCOS group (P=.087) (Table 2). As expected, compared with controls, the PCOS group had higher Ferriman-Gallwey score (P<.0001), free T (P=.01), DHEAS (P=.01), androstenedione (P=.004), and free androgen index (P=.005), and a statistical trend for lower sex hormone–binding globulin (P=.090). There was no difference between the groups in total T or E2.
TABLE 2.
Cardiovascular profile, oral glucose tolerance test, and body composition in the study population (mean ± SD).
| Measure | PCOS (n = 13) | Control (n = 11) | P value |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 106.2 ± 10.8 | 103.4 ± 6.8 | NSa |
| Diastolic blood pressure (mmHg) | 63.4 ± 6.7 | 63.7 ± 9.8 | NSa |
| Total cholesterol (mg/dL) | 149.2 ± 24.1 | 165.3 ± 32.3 | NSa |
| Triglycerides (mg/dL) | 75.1 ± 20.8 | 55.0 ± 11.7 | .003a |
| HDL cholesterol (mg/dL) | 54.2 ± 10.9 | 47.7 ± 16.2 | NSa |
| LDL cholesterol (mg/dL) | 79.4 ± 25.6 | 101.6 ± 21.8 | .03a |
| IAUC (mIU/mL) | 104.9 ± 76.1 | 63.3 ± 53.8 | .07a |
| Whole-body insulin sensitivity index | 11.3 ± 16.2 | 19.0 ± 24.9 | NSa |
| HOMA | 1.6 ± 1.0 | 1.3 ± 0.9 | NSa |
| Insulin sensitivity measure | 16.0 ± 39.3 | 30.9 ± 56.2 | NSa |
| C-peptide (ng/mL) | 2.1 ± 0.4 | 1.8 ± 0.5 | .03a |
| Waist circumference (cm) | 74.0 ± 7.5 | 72.4 ± 6.8 | NSb |
| Lean mass (kg) | 34.4 ± 4.2 | 35.6 ± 3.6 | .077b |
| Total fat (kg) | 19.4 ± 6.8 | 19.4± 5.9 | NSb |
| Trunk fat (kg) | 9.1 ± 3.5 | 8.6 ± 3.4 | NSb |
| % Body fat | 35.3 ± 7.8 | 34.8 ± 6.5 | NSb |
| Intramyocellular lipid-water ratio | 0.046 ± 0.017 | 0.041 ± 0.023 | NSb |
| Intrahepatic lipid-water ratio | 0.075 ± 0.047 | 0.055 ± 0.043 | .04b |
| VAT (L) | 0.72 ± 0.29 | 0.67 ± 0.23 | NSb |
| SAT (L) | 6.4 ± 2.3 | 6.3 ± 2.0 | NSb |
Note: IAUC = insulin area under the curve; SAT = subcutaneous abdominal adipose tissue; VAT = visceral adipose tissue.
Adjusted for total body fat mass.
Adjusted for menarcheal age.
Sopher. Young nonobese PCOS: RBP4 & ectopic fat. Fertil Steril 2012.
Cardiovascular Profile, Oral Glucose Tolerance Test, and Insulin Resistance of the Study Population
When adjusted for total body fat mass (kg), there were no differences between the groups in systolic or diastolic blood pressure, total cholesterol, or HDL. The PCOS group had higher triglycerides (P=.003) and lower LDL (P=.03) (Table 2).
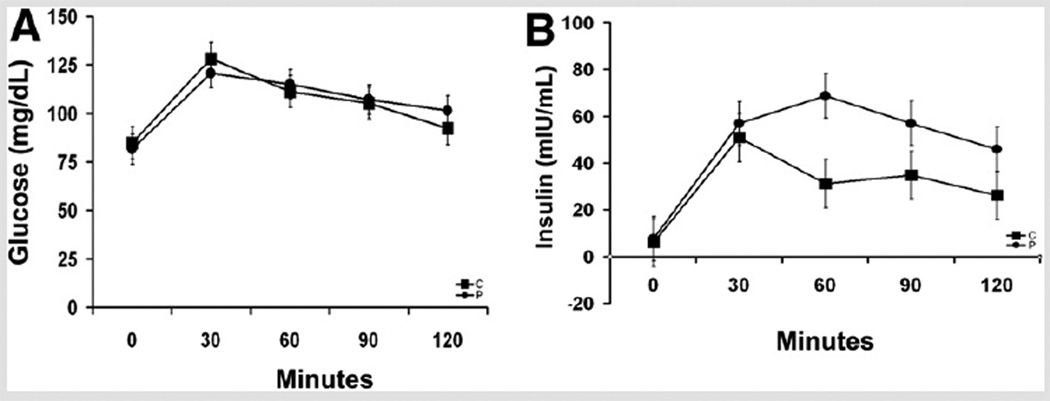
There were no subjects in the control or PCOS groups with impaired fasting glucose, and there were two PCOS subjects with impaired glucose tolerance. In the PCOS group, there was higher fasting c-peptide (P=.03) and a statistical trend for higher I60 (P=.05), I90 (P=.08), I120 (P=.06), and IAUC (P=.07) and a suggested trend for I0 (P=.14) (Fig. 1). There were no differences between the groups in HOMA, insulin sensitivity measure, whole-body insulin sensitivity index, or glucose area under the curve.
FIGURE 1.
Response to an oral glucose tolerance test. (A) Glucose response; there was no statistically significant difference in the glucose response between the nonobese adolescents with polycystic ovary syndrome (PCOS) and controls. (B) Insulin response; there was a statistical trend for higher insulin levels at 60, 90, and 120 minutes in the PCOS group. Analysis was adjusted for total body fat mass. Solid circle, PCOS; solid square, control.
Sopher. Young nonobese PCOS: RBP4 & ectopic fat. Fertil Steril 2012.
Body Composition
In a menarcheal age–adjusted analysis, the PCOS group had higher intrahepatic lipid content (P=.04) than the control group and a statistical trend for lower lean body mass (P=.08) (Table 2). There was no difference between the groups in waist circumference, lean body mass, total fat mass, trunk fat, percentage body fat, intramyocellular lipid content, visceral adipose tissue, or subcutaneous adipose tissue.
Pearson Correlation Analysis
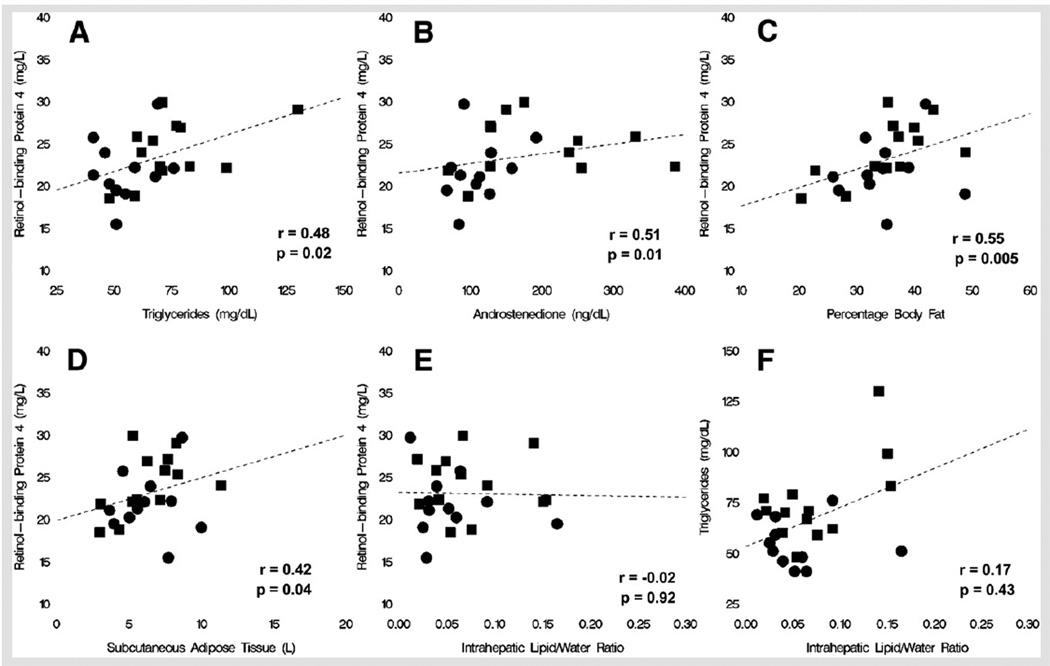
Retinol-binding protein 4 correlated with triglycerides (r = 0.48, P=.02), androstenedione (r = 0.51, P=.01), DHEAS (r = 0.41, P=.048), G90 (r = 0.41, P=.048), total fat (r = 0.47, P=.02), percentage body fat (r = 0.55, P=.005), and subcutaneous adipose tissue (r = 0.42, P=.04), and there was a negative statistical trend for correlation between RBP4 and sex hormone–binding globulin (r = −0.38, P=.064), I60 (r = 0.38, P=.064), and insulin sensitivity measure (r = −0.40, P=.054) (Fig. 2). Retinol-binding protein 4 did not correlate with intrahepatic lipid content. When adjusted for total body fat mass, the correlation of RBP4 with triglycerides weakened to a statistical trend (r = 0.40, P=.06) and was no longer statistically significant for the other parameters. Intrahepatic lipid correlated with LH (r = 0.43, P=.03) and androstenedione (r = 0.44, P=.03) and had a statistical trend for correlation with free T (r = 0.39, P=.06) and free androgen index (r = 0.36, P=.08).
FIGURE 2.
Regression lines representing relationships between metabolic parameters. (A) Retinol-binding protein 4 (RBP4) and triglycerides. (B) RBP4 and androstenedione. (C) RBP4 and percentage body fat. (D) RBP4 and subcutaneous adipose tissue. (E) Intrahepatic lipid-water ratio and triglycerides. (F) Intrahepatic lipid-water ratio and RBP4. Solid circle, PCOS; solid square, control.
Sopher. Young nonobese PCOS: RBP4 & ectopic fat. Fertil Steril 2012.
DISCUSSION
To our knowledge, this is the first study to examine RBP4 in a cohort of nonobese adolescents with PCOS, a group that is at high risk for development of the metabolic syndrome. In this small group, there was higher intrahepatic lipid and a trend for higher RBP4 in the PCOS group. In addition, RBP4 correlated with body fat, insulin resistance, androgens, and triglycerides; however, when adjusted for total body fat mass, the correlation with triglyceride weakened to a statistical trend and was no longer statistically significant for insulin resistance and androgens. Intrahepatic lipid content did not correlate with RBP4; however, it did correlate with LH and androstenedione.
Our findings of a statistical trend for higher RBP4 in the PCOS group are in accordance with several other studies on PCOS (3, 19, 22, 38, 39), suggesting that RBP4 may be involved either directly or indirectly in the pathogenesis of PCOS. In a small study, it was found that subcutaneous and omental adipocytes and adipose tissue depots expressed more RBP4 and less GLUT4 mRNA and protein in overweight women with PCOS than in obese controls (19). Estradiol, T, and insulin increased RBP4 secretion and decreased GLUT4 secretion in these tissues, although only the effect of E2 reached statistical significance (19). In another study, PCOS subjects with lower RBP4 had lower hirsutism scores and those with higher RBP4 had higher hirsutism scores. In addition, in this same study, RBP4 decreased in response to pioglitazone with a more pronounced reduction in those who started with a higher hirsutism score, but it did not correlate with parameters of glucose metabolism (40). These studies suggest that there could be interplay between RBP4 and steroid hormones that may contribute to the presentation of PCOS.
Congruent with our findings, many studies did not find an independent relationship between RBP4 and insulin resistance (3, 17–22). In one study in which RBP4 was higher in overweight women with PCOS than in controls, those with PCOS who were treated with metformin had improvement in insulin resistance compared with those treated with an oral contraceptive pill; however, RBP4 levels did not decline parallel to insulin resistance (21). Another study in adult women did demonstrate a relationship between RBP4 and HOMA that was statistically significant after adjustment for BMI and age; however, RBP4 explained only 4.6% of the variability in HOMA and, compared with HOMA, was less able to identify women with PCOS with impaired glucose tolerance or diabetes mellitus. These findings led the investigators to conclude that RBP4 has a small, but independent, impact on insulin resistance in PCOS (40). However, although many investigators agree that RBP4 does not seem to be a clinically useful marker in identifying insulin resistance in PCOS (3, 21, 41), one study of nonobese adults with PCOS showed a strong association between RBP4 and IR determined by an IV glucose tolerance test (39).
Our finding of a relationship between RBP4 and triglycerides has been shown in other studies as well (3, 17, 18, 21). This association suggests that RBP4 may be a marker of impaired hepatic fatty acid metabolism, or may be involved in the pathogenesis of dyslipidemia. One study of healthy adults demonstrated that RBP4 correlates strongly with intrahepatic lipid (8); however, this relationship was not demonstrated in our cohort. In addition, our data suggest that the relationship between RBP4 and triglycerides is at least partially driven by body fat.
This is the first report, to our knowledge, of higher intrahepatic lipid content in nonobese adolescents with PCOS than in controls. Our results support the finding of other investigators that nonalcoholic fatty liver disease may be more frequent in women with PCOS than controls (26, 42); however, in contrast with other studies, intrahepatic lipid content did not correlate with RBP4 or with triglycerides in our cohort (6, 43). The lack of correlation in our study may be explained by insufficient sample size or a PCOS population with differing degrees of insulin resistance, which may, in part, be due to the relatively young study population.
The heterogeneity of results in studies of RBP4 has been attributed to a variety of factors including different cohorts studied (pediatric vs. adult; obese vs. nonobese), differences in RBP4 assays used (ELISA vs. Western blot), and differences in the methodology used for the evaluation of insulin resistance (oral glucose tolerance test vs. IV glucose tolerance test vs. clamp studies) (21). In addition, there are different polymorphisms of RBP4, which may affect the relationship between RBP4 and insulin resistance (44).
Strengths of our study are a nonobese cohort, which minimizes the confounding effects of obesity, and the use of body composition technologies to assess body fat and ectopic fat. Limitations of our study include the small sample size, a PCOS group that was slightly younger than the control group, and an admixture of PCOS subjects who had insulin resistance and who did not have insulin resistance. Another limitation is the difficulty of diagnosis of PCOS in adolescents; thus, it is possible that subjects classified as PCOS ultimately may not develop PCOS and that control subjects eventually may develop PCOS (45). Other potential limitations include not measuring retinol or iron levels, both of which have relationships with RBP4 (44).
In summary, in this small study of nonobese adolescent girls with and without PCOS, there was significantly higher intrahepatic lipid content and a statistical trend for higher RBP4 in the PCOS group. In addition, our data suggest that there may be an independent relationship between RBP4 and triglycerides, implying a possible role of RBP4 in the pathogenesis of dyslipidemia. The findings in other studies of increased RBP4 expression in adipocytes of women with PCOS and the correlation between RBP4 and hirsutism add to the interest of RBP4 as a potential agent involved in the pathogenesis of PCOS. Future studies that involve larger cohorts, histologic and molecular analyses of the liver and adipose tissue, dividing PCOS groups to insulin resistant and insulin sensitive, longitudinal studies and interventional studies with insulin-sensitizing agents such as metformin will be invaluable in contributing to our understanding of the role of RBP4 in dyslipidemia and insulin resistance in PCOS.
Acknowledgments
Supported in part by a training grant in pediatric endocrinology (NIH/NIDDK T32 DK065522) from the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (S.E.O.); a grant (RR00645) from the General Clinical Research Center (A.T.G.); a Clinical and Translational Science Awards grant (1UL1RR024156) from the Irving Institute for Clinical and Translational Research (A.S.); a medical student research training program grant (3T32DK065522-06S1) from the NIH/NIDDK (A.T.G.); and by the Doris Duke Charitable Foundation (A.T.G.).
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources (NCRR) or the National Institutes of Health (NIH). Information on NCRR is available at the NCRR Website. Information on Reengineering the Clinical Research Enterprise can be obtained from the NIH Roadmap website.
Footnotes
A.B.S. has nothing to disclose. A.T.G. has nothing to disclose. W.S.B. has nothing to disclose. J.M.R. has nothing to disclose. D.J.M. has nothing to disclose. S.E.O. has nothing to disclose.
Presented at the annual meeting of the Pediatric Academic Societies, Denver, Colorado, 2011.
REFERENCES
- 1.Norman RJ, Dewilly D, Legro RS, Hickey TE. Polycystic ovarian syndrome. Lancet. 2008;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Dewilly D, Diamanti-Kandarakis E, Excobar-Morreal HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;912:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Chan T, Tsai Y, Chiu P, Chen Y, Lee C, Tsai E. Serum retinol-binding protein 4 levels in nonobese women with polycystic ovary syndrome. Fertil Steril. 2010;93:869–873. doi: 10.1016/j.fertnstert.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamori Y, Sakaue H, Kasuga M. RBP-4, an unexpected adipokine. Nat Med. 2006;12:30–31. doi: 10.1038/nm0106-30. [DOI] [PubMed] [Google Scholar]
- 6.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol-binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 7.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, et al. Retinol-binding protein 4 and insulin resistance in lean, obese and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 8.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Schleicher E, et al. High circulating retinol-binding protein 4 is associate with elevated liver fat but not with total, subcutaneous, visceral, or intramyocellular fat in humans. Diabetes Care. 2007;30:1173–1178. doi: 10.2337/dc06-2342. [DOI] [PubMed] [Google Scholar]
- 9.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumarad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat is linked with metabolic complications of obesity. Proc Natl Acad Sci. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aebreli I, Biebenger R, Lehmann R, L’Allemand D, Spinas GA, Zimmermann MB. Serum retinol-binding protein 4 concentration and its ratio to serum retinol are associated with obesity and metabolic syndrome components in children. J. Clin Endocrinol Metab. 2007;92:4359–4365. doi: 10.1210/jc.2007-0468. [DOI] [PubMed] [Google Scholar]
- 11.Lee D, Lee J, Im J. Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents. Metabolism. 2007;56:327–331. doi: 10.1016/j.metabol.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Sopher AB, Gerken AT, Lee E, Blaner WE, Deeds S, Gallagher D, et al. Retinol-binding protein 4 correlates with triglycerides but not insulin resistance in prepubertal children with and without premature adrenarche. J Pediatr Endocrinol Metab. 2011;24(9–10):683–687. doi: 10.1515/jpem.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baglopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin Endocrinol Metab. 2007;92:1971–1974. doi: 10.1210/jc.2006-2712. [DOI] [PubMed] [Google Scholar]
- 14.Goodman E, Graham TE, Dolan LM, Daniels SR, Goodman ER, Kahn BB. The relationship retinol binding protein 4 to changes in insulin resistance and cardiometabolic risk in overweight black adolescents. J Pediatr. 2008;154:67–73. doi: 10.1016/j.jpeds.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeste D, Vendrell J, Tomasini R, Gallart LL, Clemente M, Simon I, et al. Retinol-binding protein 4 levels in obese children and adolescents with glucose intolerance. Horm Res Paediatr. 2010;73:335–340. doi: 10.1159/000308165. [DOI] [PubMed] [Google Scholar]
- 16.Kanaka-Gantenbein C, Margeli A, Pervanidou P, Sakka S, Mastorakos G, Chrousos GP, et al. Retinol-binding protein 4 and lipocalin-2 in childhood and adolescent obesity: When children are not just “small adults.”. Clin Chem. 2008;54:1176–1182. doi: 10.1373/clinchem.2007.099002. [DOI] [PubMed] [Google Scholar]
- 17.Hahn S, Backhaus M, Broecker-Preuss M, Tan S, Dietz T, Kimmig R, et al. Retinol-binding protein 4 levels are elevated in polycystic ovary syndrome women with obesity and impaired glucose metabolism. Eur J Endocrinol. 2007;157:201–207. doi: 10.1530/EJE-07-0143. [DOI] [PubMed] [Google Scholar]
- 18.Barber TM, Hazel lM, Christodoulides C, Golding SJ, Alvey C, Burling K, et al. Serum levels of retinol-binding protein 4 and adiponectin in women with polycystic ovary syndrome: associations with visceral fat but no evidence for fat mass-independent effects on pathogenesis of this condition. J Clin Endocrinol Metab. 2008;93:2859–2865. doi: 10.1210/jc.2007-2759. [DOI] [PubMed] [Google Scholar]
- 19.Tan BK, Chen J, Lehnert H, Kennedy R, Randeva HS. Raised serum, adipocyte and adipose tissue retinol-binding protein 4 in overweight women with polycystic ovary syndrome: effects of gonadal and adrenal steroids. J Clin Endocrinol Metab. 2007;92:2764–2772. doi: 10.1210/jc.2007-0091. [DOI] [PubMed] [Google Scholar]
- 20.Yildiz BO, Bozdag G, Otegan U, Harmanci A, Boynukalin K, Vutal Z, et al. Visfatin and retinol-binding protein 4 concentrations in lean, glucose-tolerant women with PCOS. Reprod BioMed Online. 2010;20:150–155. doi: 10.1016/j.rbmo.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Hutchinson SK, Harrison C, Stepto N, Meyer C, Teede HJ. Retinol-binding protein 4 and insulin resistance in polycystic ovary syndrome. Diabetes Care. 2008;31:1427–1432. doi: 10.2337/dc07-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yildizhan R, Ilham GA, Yildizhan B, Kolusari A, ADali E, Bugdayci G. Serum retinol-binding protein 4, leptin and plasma asymmetric dimethylarginine levels in obese and nonobese young women with polycystic ovary syndrome. Fertil Steril. 2011;96:246–250. doi: 10.1016/j.fertnstert.2011.04.073. [DOI] [PubMed] [Google Scholar]
- 23.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 25.Despres J, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 26.Cerda C, Perez-Ayuso RM, Riquelme A, Soza A, Villaseca P, Sir-Petermann T, et al. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. J Hepatol. 2007;47:412–417. doi: 10.1016/j.jhep.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Gambarin-Gelwan M, Kinkhabwala S, Schiano TD, Bodian C, Yeh H, Futterweit W. Prevalence of nonalcoholic fatty liver disease in women with polycystic ovary syndr. Clin Gastroenterol Hepatol. 2007;5:496–501. doi: 10.1016/j.cgh.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 29.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic ovary syndrome. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 30.Armengaud J, Charkaluk M, Trivin C, Tardy V, Breat G, Brauner R, et al. Precocious pubarche: distinguishing late-onset congenital adrenal hyperplasia from premature adrenarche. J Clin Endocrinol Metab. 2009;94:2835–2840. doi: 10.1210/jc.2009-0314. [DOI] [PubMed] [Google Scholar]
- 31.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–27. [PubMed] [Google Scholar]
- 32.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 33.Silfen ME, Manibo AM, McMahon DJ, Levine LS, Murphy AR, Oberfield SE. Comparison of simple measures of insulin sensitivity in young girls with premature adrenarche: the fasting glucose to insulin ratio may be a simple and useful measure. J Clin Endocrinol Metab. 2001;86:2863–2868. doi: 10.1210/jcem.86.6.7537. [DOI] [PubMed] [Google Scholar]
- 34.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 35.Avignon A, Boegner C, Mariano-Goulart D, Colette C, Monnier L. Assessment of insulin sensitivity from plasma insulin and glucose in the fasting or post oral glucose-load state. Int J Obes Relat Metab Disord. 1999;23:512–517. doi: 10.1038/sj.ijo.0800864. [DOI] [PubMed] [Google Scholar]
- 36.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 37.Le Floch JP, Escuyer P, Baudin E, Baudon D, Perlemuter L. Blood glucose area under the curve. Methodological aspects. Diabetes Care. 1990;13:172–175. doi: 10.2337/diacare.13.2.172. [DOI] [PubMed] [Google Scholar]
- 38.Carmina E, Bucchieri S, Mansueto P, Rini G, Ferin M, Lobo RA. Circulating levels of adipose products and differences in fat distribution in the ovulatory and anovulatory phenotypes of polycystic ovary syndrome. Fertil Steril. 2009;91:1332–1335. doi: 10.1016/j.fertnstert.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Weiping L, Qingfeng C, Shikun M, Xiurong L, Hua Q, Xiaoshu B, et al. Elevated serum RBP4 is associated with insulin resistance in women with polycystic ovary syndrome. Endocrine. 2006;30:283–287. doi: 10.1007/s12020-006-0006-3. [DOI] [PubMed] [Google Scholar]
- 40.Aigner E, Bachofner N, Klein K, De Geyter C, Hohla F, Patsch W, et al. Retinol-binding protein 4 in polycystic ovary syndrome-association with steroid hormones and response to pioglitazone treatment. J Clin Endocrinol Metab. 2009;94:1229–1235. doi: 10.1210/jc.2008-2156. [DOI] [PubMed] [Google Scholar]
- 41.Mohlig M, Weickert MO, Ghadamgahi E, Arafat AM, Spranger J, Pfeiffer AFH, et al. Retinol-binding protein 4 is associated with insulin resistance but appears unsuited for metabolic screening in women with polycystic ovary syndrome. Eur J Endocrinol. 2008;158:517–523. doi: 10.1530/EJE-07-0833. [DOI] [PubMed] [Google Scholar]
- 42.Setji TL, Holland ND, Sanders LL, Pereira K, Diehl AM, Brown AJ. Nonalcoholic steatohepatitis and nonalcoholic fatty liver disease in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1741–1747. doi: 10.1210/jc.2005-2774. [DOI] [PubMed] [Google Scholar]
- 43.Romanowska A, Lebensztejn DM, Skiba E, Tarasow E, Kaczmarski M. Retinol binding protein-4, a serum biomarker of intrahepatic lipid content in obese children—a preliminary report. Acta Biochimica Polonica. 2011;58:35–38. [PubMed] [Google Scholar]
- 44.Kotnik P, Fischer-Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol. 2011;165:703–711. doi: 10.1530/EJE-11-0431. [DOI] [PubMed] [Google Scholar]
- 45.Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol. 2010;203:201.e1–201.e5. doi: 10.1016/j.ajog.2010.03.008. [DOI] [PubMed] [Google Scholar]