Abstract
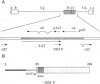
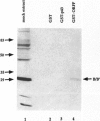
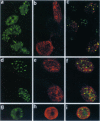
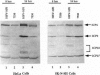
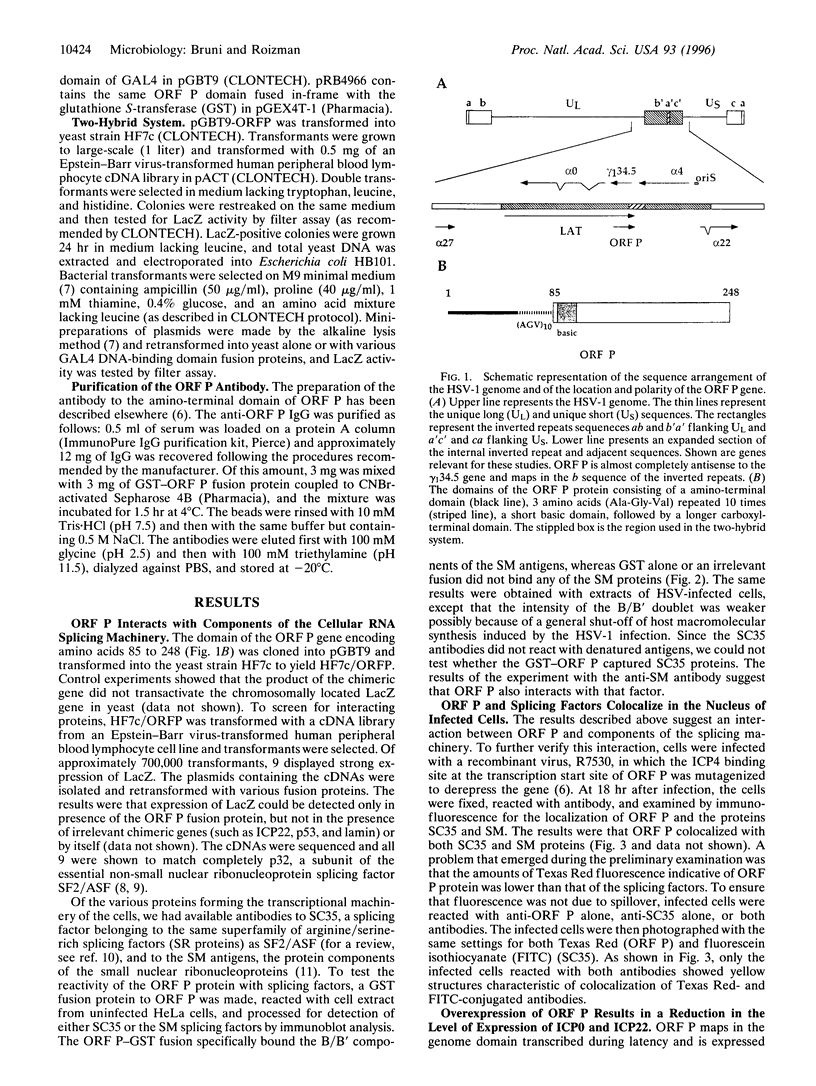
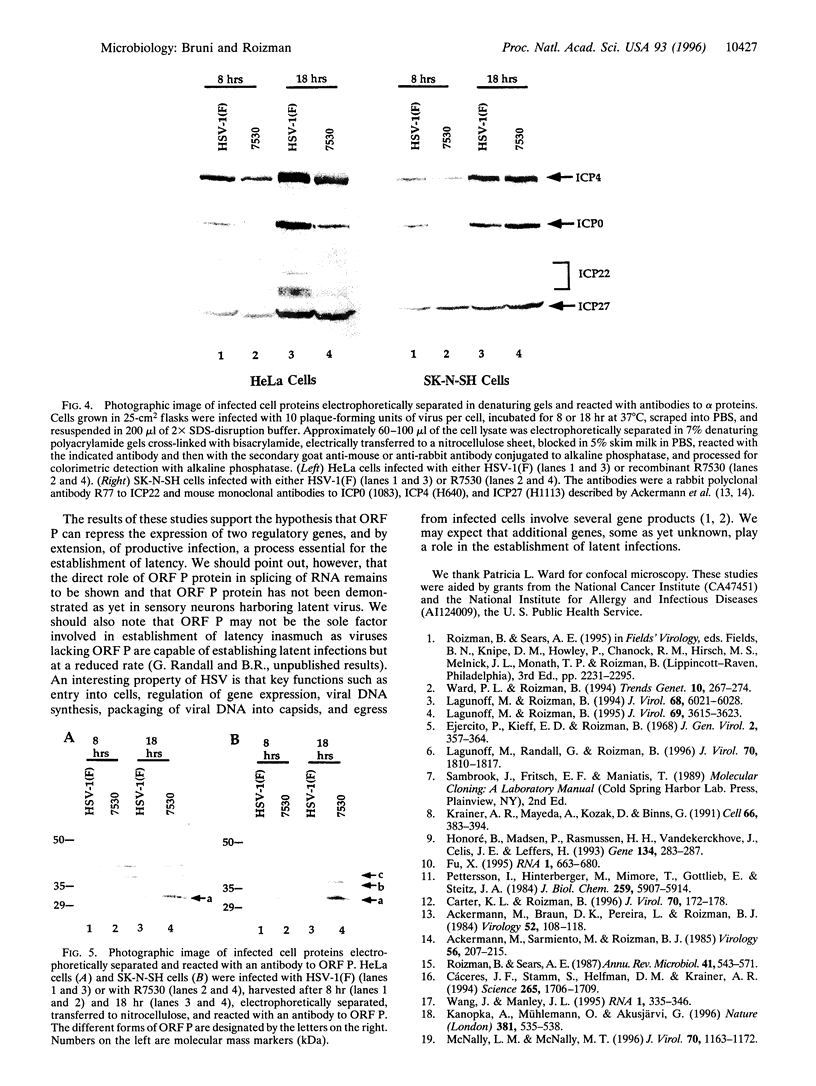
The open reading frame P (ORF P) is located in the domain and on the DNA strand of the herpes simplex virus 1 transcribed during latent infection. ORF P is not expressed in productively infected cells as a consequence of repression by the binding of the major viral regulatory protein to its high-affinity binding site. In cells infected with a mutant virus carrying a derepressed gene, ORF P protein is extensively posttranslationally processed. We report that ORF P interacts with a component of the splicing factor SF2/ASF, pulls down a component of the SM antigens, and colocalizes with splicing factors in nuclei of infected cells. The hypothesis that ORF P protein may act to regulate viral gene expression, particularly in situations such as latently infected sensory neurons in which the major regulatory protein is not expressed, is supported by the evidence that in cells infected with a mutant in which the ORF P gene was derepressed, the products of the regulatory genes alpha 0 and alpha 22 are reduced in amounts early in infection but recover late in infection. The proteins encoded by these genes are made from spliced mRNAs, and the extent of recovery of these proteins late in infection correlates with the extent of accumulation of post-translationally processed forms of ORF P protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Braun D. K., Pereira L., Roizman B. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J Virol. 1984 Oct;52(1):108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M., Sarmiento M., Roizman B. Application of antibody to synthetic peptides for characterization of the intact and truncated alpha 22 protein specified by herpes simplex virus 1 and the R325 alpha 22- deletion mutant. J Virol. 1985 Oct;56(1):207–215. doi: 10.1128/jvi.56.1.207-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. L., Roizman B. The promoter and transcriptional unit of a novel herpes simplex virus 1 alpha gene are contained in, and encode a protein in frame with, the open reading frame of the alpha 22 gene. J Virol. 1996 Jan;70(1):172–178. doi: 10.1128/jvi.70.1.172-178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres J. F., Stamm S., Helfman D. M., Krainer A. R. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science. 1994 Sep 16;265(5179):1706–1709. doi: 10.1126/science.8085156. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Fu X. D. The superfamily of arginine/serine-rich splicing factors. RNA. 1995 Sep;1(7):663–680. [PMC free article] [PubMed] [Google Scholar]
- Honoré B., Madsen P., Rasmussen H. H., Vandekerckhove J., Celis J. E., Leffers H. Cloning and expression of a cDNA covering the complete coding region of the P32 subunit of human pre-mRNA splicing factor SF2. Gene. 1993 Dec 8;134(2):283–287. doi: 10.1016/0378-1119(93)90108-f. [DOI] [PubMed] [Google Scholar]
- Kanopka A., Mühlemann O., Akusjärvi G. Inhibition by SR proteins of splicing of a regulated adenovirus pre-mRNA. Nature. 1996 Jun 6;381(6582):535–538. doi: 10.1038/381535a0. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Mayeda A., Kozak D., Binns G. Functional expression of cloned human splicing factor SF2: homology to RNA-binding proteins, U1 70K, and Drosophila splicing regulators. Cell. 1991 Jul 26;66(2):383–394. doi: 10.1016/0092-8674(91)90627-b. [DOI] [PubMed] [Google Scholar]
- Lagunoff M., Randall G., Roizman B. Phenotypic properties of herpes simplex virus 1 containing a derepressed open reading frame P gene. J Virol. 1996 Mar;70(3):1810–1817. doi: 10.1128/jvi.70.3.1810-1817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunoff M., Roizman B. Expression of a herpes simplex virus 1 open reading frame antisense to the gamma(1)34.5 gene and transcribed by an RNA 3' coterminal with the unspliced latency-associated transcript. J Virol. 1994 Sep;68(9):6021–6028. doi: 10.1128/jvi.68.9.6021-6028.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunoff M., Roizman B. The regulation of synthesis and properties of the protein product of open reading frame P of the herpes simplex virus 1 genome. J Virol. 1995 Jun;69(6):3615–3623. doi: 10.1128/jvi.69.6.3615-3623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally L. M., McNally M. T. SR protein splicing factors interact with the Rous sarcoma virus negative regulator of splicing element. J Virol. 1996 Feb;70(2):1163–1172. doi: 10.1128/jvi.70.2.1163-1172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson I., Hinterberger M., Mimori T., Gottlieb E., Steitz J. A. The structure of mammalian small nuclear ribonucleoproteins. Identification of multiple protein components reactive with anti-(U1)ribonucleoprotein and anti-Sm autoantibodies. J Biol Chem. 1984 May 10;259(9):5907–5914. [PubMed] [Google Scholar]
- Roizman B., Sears A. E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- Wang J., Manley J. L. Overexpression of the SR proteins ASF/SF2 and SC35 influences alternative splicing in vivo in diverse ways. RNA. 1995 May;1(3):335–346. [PMC free article] [PubMed] [Google Scholar]
- Ward P. L., Roizman B. Herpes simplex genes: the blueprint of a successful human pathogen. Trends Genet. 1994 Aug;10(8):267–274. doi: 10.1016/0168-9525(90)90009-u. [DOI] [PubMed] [Google Scholar]