Abstract
Intracranial EEG (icEEG) monitoring is critical in epilepsy surgical planning, but it has limitations. The advances of neuroimaging have made it possible to reveal epileptic abnormalities that could not be identified previously and improve the localization of the seizure focus and the vital cortex. A frequently asked question in the field is whether non-invasive neuroimaging could replace invasive icEEG or reduce the need for icEEG in presurgical evaluation. This review considers promising neuroimaging techniques in epilepsy presurgical assessment in order to address this question. In addition, due to large variations in the accuracies of neuroimaging across epilepsy centers, multicenter neuroimaging studies are reviewed, and there is much need for randomized controlled trials (RCTs) to better reveal the utility of presurgical neuroimaging. The results of multiple studies indicate that non-invasive neuroimaging could not replace invasive icEEG in surgical planning especially in non-lesional or extratemporal lobe epilepsies, but it could reduce the need for icEEG in certain cases. With technical advances, multimodal neuroimaging may play a greater role in presurgical evaluation to reduce the costs and risks of epilepsy surgery, and provide surgical options for more patients with drug-resistant epilepsy.
Keywords: Multimodal neuroimaging, Focus localization, Epilepsy surgery, Presurgical evaluation
Highlights
-
•
Promising neuroimaging in epilepsy presurgical evaluation is reviewed.
-
•
Frequently asked questions in the field are addressed.
-
•
Multicenter presurgical neuroimaging studies are also considered and reviewed.
-
•
Randomized controlled trials are needed to evaluate presurgical neuroimaging.
1. Introduction
Epilepsy affects approximately 50 million people worldwide (WHO, 2009). Around 30% of those with partial seizures are resistant to antiepileptic drugs and may need surgical treatment (Arroyo, 2000; Guerrini et al., 2003). Epilepsy surgery is aimed at removing the epileptogenic zone (seizure focus) as complete as possible while avoiding neurological deficits (Seeck et al., 2010), and surgical results in seizure control have reached 60% to 90% seizure-free outcome in patients with temporal lobe epilepsy (TLE) and 40% to 60% in extratemporal lobe epilepsy (ETLE) (Tellez-Zenteno et al., 2005).
Precise localization of the epileptogenic focus is a prerequisite for seizure-free outcome, but it remains a challenge, especially for non-lesional epilepsy (usually with negative or normal MRI) and ETLE. In TLE, around 30% of patients with intractable TLE have normal (or negative) MRI (Hammen and Kuzniecky, 2012). Patients with MRI-positive (i.e., a visible MRI lesion) TLE have good surgical outcomes (60–90% chance of seizure freedom), while patients with MRI-negative TLE have less favorable outcomes (40%–70%) (Brodbeck et al., 2010), and patients with MRI-negative ETLE have the worst outcome (40%–60%) (Tellez-Zenteno et al., 2010).
The main tasks in presurgical evaluation are to delineate the epileptogenic zone and localize the vital eloquent cortex, but the presurgical evaluation of patients with medically refractory epilepsy is often lengthy and expensive, and even non-localizing in a significant percentage of patients (Berg et al., 2003; Spencer, 1998; Thivard et al., 2006). At present, presurgical localization of the epileptogenic zone and functional cortex in non-lesional and extratemporal lobe epilepsies still relies largely on invasive intracranial electrodes. Compared with scalp EEG, intracranial electroencephalogram (icEEG) has high sensitivity and space specificity (Blount et al., 2008; Vulliemoz et al., 2011) and is the “gold standard” for delineating the epileptogenic zone (Blount et al., 2008). However, it is invasive, sample-limited, costly and risky with potential complications such as (subdural, epidural or intra-cerebral hematomas) bleeding and infections (Blount et al., 2008; Seeck et al, 2010).
Structural neuroimaging such as MRI and functional neuroimaging such as PET (Positron Emission Tomography) and SPECT (Single Photon Emission Computed Tomography) have become an essential part of presurgical evaluation in selecting patients and planning icEEG recordings (Whiting et al., 2006; Widjaja and Raybaud, 2008). Recent advances in neuroimaging such as MRI morphometry, DTI (Diffusion Tensor Imaging), MRS (Magnetic Resonance Spectroscopy)/MRSI (MRS Imaging), EEG–fMRI, EEG/ESI (Electronic Source Imaging) and MEG (Magnetoencephalography)/MSI (Magnetic Source Imaging) have improved patient selection and surgical decision making (Whiting et al., 2006).
However, can non-invasive neuroimaging replace invasive icEEG or reduce the need for icEEG in presurgical evaluation? This paper considered promising neuroimaging techniques in localizing the epileptogenic zone and the eloquent cortex, and reviewed multicenter studies that used neuroimaging techniques in presurgical evaluation. The paper was organized in four sections, and each section addressed one frequently-asked question in the field.
2. Can non-invasive neuroimaging techniques replace or reduce the need for invasive icEEG monitoring in focus localization?
2.1. Summary of previous findings
A comprehensive review by Whiting et al. found that ictal SPECT had the highest sensitivity (70–100%) and specificity (93–100%) in TLE and was most promising than other tests (interictal SPECT, PET, etc.) (Whiting et al., 2006). Earlier studies comparing MRI, SPECT and PET also showed that the highest diagnostic accuracy was achieved by ictal SPECT (90% in TLE and 81% in ETLE), followed by 84% for interictal PET vs. 71% for quantitative MR imaging and 66% for interictal SPECT (Spencer, 1994; Spencer et al., 1995). It has been reported that the overall sensitivity and specificity of interictal FDG-PET (Fig. 2B) and ictal SPECT are similar in large series of partial epilepsies (Engel et al., 2008). In addition, volumetric MRI and PET appear promising, while MRS (Magnetic Resonance Spectroscopy) and SISCOM (subtraction ictal single photon emission computed tomography co-registered to magnetic resonance imaging) are less promising at focus localization (Whiting et al., 2006). When MRI is normal, ictal SPECT and MRS have much lower focus localization precision than those when MRI is abnormal (ictal SPECT: 67% vs. 84%; MRS: 71% vs. 82%) in TLE (Doelken et al., 2007). Moreover, Chandra et al. (2006) demonstrated that PET and apparent diffusion coefficient (ADC) of DTI are more accurate than MRI and FA (Fractional Anisotropy) of DTI in identifying epileptogenic tubers in patients with tuberous sclerosis complex (TSC). Recent imaging techniques such as quantitative MRI, EEG–fMRI and MEG are also found helpful in capturing subtle lesions as possible neocortical or extratemporal foci.
Fig. 2.
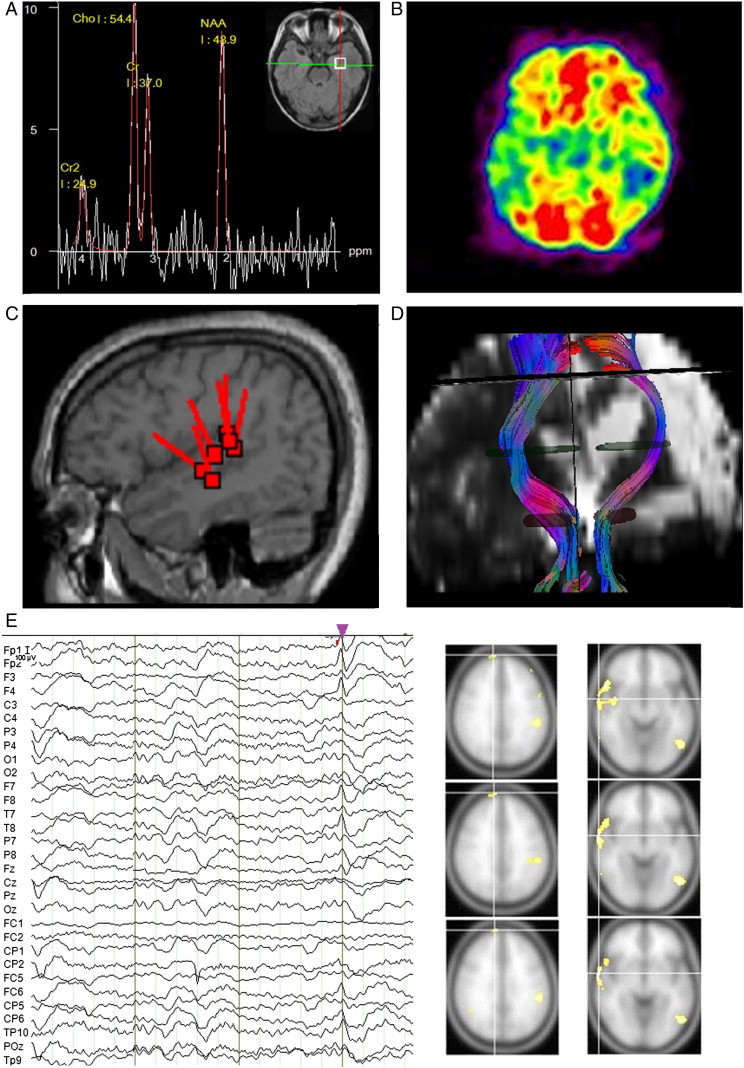
Multimodal neuroimaging (MRI, DTI, MEG, etc.) in source localization.
A. Presurgical 1H MR spectroscopy and MRI of a patient with TLE, who has hippocampal sclerosis in the left hippocampus (with decreased NAA in 1H MRS) and the seizure focus is in the left anterior temporal region.
B. FDG-PET scan of a patient with bilateral TLE. Hypometabolism is seen in the temporal lobes of both hemispheres with lower hypometabolism in the right temporal region. Other neuroimaging tests are needed to further localize the dominant seizure focus.
C. (De Tiègea et al., 2008; courtesy of Dr Xavier De Tiège; reprinted with permission from De Tiègea et al., 2008) Right posterior temporal sources detected by MEG found in a patient shown on the co-registered 3D-T1 MRI.
D. Fiber tracking from DTI scan: Corticospinal tract (CST) tracking to assess diffusion abnormalities in a patient before functional hemispherectomy.
E. Presurgical EEG–fMRI of a patient with bilateral TLE and ETLE, and EEG–fMRI identified seizure foci in both hemispheres with major right frontal and temporal sources.
2.2. Recent findings on MEG/MSI and possibility of replacing icEEG with neuroimaging
In recent years, studies have shown that the localization accuracy of MEG (Fig. 2C) might be closer to that of the “gold standard” icEEG (Knowlton et al., 2006, 2008a; Lau et al., 2008; Papanicolaou et al., 2005; Wu et al., 2006), although MEG is less available and requires more interictal epileptiform discharges (IEDs) (Knake et al., 2006; Knowlton et al., 1997; Pataraia et al., 2004; Stefan et al., 2003). A study with a large sample (n = 455) showed that the sensitivity of MEG/MSI was around 70% (72% in TLE, 67% in ETLE); while compared with surgical site (n = 131), MEG/MSI correctly localized seizure focus in 86% TLE and 89% ETLE cases (Stefan et al., 2003). Since MEG covers the whole head (e.g., cortices) while icEEG is sample-limited, MEG might be more advantageous in detecting the seizure focus than icEEG in patients with normal MRI. Papanicolaou et al. (2005) compared the localization accuracy of interictal MEG with ictal and interictal invasive video-EEG in identifying the epileptogenic zone in 41 epilepsy surgery candidates (29 TLE, 12 ETLE) and found that the overall localization accuracy was 54% of icEEG vs. 56% of MEG (in TLE, icEEG 55.2% vs. MEG 65.5%; in ETLE, icEEG 50.0% vs. MEG 33.3%). Thus, the authors concluded that MEG was statistically equivalent accurate to icEEG. Knowlton et al. prospectively evaluated the results of MSI and icEEG in 49 patients with partial epilepsy (most had normal or non-localizing MRI). They found that MEG/MSI could correctly localize the epileptogenic source at sublobar level in 65.3% (32/49) patients and icEEG in 69.4% (34/49), and the ratios of MEG vs. icEEG were nearly the same in TLE and ETLE (Knowlton et al., 2006). However, spikes were localized by icEEG (not by MEG) in 14.3% of the cases, and vice versa in 6.1% of the cases (Knowlton et al., 2006), indicating that icEEG could not be replaced in these cases.
The Ontario Health Technology Advisory Committee (OHTAC) reported that there was some “limited observational data (five studies, n = 190) to suggest that MEG may be as accurate as invasive EEG at localizing the seizure foci.” and called for a field evaluation to determine the potential substitutive role of MEG vs. icEEG (OHTAC, 2007). Knowlton et al. investigated presurgical neuroimaging (including 148-channel MEG/MSI, 18FDG-PET and ictal SPECT) in comparison to icEEG (Knowlton et al., 2008a,b). They examined 77 patients with normal MRI or ambiguous MRI abnormalities (39 TLE, 33 ETLE, 5 non-localized), and found that icEEG localized the seizure focus in 54 (70.1%) cases while MEG/MSI in 47 (61%) cases; icEEG indicated non-localized seizure onsets in 18 cases while MEG/MSI in 14; and icEEG did not capture seizures in 5 (6.5%) cases while MEG/MSI in 16 (20.8%) (Knowlton et al., 2008a). The results indicated that MEG/MSI could not replace icEEG in focus localization.
When MRI and/or ictal scalp EEG is not localizing, MEG/MSI can detect medial temporal spikes and it may provide important localizing information in patients with medial TLE (MTLE) (Kaiboriboon et al., 2010). Using 151-channel MEG, Agirre-Arrizubieta et al. (2009) found that 56% of all interictal icEEG spikes had an interictal MEG counterpart, the association between the two varied (≥90% in the interhemispheric and frontal orbital region; ~ 75% in the superior frontal, central and lateral temporal regions; ~ 25% in the mesial temporal region), and a large number of interictal icEEG spikes were not detected by MEG indicating that MEG cannot substitute for icEEG in localization of seizure onsets (Wennberg, 2006). Further, Knowlton et al. (2008a) demonstrated that MEG/MSI, PET and ictal SPECT alone or in combination could not replace icEEG. These findings showed that current neuroimaging, either single modality or combined multimodalities, could not be an alternative to icEEG in presurgical focus localization, especially in difficult epilepsy cases such as non-lesional or bilateral TLE or ETLE.
2.3. Limitations of icEEG as a “gold standard”
Nevertheless, the limitations of the icEEG as a “gold standard” in focus localization cannot be ignored. Even with widespread cortical coverage, sampling errors (due to limited-sampling) may occur (Duchowny, 2009) and sometimes, the electrodes have to be removed with non-localizing results (Wetjen et al., 2009). Because of such limitations, the diagnostic values of neuroimaging tests are lower than “true values” (Knowlton et al., 2008a). Knowlton reported that seven patients had non-localizing icEEG findings, underwent surgery anyway (based on neuroimaging findings), and became seizure-free (Knowlton et al., 2008a). Such false-negative icEEG cases demonstrated that icEEG may potentially be skipped in these cases, while neuroimaging findings could lead to correct surgical decisions and good surgical outcomes. Further, the time required for presurgical icEEG monitoring is long (from several days to several weeks depending on the need of seizure monitoring), while the time for neuroimaging is relatively short (from several minutes to several hours (e.g., MRI or PET/MEG)) which is advantageous.
2.4. Multimodal neuroimaging
On the other hand, it was found that MEG spatio-temporal analysis is more adequate in modeling frontotemporal spikes on icEEG than that of EEG (Tanaka et al., 2010), MEG/MSI has higher diagnostic accuracy than PET or ictal SPECT and diagnostic gain may be achieved by adding either PET or ictal SPECT to MEG/MSI (Knowlton et al., 2008a). There is an evolving consensus that the combined use of these imaging techniques improves the accuracy of focus localization (Barkley and Baumgartner, 2003; Fuchs et al., 1998; Knowlton et al., 2008a,b; Madan and Grant, 2009). In other words, a multimodal imaging approach could use concordant imaging findings to achieve better focus localization (Knake et al., 2006; Moeller et al., 2009; Stefan et al., 2003; Zijlmans et al., 2007).
If the findings of two modalities (e.g., MRI and EEG/ESI) are concordant or complementary, then the localization confidence is increased, but if they are discordant, another imaging modality (e.g., PET) or icEEG is needed. The concordance between quantitative MRI, PET and MRS is around 73% (Kuzniecky et al., 1998; Park et al, 2001) and the concordance between pathological diagnosis and MRI, PET, ictal SPECT and icEEG varies from 55% to 85% (Won et al., 1999). In addition, concordance rates of interictal PET and ictal SPECT for temporal lesions are 96% and 100% respectively and respective rates for extratemporal lesions were 68% and 92% (Kim et al., 2009). Moreover, MRS (Fig. 2A) markers such as unilateral decrease of NAA/Cr, NAA/Cho, or NAA/Cr + Cho ratios demonstrated good concordance with localization of the epileptogenic zone identified by EEG, MRI, and FDG-PET (Cendes et al., 1997a, 1997b; Guye et al., 2002; Hajek et al., 2009; Hammen et al., 2006; Kantarci et al., 2002; Maton et al., 2001; Meyer et al., 2001; Park et al., 2001; Someya et al., 2000). Since concordant imaging findings could achieve better focus localization, using multimodal neuroimaging might lead to fewer patients undergoing invasive icEEG (Health Net, 2010). Further, it was found that MEG/MSI, PET and ictal SPECT each have clinical value in predicting surgical outcome for patients with non-localized MRI or video-EEG, and MEG/MSI was close to ictal icEEG in predicting a good surgical outcome (Knowlton et al., 2008b).
2.5. Possibility of reducing the need for icEEG
Although neuroimaging could not replace icEEG in focus localization, a number of studies have reported that non-invasive neuroimaging tests could reduce the need for icEEG monitoring (Knowlton et al., 2006, 2008a,b; Papanicolaou et al., 2005; Thomas et al., 2002). In mesial temporal lobe epilepsy (MTLE), precise localization of the epileptogenic focus to a very small brain region with icEEG is not necessary (Tran et al., 1995; Zumsteg et al., 2006). Similarly, in epilepsies of clear structural abnormalities (such as hippocampal sclerosis or atrophy) detected by (high-resolution) MRI, plus concordant symptomatology of partial seizures and good lateralization of scalp EEG, icEEG is not necessary (Blount et al., 2008). However, the situations where invasive icEEG can be skipped are yet to be identified.
Recent advances in neuroimaging improve clinical yields and make it possible to capture formerly “non-lesional” subtle lesions and localize seizure focus in extratemporal lobe regions with improved accuracy. Structural neuroimaging such as high-resolution MRI, MRI morphometry, voxel-based intensity analysis and DTI increasingly revealed previously undetected “non-lesional” dysplastic lesions (Bernasconi et al., 2011; Chen et al., 2008; Guye et al., 2007; Rugg-Gunn et al., 2001; Thivard et al., 2006). For example, MRI voxel-based morphometry (VBM) analysis could detect focal cortical dysplasia (FCD) with high accuracy (sensitivity: 63–95%, specificity: 91–100%) (Bruggemann et al., 2007; Colliot et al., 2006a), while DTI (Fig. 2D) could capture “non-lesional” diffusion abnormalities (Guye et al., 2007; Thivard et al., 2006). These techniques have provided surgical options for more patients with drug-resistant epilepsy (Bernasconi et al., 2011). In functional neuroimaging, ictal SISCOM is found more reliable on the diagnosis of the epileptogenic focus than ictal SPECT, it has high concordance (92.5%) with the surgical site (n = 123) (Matsuda et al., 2009) and has remarkable predictive value for surgical outcome (La Fougère et al., 2009). In addition, the clinical yield of 128-channel EEG/ESI (EEG Source Imaging) (n = 32, 17 TLE, 15 ETLE) has reached high focus localization precision (93.7% [100% in TLE, 86.7% in ETLE] on the lobar level; and 79% in the resected area) (Michel et al., 2004). In non-lesional (normal MRI) epilepsy, EEG/ESI could correctly localize the epileptic focus in 80% of the patients (n = 10) (Brodbeck et al., 2010). Comparison between EEG and MEG showed that MEG findings correlated more with the surgical sites than those of EEG (72.3% vs. 40%) (Pataraia et al., 2004), and in patients who had non-localizing findings with EEG (n = 25), MEG identified the seizure focus (in the resection lobe) in 11 (44%) of them (Paulini et al., 2007). For details on high-density EEG/ESI in spike detection compared with MEG/MSI, see a review by Shibasaki et al. (2007). Moreover, a number of EEG–fMRI (Fig. 2E) validation studies compared EEG–fMRI findings with those of icEEG (Bagshaw et al., 2004; Bénar et al., 2006; Lazeyras et al., 2000; Moeller et al., 2009). Some showed that EEG–fMRI data were convincing (Groening et al., 2009; Grouiller et al., 2011; Thornton et al., 2010; Zhang et al., 2012; Zijlmans et al., 2007) and EEG–fMRI analysis could obtain a sensitivity comparable to PET and SPECT in focus localization (Hauf et al., 2012). MRS (Fig. 2A) is helpful in focus lateralization in non-lesional TLE, and NAA reduction in the affected hemisphere was found in 66–71% of patients with unilateral TLE, indicating that 1H MRS can provide valuable information for hemispheric lateralization and focus localization in such patients (Hammen and Kuzniecky, 2012; Hammen et al., 2006). Further, multimodal neuroimaging is needed not only in presurgical evaluation, but also in functional navigation in epilepsy surgery (Duncan, 2010; Kamada et al., 2003). As a result of advances in neuroimaging, invasive icEEG is used in ~ 25–40% of surgical cases in most large epilepsy centers (Faught and Blount, 2008).
2.6. Presurgical neuroimaging in clinical settings
In practice, a multimodal imaging approach for presurgical evaluation has been taken by various epilepsy centers and concordant neuroimaging findings often reduce the need for icEEG in presurgical planning. For example, in the protocol of drug-resistant epilepsy presurgical evaluation in Kyoto University Hospital (Fig. 1A) (Shibasaki et al., 2007), if the findings of non-invasive techniques such as long-term video-EEG, ESI (EEG Source Imaging), MRI, FDG-PET, ictal SPECT and/or MEG are convergent, then presurgical icEEG monitoring is unnecessary and surgical treatment with ECoG (electro-corticography) is performed; otherwise, presurgical icEEG monitoring is performed. Another example, in a presurgical evaluation protocol (Fig. 1B) adopted by seven epilepsy centers (Haut et al., 2002), if space occupying lesion is found on MRI and there are no conflicting EEG findings, then surgery (for TLE or ETLE) will be performed (without presurgical icEEG monitoring); otherwise, if findings from MRI, ictal or interictal EEG, PET and/or SPECT, etc. meet criteria for medial temporal lobe onset, then anterior temporal lobectomy will be performed (without presurgical icEEG monitoring); otherwise, invasive presurgical monitoring is required. It has been reported that non-invasive presurgical evaluation can be achieved in a safe and cost-effective manner in ~ 25–50% of patients with drug-resistant seizures, while ~ 50–75% presurgical evaluation remains invasive (Health Net, 2010).
Fig. 1.
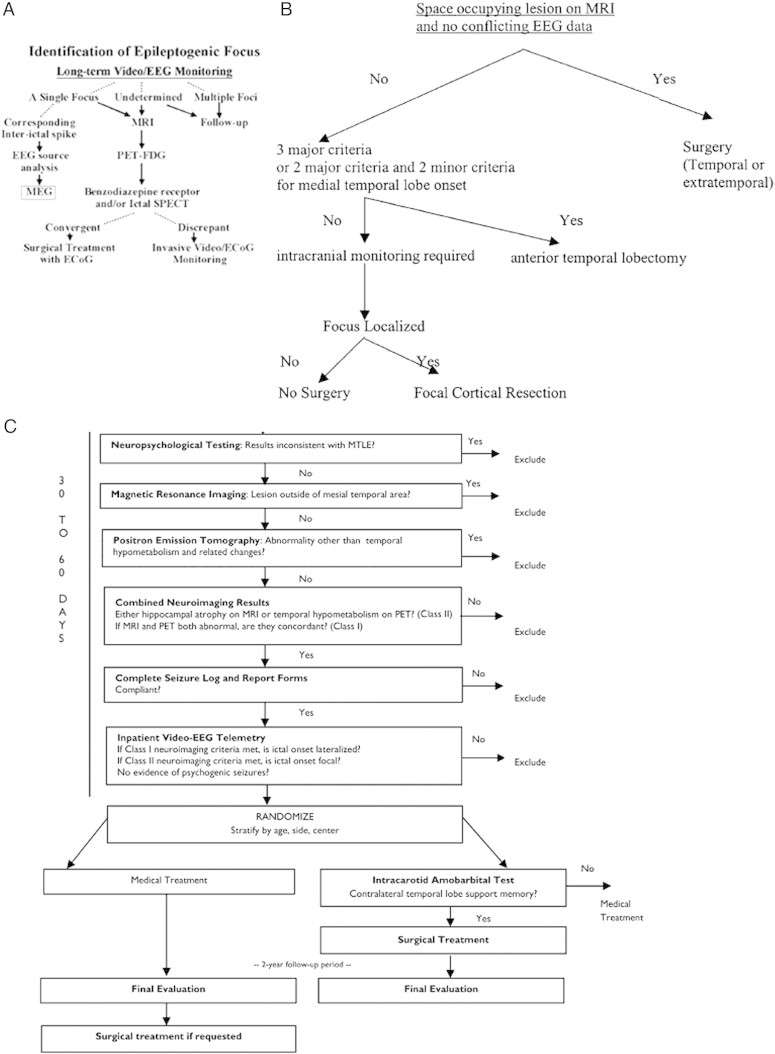
Flow charts indicating the decision making process in epilepsy presurgical evaluation.
A. Decision tree for source localization in Kyoto University Hospital (Shibasaki et al., 2007; courtesy of Dr. Shibasaki; reprinted with permission from Shibasaki et al., 2007). The branch after MEG analysis was in parallel with the main stream of evaluation (i.e., if the findings of EEG/ESI and MEG/MSI in focus localization are convergent, then perform surgery with ECoG; otherwise, perform invasive monitoring).
B. Decision tree for invasive monitoring across 7 epilepsy centers (Haut et al., 2002; courtesy of Dr. Haut; reprinted with permission from Haut et al., 2002).
Major and minor criteria supporting medial temporal lobe onset:
1. Major criteria:
(1) Interictal EEG: At least 70% of interictal discharges with a single anterior temporal field, in a sample of ≥ 50 discharges.
(2) Ictal EEG: Seizure with rhythmic theta or alpha discharge confined to one temporal lobe at least one third of seizures, with no conflicting data.
(3) MRI: Mesial temporal sclerosis.
2. Minor criteria:
(1) Interictal focal temporal EEG slowing present ≥ 50% of the time during wakefulness.
(2) PET: medial temporal hypometabolism (required if major criteria are 1 and 2).
(3) SPECT: temporal hypoperfusion.
(4) Wada test lateralized (percentage of items recalled after each injection differed by > 20%).
(5)Neuropsychological testing: medial temporal deficits present.
C. Multicenter ERSET protocol flow chart (Engel et al., 2010; courtesy of Dr. Engel; reprinted with permission from Engel et al., 2010).
2.7. Summary
Taken together, promising non-invasive neuroimaging such as MEG/MSI, PET and ictal SPECT alone or in combination so far still cannot replace invasive icEEG in localizing seizure focus especially in non-lesional epilepsy or ETLE, but neuroimaging could reduce the need for invasive presurgical monitoring in certain cases.
3. Can neuroimaging identify subtle lesions such as those in dual pathology which often causes surgical failure?
3.1. Identifying lesions in dual pathology
Dual pathology refers to the coexistence of mesial temporal sclerosis (MTS) and extrahippocampal lesion (Harroud et al., 2012). The lesions could be temporal or extratemporal, and the most common lesions are developmental abnormalities such as cortical dysplasia (Harroud et al., 2012; Vale et al., 2012). In epilepsy with dual pathology, the contribution of hippocampus to seizure generation corresponds to the degree of hippocampal pathology, while even mild cortical dysplasia could be epileptogenic (Fauser and Schulze-Bonhage, 2006). It has been estimated that around 90% patients with developmental abnormalities in the temporal lobe have concurrent atrophy of the mesial structures (Ho et al., 1998; Schwartz and Spencer, 2001), and dual pathology has been considered the main cause of surgical failures by some authors (Schwartz and Spencer, 2001; Spencer and Huh, 2008). A multi-center study by Li et al. reported that resection of both the lesion and the mesial temporal structures resulted in seizure-free outcome in 73% of the patients with dual pathology, while resection of the lesion or the mesial temporal structures alone resulted in seizure-free outcome in only 12.5%–20% of the patients (Li et al., 1999). Similar findings were obtained in another study by this group (Li et al., 1997).
Identification of the lesion in dual pathology is a challenge because mild cortical dysplasia can hardly be revealed by current neuroimaging techniques (Abosch et al., 2002; Hennessy et al., 2000). In addition, early signs of mesial temporal sclerosis such as mild hippocampal atrophy can be missed by MRI visual assessment. However, quantitative MRI is of value in identifying such subtle changes (Li et al., 1999).
Compared with the detection of MTS, identification of developmental lesions in dual pathology is more challenging. 87% of patients with focal cortical dysplasia (FCD) type I and 33% of those with FCD type II have unremarkable MRI, which indicated the limited power of conventional MRI to detect subtle dysplastic changes (Krsek et al., 2009). Quantitative MRI morphometry techniques such as VBM, voxel-based intensity analysis and sulcal morphometry have demonstrated increased sensitivity in FCD detection, compared with MRI visual inspection (Bruggemann et al., 2007; Colliot et al., 2006a). Each of these analyses has strengths and limitations, and the combination of such techniques could improve the detection of subtle dysplastic lesions overlapping with seizure focus undetected by MRI (Bernasconi, 2004; Bernasconi et al., 2011). Antel et al. developed an automated FCD detection system that combined multiple computational models of MRI characteristics and texture analysis, and the system detected 83% (15/18) of cases including 4 of 7 lesions that had eluded MRI assessment (Antel et al., 2003; Bernasconi, 2004). The system was further improved in FCD coverage (Colliot et al., 2006b), but it lacked the sensitivity in detecting subtle dysplastic lesions due to the absence of cortical topological information. Besson et al. used a surfaced-based texture approach (which preserves cortical topology) combined with morphometry analysis (which captures sulcal depth and curvature features of FCD), and identified 89% (17/19) of small, histologically proven FCDs undetected on MRI (Besson et al., 2008), while the specificity of such methods needed to be improved.
Since the lesions in dual pathology are usually mild and the abnormalities of lesional tissue are subtle, the detection power of MRI imaging with the most advanced quantitative techniques sometimes is not sufficient to detect such subtle changes (Abosch et al., 2002; Harroud et al., 2012). Focus localization could be very difficult when lesions or epileptic abnormalities are so subtle that they appear to be nonlesional and elude from quantitative MRI and other imaging modalities (PET, SPECT, etc.). In addition, multiple seizure foci may be in an epileptic network in the temporal or extra-temporal regions, and neuroimaging tests may fail to capture or distinguish epileptic discharges of the dominant seizure focus that initiates most seizures, ending up with contradictory or non-localizing imaging findings. In bilateral epilepsy, the hemisphere with the most abnormality detected by neuroimaging such as MRS might not be the side of seizure origin (Hammen and Kuzniecky, 2012). Failing to identify the focus of seizure origin might either exclude the surgical candidate from surgery, or result in resection error or incomplete resection (leaving the dominant seizure focus more or less intact) which causes poor outcome and surgical failure. Therefore, invasive icEEG is needed especially in these challenging cases to further detect epileptic abnormality and improve focus localization.
3.2. Summary
Taken together, a multimodality approach including neuroimaging and icEEG is needed to identify subtle lesions in presurgical evaluation which may enhance surgical outcome and reduce surgical failure.
4. Can neuroimaging replace invasive tests in localizing vital eloquent cortex?
4.1. Localizing vital eloquent cortex with neuroimaging
In order to protect neurological functions, mapping the motor, sensory and language areas and assessing the language-dominant hemisphere are the main tasks of localizing vital eloquent cortex in presurgical planning. Traditionally, these tasks are performed by invasive tests such as presurgical Wada test and intraoperative ECoG (Rutten and Ramsey, 2010). Intraoperative ECoG could identify motor, sensory and language areas, but it has limited field of view, requires an awake patient (via local anesthesia), and sometimes can only be performed in adolescents (Tripathi et al., 2010). Functional MRI (fMRI) is helpful in mapping motor, sensory and memory functions, and assessing the language-dominant hemisphere (Binder et al., 2002; Bizzi et al., 2008; Roessler et al., 2005; Rutten et al., 2010). Lateralization of the language function with fMRI is reliable and fMRI studies of language lateralization have demonstrated an 80–90% concordance with Wada test (Binder et al., 2002; Bizzi et al., 2008; Detre et al., 1998; Golby et al., 2002; Lehéricy et al., 2000; Pouratian et al., 2002; Rutten et al., 2002; Sabsevitz et al., 2003; Yetkin et al., 1998). It has been found that language lateralization with fMRI is as reliable as with Wada test and maybe more reliable than speech arrest following ECoG (Carpentier et al., 2001; Fernández et al., 2003), which supports that fMRI could be a non-invasive alternative to Wada test in language lateralization (Binder, 2011a). Further, presurgical fMRI is useful for predicting verbal memory decline after left anterior temporal lobectomy (Binder et al., 2008) and selective reminding test (i.e., word list learning and delayed recall) is often used as the memory measure (Binder et al., 2008; Binder, 2011; Binder et al., 2011). However, memory lateralization with fMRI is not clinically validated and fMRI is less promising in localizing language area (Rutten and Ramsey, 2010; Seeck et al., 2010), the accuracy is relatively low (sensitivity: 80%, specificity: 78%) (Bizzi et al., 2008), and a validated fMRI protocol is needed to map language area (Binder, 2011b). For details on mapping language area with fMRI, see reviews by Rutten and Ramsey (2010), and Binder (2011a).
When a patient has difficulty to undergo fMRI, MEG/MSI (or EEG/ESI) could be an alternative to mapping functional cortex in surgical planning (Bast et al., 2007; Seeck et al., 2010). High resolution MEG/MSI has been used to localize sensory and motor cortex. Studies have shown that MEG/MSI results in sensory and motor evoked fields are well correlated with those of ECoG (Ganslandt et al., 1999; Rezai et al., 1996). In addition, the use of MEG/MSI or EEG/ESI in language localization might be promising (Doss et al., 2009; Hirata et al., 2010; McDonald et al., 2009; Seeck et al., 2010). Further, EEG/ESI has been applied to localizing the somatosensory cortex and the results are comparable to those of MEG/MSI (Bast et al., 2007).
4.2. Summary
Taken together, although non-invasive neuroimaging such as fMRI, MEG/MSI and EEG/ESI still cannot replace invasive tests, they have reduced the need for ECoG in mapping the eloquent cortex, and fMRI might replace invasive Wada test in language lateralization.
5. How to assess the utility of presurgical neuroimaging more accurately?
One drawback of the neuroimaging validation studies (using invasive tests or the surgical site as reference standard) conducted by individual groups (or epilepsy centers) is the large variability across studies/centers. The varied accuracy (measured by sensitivity and specificity) of neuroimaging tests across studies may be due to a number of factors such as the different subject samples, the criteria and protocols of presurgical evaluation among centers, the imaging equipment and parameters used at each center, and pathological substrates of patients in these studies. Differences in pathological substrates such as mesial temporal sclerosis (MTS), developmental lesion and tumor may result in the wide range of sensitivity and specificity of neuroimaging modalities (Spencer et al., 1995). For example, PET and MRI are more sensitive to MTS than SPECT (100%, 95% vs. 70%), while PET, MRI and SPECT are equally sensitive to developmental lesions (88–92%), and MRI is most sensitive (96%) and SPECT least (82%) to tumors (Spencer et al., 1995). Further, the protocols of presurgical evaluation and criteria for focus localization using intracranial monitoring differ among centers, which may result in varied presurgical findings, surgical decisions and outcomes.
The true utility of presurgical neuroimaging often eludes from studies at individual centers due to such high variability. Thus, multicenter studies using consistent protocols and criteria for surgical planning have the advantage to reduce the variation across centers and reveal the true utility of neuroimaging in presurgical evaluation.
5.1. A review of multicenter studies
Pioneering work of multicenter research in this area first appeared one and a half decades ago. Silander et al. studied 152 patients in 3 epilepsy centers across Sweden, and found that non-invasive tools such as EEG, CT/MRI, and PET/SPECT localized the seizure focus in 85% of the young patients and 95% of the adult patients in presurgical evaluation (Silander et al., 1997).
In the early 2000s, Haut et al. conducted a 7-center study on the interrater reliability of presurgical testing and surgical decisions, and found that agreement was excellent for extracranial EEG lateralization (intraclass correlation coefficient: 0.80), MRI lateralization (0.95) and localization (0.91), Wada lateralization (0.95), icEEG localization (0.79), decision on whether to perform surgery (0.83); but the agreement on the decision to perform intracranial monitoring was poor (0.54) (Haut et al., 2002). In the same multicenter study, Berg et al. studied the localizing evidence of seizure focus and found that among the 565 surgical candidates, 34% underwent intracranial monitoring and 85% had surgery, while up to 30% of patients did not have surgery due to lack of clear localizing evidence (Berg et al., 2003). Spencer et al. (2003) further reported that among the 355 patients who underwent surgery, medial temporal resection significantly reduced seizures (77% 1-year remission) compared with neocortical resection (56% 1-year remission). These results have revealed a rough picture on the variability in the interpretation of neuroimaging in presurgical evaluation across centers and demonstrated the consensus on neuroimaging tests, surgical decisions, and overall outcomes across these centers.
In recent years, Zaknun et al. (2008) investigated 74 patients with TLE in a four-center study, and reported that the localization sensitivity for MRI was 86%, ictal SPECT 84% and ictal EEG/ESI 70%, and the seizure free outcome was 89%. They concluded that compared with MRI and EEG/ESI, ictal SPECT is an effective diagnostic modality for identifying seizure origin in TLE (Zaknun et al., 2008). In a more recent multicenter study, Matsuda et al. (2009) compared SISCOM with regular ictal SPECT and found that SISCOM provides higher predictive value of good surgical outcome and more reliability on the diagnosis of the epileptogenic focus than side-by-side comparison in medically intractable partial epilepsy. Further, multicenter clinical trials such as Early Randomized Surgical Epilepsy Trial (ERSET) (Engel et al., 2010) with rigorous presurgical protocols and criteria might be the best organized and most effective multicenter studies.
In addition to source localization, efforts have been made to localize the vital eloquent cortex in surgical planning across centers and proved the value of non-invasive imaging techniques as well as new protocols. For example, to map language cortex, Binder et al. (2011b) applied an fMRI (Story–Math task) protocol to seven centers, and found that the protocol provides a reliable method for activation of surgical regions of interest in the anterior TLE.
Taken together, multicenter studies have reduced the variability across epilepsy centers and revealed a more close-to-truth utility of neuroimaging in surgical planning.
5.2. The call for randomized controlled trials
Most comparative validation studies and multicenter studies assessing the diagnostic accuracy of presurgical neuroimaging tests are retrospective studies. Such retrospective studies have limitations in study design (e.g., limited statistical power to draw strong conclusions) and cannot provide much useful clinical information (Burch et al., 2012; Whiting et al., 2006). To assess the clinical utility of neuroimaging more accurately, it would be useful to randomize patients to groups using neuroimaging techniques and to other groups using invasive techniques. Whiting et al. (2006) realized such need and called for randomized controlled trials (RCTs). RCTs were regarded as the most reliable method to investigate the utility of neuroimaging in the workup for epilepsy surgery and it could examine single tests or combinations of tests on patient outcome. The efficacy of surgical treatment for drug-resistant TLE was demonstrated convincingly by a RCT in 2001 where 80 patients were randomly assigned to surgery (n = 40) and therapy of anti-epilepsy drugs (AED) (n = 40) and at one year, patients in the surgical group had significantly higher percentage (58% vs. 8%) of seizure-free outcome, fewer cognitive impairments and better quality of life than those in the AED group (Wiebe et al., 2001). Accordingly, Chernov et al. (2009) pointed out that carefully designed multi-center prospective trials can clarify the usefulness of neuroimaging in seizure investigation. Further, Okonma et al. (2011) proposed that multicenter RCTs are needed to incorporate technical advances for identifying the seizure focus and tissue at risk to identify the extent of epilepsy resection.
One key difference causing the variation of epilepsy surgical outcome across various epilepsy centers lies in the clinical decision-making process. The decision process of determining whether to perform invasive intracranial EEG monitoring varies among centers (Fig. 1A (Shibasaki et al., 2007) vs. Fig. 1B (Haut et al., 2002)), which inevitably affects the surgical outcome. Engel et al. (2010) have outlined the design considerations for ERSET, a multicenter RCT of early surgical intervention (Fig. 1C): the patients with TLE are randomized by center, age (12–16 or ≥ 17) and the side of ictal onset, and the randomization includes blocking within each stratum. The rigorous trial designs to assess surgical interventions in epilepsy provide evidence to guide treatment across 16 centers in the ERSET. To determine whether early surgical intervention could better control seizures, Engel et al. (2012) randomized 38 patients (who had seizures for no more than 2 years following trials of 2 AEDs) to medical (AED) group (n = 23) and surgical (anteromesial temporal resection) plus AED group (n = 15), and found that surgery plus AED treatment resulted in a higher percentage of seizure-free outcome (73.3% vs. 0%) and improved quality of life during the 2nd year follow-up than continued AED treatment alone. The preliminary results of the multicenter trial ERSET indicate that early surgical intervention is more effective in seizure control than continued AED treatment. Similar multicenter RCTs could be performed to reveal the efficacy and relative effectiveness of presurgical neuroimaging and icEEG.
5.3. Summary
Taken together, the clinical utility of neuroimaging could be assessed more accurately by multicenter studies and RCTs, which could standardize the decision-making process in presurgical evaluation and reveal the efficacy of neuroimaging and icEEG.
6. Conclusions
In summary, over the years, the advances of non-invasive neuroimaging techniques have provided promising tools for epilepsy surgical planning. Presurgical neuroimaging techniques such as MRI morphometry, DTI, fMRI, EEG/ESI, ictal SPECT/SISCOM and MEG/MSI have made it possible to capture previously undetected dysplastic lesions and other epileptic abnormalities, improve the localization of the epileptogenic zone and the eloquent cortex, and reduce the need for icEEG, but they still cannot replace icEEG in surgical planning. Thus, a multimodality approach including neuroimaging and invasive icEEG is needed (e.g., to identify subtle abnormalities) in presurgical evaluation especially in non-lesional and extratemporal lobe epilepsies. In addition, in localization or lateralization of vital eloquent cortex, fMRI may be a non-invasive alternative to Wada test in language lateralization. Further, due to large variability across epilepsy centers and study design problems, multicenter studies and RCTs are needed to reveal the true value of presurgical neuroimaging. With technical advances (e.g., higher resolution), neuroimaging may play a greater role in presurgical evaluation, reduce the costs and risks of epilepsy surgery and provide surgical options for more patients with drug-resistant epilepsy.
Acknowledgements
This work was partially supported by the Natural Science Foundation of China (Grant No. 81071211).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Abosch A., Bernasconi N., Boling W. Factors predictive of suboptimal seizure control following selective amygdalohippocampectomy. J. Neurosurg. 2002;97(5):1142–1151. doi: 10.3171/jns.2002.97.5.1142. [DOI] [PubMed] [Google Scholar]
- Agirre-Arrizubieta Z., Huiskamp G.J., Ferrier C.H. Interictal magnetoencephalography and the irritative zone in the electrocorticogram. Brain. 2009;132:3060–3071. doi: 10.1093/brain/awp137. [DOI] [PubMed] [Google Scholar]
- Antel S.B. Automated detection of focal cortical dysplasia lesions using computational models of their MRI characteristics and texture analysis. Neuroimage. 2003;19:1748–1759. doi: 10.1016/s1053-8119(03)00226-x. [DOI] [PubMed] [Google Scholar]
- Arroyo S. Evaluation of drug-resistant epilepsy. Rev. Neurol. 2000;30:881–886. [PubMed] [Google Scholar]
- Bagshaw A.P., Aghakhani Y., Bénar C.G., Kobayashi E., Hawco C., Dubeau F., Pike G.B., Gotman J. EEG–fMRI of focal epileptic spikes: analysis with multiple haemodynamic functions and comparison with gadolimium-enhanced MR angiograms. Hum. Brain Mapp. 2004;22:179–192. doi: 10.1002/hbm.20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley G.L., Baumgartner C. MEG and EEG in epilepsy. J. Clin. Neurophysiol. 2003;20:163–178. doi: 10.1097/00004691-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Bast T., Wright T., Boor R. Combined EEG and MEG analysis of early somatosensory evoked activity in children and adolescents with focal epilepsies. Clin. Neurophysiol. 2007;118:1721–1735. doi: 10.1016/j.clinph.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Bénar C.G., Grova C., Kobayashi E., Bagshaw A.P., Aghakhani Y., Dubeau F., Gotman J. EEG–fMRI of epileptic spikes: concordance with EEG source localization and intracranial EEG. Neuroimage. 2006;30:1161–1170. doi: 10.1016/j.neuroimage.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Berg A.T., Vickrey B.G., Langfitt J.T., Sperling M.R., Walczak T.S., Shinnar S., Bazil C.W., Pacia S.V., Spencer S.S. The multicenter study of epilepsy surgery: recruitment and selection for surgery. Epilepsia. 2003;44:1425–1433. doi: 10.1046/j.1528-1157.2003.24203.x. [DOI] [PubMed] [Google Scholar]
- Bernasconi A. Quantitative MR, imaging of the neocortex. Neuroimaging Clin. N. Am. 2004;14:425–436. doi: 10.1016/j.nic.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Bernasconi A., Bernasconi N., Bernhardt B.C., Schrader D. Advances in MRI for ‘cryptogenic’ epilepsies. Nat. Rev. Neurol. 2011;7:99–108. doi: 10.1038/nrneurol.2010.199. [DOI] [PubMed] [Google Scholar]
- Besson P., Bernasconi N., Colliot O., Evans A., Bernasconi A. Surface-based texture and morphological analysis detects subtle cortical dysplasia. Med. Image Comput. Comput. Assist Interv. 2008;11:645–652. doi: 10.1007/978-3-540-85988-8_77. [DOI] [PubMed] [Google Scholar]
- Binder J.R. Functional MRI is a valid noninvasive alternative to Wada testing. Epilepsy Behav. 2011;20:214–222. doi: 10.1016/j.yebeh.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Detre J.A., Jones-Gotman M., Swanson S.J. Functional MRI of episodic memory in temporal lobe epilepsy. Epilepsia. 2002;43:2. [Google Scholar]
- Binder J.R., Sabsevitz D.S., Swanson S.J., Hammeke T.A., Raghavan M., Mueller W.M. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008;49:1377–1394. doi: 10.1111/j.1528-1167.2008.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Gross W.L., Allendorfer J.B., Bonilha L., Chapin J., Edwards J.C., Grabowski T.J., Langfitt J.T., Loring D.W., Lowe M.J., Koenig K., Morgan P.S., Ojemann J.G., Rorden C., Szaflarski J.P., Tivarus M.E., Weaver K.E. Mapping anterior temporal lobe language areas with fMRI: a multicenter normative study. Neuroimage. 2011;54:1465–1475. doi: 10.1016/j.neuroimage.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi A., Blasi V., Falini A., Ferroli P., Cadioli M., Danesi U., Aquino D., Marras C., Caldiroli D., Broggi G. Presurgical functional MR imaging of language and motor functions: validation with intraoperative electrocortical mapping. Radiology. 2008;248:579–589. doi: 10.1148/radiol.2482071214. [DOI] [PubMed] [Google Scholar]
- Blount J.P., Cormier J., Kim H., Kankirawatana P., Riley K.O., Knowlton R.C. Advances in intracranial monitoring. Neurosurg. Focus. 2008;25:E18. doi: 10.3171/FOC/2008/25/9/E18. [DOI] [PubMed] [Google Scholar]
- Brodbeck V., Spinelli L., Lascano A.M., Pollo C., Schaller K., Vargas M.I., Wissmeyer M., Michel C.M., Seeck M. Electrical source imaging for presurgical focus localization in epilepsy patients with normal MRI. Epilepsia. 2010;51:583–591. doi: 10.1111/j.1528-1167.2010.02521.x. [DOI] [PubMed] [Google Scholar]
- Bruggemann J.M., Wilke M., Som S.S., Bye A.M., Bleasel A., Lawson J.A. Voxel-based morphometry in the detection of dysplasia and neoplasia in childhood epilepsy: combined grey/white matter analysis augments detection. Epilepsy Res. 2007;77:93–101. doi: 10.1016/j.eplepsyres.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Burch J., Marson A., Beyer F., Soares M., Hinde S., Wieshmann U., Woolacott N. Dilemmas in the interpretation of diagnostic accuracy studies on presurgical workup for epilepsy surgery. Epilepsia. 2012;53:1294–1302. doi: 10.1111/j.1528-1167.2012.03534.x. [DOI] [PubMed] [Google Scholar]
- Carpentier A., Pugh K.R., Westerveld M., Studholme C., Skrinjar O., Thompson J.L., Spencer D.D., Constable R.T. Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia. 2001;42:1241–1254. doi: 10.1046/j.1528-1157.2001.35500.x. [DOI] [PubMed] [Google Scholar]
- Cendes F., Andermann F., Dubeau F., Matthews P.M., Arnold D.L. Normalization of neuronal metabolic dysfunction after surgery for temporal lobe epilepsy: evidence from proton MR spectroscopic imaging. Neurology. 1997;49:1525–1533. doi: 10.1212/wnl.49.6.1525. [DOI] [PubMed] [Google Scholar]
- Cendes F., Caramanos Z., Andermann F., Dubeau F., Arnold D.L. Proton magnetic resonance spectroscopic imaging and magnetic resonance imaging volumetry in the lateralization of temporal lobe epilepsy: a series of 100 patients. Ann. Neurol. 1997;42:737–746. doi: 10.1002/ana.410420510. [DOI] [PubMed] [Google Scholar]
- Chandra P.S., Salamon N., Huang J., Wu J.Y., Koh S., Vinters H.V., Mathern G.W. FDG-PET/MRI coregistration and diffusion-tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: a preliminary report. Epilepsia. 2006;47:1543–1549. doi: 10.1111/j.1528-1167.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- Chen Q., Lui S., Li C.X., Jiang L.J., Ou-Yang L., Tang H.H., Shang H.F., Huang X.Q., Gong Q.Y., Zhou D. MRI-negative refractory partial epilepsy: role for diffusion tensor imaging in high field MRI. Epilepsy Res. 2008;80:83–89. doi: 10.1016/j.eplepsyres.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Chernov M.F., Ochiaib Taku, Onoc Yuko, Muragakib Yoshihiro, Yamanee Fumitaka, Tairab Takaomi, Maruyamab Takashi, Tanakab Masahiko, Isekia Hiroshi, Kubob Osami, Okadab Yoshikazu, Horib Tomokatsu, Takakura Kintomo. Role of proton magnetic resonance spectroscopy in preoperative evaluation of patients with mesial temporal lobe epilepsy. J. Neurol. Sci. 2009;285:212–219. doi: 10.1016/j.jns.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Colliot O., Bernasconi N., Khalili N., Antel S.B., Naessens V., Bernasconi A. Individual voxel-based analysis of gray matter in focal cortical dysplasia. Neuroimage. 2006;29:162–171. doi: 10.1016/j.neuroimage.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Colliot O. Segmentation of focal cortical dysplasia lesions on MRI using level set evolution. Neuroimage. 2006;32:1621–1630. doi: 10.1016/j.neuroimage.2006.04.225. [DOI] [PubMed] [Google Scholar]
- De Tiègea X., Op de Beecka M., Funkeb M., Legrosc B., Parkkonend L., Goldmana S., Van Bogaerta P. Recording epileptic activity with MEG in a light-weight magnetic shield. Epilepsy Res. 2008;82:227–231. doi: 10.1016/j.eplepsyres.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Detre J.A., Maccotta L., King D., Alsop D.C., Glosser G., D'Esposito M., Zarahn E., Aguirre G.K., French J.A. Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology. 1998;50:926–932. doi: 10.1212/wnl.50.4.926. [DOI] [PubMed] [Google Scholar]
- Doelken M.T., Richter G., Stefan H., Doerfler A., Noemayr A., Kuwert T., Ganslandt O., Nimsky C.H., Hammen T. Multimodal coregistration in patients with temporal lobe epilepsy—results of different imaging modalities in lateralization of the affected hemisphere in MR imaging positive and negative subgroups. Am. J. Neuroradiol. 2007;28:449–454. [PMC free article] [PubMed] [Google Scholar]
- Doss R.C., Zhang W., Risse G.L., Dickens D.L. Lateralizing language with magnetic source imaging: validation based on the Wada test. Epilepsia. 2009;50:2242–2248. doi: 10.1111/j.1528-1167.2009.02242.x. [DOI] [PubMed] [Google Scholar]
- Duchowny M. Clinical, functional, and neurophysiologic assessment of dysplastic cortical networks: implications for cortical functioning and surgical management. Epilepsia. 2009;50(Suppl. 9):19–27. doi: 10.1111/j.1528-1167.2009.02291.x. [DOI] [PubMed] [Google Scholar]
- Duncan J.S. Imaging the surgical treatment of epilepsy. Nat. Rev. Neurol. 2010;6:537–550. doi: 10.1038/nrneurol.2010.131. [DOI] [PubMed] [Google Scholar]
- Engel J., Pedley T.A., Engel J. Jr., editors. Epilepsy: A Comprehensive Textbook. vol. 1. Lippincott-Raven; Philadelphia, New York: 2008. pp. 517–524. [Google Scholar]
- Engel J., Jr., McDermott M.P., Wiebe S., Langfitt J.T., Erba G., Gardiner I., Stern J., Dewar S., Sperling M.R., Jacobs M., Kieburtz K., Early Randomized Surgical Epilepsy Trial (ERSET) Study Group Design considerations for a multicenter randomized controlled trial of early surgery for mesial temporal lobe epilepsy. Epilepsia. 2010;51:1978–1986. doi: 10.1111/j.1528-1167.2010.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., McDermott M.P., Wiebe S., Langfitt J.T., Stern J.M., Dewar S., Sperling M.R., Gardiner I., Erba G., Fried I., Jacobs M., Vinters H.V., Mintzer S., Kieburtz K. Early surgical therapy for drug-resistant temporal lobe epilepsy, a randomized trial. JAMA. 2012;307(9):922–930. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faught E., Blount J. Clinical neurophysiology III: intracranial electrodes. In: Wheless J., Willmore L.J., Brumback R.A., editors. Advanced Epilepsy. BC Decker; Hamilton, Ontario: 2008. [Google Scholar]
- Fauser S., Schulze-Bonhage A. Epileptogenicity of cortical dysplasia in temporal lobe dual pathology: an electrophysiological study with invasive recordings. Brain. 2006;129:82–95. doi: 10.1093/brain/awh687. [DOI] [PubMed] [Google Scholar]
- Fernández G., Specht K., Weis S., Tendolkar I., Reuber M., Fell J., Klaver P., Ruhlmann J., Reul J., Elger C.E. Intrasubject reproducibility of presurgical language lateralization and mapping using fMRI. Neurology. 2003;60:969–975. doi: 10.1212/01.wnl.0000049934.34209.2e. [DOI] [PubMed] [Google Scholar]
- Fuchs M., Wagner M., Wischmann H.A., Köhler T., Theissen A., Drenckhahn R., Buchner H. Improving source reconstructions by combining bioelectric and biomagnetic data. Electroencephalogr. Clin. Neurophysiol. 1998;107:93–111. doi: 10.1016/s0013-4694(98)00046-7. [DOI] [PubMed] [Google Scholar]
- Ganslandt O., Fahlbusch R., Nimsky C., Kober H., Möller M., Steinmeier R., Romstöck J., Vieth J. Functional neuronavigation with magnetoencephalography: outcome in 50 patients with lesions around the motor cortex. J. Neurosurg. 1999;91:73–79. doi: 10.3171/jns.1999.91.1.0073. [DOI] [PubMed] [Google Scholar]
- Golby A.J., Poldrack R.A., Illes J., Chen D., Desmond J.E., Gabrieli J.D. Memory lateralization in medial temporal lobe epilepsy assessed by functional MRI. Epilepsia. 2002;43:855–863. doi: 10.1046/j.1528-1157.2002.20501.x. [DOI] [PubMed] [Google Scholar]
- Groening K., Brodbeck V., Moeller F., Wolff S., van Baalen A., Michel C.M., Jansen O., Boor R., Wiegand G., Stephani U., Siniatchkin M. Combination of EEG–fMRI and EEG source analysis improves interpretation of spike-associated activation networks in pediatric pharmacoresistant focal epilepsies. NeuroImage. 2009;46:827–833. doi: 10.1016/j.neuroimage.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Grouiller F., Thornton R.C., Groening K., Spinelli L., Duncan J.S., Schaller K., Siniatchkin M., Lemieux L., Seeck M., Michel C.M., Vulliemoz Serge. With or without spikes: localization of focal epileptic activity by simultaneous electroencephalography and functional magnetic resonance imaging. Brain. 2011;134:2868–2886. doi: 10.1093/brain/awr156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini R., Sicca F., Parmeggiani L. Epilepsy and malformations of the cerebral cortex. Epileptic. Disord. 2003;5:S9–S26. [PubMed] [Google Scholar]
- Guye M., Le Fur Y., Confort-Gouny S., Ranjeva J.P., Bartolomei F., Regis J. Metabolic and electrophysiological alterations in subtypes of temporal lobe epilepsy: a combined proton magnetic resonance spectroscopic imaging and depth electrodes study. Epilepsia. 2002;43:1197–1209. doi: 10.1046/j.1528-1157.2002.05102.x. [DOI] [PubMed] [Google Scholar]
- Guye M., Ranjeva J.P., Bartolomei F., Confort-Gouny S., McGonigal A., Régis J. What is the significance of interictal water diffusion changes in frontal lobe epilepsies? Neuroimage. 2007;35:28–37. doi: 10.1016/j.neuroimage.2006.11.049. [DOI] [PubMed] [Google Scholar]
- Hajek M., Krsek P., Dezortova M., Marusic P., Zamecnik J., Kyncl M., Tomasek M., Krijtova H., Komarek V. 1H MR spectroscopy in histopathological subgroups of mesial temporal lobe epilepsy. Eur. Radiol. 2009;19:400–408. doi: 10.1007/s00330-008-1156-x. [DOI] [PubMed] [Google Scholar]
- Hammen T., Kuzniecky R. Magnetic resonance spectroscopy in epilepsy. In: Stefan H., Theodore W.H., editors. Handbook of Clinical Neurology. Vol. 107 (3rd series); 2012. pp. 399–408. (Epilepsy, Part I). [DOI] [PubMed] [Google Scholar]
- Hammen T., Kerling F., Schwarz M. Identifying the affected hemisphere by (1)H-MR spectroscopy in patients with temporal lobe epilepsy and no pathological findings in high resolution MRI. Eur. J. Neurol. 2006;13:482–490. doi: 10.1111/j.1468-1331.2006.01293.x. [DOI] [PubMed] [Google Scholar]
- Harroud A., Bouthillier A., Weil A.G., Nguyen D.K. Temporal lobe epilepsy surgery failures: a review. Epilepsy Res. Treat. 2012:10. doi: 10.1155/2012/201651. ( http://www.hindawi.com/journals/ert/2012/201651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf M., Scheidegger O., Rummel C., Rahman A., Wiest R. Recent developments of simultaneous EEG/fMRI in epilepsy—towards clinical application. Epileptologie. 2012;29:90–98. [Google Scholar]
- Haut S.R., Berg A.T., Shinnar S., Cohen H.W., Bazil C.W., Sperling M.R., Langfitt J.T., Pacia S.V., Walczak T.S., Spencer S.S. Interrater reliability among epilepsy centers: multicenter study of epilepsy surgery. Epilepsia. 2002;43:1396–1401. doi: 10.1046/j.1528-1157.2002.20902.x. [DOI] [PubMed] [Google Scholar]
- Health Net Inc. 2010. National Medical Policy (NMP 328) on MEG MSI. [Google Scholar]
- Hennessy M.J., Elwes R.D., Binnie C.D., Polkey C.E. Failed surgery for epilepsy: a study of persistence and recurrence of seizures following temporal resection. Brain. 2000;123(12):2445–2466. doi: 10.1093/brain/123.12.2445. [DOI] [PubMed] [Google Scholar]
- Hirata M., Goto T., Barnes G., Umekawa Y., Yanagisawa T., Kato A., Oshino S., Kishima H., Hashimoto N., Saitoh Y., Tani N., Yorifuji S., Yoshimine T. Language dominance and mapping based on neuromagnetic oscillatory changes: comparison with invasive procedures. J. Neurosurg. 2010;112:528–538. doi: 10.3171/2009.7.JNS09239. [DOI] [PubMed] [Google Scholar]
- Ho S.S., Kuzniecky R.I., Gilliam F., Faught E., Morawetz R. Temporal lobe developmental malformations and epilepsy: dual pathology and bilateral hippocampal abnormalities. Neurology. 1998;50(3):748–754. doi: 10.1212/wnl.50.3.748. [DOI] [PubMed] [Google Scholar]
- Kaiboriboon K., Nagarajan S., Mantle M. Interictal MEG/MSI in intractable mesial temporal lobe epilepsy: spike yield and characterization. Clin. Neurophysiol. 2010;121:325–331. doi: 10.1016/j.clinph.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada K., Houkin K., Takeuchi F., Ishii N., Ikeda J., Sawamura Y., Kuriki S., Kawaguchi H., Iwasaki Y. Visualization of the eloquent motor system by integration of MEG, functional, and anisotropic diffusion-weighted MRI in functional neuronavigation. Surg. Neurol. 2003;59:352–361. doi: 10.1016/s0090-3019(03)00018-1. [DOI] [PubMed] [Google Scholar]
- Kantarci K., Shin C., Britton J.W., So E.L., Cascino G.D., Jack C.R., Jr. Comparative diagnostic utility of 1H MRS and DWI in evaluation of temporal lobe epilepsy. Neurology. 2002;58:1745–1753. doi: 10.1212/wnl.58.12.1745. [DOI] [PubMed] [Google Scholar]
- Kim J.T., Bai S.J., Choi K.O. Comparison of various imaging modalities in localization of epileptogenic lesion using epilepsy surgery outcome in pediatric patients. Seizure. 2009;18:504–510. doi: 10.1016/j.seizure.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Knake S., Halgren E., Shiraishi H., Hara K., Hamer H.M., Grant P.E., Carr V.A., Foxe D., Camposano S., Busa E., Witzel T., Hämäläinen M.S., Ahlfors S.P., Bromfield E.B., Black P.M., Bourgeois B.F., Cole A.J., Cosgrove G.R., Dworetzky B.A., Madsen J.R., Larsson P.G., Schomer D.L., Thiele E.A., Dale A.M., Rosen B.R., Stufflebeam S.M. The value of multichannel MEG and EEG in the presurgical evaluation of 70 epilepsy patients. Epilepsy Res. 2006;69:80–86. doi: 10.1016/j.eplepsyres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Knowlton R.C., Laxer K.D., Aminoff M.J., Roberts T.P., Wong S.T., Rowley H.A. Magnetoencephalography in partial epilepsy: clinical yield and localization accuracy. Ann. Neurol. 1997;42:622–631. doi: 10.1002/ana.410420413. [DOI] [PubMed] [Google Scholar]
- Knowlton R.C., Elgavish R., Howell J., Blount J., Burneo J.G., Faught E., Kankirawatana P., Riley K., Morawetz R., Worthington J., Kuzniecky R.I. Magnetic source imaging versus intracranial electroencephalogram in epilepsy surgery: a prospective study. Ann. Neurol. 2006;59:835–842. doi: 10.1002/ana.20857. [DOI] [PubMed] [Google Scholar]
- Knowlton R.C., Elgavish R.A., Limdi N., Bartolucci A., Ojha B., Blount J., Burneo J.G., Ver Hoef L., Paige L., Faught E., Kankirawatana R., Riley K., Kuzniecky R. Functional imaging: I. Relative predictive value of intracranial electroencephalography. Ann. Neurol. 2008;64:25–34. doi: 10.1002/ana.21389. [DOI] [PubMed] [Google Scholar]
- Knowlton R.C., Elgavish R.A., Bartolucci A., Ojha B., Limdi N., Blount J., Burneo J.G., Ver Hoef L., Paige L., Faught E., Kankirawatana P., Riley K., Kuzniecky R. Functional Imaging: II. Prediction of epilepsy surgery outcome. Ann. Neurol. 2008;64:35–41. doi: 10.1002/ana.21419. [DOI] [PubMed] [Google Scholar]
- Krsek P. Incomplete resection of focal cortical dysplasia is the main predictor of poor postsurgical outcome. Neurology. 2009;72:217–223. doi: 10.1212/01.wnl.0000334365.22854.d3. [DOI] [PubMed] [Google Scholar]
- Kuzniecky R., Hugg J.W., Hetherington H., Butterworth E., Bilir E., Faught E., Gilliam F. Proton magnetic resonance spectroscopy in MRI-negative temporal lobe epilepsy. Neurology. 1998;51:66–71. doi: 10.1212/wnl.51.1.66. [DOI] [PubMed] [Google Scholar]
- La Fougère C., Rominger A., Förster S., Geisler J., Bartenstein P. PET and SPECT in epilepsy: a critical review. Epilepsy Behav. 2009;15:50–55. doi: 10.1016/j.yebeh.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Lau M., Yam D., Burneo J.G. A systematic review on MEG and its use in the presurgical evaluation of localization-related epilepsy. Epilepsy Res. 2008;79:97–104. doi: 10.1016/j.eplepsyres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Lazeyras F., Blanke O., Perrig S., Zimine I., Golay X., Delavelle J., Michel C.M., de Tribolet N., Villemure J.G., Seeck M. EEG-triggered functional MRI in patients with pharmacoresistant epilepsy. J. Magn. Reson. Imaging. 2000;12:177–185. doi: 10.1002/1522-2586(200007)12:1<177::aid-jmri20>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lehéricy S., Cohen L., Bazin B., Samson S., Giacomini E., Rougetet R. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- Li L.M., Cendes F., Watson C. Surgical treatment of patients with single and dual pathology: relevance of lesion and of hippocampal atrophy to seizure outcome. Neurology. 1997;48(2):437–444. doi: 10.1212/wnl.48.2.437. [DOI] [PubMed] [Google Scholar]
- Li L.M., Cendes F., Andermann F. Surgical outcome in patients with epilepsy and dual pathology. Brain. 1999;122(5):799–805. doi: 10.1093/brain/122.5.799. [DOI] [PubMed] [Google Scholar]
- Madan N., Grant P.E. New directions in clinical imaging of cortical dysplasias. Epilepsia. 2009;50:9–18. doi: 10.1111/j.1528-1167.2009.02292.x. [DOI] [PubMed] [Google Scholar]
- Maton B., Gilliam F., Sawrie S., Faught E., Hugg J., Kuzniecky R. Correlation of scalp EEG and 1H-MRS metabolic abnormalities in temporal lobe epilepsy. Epilepsia. 2001;42:417–422. doi: 10.1046/j.1528-1157.2001.25999.x. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Matsuda K., Nakamura F., Kameyama S., Masuda H., Otsuki T. Contribution of subtraction ictal SPECT coregistered to MRI to epilepsy surgery: a multicenter study. Ann. Nucl. Med. 2009;23:283–291. doi: 10.1007/s12149-009-0236-6. [DOI] [PubMed] [Google Scholar]
- McDonald C.R., Thesen T., Hagler D.J., Jr., Carlson C., Devinksy O., Kuzniecky R., Barr W., Gharapetian L., Trongnetrpunya A., Dale A.M., Halgren E. Distributed source modeling of language with magnetoencephalography: application to patients with intractable epilepsy. Epilepsia. 2009;50:2256–2266. doi: 10.1111/j.1528-1167.2009.02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P.T., Cortes-Blanco A., Pourdehnad M., Levy-Reis I., Desiderio L., Jang S. Inter-modality comparisons of seizure focus lateralization in complex partial seizures. Eur. J. Nucl. Med. 2001;28:1529–1540. doi: 10.1007/s002590100602. [DOI] [PubMed] [Google Scholar]
- Michel C.M., Lantz G., Spinelli L., De Peralta R.G., Landis T., Seeck M. 128-Channel EEG source imaging in epilepsy: clinical yield and localization precision. J. Clin. Neurophysiol. 2004;21:71–83. doi: 10.1097/00004691-200403000-00001. [DOI] [PubMed] [Google Scholar]
- Moeller F., Tyvaert L., Nguyen D.K., LeVan P., Bouthillier A., Kobayashi E., Tampieri D., Dubeau F., Gotman J. EEG–fMRI: adding to standard evaluations of patients with nonlesional frontal lobe epilepsy. Neurology. 2009;73:2023–2030. doi: 10.1212/WNL.0b013e3181c55d17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHTAC (The Ontario Health Technology Advisory Committee) 2007. OHTAC Recommendation Functional Brain Imaging. [Google Scholar]
- Okonma S.V., Blount J.P., Gross R.E. Planning extent of resection in epilepsy: limited versus large resections. Epilepsy Behav. 2011;20:233–240. doi: 10.1016/j.yebeh.2010.09.036. [DOI] [PubMed] [Google Scholar]
- Papanicolaou A.C., Pataraia E., Billingsley-Marchall R., Castillo E.M., Wheless J.W., Swank P. Toward the substitution of invasive electroencephalography in epilepsy surgery. J. Clin. Neurophysiol. 2005;22:231–237. doi: 10.1097/01.wnp.0000172255.62072.e8. [DOI] [PubMed] [Google Scholar]
- Park S.W., Chang K.H., Kim H.D., Song I.C., Lee D.S., Lee S.K. Lateralizing ability of single-voxel proton MR spectroscopy in hippocampal sclerosis: comparison with MR imaging and positron emission tomography. AJNR Am. J. Neuroradiol. 2001;22:625–631. [PMC free article] [PubMed] [Google Scholar]
- Pataraia E., Simos P.G., Castillo E.M., Billingsley R.L., Sarkari S., Wheless J.W., Maggio V., Maggio W., Baumgartner J.E., Swank P.R., Breier J.I., Papanicolaou A.C. Does magnetoencephalography add to scalp video-EEG as a diagnostic tool in epilepsy surgery? Neurology. 2004;62:943–948. doi: 10.1212/01.wnl.0000115122.81621.fe. [DOI] [PubMed] [Google Scholar]
- Paulini A., Fischer M., Rampp S. Lobar localization information in epilepsy patients: MEG—a useful tool in routine presurgical diagnosis. Epilepsy Res. 2007;76:124–130. doi: 10.1016/j.eplepsyres.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Pouratian N., Bookheimer S.Y., Rex D.E., Martin N.A., Toga A.W. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. J. Neurosurg. 2002;97:21–32. doi: 10.3171/jns.2002.97.1.0021. [DOI] [PubMed] [Google Scholar]
- Rezai A.R., Hund M., Kronberg E., Zonenshayn M., Cappell J., Ribary U., Kall B., Llinás R., Kelly P.J. The interactive use of magnetoencephalography in stereotactic image-guided neurosurgery. Neurosurgery. 1996;39:92–102. doi: 10.1097/00006123-199607000-00018. [DOI] [PubMed] [Google Scholar]
- Roessler K., Donat M., Lanzenberger R., Novak K., Geissler A., Gartus A., Tahamtan A.R., Milakara D., Czech T., Barth M., Knosp E., Beisteiner R. Evaluation of preoperative high magnetic field motor functional MRI (3 Tesla) in glioma patients by navigated electrocortical stimulation and postoperative outcome. J. Neurol. Neurosurg. Psychiatry. 2005;76:1152–1157. doi: 10.1136/jnnp.2004.050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg-Gunn F.J., Eriksson S.H., Symms M.R., Barker G.J., Duncan J.S. Diffusion tensor imaging of cryptogenic and acquired partial epilepsies. Brain. 2001;124:627–636. doi: 10.1093/brain/124.3.627. [DOI] [PubMed] [Google Scholar]
- Rutten G.J., Ramsey N.F. The role of functional magnetic resonance imaging in brain surgery. Neurosurg. Focus. 2010;28:E4. doi: 10.3171/2009.12.FOCUS09251. [DOI] [PubMed] [Google Scholar]
- Rutten G.J., Ramsey N.F., van Rijen P.C., Noordmans H.J., van Veelen W.M. Development of a functional magnetic resonance imaging protocol for intraoperative localization of critical temporoparietal language areas. Ann. Neurol. 2002;51:350–360. doi: 10.1002/ana.10117. [DOI] [PubMed] [Google Scholar]
- Sabsevitz D.S., Swanson S.J., Hammeke T.A. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- Schwartz T.H., Spencer D.D. Strategies for reoperation after comprehensive epilepsy surgery. J. Neurosurg. 2001;95:615–623. doi: 10.3171/jns.2001.95.4.0615. [DOI] [PubMed] [Google Scholar]
- Seeck M., Michel C.M., Vulliémoz S. Non-invasive work-up in presurgical evaluation of extratemporal lobe epilepsy. Epileptologie. 2010;27:151–163. [Google Scholar]
- Shibasaki H., Ikeda A., Nagamine T. Use of magnetoencephalography in the presurgical evaluation of epilepsy patients. Clin. Neurophysiol. 2007;118:1438–1448. doi: 10.1016/j.clinph.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Silander HCson, Blom S., Malmgren K., RosCn Uvebrant F. Surgical treatment for epilepsy: a retrospective Swedish multicenter study. Acta Neurol. Scand. 1997;95:321–330. doi: 10.1111/j.1600-0404.1997.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Someya Y., Obata T., Suhara T., Ota Y., Ikehira H., Tanada S. Seizure frequency and bilateral temporal abnormalities: a proton magnetic resonance spectroscopy of temporal lobe epilepsy. Seizure. 2000;9:274–279. doi: 10.1053/seiz.2000.0396. [DOI] [PubMed] [Google Scholar]
- Spencer S.S. The relative contributions of MRI, SPECT, and PET imaging in epilepsy. Epilepsia. 1994;35:S72–S89. doi: 10.1111/j.1528-1157.1994.tb05990.x. [DOI] [PubMed] [Google Scholar]
- Spencer S.S. Substrates of localization-related epilepsies: biologic implications of localizing findings in humans. Epilepsia. 1998;39:114–123. doi: 10.1111/j.1528-1157.1998.tb01349.x. [DOI] [PubMed] [Google Scholar]
- Spencer S.S., Berg A.T., Vickrey B.G., Sperling M.R., Bazil C.W., Shinnar S., Langfitt J.T., Walczak T.S., Pacia S.V., Ebrahimi N., Frobish D. Multicenter Study of Epilepsy Surgery. Initial outcomes in the Multicenter Study of Epilepsy Surgery. Neurology. 2003;61(12):1680–1685. doi: 10.1212/01.wnl.0000098937.35486.a3. [DOI] [PubMed] [Google Scholar]
- Spencer S., Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7(6):525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- Spencer S.S., Theodore W.H., Berkovic S.F. Clinical applications: MRI, SPECT, and PET. Magn. Reson. Imaging. 1995;13:1119–1124. doi: 10.1016/0730-725x(95)02021-k. [DOI] [PubMed] [Google Scholar]
- Stefan H., Hummel C., Scheler G., Genow A., Druschky K., Tilz C., Kaltenhäuser M., Hopfengärtner R., Buchfelder M., Romstöck J. Magnetic brain source imaging of focal epileptic activity: a synopsis of 455 cases. Brain. 2003;126:2396–2405. doi: 10.1093/brain/awg239. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Hamalainen M.S., Ahlfors S.P. Propagation of epileptic spikes reconstructed from spatiotemporal magnetoencephalographic and electroencephalographic source analysis. Neuroimage. 2010;50:217–222. doi: 10.1016/j.neuroimage.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Zenteno J.F., Dhar R., Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- Tellez-Zenteno J.F., Hernandez Ronquillo L., Moien-Afshari F., Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89:310–318. doi: 10.1016/j.eplepsyres.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Thivard L., Adam C., Hasboun D., Clémenceau S., Dezamis E., Lehéricy S., Dormont D., Chiras J., Baulac M., Dupont S. Interictal diffusion MRI in partial epilepsies explored with intracerebral electrodes. Brain. 2006;129:375–385. doi: 10.1093/brain/awh709. [DOI] [PubMed] [Google Scholar]
- Thomas R., Bhatia M., Bal C.S., Gaikwad S.B., Singh V.P., Jain S. Correlation of ictal EEG and SPECT studies in patients of intractable epilepsy with normal MRI. Neurol. India. 2002;50:440–443. [PubMed] [Google Scholar]
- Thornton R.C., Rodionov R., Laufs H., Vulliemoz S., Vaudano A., Carmichael D., Cannadathu S., Guye M., McEvoy A., Lhatoo S., Bartolomei F., Chauvel P., Diehl B., De Martino F., Elwes R.D., Walker M.C., Duncan J.S., Lemieux L. Imaging haemodynamic changes related to seizures: comparison of EEG-based general linear model, independent component analysis of fMRI and intracranial EEG. NeuroImage. 2010;53:196–205. doi: 10.1016/j.neuroimage.2010.05.064. [DOI] [PubMed] [Google Scholar]
- Tran T.A., Spencer S.S., Marks D., Javidan M., Pacia S., Spencer D.D. Significance of spikes recorded on electrocorticography in nonlesional medial temporal lobe epilepsy. Ann. Neurol. 1995;38:763–770. doi: 10.1002/ana.410380511. [DOI] [PubMed] [Google Scholar]
- Tripathi M., Garg A., Gaikwad S., Bal C.S., Chitra S., Prasad K., Dash H.H., Sharma B.S., Sarat Chandra P. Intra-operative electrocorticography in lesional epilepsy. Epilepsy Res. 2010;89(1):133–141. doi: 10.1016/j.eplepsyres.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Vale F.L., Pollock G., Benbadis S.R. Failed epilepsy surgery for mesial temporal lobe sclerosis: a review of the pathophysiology. Neurosurg. Focus. 2012;32(3):E9. doi: 10.3171/2011.12.FOCUS11318. [DOI] [PubMed] [Google Scholar]
- Vulliemoz S., Carmichael D.W., Rosenkranz K., Diehl B., Rodionov R., Walker M.C., McEvoy A.W., Lemieux L. Simultaneous intracranial EEG and fMRI of interictal epileptic discharges in humans. Neuroimage. 2011;54:182–190. doi: 10.1016/j.neuroimage.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Wennberg R. Magnetic source imaging versus intracranial electroencephalogram: neocortical versus temporolimbic epilepsy surgery. Ann. Neurol. 2006;60:271. doi: 10.1002/ana.20924. [DOI] [PubMed] [Google Scholar]
- Wetjen N.M. Intracranial electroencephalography seizure onset patterns and surgical outcomes in nonlesional extratemporal epilepsy. J. Neurosurg. 2009;110:1147–1152. doi: 10.3171/2008.8.JNS17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P., Gupta R., Burch J., Mujica Mota R.E., Wright K., Marson A. A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery. Health Technol. Assess. 2006;10:1–250. doi: 10.3310/hta10040. [DOI] [PubMed] [Google Scholar]
- WHO Epilepsy fact sheet no. 999. 2009. http://www.who.int/mediacentre/factsheets/fs999/en/index.html
- Widjaja E., Raybaud C. Advances in neuroimaging in patients with epilepsy. Neurosurg. Focus. 2008;25:E3. doi: 10.3171/FOC/2008/25/9/E3. [DOI] [PubMed] [Google Scholar]
- Wiebe S., Blume W.T., Girvin J.P., Eliasziw M., The Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group A randomized, controlled trial of surgery for temporal-lobe epilepsy. N. Engl. J. Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Won H.J., Chang K.H., Cheon J.E., Kim H.D., Lee D.S., Han M.H., Kim I.O., Lee S.K., Chung C.K. Comparison of MR imaging with PET and ictal SPECT in 118 patients with intractable epilepsy. AJNR Am. J. Neuroradiol. 1999;20:593–599. [PMC free article] [PubMed] [Google Scholar]
- Wu J.Y., Sutherling W.W., Koh S. Magnetic source imaging localizes epileptogenic zone in children with tuberous sclerosis complex. Neurology. 2006;66:1270–1272. doi: 10.1212/01.wnl.0000208412.59491.9b. [DOI] [PubMed] [Google Scholar]
- Yetkin F.Z., Swanson S., Fischer M., Akansel G., Morris G., Mueller W. Functional MR of frontal lobe activation: comparison with Wada language results. AJNR Am. J. Neuroradiol. 1998;19:1095–1098. [PMC free article] [PubMed] [Google Scholar]
- Zaknun J.J., Bal C., Maes A., Tepmongkol S., Vazquez S., Dupont P., Dondi M. Comparative analysis of MR imaging, ictal SPECT and EEG in temporal lobe epilepsy: a prospective IAEA multi-center study. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:107–115. doi: 10.1007/s00259-007-0526-y. [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu W., Chen H., Xia H., Zhou Z., Wang L., Mei S., Liu Q., Li Y. EEG–fMRI validation studies in comparison with icEEG: a review. Int. J. Psychophysiol. 2012;84:233–239. doi: 10.1016/j.ijpsycho.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Zijlmans M., Huiskamp G., Hersevoort M., Seppenwoolde J.H., van Huffelen A.C., Leijten EEG–fMRI in the preoperative work-up for epilepsy surgery. Brain. 2007;130:2343–2353. doi: 10.1093/brain/awm141. [DOI] [PubMed] [Google Scholar]
- Zumsteg D., Friedman A., Wieser H.G., Wennberg R.A. Propagation of interictal discharges in temporal lobe epilepsy: correlation of spatiotemporal mapping with intracranial foramen ovale electrode recordings. Clin. Neurophysiol. 2006;117:2615–2626. doi: 10.1016/j.clinph.2006.07.319. [DOI] [PubMed] [Google Scholar]


