Abstract
Objectives
A lifespan approach was used to evaluate age at menopause, and determinants of surgical and natural menopause, in the multi-ethnic community of Hilo, Hawaii.
Study design
Participants aged 40–60 years (n=898) were drawn from a larger, randomly-generated sample recruited by postal questionnaires. Median age at natural menopause was computed by probit analysis. Logistic regression analysis was applied to examine determinants of hysterectomy, and Cox regression analysis was used to examine risk factors for an earlier age at menopause.
Main outcome measures
History of hysterectomy, Age at menopause
Results
Frequency of hysterectomy was 19.2% at a mean age of 40.5 years. The likelihood of hysterectomy increased with older ages, lower education, mixed ancestry, having been overweight at age 30, and married 20 years prior to survey. Median age at natural menopause was 53.0 years. Smoking and not being married 10 years before survey were associated with an earlier age at menopause.
Conclusions
Median age at menopause was later than the national average. Ethnicity and education were determinants of hysterectomy, but not associated with age at natural menopause. Events later in the lifespan (e.g., smoking and not being married 10 years prior to the survey) were more important than earlier events (e.g., childhood residence) in relation to age at menopause. The timing of weight gain and marital status appear to be important in relation to surgical menopause, and the timing of marital status appears to be important in relation to the timing of natural menopause.
Keywords: Hawaii, age at menopause, hysterectomy, BMI, ethnicity, smoking
1. Introduction
Ages at surgical or natural menopause are associated with long term consequences for health during the post-reproductive years. For example, earlier ages at hysterectomy with bilateral oophorectomy are associated with an elevated risk of cardiovascular mortality,1–2 and a later age at natural menopause is associated with a higher risk of breast cancer.3–4 A later age at natural menopause is also associated with increased longevity5–6 and lower all-cause mortality.7
The state of Hawaii boasts the longest life expectancy in the nation, with a mean life expectancy of 80.5 years8 vs. a national average of 78.7 years;9 thus, median age at menopause in Hawaii may also be later, relative to the median ages of 51 to 52.5 years in the United States.10–12 The purpose of this study was to establish the median age at natural menopause in Hilo, Hawaii, as well as the determinants of surgical and natural menopause among this multi-ethnic population. Confirming determinants in this population contributes to our knowledge about risk for health concerns in Hawaii so that women can be targeted for screening and other preventative measures.
Variation in age at menopause, both within and across populations, is related to differences in genes, early environment, adult lifestyle, and reproductive history. Estimates for heritability in age at menopause vary from 31 to 85%.13–16 A number of studies have demonstrated a relationship between early ages at menopause and adverse early life events, such as severe caloric restriction during early childhood,17 low weight gain during early childhood,18–19 socioeconomic disadvantage,20 or infectious disease burden.21 Early ages at menopause are also associated with smoking,10,22 nulliparity,10,23 low levels of education,11 being single or otherwise unmarried,24 and low BMI at midlife.22,23
Ethnicity has been associated with age at menopause in some studies, but not others. In the longitudinal Study of Women’s Health Across the Nation (SWAN), Japanese ancestry was at first significantly associated with a later age at natural menopause compared to non-Hispanic whites (51.8 years vs. 51.4 years).10 The most recent study by SWAN researchers, using data from 1996 through 2007 (n=3253), found that Japanese participants had a later age at menopause than African-American, Chinese, and Hispanic participants in unadjusted results. However, after adjusting for smoking, self-reported health, education level, use of oral contraceptives, alcohol intake, employment, physical activity, and baseline weight, ethic differences were no longer significant.11 An independent effect of race/ethnicity on the timing of natural menopause was shown in the Multiethnic Cohort Study of women aged 45–74 (n=95,704). After adjusting for smoking status, age at menarche, parity, and BMI, native Hawaiians had an earlier menopause than non-Hispanic whites (HR 1.05, 95% CI 1.01–1.09). Japanese-Americans experienced a later menopause than non-Hispanic whites (HR = 0.93, 95% CI 0.90–0.95).23
Ethnicity has also been associated with variation in rates of hysterectomy. In a 1977 study of menopause among women aged 35 to 60 in Hawaii, menopausal women of European descent were more likely to have undergone “surgery for female disorders” compared to Japanese-Americans (52% vs. 37%).25 More recently, SWAN results found that Asian-Americans reported a lower rate of hysterectomy compared with non-Hispanic whites.26 In a study of California school teachers, Asians/Pacific Islanders were not more likely to have surgery for uterine fibroids than non-Hispanic whites.27
Hilo, on the Big Island of Hawaii, was once a port associated with a sugar cane industry that attracted thousands of workers of different ethnicities to Hawaii.28 Hilo is now a multi-ethnic community of 46,165 residents. Japanese-Americans are the largest ethnic group (21%), followed by European-Americans (19%), Native Hawaiians (11%), and Filipinos (7%). Thirty-two percent of people classify themselves as members of two or more ethnic groups (2010 census). This population provides the opportunity to study different ethnic groups residing in the same general locality, although there are socioeconomic and cultural differences, e.g., in diet and activity patterns.29 Hilo is also of interest because of a previous study that showed a high frequency of hysterectomies. In a study of ambulatory blood pressure among Japanese-American and European-American school teachers (n=120), 66% of the 47 postmenopausal participants (29 Japanese-Americans and 18 European-Americans) had undergone a hysterectomy, 15 with ovaries left intact and 16 with ovaries removed.30
The purpose of this study was to consider age at menopause in the multi-ethnic community of Hilo, HI, and to examine determinants of surgical and natural menopause. This study applied a lifespan approach31,32 to examine early life events (e.g., where women grew up – Hawaii, U.S. mainland, not U.S.), level of education, and later life events such as smoking habits and marital status. In a lifespan perspective, biological and socio-cultural trajectories are intertwined across time,33–35 and every point in the lifespan can be studied as both predictive of later aging and the result of cumulative processes.36
Age at hysterectomy and age at natural menopause are both influenced by biological and socio-cultural variables. For example, a twin study carried out in the UK found similar estimates of heritability for hysterectomy (0.59, 95% CI 0.43–0.72) and for the main indications for hysterectomy, fibroids (0.69) and menorrhagia (0.55).15 In a more recent study, the decision to undergo a hysterectomy was associated with fibroids and uterine bleeding, and also with the importance of sexual relationships and attitudes, e.g., whether the uterus is “useless” to women who have completed childbearing.37 A lower level of education and income has also been associated with increased rates of hysterectomy.38
Age at natural menopause is determined by the number of undeveloped eggs present in ovaries at birth and the rate of loss of ovarian follicles across the lifespan.39,40 We do not have birth weight to assess possible effects of maternal health on the number of undeveloped eggs present at birth; therefore, we focus on determinants that may influence the rate of loss of ovarian follicles across the lifespan. In addition to identifying determinants of age at surgical and natural menopause, we are interested in when, during the lifespan, such variables may have had an influence.
2. Methods
2.1 Study sample
For this study, women aged 40 to 60 (n=898) were drawn from the larger Hilo Women’s Health Study. This study involved two phases: a postal survey about general health and menopause, and a follow up clinical study of hot flashes and blood pressure among a subsample of 200 women. The current analysis is based on the postal survey.
Eight-page health questionnaires were mailed to property lots in Hilo based on tax map key numbers chosen by random assignment (see Sievert et al. for further details).41 Surveys were mailed to 7,207 households, and 1824 surveys were completed and returned for a household return rate of 28.5%. All women gave written informed consent and the study was approved by the University of Hawaii Human Studies Program.
To determine ethnicity, women were asked to check off all groups “that are included in your background” from a list of ethnicities, and were asked to include the percentage of each ethnicity where this was known. For these analyses, ethnicity was categorized into 4 groups: European-American, Japanese-American, Mixed, and Other. The category European-American was made up of women who claimed 100% European ancestry on the questionnaire. The category Japanese-American was limited to women who claimed 100% Japanese ancestry. Although Hawaiian Japanese-Americans are primarily third and fourth generation, and native English-speakers, the Japanese-American community in Hawaii has intermarried less than other ethnic groups.42 Therefore, a high proportion of women with 100% Japanese ancestry is present.41 In the mixed ancestry group, the majority indicated Hawaiian (60%) and/or European (66%) ancestry. Mixed ancestry also included Chinese (43%), Japanese (25%), Filipino (23%), Hispanic (12%), Native American (12%), Korean (4%), Pacific Islander (4%), and African-American (2%) backgrounds. Women were categorized as “Other” if they described their background as 100% one ethnicity besides European or Japanese.
2.2 Variables of interest
Body mass index (BMI) at time of survey was calculated from self-reported height and weight. Past BMI was calculated from recalled, self-reported weight at ages 20, 30, and 40 in combination with self-reported height at time of survey. For multivariate models, BMI was categorized as underweight/normal (<25 kg/m2), overweight (25 to 30 kg/m2), and obese (>30 kg/m2).
Variables examined in relation to ages at surgical and natural menopause included: age at time of survey; ethnicity; where a woman grew up (Hawaii, U.S. mainland, not U.S.); level of education (some or completed high school, some or completed two year or four year college, some or completed postgraduate degree); financial comfort (struggling, OK, comfortable, well-off); marital status at time of survey, marital status 10 years ago, marital status 20 years ago; parity (parous or nulliparous); smoking habits at time of survey (yes/no); BMI at time of survey and at ages 20, 30, and 40; age at menarche; use of birth control pills; and use of hormone therapy (HT).
2.3 Age at hysterectomy and determinants
Recalled age at hysterectomy was used to determine mean age at hysterectomy. Across all women aged 40 to 60 years, history of hysterectomy (yes/no) was examined through bivariate logistic regression in relation to ethnicity, demographic variables, lifestyle factors, BMI, weight change, and hormone use. Choice of variables was guided by univariate analyses and review of the literature. Women classified as other ethnicity (N=54) were excluded from the multivariable analyses.
Marital status at time of survey, marital status 10 years ago, and marital status 20 years ago were each examined in the model along with other demographic variables. Only marital status 20 years ago was significant in the univariate analyses. After considering models with BMI at ages 30, 40, and at the time of the survey, the best fit was the model that included BMI at age 30. The final model included ethnicity, age at time of survey, age at menarche, BMI at age 30, marital status 20 years ago, parity, level of education, and smoking status.
Where a woman grew up was not included in the model because it was highly associated with ethnicity (p<0.001), e.g., 66% of the European-Americans grew up on the mainland, while 96% of the Japanese-Americans and 87% of the women of mixed descent grew up in Hawaii. Use of HT was not included in the model because HT use was viewed as a consequence, not determinant, of having had a hysterectomy. Based on the results of the logistic regression, the analysis was repeated among the mixed ethnicity subsample to examine whether percent Hawaiian ancestry was a determinant of having undergone a hysterectomy while controlling for demographic and lifestyle variables. Hosmer and Lemeshow tests for goodness of fit were carried out for all logistic regression models.
2.4 Age at natural menopause and determinants
For comparisons within this study, and across other studies, mean recalled ages at natural menopause for the entire sample and for women of European, Japanese, and mixed descent were calculated. In addition, median ages at natural menopause were computed by probit analysis. Post-menopausal women were defined as those who had not menstruated for at least 12 months for the calculation of mean recalled age at menopause, for median ages at menopause, and for Cox regression analyses carried out to determine risk of an earlier age at menopause.11,43 Women with recalled ages at menopause prior to 40 years of age (n=6) were excluded from this and other analyses.
Mean recalled ages at natural menopause were examined by t-test or ANOVA analyses in relation to where a woman grew up (HI, US mainland, not US); level of education; financial comfort; current marital status (married, not married), marital status 10 years ago, and marital status 20 years ago; parous or nulliparous status; smoking status; BMI (normal, overweight, obese) at time of survey, at age 20, at age 30, and at age 40; change in BMI from age 20 to 40 (<5 kg/m2, 5+ kg/m2); and use of birth control or HT. Pearson’s correlation was used to examine recalled ages at natural menopause in relation to height, age at menarche, age at first pregnancy, and age at last pregnancy.
Cox regression analyses were used to calculate hazard ratios (HR) for the event of not having menstruated for at least 12 months (postmenopausal status). Variables included in a Cox regression analysis precede the event, or are proxies for behaviors that most likely preceded the event (e.g., smoking). Pre-menopausal and naturally post-menopausal women were included in the analysis, with age at most recent menstrual period entered as the covariate of interest. BMI at ages 20, 30, and 40 were examined in separate models. Marital status 10 years ago, 20 years ago, and at time of survey were examined in separate models. The models with current marital status and marital status 20 years ago were not significant; therefore, we included marital status 10 years ago in the final model. Level of education and financial status were also examined separately. Neither were significant variables, although financial status somewhat improved the significance of the model. In addition to age at most recent menstrual period, the final model included ethnicity, financial comfort, smoking status, parity, BMI at age 30, and marital status 10 years ago. All analyses were carried out using SPSS 16.0 GP.
3. Results
3.1 Sample characteristics
The sample was characterized by women of European-American (24%), Japanese-American (29%), and mixed ethnic descent (41%) who grew up in Hawaii (73%). The majority attended or completed college, described themselves to be financially OK or comfortable, married, with an average of 2 children. Sample characteristics are shown in Table 1.
Table I.
Sample characteristics with (1) comparison of pre-menopausal vs. naturally post-menopausal and (2) comparison of naturally post-menopausal vs. hysterectomy status
| All N=898a |
Pre-menopausal N=468 |
Naturally post-menopausal N=225 |
History of hysterectomy N=165 |
|
|---|---|---|---|---|
|
| ||||
| Age at time of survey (yrs) Mean and (s.d.) | 50.0 (5.4) | 47.0 (4.3)** b | 54.5 (3.7) | 51.7 (5.4)** c |
|
| ||||
| Ethnicity | ||||
| European | 24% | 23%* b | 29% | 16% |
| Japanese | 29% | 28% | 34% | 29% |
| Mixed (Hawaiian) | 41% | 43% | 30% | 49% |
| Other | 6% | 6% | 6% | 6% |
|
| ||||
| Where she grew up | ||||
| Hawaii | 73% | 72% | 67% | 81%** c |
| Mainland, U.S. | 21% | 21% | 27% | 15% |
| Not U.S. | 6% | 7% | 6% | 4% |
|
| ||||
| Level of education | ||||
| Some or completed HS | 16% | 15% | 13% | 19%** c |
| Some or completed college | 60% | 60% | 56% | 66% |
| Some or completed grad sch | 24% | 25% | 31% | 16% |
|
| ||||
| Financial Status | ||||
| Struggling | 16% | 15% | 16% | 17% |
| OK | 46% | 47% | 43% | 48% |
| Comfortable | 35% | 35% | 38% | 33% |
| Well-off | 3% | 3% | 4% | 2% |
|
| ||||
| Percent married | ||||
| At time of survey | 72% | 75%* b | 67% | 72% |
| 10 years ago | 76% | 77%* b | 69% | 79%* c |
| 20 years ago | 67% | 57%** b | 75% | 81% |
|
| ||||
| Age at menarche (yrs) | ||||
| Mean and (s.d.) | 12.5 (1.7) | 12.6 (1.7)* b | 12.3 (1.6) | 12.2 (1.6) |
|
| ||||
| Mean parity | ||||
| Mean and (s.d.) | 2.1 (1.3) | 2.1 (1.4) | 2.1 (1.4) | 2.2 (1.1) |
|
| ||||
| Percent nulliparous | 13% | 13% | 13% | 7% |
|
| ||||
| Smoking | 16% | 17% | 14% | 15% |
|
| ||||
| Height at time of survey (cm) Mean and (s.d.) | 63.0 (2.9) | 63.1 (2.8) | 62.8 (2.9) | 62.8 (3.1) |
|
| ||||
| BMI | ||||
| At age 20 | 21.4 (3.3) | 21.4 (3.4) | 21.3 (3.1) | 21.9 (3.2) |
| At age 30 | 23.2 (4.3) | 23.2 (4.3) | 22.7 (4.3) | 24.1 (4.3)** c |
| At age 40 | 25.1 (5.4) | 25.3 (5.6)** b | 24.1 (4.6) | 26.1 (5.7)** c |
| At time of survey | 26.7 (6.2) | 26.5 (5.9) | 26.5 (6.2) | 27.9 (7.0)* c |
|
| ||||
| Birth control pill | ||||
| Never | 19% | 18%** b | 20% | 20% |
| Past | 77% | 75% | 79% | 80% |
| Current | 4% | 7% | 1% | 1% |
|
| ||||
| Hormone therapy | ||||
| Never | 71% | 92%** b | 52% | 41% |
| Past | 18% | 4% | 34% | 36% |
| Current | 10% | 4% | 14% | 24% |
p<0.05
p<0.01
unable to classify all participants by hysterectomy and menopausal status
Comparison of pre-menopausal vs. naturally post-menopausal status
Comparison of naturally post-menopausal vs. history of hysterectomy
3.2 Age and determinants of hysterectomy
Nineteen percent of women reported a history of hysterectomy. Mean age at hysterectomy was 40.5 years (s.d. 6.8) with a range in age from 22.7 to 58.4 years (n=148). Ninety-four percent of women were still menstruating at the time of their hysterectomy, 22% had one ovary removed and 43% reported the removal of both ovaries.
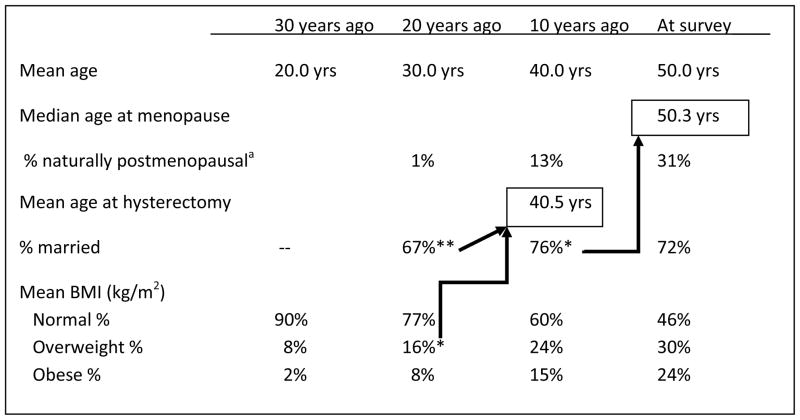
In univariate analyses, women with a history of hysterectomy were more likely to have grown up in Hawaii, had lower levels of education, were more likely to have been married 20 years ago, were more likely to have children, more likely to be overweight in the past and at the time of survey, and more likely to be taking HT (Table II). In addition, women with a history of hysterectomy were older at time of survey (51.7 years, s.d. 5.4, vs. 49.4 years, s.d. 5.4, p<0.001) and reported an earlier age at menarche (12.2 years, s.d. 1.6, vs. 12.5 years, s.d. 1.7, p=0.006). See also Figure I.
Table II.
History of hysterectomy in relation to potential determinants among women aged 40 to 60 in Hilo, Hawaii
| N | % having had a hysterectomy | P value* | |
|---|---|---|---|
|
| |||
| Ethnicity | |||
| European | 201 | 12.9 | |
| Japanese | 250 | 18.8 | |
| Mixed (Hawaiian) | 347 | 23.3 | 0.012 |
|
| |||
| Where she grew up | |||
| Hawaii | 623 | 21.3 | |
| Mainland, U.S. | 183 | 13.1 | |
| Not U.S. | 51 | 13.7 | 0.027 |
|
| |||
| Level of education | |||
| Low | 132 | 23.5 | |
| Medium | 518 | 20.8 | |
| High | 211 | 12.3 | 0.012 |
|
| |||
| Financial Status | |||
| Struggling | 132 | 20.5 | |
| OK | 396 | 19.7 | |
| Comfortable | 297 | 17.8 | |
| Well-off | 26 | 11.5 | 0.681 |
|
| |||
| Married 20 years ago | 548 | 23.4 | |
| Not married 20 years ago | 275 | 11.3 | <0.001 |
|
| |||
| Married 10 years ago | 613 | 19.7 | |
| Not married 10 years ago | 197 | 16.2 | 0.276 |
|
| |||
| Married at time of survey | 621 | 19.3 | |
| Not married at time of survey | 238 | 19.2 | 0.956 |
|
| |||
| Parous | 755 | 20.3 | |
| Nulliparous | 106 | 11.3 | 0.028 |
|
| |||
| Not smoking | 719 | 19.3 | |
| Smoking | 136 | 18.4 | 0.796 |
|
| |||
| BMI at age 20 | |||
| Normal | 732 | 18.9 | |
| Overweight | 70 | 28.6 | |
| Obese | 15 | 13.3 | 0.122 |
|
| |||
| BMI at age 30 | |||
| Normal | 608 | 17.6 | |
| Overweight | 129 | 27.9 | |
| Obese | 60 | 25.0 | 0.017 |
|
| |||
| BMI at age 40 | |||
| Normal | 467 | 16.5 | |
| Overweight | 193 | 23.3 | |
| Obese | 121 | 24.8 | 0.036 |
|
| |||
| BMI at time of survey | 372 | 15.9 | |
| Normal | 246 | 19.5 | 0.049 |
| Overweight | 202 | 24.3 | |
| Obese | |||
|
| |||
| BMI change from 20 to 40 | |||
| <5 kg/m2 | 566 | 18.4 | |
| 5+ kg/m2 | 220 | 22.3 | 0.215 |
|
| |||
| Birth control pill use | |||
| Never | 158 | 20.3 | |
| Past | 661 | 19.8 | 0.902 |
|
| |||
| Hormone therapy use | |||
| Never | 597 | 11.1 | |
| Past | 150 | 38.7 | |
| Current | 90 | 43.3 | <0.001 |
ANOVAs or t-tests
Figure I.
The timing of various life events among women in Hilo, Hawaii aged 40 to 60 at survey (n=898).
* p<0.05, ** p<0.01
Significance based on logistic regression results.
a Among naturally post-menopausal women at time of survey (n=191), for whom age at menopause is known. Twenty years ago, all but 2 women were pre-menopausal. Ten years ago 82% were pre-menopausal, 5% were peri-menopausal, and 13% were postmenopausal.
Results from the final logistic regression analysis are shown in Table III. In this model, women with mixed ancestry were more likely to have undergone a hysterectomy compared to women of European descent (OR 2.10, 95% CI 1.23–3.59). Probability of hysterectomy increased with age (OR 1.09, 95% CI 1.04–1.13), and women who were overweight at age 30 were almost twice as likely to have undergone a hysterectomy (OR 1.87, 95% CI 1.13–3.09). Women who were married 20 years ago were also nearly twice as likely to have undergone a hysterectomy (OR 1.94, 95% CI 1.29–3.16). Lower levels of education increased the likelihood of a hysterectomy, but smoking, parity and age at menarche did not (Table III).
Table III.
Results of logistic regression for history of hysterectomy (n=712)
| Odds ratio | 95% CI | p-value | |
|---|---|---|---|
|
| |||
| Ethnicity | |||
| European (ref) | |||
| Japanese | 1.360 | 0.766–2.415 | 0.294 |
| Mixed (Hawaiian) | 2.102 | 1.232–3.587 | 0.006 |
|
| |||
| Age | 1.087 | 1.043–1.132 | <0.001 |
|
| |||
| Age at menarche | 0.901 | 0.792–1.025 | 0.113 |
|
| |||
| BMI at age 30 | |||
| Normal (ref) | |||
| Overweight | 1.868 | 1.130–3.088 | 0.015 |
| Obese | 1.705 | 0.860–3.380 | 0.126 |
|
| |||
| Married 20 years ago | 1.943 | 1.195–3.159 | 0.007 |
| Not married 20 years ago (ref) | |||
|
| |||
| Nulliparous (ref) | |||
| Parous | 1.377 | 0.682–2.780 | 0.372 |
|
| |||
| Education | |||
| High school or less | 1.871 | 0.903–3.877 | 0.092 |
| Some college or degree | 1.932 | 1.067–3.253 | 0.013 |
| Some post-grad or degree (ref) | |||
|
| |||
| Current smoker no (ref) | |||
| Current smoker yes | 0.847 | 0.534–1.673 | 0.945 |
Mixed ethnicity was a determinant of hysterectomy and 60% of women of mixed ethnicity claimed Hawaiian background. Therefore, we examined the determinants of hysterectomy among only women of mixed ethnicity to assess whether self-reported percent Hawaiian ancestry was a determinant of hysterectomy, controlling for variables significant in the larger model. Age and marital status 20 years ago remained significant in this second model; however, Hawaiian ancestry examined as a continuous variable or in quartiles, was not a significant determinant of the likelihood of having undergone a hysterectomy (not shown.)
3.3 Age and determinants of natural menopause
Mean recalled age at natural menopause was 49.4 years (s.d. 3.4). Median age at natural menopause by probit analysis was 53.0 years (n=568). As Table IV shows, there were no significant differences across ethnic groups in mean or median ages at menopause.
Table IV.
Age at natural menopause among women of European, Japanese, and Mixed ancestry (age at menopause >40). No significant differences across ethnic groups in mean or median ages at menopause.
| Group | N | Mean recalled age at menopause (years, s.d.) | N | Median age at menopause by probit analysis (95% Fieller Bounds) |
|---|---|---|---|---|
|
| ||||
| European | 53 | 49.3 (3.3) | 153 | 53.1 (52.0–54.3) |
| Japanese | 61 | 50.1 (3.4) | 179 | 53.0 (52.1–54.0) |
| Mixed | 57 | 48.9 (3.4) | 236 | 52.8 (52.0–53.8) |
| All | 171 | 49.4 (3.4) | 568 | 53.0 (52.4–53.6) |
In univariate analyses, post-menopausal women were older, less likely to be of mixed ethnic descent, and had an earlier age at menarche (Table I). An earlier recalled age at natural menopause was associated with lower levels of financial comfort, smoking, and unmarried status 20 years ago (but not unmarried status 10 years ago or at time of survey) (Table V). Change in BMI from ages 20 to 40 approached significance, so that women who gained 5 or more kg/m2 had an earlier age at natural menopause (p=0.051). There was no significant correlation between mean age at natural menopause and height, age at menarche, age at first birth, or age at last birth. The correlation between age at natural menopause and age at birth of last child approached significance (r=−.157, p=0.080, n=125).
Table V.
Recalled age at natural menopause in relation to potential determinants (Women with menopause before age 40 excluded)
| N | Mean age at menopause (s.d) | p-value | |
|---|---|---|---|
|
| |||
| Ethnicity | |||
| European | 53 | 49.0 (3.3) | |
| Japanese | 61 | 50.1 (3.4) | |
| Mixed (Hawaiian) | 57 | 48.9 (3.4) | |
| Other | 11 | 48.4 (2.9) | 0.167 |
|
| |||
| Where she grew up | |||
| Hawaii | 122 | 49.4 (3.5) | |
| Mainland, U.S. | 50 | 49.3 (3.3) | |
| Not U.S. | 12 | 49.6 (2.0) | 0.965 |
|
| |||
| Level of education | |||
| Low | 22 | 49.4 (2.4) | |
| Medium | 102 | 49.1 (3.6) | |
| High | 61 | 49.8 (3.2) | 0.452 |
|
| |||
| Financial Status | |||
| Struggling | 27 | 48.1 (3.8) | |
| OK | 77 | 49.0 (3.4) | |
| Comfortable | 71 | 50.1 (3.2) | |
| Well-off | 8 | 49.9 (1.6) | 0.033 |
|
| |||
| Married 20 years ago | 133 | 49.7 (3.2) | |
| Not married 20 years ago | 45 | 48.3 (3.7) | 0.012 |
|
| |||
| Married 10 years ago | 122 | 49.6 (3.3) | |
| Not married 10 years ago | 53 | 49.0 (3.4) | 0.227 |
|
| |||
| Married at time of survey | 123 | 49.5 (3.2) | |
| Not married at survey | 61 | 49.1 (3.6) | 0.462 |
|
| |||
| Parous | 161 | 49.5 (3.3) | |
| Nulliparous | 24 | 48.7 (3.8) | 0.273 |
|
| |||
| Not smoking | 159 | 49.6 (3.3) | |
| Smoking | 24 | 47.8 (3.6) | 0.015 |
|
| |||
| BMI at age 20 | |||
| Normal | 163 | 49.5 (3.4) | |
| Overweight | 11 | 48.5 (2.4) | |
| Obese | 2 | 46.6 (7.4) | 0.357 |
|
| |||
| BMI at age 30 | |||
| Normal | 139 | 49.4 (3.4) | |
| Overweight | 25 | 49.5 (2.7) | |
| Obese | 9 | 48.2 (3.5) | 0.566 |
|
| |||
| BMI at age 40 | |||
| Normal | 121 | 49.6 (3.1) | |
| Overweight | 35 | 49.1 (3.2) | |
| Obese | 16 | 47.6 (3.8) | 0.073 |
|
| |||
| BMI at time of survey | |||
| Normal | 89 | 49.6 (3.1) | |
| Overweight | 48 | 49.3 (3.4) | |
| Obese | 39 | 49.0 (3.9) | .591 |
|
| |||
| BMI change from 20 to 40 | |||
| <5 kg/m2 | 139 | 49.6 (3.1) | |
| 5+ kg/m2 | 35 | 48.3 (4.0) | 0.051 |
|
| |||
| Birth control pill use | |||
| Never | 33 | 48.7 (4.0) | |
| Past | 148 | 49.5 (3.2) | 0.216 |
|
| |||
| Hormone therapy use | |||
| Never | 93 | 49.4 (3.4) | |
| Past | 63 | 49.6 (3.0) | |
| Current | 26 | 48.5 (4.1) | 0.377 |
In the final Cox survival model for age at natural menopause (Table VI), only smoking (HR 1.75, 95% CI 1.12–2.74) and not being married 10 years ago (HR 1.54, 95% CI 1.06–2.25) were significantly associated with the likelihood of an earlier age at menopause. Being overweight at age 30 approached significance for an association with an earlier age at natural menopause (HR 1.50, 95% CI 0.962–2.338).
Table VI.
Cox survival model for age at natural menopause (n=545)
| Unadjusted HR | Adjusted HR | 95% Confidence interval | P-value | P-trend | |
|---|---|---|---|---|---|
|
| |||||
| Ethnic group | |||||
| European (ref) | |||||
| Japanese | 0.86 | 0.951 | 0.646–1.402 | 0.802 | |
| Mixed (Hawaiian) | 0.89 | 0.874 | 0.587–1.301 | 0.506 | |
|
| |||||
| Financial comfort | 0.407 | ||||
| Struggling | 1.112 | 0.907 | 0.370–2.222 | 0.831 | |
| OK | 0.660 | 0.630 | 0.279–1.425 | 0.267 | |
| Comfortable | 0.691 | 0.703 | 0.309–1.601 | 0.402 | |
| Well off (ref) | |||||
|
| |||||
| BMI at age 30 | 0.197 | ||||
| Normal (ref) | |||||
| Overweight | 1.501 | 1.500 | 0.962–2.338 | 0.073 | |
| Obese | 1.185 | 1.168 | 0.584–2.333 | 0.661 | |
|
| |||||
| Smoking | |||||
| No (ref) | |||||
| Yes | 1.810 | 1.750 | 1.116–2.744 | 0.015 | |
|
| |||||
| Marital status 10 yrs ago | |||||
| No | 1.719 | 1.543 | 1.060–2.245 | 0.024 | |
| Yes (ref) | |||||
|
| |||||
| Parous (ref) | |||||
| Nulliparous | 1.383 | 1.209 | 0.761–1.919 | 0.422 | |
4. Discussion
4.1 Age and determinants of hysterectomy
In this study of ages at surgical and natural menopause in the multi-ethnic community of Hilo, Hawaii, frequency of hysterectomy was 19.2%. This frequency of hysterectomy is somewhat higher than the 17.3% found in a survey carried out in 2005, the same year as our survey, in the county of Hawaii, where Hilo is located.44 It could be that the relatively low rate of questionnaire return (28.5%), and the large percentage of respondents with mixed ancestry (41%), may have biased our sample toward women with hysterectomies. Among women in Hilo, Hawaii, we found that the likelihood of having undergone a hysterectomy increased with older ages, lower levels of education, mixed ancestry, having been overweight at age 30, and having been married 20 years before survey.
The lifespan perspective provides a framework for looking at early life events in relation to later health outcomes. In addition, it offers clues with regard to cohort effects.33 When the Hilo sample was limited to post-menopausal women (n=390), 42.3% had undergone a hysterectomy. This frequency is lower than the frequency reported in an earlier study among school teachers in Hilo where Brown et al.30 found that 66% of postmenopausal women had undergone a hysterectomy. The difference in rates of hysterectomy between the two surveys may reflect changes in the delivery of health care over time among the few gynecologists practicing in the Hilo area. Alternatively, the difference may represent an occupational or socioeconomic effect because the earlier study was limited to school teachers.30
With a focus on early life events, mixed ancestry, growing up in Hawaii, and lower levels of education were associated with having had a hysterectomy. Compared to European-Americans, women of mixed ancestry were more than twice as likely to have undergone a hysterectomy (OR 2.10, 95% CI 1.23–3.59). There may be a genetic component to risk of hysterectomy;15 however, a relationship between percent Hawaiian ancestry and likelihood of hysterectomy could not be demonstrated. Instead, in this population, it is more likely that ancestry, place of birth, and education are associated. Women of mixed ethnicity were more likely to have grown up in Hawaii. In addition, women of mixed ancestry have significantly lower levels of education compared to European- and Japanese-American women (p<0.001). Women who grew up in Hawaii have significantly lower levels of education compared to women who grew up outside of Hawaii (p<0.001). For example, only 19% of women who grew up in Hawaii have some post-graduate education compared to 36% of women who grew up on the U.S. mainland.
The effect of mixed ethnicity and lower levels of education persisted in logistic regression results after controlling for age at time of survey and other demographic and lifestyle variables. The relationship between lower levels of education and higher frequencies of hysterectomy has been found in studies in a number of countries.45,46 In a U.S. study of 7139 women aged 25 to 54 in 15 states, frequency of hysterectomy ranged from 26.1% among women with some high school education to 10.1% among college graduates. After controlling for income, women without a high school degree were twice as likely to have undergone a hysterectomy compared to women with a college degree or beyond (OR 2.1, 95% CI 1.7–2.7).38 Factors that may influence hysterectomy rates in relation to levels of education include access to information, access to health care resources, ability to communicate with physicians, and attitudes toward surgical procedures.25,45
With regard to later life events, marital status 20 years ago, but not marital status at time of survey or 10 years prior to survey, was associated with history of hysterectomy. Women who were married 20 years ago were twice as likely to have undergone a hysterectomy (OR 1.94, 95% CI 1.20–3.16). The women in this study have a mean age of 50.0 years. Mean age at hysterectomy was 40.5 years. It is interesting, then, that it was not marital status at the time of hysterectomy, but marital status on average 10 years prior to hysterectomy – around the age of 30 – that was associated with a higher likelihood of hysterectomy. As Figure I shows, fewer women were married 20 years prior to the survey. Marriage may have increased the likelihood of health insurance. In addition, if heavy bleeding or pain from fibroids affected sexual relationships,37 then married women may have been more likely to seek a surgical treatment. The answer is not that women married 20 years prior to the survey were more likely to have had children, because women married 10 years ago and at the time of survey were also significantly more likely to have had children compared to unmarried women at those periods of time. Parity did not remain a significant determinant of hysterectomy after controlling for other demographic and lifestyle factors.
Finally, with regard to hysterectomy, women who were overweight at the age of 30 were more likely to have undergone a hysterectomy. Univariate and logistic regression results point to BMI at age 30, rather than ages 20, 40, or at time of survey, as having relevance for the likelihood of undergoing a hysterectomy. This indicates BMI 10 years prior to the mean age at hysterectomy (Figure I). BMI is related to various health concerns that result in hysterectomy. For example, in a study of 80,204 California teachers, the risk of surgery for fibroids was higher among overweight women (RR 1.23, 95% CI 1.10–1.38) and obese women (RR 1.27, 95% CI 1.11–1.46) compared to women of normal body size, after controlling for numerous demographic and lifestyle factors.27 There is also a strong association between obesity and endometrial cancer,47 and an association between obesity and pelvic organ prolapse.48
4.2 Age and determinants of natural menopause
Mean recalled ages at menopause are generally earlier than median ages at menopause computed by probit analysis, as is the case here (49.4 years vs. 53.0 years.) This is to be expected because of the truncation of data in a cross-sectional study. Among women aged 40 to 60, those who will have a later age at menopause continue to menstruate and, therefore, cannot contribute their age at menopause to the sample. In a cross-sectional study, probit analysis is the best measure for determining age at menopause because it does not rely on recall. Women are asked their age at time of survey and whether or not they have menstruated within the past 12 months. The age at which 50% of the women have menstruated in the past 12 months, and 50% of the women have not is the median age at menopause.43
In Hilo, Hawaii, the median age at menopause is 53.0 years, which is higher than the median reported for the U.S.11,12 This later age at menopause is consistent with a higher life expectancy in Hawaii, and consistent with the relatively high level of education seen in the sample.
There were no significant differences across ethnic groups in mean or median ages at menopause, consistent with the most recent finding from SWAN.11 From a lifespan perspective, where a woman grew up was also without significance, although this may be because migrants to Hawaii tend to be of equal or greater socioeconomic status than women living in Hawaii. Quite different results are found when migration takes place, for example, between Bangladesh and London. In a recent study, Bangladeshi immigrants had a significantly earlier age at natural menopause compared to their London neighbors of European ancestry.9 In Hilo, smoking was significantly associated with the likelihood of an earlier age at natural menopause. The relationship between smoking and age at menopause has been well-substantiated.49 In Cox survival analyses we found no association between ethnicity, financial comfort, parity, or BMI and age at natural menopause.
From a lifespan perspective, marriage 20 years before survey was related to age at menopause in univariate results, and marriage 10 years ago was related to age at menopause in the Cox regression analysis after controlling for other demographic and lifestyle variables. Marriage at all points in time was significantly related to financial comfort, with married women significantly more likely to describe themselves as comfortable. Married women may have a later age at menopause because of the relationship between higher income and marital status,12 because of sexual activity, or because of the pheromonal influence of living with a man.24 One difficulty in pinpointing the relationship between marital status and age at menopause is the fluidity of the former. For example, 21% of the women who were married 20 years ago were not married at the time of the survey, and 56% of the women who were not married 20 years were married at the time of the survey.
4.3 Limitations
One limitation of this postal survey is the need to rely on self-reported heights and weights. In general women tend to over-report their height and under-report their weight;50 however, if everyone tends to under- or over-report in the same direction, then the error will not substantially affect study results. Weight tends to increase with age,51 and this increase is apparent in Table I and Figure I. As shown in Table I, BMI increases with age (21.4 kg/m2, 23.2 kg/m2, 25.1 kg/m2, and 26.7 kg/m2) as would be expected if we had access to measurements rather than self-report.
As noted, the relatively low rate of questionnaire return (28.5%), and the large percentage of respondents with mixed ancestry (41%), may have biased our sample toward women with hysterectomies. With regard to the determinants of hysterectomy, these results may not be generalizable outside of Hilo or Hawaii because of regional variation in rates of hysterectomy.52
5. Conclusions
The purpose of this study was to consider age at menopause in the multi-ethnic community of Hilo, Hawaii, and to examine determinants of surgical and natural menopause. This study applied a lifespan approach to examine early life events (e.g., where women grew up) and later life events such as the timing of weight gain and the timing of marriage.
Women who were older, married 20 years before the survey, overweight, or of mixed ethnicity were more likely to have undergone a hysterectomy, perhaps because of factors related to a lower level of education such as access to information, access to health care resources, better communication with physicians, or attitudes toward surgical procedures.25,45 The rate of hysterectomies may also be a reflection of the dominant healthcare paradigm in Hilo one to two decades ago.
With regard to age at menopause, it appears that events later in the lifespan (e.g., smoking and not being married 10 years prior to the survey) were more important than events earlier in the lifespan (e.g., childhood residence) in relation to age at menopause.
Acknowledgments
Funding: NIH (No. S06-GM08073-35)
Our thanks to Akolea Ioane, Erin Kalua, Phoebe Mills, Nicola Nicolaisen, Giselle O’Connor, Nichole Rahberg, Harold Tefft, Amber Goodloe, and to the women of Hilo who generously took the time to return the survey.
Footnotes
Contributions:
Lynnette Leidy Sievert, authored the publication and carried out all analyses in close collaboration with Lorna Murphy who supervised logistic regression and Cox regression analyses; Lynn Morrison, supervised data collection and substantially edited the paper; Angela Reza, project manager and edited the paper; Daniel E. Brown, PI of the grant and substantially edited the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lynnette Leidy Sievert, Email: leidy@anthro.umass.edu.
Lorna Murphy, Email: lorna@munroglobal.com.
Lynn Morrison, Email: lmorriso@hawaii.edu.
Angela Reza, Email: angelareza@yahoo.com.
Daniel E. Brown, Email: dbrown@hawaii.edu.
References
- 1.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–10. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 2.Rocca W, Grossardt B, de Andrade M, Malkasian G, Melton J. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821–8. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- 3.Hankinson SE, Colditz GA, Willett WC. Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res. 2004;6:213–8. doi: 10.1186/bcr921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pike MC, Krailo MD, Henderson BE, Casgrande JT, Hoel DG. Hormonal risk factors, breast tissue age and the age-incidence of breast cancer. Nature. 1983;303(5920):767–70. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 5.Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobbee DE, van der Schouw YT. Age at menopause, cause-specific mortality and total life expectancy. Epidemiol. 2005;16(4):556–62. doi: 10.1097/01.ede.0000165392.35273.d4. [DOI] [PubMed] [Google Scholar]
- 6.Snowdon DA, Kane RL, Beeson WL, Burke GL, Sprafka JM, Potter J, Iso H, Jacobs DR, Jr, Phillips RL. Is early natural menopause a biologic marker of health and aging? Am J Public Health. 1989;79(6):709–14. doi: 10.2105/ajph.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobsen BK, Heuch I, Kvale G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol. 2003;157(10):923–9. doi: 10.1093/aje/kwg066. [DOI] [PubMed] [Google Scholar]
- 8.Park CB, Braun KL, Horiuchi BY, Tottori C, Onaka AT. Longevity disparities in multiethnic HI: An analysis of 2000 life tables. Public Health Rep. 2009;124(4):579–84. doi: 10.1177/003335490912400415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4) [PubMed] [Google Scholar]
- 10.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–74. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 11.Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, Lee JS, Thurston R, Vuga M, Harlow SD. Factors related to age at natural menopause: longitudinal analyses from SWAN. [advance access 6/20/13];Am J Epidemiol. 2013 doi: 10.1093/aje/kws421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanford JL, Hartge P, Brinton LA, Hoover RN, Brookmeyer R. Factors influencing the age at natural menopause. J Chronic Dis. 1987;40:995–1002. doi: 10.1016/0021-9681(87)90113-5. [DOI] [PubMed] [Google Scholar]
- 13.de Bruin JP, Bovenhuis H, van Noord PAH, Pearson PL, van Arendonk JAM, te Velde ER, Kuurman WW, Dorland M. The role of genetic factors in age at natural menopause. Hum Repro. 2001;16(9):2014–8. doi: 10.1093/humrep/16.9.2014. [DOI] [PubMed] [Google Scholar]
- 14.Peccei JS. First estimates of heritability in the age of menopause. Current Anthropol. 1999;40:553–58. [Google Scholar]
- 15.Sneider H, MacGregor AJ, Spector TD. Genes control the cessation of a woman’s reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metabol. 1998;83:1875–80. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- 16.Treloar SA, Do K-A, Martin NG. Genetic influences on the age at menopause. Lancet. 1998;352:1084–5. doi: 10.1016/S0140-6736(05)79753-1. [DOI] [PubMed] [Google Scholar]
- 17.Elias SG, van Noord PA, Peeters PH, den Tonkelaar I, Grobbee DE. Caloric restriction reduces age at menopause: the effect of the 1944–1945 Dutch famine. Menopause. 2003;10(5):399–405. doi: 10.1097/01.GME.0000059862.93639.C1. [DOI] [PubMed] [Google Scholar]
- 18.Cresswell JL, Egger P, Fall CHD, Osmond C, Fraser RB, Barker DJP. Is the age of menopause determined in-utero? Early Hum Dev. 1997;49:143–8. doi: 10.1016/s0378-3782(97)00028-5. [DOI] [PubMed] [Google Scholar]
- 19.Lawlor DA, Ebrahim S, Smith GD. The association of socio-economic position across the life course and age at menopause: the British Women’s Heart and Health Study. BJOG. 110(2):1078–87. [PubMed] [Google Scholar]
- 20.Hardy R, Kuh D. Does early growth influence timing of the menopause? Evidence from a British birth cohort. Hum Reprod. 2002;17:2474–9. doi: 10.1093/humrep/17.9.2474. [DOI] [PubMed] [Google Scholar]
- 21.Murphy L, Sievert LL, Begum K, Sharmeen T, Chowdhury O, Puleo E, Muttukrishna S, Bentley GR. Life course effects on age at menopause among Bangladeshi sedentees and migrants to the UK. Am J Hum Biol. 2013;25(1):83–93. doi: 10.1002/ajhb.22345. [DOI] [PubMed] [Google Scholar]
- 22.Morris DH, Jones MK, Schoemaker MJ, McFadden E, Ashworth A, Swerdlow AJ. Body mass index, exercise, and other lifestyle factors in relation to age at natural menopause: analyses from the breakthrough generations study. Am J Epidemiol. 2012;175(10):998–1005. doi: 10.1093/aje/kwr447. [DOI] [PubMed] [Google Scholar]
- 23.Henderson KD, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the multiethnic cohort study. Am J Epidemiol. 2008;167(11):1287–94. doi: 10.1093/aje/kwn046. [DOI] [PubMed] [Google Scholar]
- 24.Sievert LL, Waddle D, Canali K. Marital status and age at natural menopause: considering pheromonal influence. Am J Hum Biol. 2001;13:479–85. doi: 10.1002/ajhb.1079. [DOI] [PubMed] [Google Scholar]
- 25.Goodman MJ, Stewart CJ, Gilbert F. Patterns of menopause: a study of certain medical and physiological variables among Caucasian and Japanese women living in Hawaii. J Gerontol. 1977;32(3):291–8. doi: 10.1093/geronj/32.3.291. [DOI] [PubMed] [Google Scholar]
- 26.Gold EB, Sternfeld B, Kelsey JL, et al. The relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol. 2000;152:463–67. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 27.Templeman C, Marshall SF, Clarke CA, Henderson KD, Largent J, Neuhausen S, et al. Risk factors for surgically removed fibroids in a large cohort of teachers. Fertil Steril. 2009;92:1436–46. doi: 10.1016/j.fertnstert.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lind AW. Hawaii’s People. 4. Honolulu: University Press of Hawaii; 1980. [Google Scholar]
- 29.McDermott JF, Andrade NN, editors. People and Cultures of Hawaii: The Evolution of Culture and Ethnicity. Honolulu: University of Hawaii Press; 2011. [Google Scholar]
- 30.Brown DE, Leidy Sievert L, Aki SL, Mills PS, Etrata MB, Kohagura RN, James GD. The effects of age, ethnicity and menopause on ambulatory blood pressure: Japanese-American and Caucasian school teachers in Hawaii. Am J Hum Biol. 2001;13:486–93. doi: 10.1002/ajhb.1080. [DOI] [PubMed] [Google Scholar]
- 31.Kuh D, Hardy R, editors. A Life Course Approach to Women’s Health. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- 32.Mishra GD, Cooper R, Kuh D. A life course approach to reproductive health: theory and methods. Maturitas. 2010;65(2):92–7. doi: 10.1016/j.maturitas.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elder GH. Perspective on the life course. In: Elder GH, editor. Life Course Dynamics: Trajectories and Transitions, 1968–1980. Ithaca: Cornell University Press; 1985. pp. 23–49. [Google Scholar]
- 34.Riley MW. Introduction: Life-course perspectives. In: Riley MW, editor. Aging from Birth to Death: Interdisciplinary Perspectives. Boulder, CO: Westview Press; 1979. pp. 3–13. [Google Scholar]
- 35.Sorensen A, Weinert F, Sherrod L. Human Development and the Life Course: Multidisciplinary Perspectives. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1986. [Google Scholar]
- 36.Sievert LL. Menopause: A Biocultural Perspective. Rutgers University Press; 2006. [Google Scholar]
- 37.Kupperman M, Learman LA, Schembri M, Gregorich SE, Jackson R, Jacoby A, Lewis J, Washington AE. Predictors of hysterectomy use and satisfaction. Obstet Gynecol. 2010;115(3):543–51. doi: 10.1097/AOG.0b013e3181cf46a0. [DOI] [PubMed] [Google Scholar]
- 38.Kjerulff, Langenberg P, Guzinski G. The socioeconomic correlates of hysterectomies in the United States. Am J Public Health. 1993;83(1):106–8. doi: 10.2105/ajph.83.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson JF, Felicio LS. Reproductive aging in the female: an etiological perspective. In: Rothstein M, editor. Review of Biological Research in Aging. Vol. 2. New York: Alan R. Liss; 1985. pp. 251–314. [Google Scholar]
- 40.Thomford PJ, Jelovsek FR, Mattison DR. Effect of oocyte number and rate of atresia on the age of menopause. Reprod Toxicol. 1987;1:41–51. doi: 10.1016/0890-6238(87)90070-0. [DOI] [PubMed] [Google Scholar]
- 41.Sievert LL, Morrison L, Brown DE, Reza AM. Vasomotor symptoms among Japanese-American and European-American women living in Hilo, Hawaii. Menopause. 2007;14(2):261–9. doi: 10.1097/01.gme.0000233496.13088.24. [DOI] [PubMed] [Google Scholar]
- 42.Tinker JN. Intermarriage and assimilation in a plural society: Japanese-Americans in the United States. In: Cretser GA, Leon JJ, editors. Intermarriage in the United States. Vol. 5. Binghamton, N.Y: Hawthorn Press; 1984. pp. 61–74. [Google Scholar]
- 43.Sievert LL, Hautaniemi SI. Age at menopause in Puebla, Mexico. Hum Biol. 2003;75(2):205–26. doi: 10.1353/hub.2003.0037. [DOI] [PubMed] [Google Scholar]
- 44.Hawaii Health. The Hawaii Health Data Warehouse. State of Hawaii Department of Health Behavioral Risk Factor Surveillance System (BRFSS) :49. Report Date 01/23/2012. http://www.hhdw.org/cms/uploads/Data%20Source_%20BRFFS/Hysterectomy/BRFSS_Hysterectomy_IND_00001.pdf.
- 45.Dharmalingam A, Pool I, Dickson J. Biosocial Determinants of Hysterectomy in New Zealand. Am J Public Health. 2000;90:1455–8. doi: 10.2105/ajph.90.9.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parazzini F. Determinants of hysterectomy and oophorectomy in women attending menopause clinics in Italy. Maturitas. 2000;36(1):19–25. doi: 10.1016/s0378-5122(00)00135-3. [DOI] [PubMed] [Google Scholar]
- 47.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 48.Awwad J, Savegh R, Yeretzian J, Deeb ME. Prevalence, risk factors, and predictors of pelvic organ prolapse: a community-based study. Menopause. 2012;19(11):1235–41. doi: 10.1097/gme.0b013e31826d2d94. [DOI] [PubMed] [Google Scholar]
- 49.Midgett AS, Baron JA. Cigarette smoking and risk of natural menopause. Epidemiol. 1990;1:474–80. doi: 10.1097/00001648-199011000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obesity Rev. 2007;8(4):307–26. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 51.Ogden CL, Yanovski SZ, Carroll MD, et al. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 52.Lepine LA, Hillis SD, Marchbanks PA, Koonin LM, Morrow B, Kieke BA, Wilcox LS. Hysterectomy surveillance—United States, 1980–1993. MMWR CDC Surveill Summ. 1997;46:1–15. [PubMed] [Google Scholar]