Abstract
Rationale
The serotonin (5-hydroxytryptamine, 5-HT) system plays an important role in stress related psychiatric disorders and substance abuse. Our data indicate that stress inhibits the dorsal raphe nucleus (DR)-5-HT system via stimulation of GABA synaptic activity by the stress neurohormone corticotropin-releasing factor and more recently that morphine history sensitizes 5-HT DR neurons to GABAergic inhibitory effects of stress.
Objectives
We tested the hypothesis that GABAA receptors on 5-HT neurons contribute to stress-induced reinstatement of morphine conditioned place-preference (CPP).
Methods
First, we tested if activation of GABAA receptors in the DR would reinstate morphine CPP. Second, we tested if blockade of GABAA receptors in the DR would attenuate swim stress-induced reinstatement of morphine CPP. CPP was induced by morphine (5 mg/kg) in a 4-day conditioning phase followed by a conditioning test. Upon acquiring conditioning criteria, subjects underwent 4 days of extinction training followed by an extinction test. Upon acquiring extinction criteria, animals underwent a reinstatement test. For the first experiment, the GABAA receptor agonist muscimol (50 ng) or vehicle was injected into DR prior to the reinstatement test. For the second experiment, the GABAA receptor antagonist bicuculline (75 ng) or vehicle was injected into the DR prior to a forced swim stress, and then animals were tested for reinstatement of CPP.
Results
Intraraphe injection of muscimol reinstated of morphine CPP, while intraraphe injection of bicuculline attenuated swim stress-induced reinstatement.
Conclusions
These data provide evidence that GABAA receptor-mediated inhibition of the serotonergic DR contributes to stress-induced reinstatement of morphine CPP.
Keywords: Addiction, stressor, relapse, morphine, conditioned place-preference, stress-induced reinstatement, swim, corticotropin-releasing factor (CRF), dorsal raphe, GABAA
1. Introduction
Drug addiction is characterized by repeated relapse to drug use even after a prolonged period of abstinence. This relapse is often triggered by exposure to stress (Goeders 2003; Sinha 2008). Stress-induced relapse has been modeled in animal models such as conditioned place-preference (CPP) and self-administration, in which stress, e.g. forced swim, can reinstate drug-seeking behavior in animals with a history of drug-taking (Conrad et al. 2010; Katz and Higgins 2003; Shaham et al. 2003; Staub et al. 2012). Understanding the neurobiological mechanisms underlying stress-induced drug relapse could contribute to the development of novel therapeutic strategies for drug addiction.
In response to stress, corticotropin-releasing factor (CRF) is released by hypothalamus as the primary neurohormone which activates the hypothalamic-pituitary-adrenal (HPA) axis (Habib et al. 2001; Strohle and Holsboer 2003). The critical role of CRF in stress-induced reinstatement has been identified for several addictive drugs including heroin, cocaine and alcohol (Buffalari et al. 2012; Le et al. 2011; Shaham et al. 1997; Shalev et al. 2010). Interestingly, evidence suggests that the effect of CRF in stress-induced reinstatement may be independent of the HPA axis but instead is related to its actions at extrahypothalamic sites (Erb et al. 1998; Marinelli et al. 2007; Shaham et al. 1997; Shalev et al. 2010), such as the dorsal raphe nucleus (DR).
The DR, which contains the majority of the serotonin (5-hydroxytryptamine, 5-HT) neurons projecting to the forebrain (Jacobs and Azmitia 1992), plays an important role in stress-related psychiatric disorders (Baldwin and Rudge 1995; Mann 1999). DR-5-HT neurons are strongly regulated by CRF in a bimodal manner, in which activation of difference CRF receptor subtypes can have opposing effects on DR-5-HT neurons (Valentino et al. 2010). Although there is evidence that 24 hours after exposure to swim stress DR-5-HT neurons exhibited increased excitability (Lamy and Beck 2010), acute stress as well as intraDR injection of low doses of CRF inhibits DR-5-HT activity (Kirby et al. 2000; Price et al. 1998; Price et al. 2002) resulting in changes of 5-HT release in targeted brain regions, e.g. decreased 5-HT levels in the lateral septum, amygdala and thalamus-hypothalamus but increased 5-HT in the striatum (Briones-Aranda et al. 2005; Kirby et al. 1995; Kirby and Lucki 1998).
The inhibitory effect of acute stress and CRF on 5-HT neurons is indirectly mediated by GABAergic interneurons in DR. Anatomical studies showed that DR GABAergic interneurons receive strong CRF-containing projections and have dense expression of CRF-R1 receptors (Roche et al. 2003). Electrophysiological studies showed direct evidence that CRF activates CRF-R1 receptors on GABAergic neurons causing increased presynaptic release of GABA onto 5-HT DR neurons (Kirby et al. 2008). Behavioral studies further confirm the involvement of GABA transmission in DR in anxiety-related and defensive responses (Takahashi et al. 2010b; Zangrossi et al. 2001).
The role of GABA transmission in DR-5-HT system in drug-related stress disorders is also supported by emerging evidence. For example, infusion of GABA agonists into DR reduces cocaine-induced anxiety (Ettenberg et al. 2011). Infusion of a GABAA, but not GABAB, receptor agonist muscimol in the DRN escalates alcohol-heightened aggression (Takahashi et al. 2010a). Moreover, injecting muscimol into medial raphe nucleus (MRN), another major source of 5-HT projections to forebrain sites with shared as well as distinct projections compared to DRN (Vertes et al. 1999), reinstates alcohol seeking (Le et al. 2008). Recent data from our laboratory indicate that morphine history sensitizes 5-HT DR neurons to the GABAergic inhibitory effects of stress (Staub et al. 2012), suggesting that the subjects with morphine history may be more vulnerable to the effects of stress. However, there is no direct evidence to date showing that activation of the DR GABA system causes stress-induced reinstatement of morphine seeking. Therefore, in our current experiment we hypothesized that GABAA receptors in the DR contribute to stress-induced reinstatement of morphine conditioned place-preference (CPP). To test this hypothesis, in the first experiment, we tested if activation of GABAA receptors in the DR would induce reinstatement in abstinent subjects with a history of morphine CPP. In the second experiment, we tested if blockade of GABAA receptors in the DR would attenuate swim stress-induced reinstatement of morphine CPP.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats (Taconic Farms, Germantown, NY) arrived in the laboratory at the weight of 225–250g and were housed 2–3 per cage under standard temperature (20°C) and humidity (40%) on a 12 h light/dark cycle (lights on at 7:00 AM). After 6–7 days of acclimation, rats received intracranial implantation of a guide cannula into dorsal raphe nucleus, after that they were singly housed throughout the rest of experiment. Food and water were provided ad libitum. All subjects were observed and/or weighed daily to assess general health and responsiveness to drug exposure. Animal protocols were approved by the Temple University Institutional Animal Care and Use Committee and were conducted in accordance to the National Research Council Guide for the Care and Use of Laboratory Animals.
2.2. Surgery
A guide cannula was implanted with a stereotaxic apparatus aimed at the mid-line of the dorsal raphe nucleus. Rats were anesthetized with a ketamine/acepromazine mixture (40.5 mg/kg/0.4 mg/kg, intramuscular), the skull was immobilized. A 26-gauge guide cannula (Plastics One, Roanoke, Virginia, USA) was implanted using the coordinates: −7.8 mm caudal to bregma, −2.2 mm from the midline, and −4.6 mm ventral from the brain surface at a 25° angle to bypass the sagittal sinus. The cannula was secured to the skull with the use of dental cement and three stainless steel screws. An obdurator was inserted into the guide cannula to maintain patency of the cannula and prevent infection. Animals were allowed to recover for 6–7 days before beginning of behavioral testing.
2.3. Drugs
Morphine (made by Research Triangle Institute and generously supplied by the National Institute on Drug Abuse) was dissolved in 0.9% saline and administered subcutaneously (s.c.) in a dose of 5 mg/kg. This dose was selected because it induced a robust CPP that could be extinguished and reinstated by exposure to a forced swim stressor in our previous study (Staub et al. 2012). To activate GABAA receptors in DR, a GABAA agonist muscimol (3mM) (Sigma-Aldrich, St. Louis, MO) was prepared in 0.9% saline in a final dose of 50ng/0.5µl (Ferreira and Menescal-de-Oliveira 2012; Tomkins and Fletcher 1996; Tomkins et al. 1994a; b) and infused into the DR. To block GABAA receptors, a water soluble GABAA antagonist bicuculline methobromide (3.25mM) (Sigma-Aldrich, St. Louis, MO) was prepared in 0.9% saline in a final dose of 75ng/0.5µl and infused into the DR (Duncan and Congleton 2010; Tao and Ma 2012). The doses were chosen on the basis of previous publications as well as pilot studies, using the criteria of behavioral efficacy with few confounding side effects. For muscimol, a lower dose (25ng) than current selected one was ineffective at inducing reinstatement, whereas the selected dose of muscimol (50 ng) was at the very edge of sedation-producing effects. At the dose of 50ng, 2 out of 10 animals from saline-conditioned and 2 out of 10 animals from morphine-conditioned groups showed significant impairment in locomotion, making fewer than 5 crosses between the two chambers of the CPP apparatus in 15 min and were removed from the study. For bicuculline, about 25% of the animals demonstrated turning behaviors as other labs has described in mice for intra-DR bicuculline in the upper range of the dose-response curve (Takahashi et al. 2010a; Takahashi et al. 2010b). This behavior was observed immediately following the drug injection but largely resolved by the reinstatement session and did not seem interfere with their exploration, so no rats were excluded from the study. Infusions were made over 1 min and the infusion needle left in place for an additional 2 min before it was removed.
2.4. Conditioned Place Preference and Extinction
The CPP procedure was conducted as described previously (Staub et al. 2012). The CPP chambers consisted of two distinct boxes, and were designed and tested to ensure that animals would show no inherent preference for one side or another; all CPP experiments were conducted using an unbiased procedure. All experimental work was performed during the light phase under low lighting conditions.
For morphine-conditioned subjects, the conditioning phase consisted of 4 days (days 1–4) of subcutaneous injections of morphine (5 mg/kg) or saline given alternately in morning (10am) and afternoon (4pm) sessions (6h apart), each injection was followed by immediate confinement of the rat to its drug-paired or saline-paired chamber for 45 min (Fig. 1). For saline-conditioned controls, rats were given only saline. Injections were counterbalanced for chamber and injection time of day. On the 5th day rats were tested for conditioning by placing subjects in the CPP chambers for 15 min with free access to both sides. Successful conditioned place preference was defined as time in drug-paired minus saline-paired chamber >100 s (Herzig and Schmidt 2004; 2005). If the difference was below 100 s then the rat was removed from the experiment.
Fig. 1.
Experimental design time-line for morphine CPP paradigm with extinction and stress-induced reinstatement. Mor: morphine, Sal: saline, No inj: no injections.
For extinction of morphine CPP, all rats first underwent 4 days of extinction sessions (day 6–9), which had the same design as the conditioning phase (Fig. 1) except that no morphine was administered. On day 10 rats were tested for extinction by placing them in the CPP chambers for 15 min with free access to both sides. Successful extinction of CPP was defined as the time in the drug-paired minus saline-paired chamber <100 s. Rats that passed the extinction test were tested with reinstatement on the next day. Rats that did not pass the first extinction test were given additional extinction sessions by placing them in the CPP chambers and testing with an extinction test after each extinction session until they reached the successful extinction criteria (day 11–15). If the rats could not reach the extinction criteria on the fourth extinction test (day 16), they were removed from the experiment. The reinstatement test was performed on the day following a successful extinction test.
2.4.1. Experiment 1: Effect of GABAA receptor agonist in the DR on reinstatement of morphine CPP
To test the hypothesis that activation of GABAA receptors in the DR would induce reinstatement in abstinent subjects with a history of morphine CPP, the GABAA receptor agonist muscimol or vehicle was injected into the DR 20 min prior to the reinstatement test. For the reinstatement test, rats were placed in the CPP chambers for 15 min with free access to both sides, and their behavior was videotaped for analysis.
2.4.2. Experiment 2: Effect of GABAA receptor antagonist in the DR on swim stress-induced reinstatement of morphine CPP
To test the hypothesis that blockade of GABAA receptors in the DR would attenuate swim stress-induced reinstatement of morphine CPP, the GABAA receptor antagonist bicuculline or vehicle was injected into the DR 15 min prior to a 6-min forced swim stress (placed in a swim tank that is 20 cm in diameter and filled with 21—22 °C water to a depth of 30 cm), then after 20 min drying off period, animals were tested for reinstatement of CPP as described above.
2.5. Histology
After completion of behavioral testing all animals were euthanized with carbon dioxide overdose, and 0.5 µl of Evans blue dye (Sigma-Aldrich, St. Louis, MO) was injected through the guide cannula. Brains were removed and sliced in 30-µm frozen sections. Placements of cannulae were verified by comparing the slides with a rat brain atlas by Paxinos and Watson (Paxinos and Watson 1998).
2.6. Data analysis
For experiment 1, the time spent on the drug-paired side minus the unpaired side in morphine- or saline-conditioned groups was analyzed using a two-way mixed-measures ANOVA with phase (conditioning, extinction vs. reinstatement) as a within-subjects factor and reinstatement drug (intraDR muscimol vs. intraDR saline) as a between-subjects factor. Paired-samples t-tests (two-tailed) with Bonferroni correction were conducted as follow-up tests. According to our hypothesis, a pre-planned t-test was conducted to compare reinstatement of morphine CPP between the intraDR muscimol and intraDR saline group since the two groups of animals were treated identically in conditioning and extinction tests.
For experiment 2, the time spent on the drug-paired side minus the unpaired side in morphine- or saline-conditioned groups was analyzed using a two-way mixed-measures ANOVA with phase (conditioning, extinction vs. reinstatement) as a within-subjects factor and reinstatement treatment (bicuculline+swim, bicuculline vs. saline+swim) as a betweensubjects factor. One-way ANOVA and post hoc Tukey tests as well as one-way repeatedmeasures ANOVA and paired-sample t-tests with Bonferroni correction were conducted as follow-up tests.
3. Results
3.1. Histology
Each rat received an injection of muscimol, bicuculline or saline into the DR through a permanent guide cannula. The site of injection was confirmed by histological analysis after behavioral tests (Fig. 2). For each subject, an identical volume and procedure was used for injection of Evans blue dye to confirm that there was no diffusion beyond the boundaries of the DR. Only the animals with correct location of injection were included in the behavioral analysis (76 out of 132 for morphine- and saline-conditioned).
Fig. 2.
Sites of injection in the DR. a. Representative rat coronal brain section with Evans Blue dye infused through the guide cannula. b. Schematic representation of injection sites for experiment 1 and 2 at 4 different rostro-caudal levels (−7.3 to −8.3mm from Bregma) of the DR (shaded in grey). Each black dot represents an individual animal. Aq: cerebral aqueduct.
3.2. Behavior
CPP was used to evaluate drug reward during conditioning, extinction, and reinstatement tests. As we described previously (Staub et al. 2012), animals that did not reach successful criteria for conditioning or extinction were eliminated from this study (see Methods, Section 2.4) because the study focused on the role of GABAA receptors in DRN in stress-induced reinstatement. The success rate for conditioning was 83% (81 out of 98) and for extinction was 91% (74 out of 81).
3.2.1. Experiment 1: GABAA receptor agonist in the DR reinstated morphine CPP
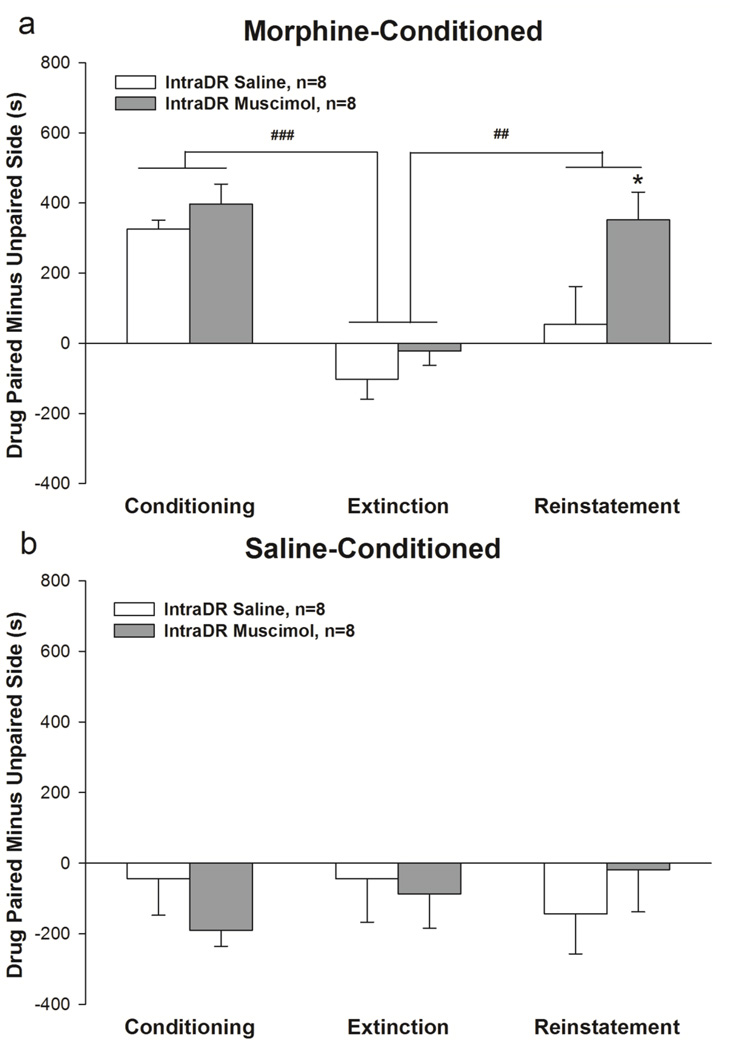
The morphine-conditioned group spent significantly more time on the drug-paired side than the saline-paired side in conditioning test, and this preference was extinguished after repeated extinction training. IntraDR injection of muscimol but not saline reinstated the preference for the drug-paired side (Fig. 3a, n=8/group). The two-way phase × reinstatement drug ANOVA revealed main effects of phase [F(2,28)=19.386, p < 0.001] and reinstatement drug [F(1,14)=8.421, p < 0.05] but no interaction. Post hoc paired-samples t-tests with Bonferroni correction revealed that conditioning vs. extinction (t(15)=10.629, p<0.001) and reinstatement vs. extinction (t(15)=3.536, p<0.01) were different. The pre-planned t-tests (see Methods, Section 2.6) revealed that reinstatement in the intraDR muscimol group was different from the intraDR saline group (t(14)=2.221, p<0.05), and reinstatement was different from extinction in the intraDR muscimol group (t(7)=4.887, p<0.01) but not in the intraDR saline group. For saline-conditioned groups the two-way phase×reinstatement drug ANOVA revealed no main effects or interaction (Fig. 3b).
Fig. 3.
Conditioned place preference, extinction and reinstatement in morphine-conditioned (a) and saline-conditioned subjects (b). Data represent mean±SEM time spent in drug paired side minus unpaired side. IntraDR injection of muscimol induced reinstatement of previously extinguished morphine CPP (* indicates p<0.05 vs. IntraDR saline group on the targeted t-test, ## p<0.01, ### p<0.001 vs. extinction in IntraDR Saline and IntraDR Muscimol groups combined).
3.2.2. Experiment 2: GABAA receptor antagonist in the DR prevented swim stressinduced reinstatement of morphine CPP
The morphine-conditioned group spent significantly more time on the drug-paired side than the saline-paired side in the conditioning test, and this preference was extinguished after repeated extinction training. Swim stress reinstated morphine CPP in animals that received intraDR injection of saline but was this effect was blocked by intraDR injection of bicuculline. IntraDR injection of bicuculline itself did not reinstate previously extinguished morphine CPP (Fig. 4a), similar to saline controls from experiment 1 (Fig 3a). The two-way phase × reinstatement drug ANOVA revealed main effects of phase [F(2,46)=17.567, p < 0.001] and reinstatement treatment [F(2,46)=5.306, p <0.05] as well as interaction [F(4,46)=3.35, p <0.05]. One-way repeated-measures ANOVAs revealed main effects of phase in all three reinstatement treatment groups [saline+swim: F(2,18) =12.541, p<0.001; bicuculline: F(2,14)=9.327, p<0.01, bicuculline+swim: F(2, 14)=5.249, p<0.05)]. Post hoc analysis showed that in the saline+swim group both conditioning [t(9)=8.047, p<0.001] and reinstatement [t(9)=−3.795, p<0.01] were different from extinction. In the bicuculline and bicuculline+swim groups conditioning was different from extinction [bicuculline: t(7)=6.317, p<0.001; bicuculline+swim: t(7)=10.423, p<0.001] but no other comparisons showed significant differences. One-way ANOVAs revealed a main effect of reinstatement treatment in reinstatement [F(2,23)= 4.433, p< 0.05] but not other phases. Post hoc analysis showed that reinstatement in the intraDR bicuculline+swim group was different from the intraDR saline+swim group (p<0.05) but reinstatement did not differ between the other two groups. For saline-conditioned groups the two-way phase × reinstatement treatment ANOVA revealed no main effects or interaction (Fig 4b). Moreover, from experiment 1, intraDR injection of saline did not reinstate CPP in either morphine or saline-conditioned animals (Fig 3).
Fig. 4.
Conditioned place preference, extinction and swim stress-induced reinstatement in morphine-conditioned (a) and saline-conditioned subjects (b). Data represent mean±SEM time spent in drug paired side minus unpaired side. IntraDR injection of bicuculline blocked swim stress-induced reinstatement of previously extinguished morphine CPP (* indicates p<0.05 vs. IntraDR saline group, ## p<0.01, ### p<0.001 vs. extinction).
4. Discussion
The primary finding in current study is that GABA transmission in DR-5-HT system is critical to stress-induced reinstatement of morphine CPP. IntraDR injection of a GABAA receptor agonist induced reinstatement of morphine CPP, while intraDR injection of a GABAA receptor antagonist blocked swim stress-induced reinstatement. These data provide behavioral evidence that stress-induced inhibition of DR-5-HT neurons could trigger reinstatement of drug seeking.
While the roles of dopaminergic and glutamatergic circuits in drug addiction and relapse are well-established, these data add evidence to a growing literature implicating the serotonin system in the neurobiological mechanisms underlying reinstatement of drug seeking (For reviews Filip et al. 2010; Kirby et al. 2011). For example, systemic stimulation of the 5-HT system with 5-HT-selective reuptake inhibitors or 5-HT releasing agents suppresses both cue-elicited cocaine reinstatement (Baker et al. 2001; Burmeister et al. 2003) and also stress-induced reinstatement of alcohol seeking behavior in rats (Le et al. 1999). Studies focusing on serotonin receptor types suggest that different types of serotonin receptors may play opposite roles in reinstatement (Filip et al. 2010). For example, blockade of 5-HT2A receptors attenuates both drug- and cue-induced reinstatement (Filip 2005; Fletcher et al. 2002; Nic Dhonnchadha et al. 2009), whereas stimulation of 5-HT2C receptors attenuates cocaine-, cue- (Fletcher et al. 2002; Fletcher et al. 2008; Neisewander and Acosta 2007) and stress-induced cocaine reinstatement (Fletcher et al. 2008). Although only a few studies have investigated the role of the sources of the serotonergic projections in drug (alcohol and cocaine) reinstatement, their results are consistent with our findings suggesting that inhibition of 5-HT cell bodies reinstates drug seeking whereas stimulation of 5-HT cell bodies blocks stress-induced reinstatement (Land et al. 2009; Le et al. 2008; Le et al. 2002). Therefore, the serotonergic system could be a novel target for the treatment of addiction and the prevention of relapse to multiple drugs of abuse.
Together with the evidence that opioid history also influences the GABAergic transmission in DR (For review Kirby et al. 2011), the current data provide a potential mechanism to explain the high degree of comorbidity of affective disorders with opioid dependence and high vulnerability to stress- induced reinstatement in opioid-dependent subjects (Sinha 2008). The primary functional target of opioid compounds in DR is mu–opioid receptors on GABAergic interneurons (Kalyuzhny and Wessendorf 1997). Acute opioid administration inhibits GABAergic neurons via mu-opioid receptors thus indirectly excites DR-5-HT neurons, increasing 5-HT levels in projection brain regions (Jolas and Aghajanian 1997; Tao and Auerbach 2002). Although there is no direct evidence showing that excitation of DR-5-HT by opioids produces euphoria, the fact that the DR supports intracranial self-stimulation suggests that the DR-5-HT system may play a role in reward and reinforcement (Rompre and Boye 1989; Simon et al. 1976) (But see Houdouin et al. 1991). However, after repeated opioid exposure, the stimulatory effect of morphine on DR-5-HT neurons is largely reduced or absent due to tolerance to the effect of mu-receptor activation on GABAergic neurons (Tao et al. 1998). Moreover, during withdrawal the activity of DR-5-HT neurons is also reduced due to an increase of GABA synaptic activity (Jolas et al. 2000). More recently, our data indicated that morphine history sensitizes DR-5-HT neurons to GABA inhibition in response to stress, and this sensitization may explain the vulnerability of subjects with an opioid history to stress-induced relapse (Staub et al. 2012). In support the above hypothesis, our current experiments establish a causal relationship between GABAA receptors in the DR and stress-induced reinstatement.
5-HT hypofunction is known to increase impulsive behaviors including motor impulsivity and choice impulsivity (Cardinal 2006) and impulsivity is an established risk factor for drug addiction and relapse in both human and animal studies (For review Kirby et al. 2011). For example, inhibition of DR-5-HT neurons with a 5-HT1A receptor agonist impairs an animal’s ability to wait for long-delayed rewards (Miyazaki et al. 2012). Moreover, rats receiving intraMRN infusion of muscimol show impaired performance in a five-choice serial reaction time task (5-CSRTT) (Le et al., 2007), which is a test for sustained attention and impulsivity. In addition, rats showing high impulsivity in the 5-CSRTT have persistent relapse behavior compared with low impulsive counterparts (Economidou et al. 2009). Therefore, inactivation of DR-5-HT neurons by muscimol in our study may increase impulsivity, contributing to reinstatement of morphine CPP.
A few limitations of the present study need to be mentioned, and future studies will be required to fully characterize the role of GABAA receptors the DR in stress-induced reinstatement. First, when the GABAA receptor agonist and antagonist were injected into the DR, they influenced not only 5-HT neurons but also other cell types and neuronal fibers expressing GABAA receptors. To resolve this limitation, we plan to use a conditional knockout approach in future studies to silence or activate GABAA receptors in only DR-5-HT neurons. Second, the DR is not a homogenous structure but can be divided into ventromedial, dorsomedial and lateral wing subregions based on their anatomical, neurochemical and functional characteristics (Calizo et al. 2011). In our current study, we did not distinguish the subregions of the DR mainly due to surgical difficulties and diffusion of injecting solutions. To resolve this limitation, smaller injection volumes targeted to individual DR subdivisions can be used to dissect their distinct contributions to stress-induced reinstatement. Third, the roles of CRF and its receptor subtypes in the DR in stress-induced reinstatement have not been tested. Our current study was based on the rationale that stress indirectly inhibits of DR-5-HT neurons by activation of CRF-R1 receptors expressed on GABAergic interneurons (Valentino et al. 2010). Although we showed that activation of GABAA receptors in the DR was critical for stress-induced reinstatement of CPP, understanding the role of CRF in the DR in stress-induced reinstatement is necessary for fully supporting the hypothesis, especially considered the bimodal influence of CRF on 5-HT DR neurons (Valentino et al. 2010). Therefore, to resolve this limitation, the effects of intra-DRN injection of CRF-R1 and -R2 receptor-selective agonists and antagonists on swim stress-induced reinstatement of morphine CPP will be tested in our future studies. Last, Although CPP model is widely used for inferring the hedonic value of addictive drugs, questions remain whether it is an adequate procedure to represent active drug-seeking behaviors that characterize drug addiction in humans (Sanchis-Segura and Spanagel 2006). To resolve this limitation, self-administration, which can measure voluntary drug intake in animals, is currently under development in our laboratory and will be used to confirm the current findings. Self-administration can better represent addiction-like behavior, (Sanchis-Segura and Spanagel 2006), such as progressive ratio schedule to assess motivation to seek drug (Roberts et al. 1989), punished responding to model compulsivity (Vanderschuren and Everitt 2004), and extended access to model binge use (Mantsch et al. 2004).
In summary, this study shows that forced swim stress induced reinstatement of previously extinguished morphine CPP, and GABA transmission in DR-5-HT system is critical for this reinstatement. Together with our previous data, it is suggested that adaptations of GABAergic synaptic transmission caused by opioid history may contribute to enhanced relapse vulnerability in these subjects.
Acknowledgments
This research was supported by NIH R01 DA 20126 and NIH P30 DA 13429.
Footnotes
Conflict of interest: All authors declare no conflict of interests and have no disclosures.
References
- Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL. Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2001;155:18–26. doi: 10.1007/s002130000676. [DOI] [PubMed] [Google Scholar]
- Baldwin D, Rudge S. The role of serotonin in depression and anxiety. Int Clin Psychopharmacol. 1995;9(Suppl 4):41–45. doi: 10.1097/00004850-199501004-00006. [DOI] [PubMed] [Google Scholar]
- Briones-Aranda A, Rocha L, Picazo O. Influence of forced swimming stress on 5-HT1A receptors and serotonin levels in mouse brain. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:275–281. doi: 10.1016/j.pnpbp.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE. Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav. 2012;105:209–214. doi: 10.1016/j.physbeh.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:146–154. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw. 2006;19:1277–1301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Conrad KL, McCutcheon JE, Cotterly LM, Ford KA, Beales M, Marinelli M. Persistent increases in cocaine-seeking behavior after acute exposure to cold swim stress. Biol Psychiatry. 2010;68:303–305. doi: 10.1016/j.biopsych.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Congleton MR. Neural mechanisms mediating circadian phase resetting by activation of 5-HT(7) receptors in the dorsal raphe: roles of GABAergic and glutamatergic neurotransmission. Brain Res. 2010;1366:110–119. doi: 10.1016/j.brainres.2010.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Ofer OA, Mueller CL, Waldroup S, Cohen A, Ben-Shahar O. Inactivation of the dorsal raphe nucleus reduces the anxiogenic response of rats running an alley for intravenous cocaine. Pharmacol Biochem Behav. 2011;97:632–639. doi: 10.1016/j.pbb.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MD, Menescal-de-Oliveira L. Opioidergic, GABAergic and serotonergic neurotransmission in the dorsal raphe nucleus modulates tonic immobility in guinea pigs. Physiol Behav. 2012;106:109–116. doi: 10.1016/j.physbeh.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Filip M. Role of serotonin (5-HT)2 receptors in cocaine self-administration and seeking behavior in rats. Pharmacol Rep. 2005;57:35–46. [PubMed] [Google Scholar]
- Filip M, Alenina N, Bader M, Przegalinski E. Behavioral evidence for the significance of serotoninergic (5-HT) receptors in cocaine addiction. Addict Biol. 2010;15:227–249. doi: 10.1111/j.1369-1600.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT2C receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The impact of stress on addiction. Eur Neuropsychopharmacol. 2003;13:435–441. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001;30:695–728. vii–viii. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- Herzig V, Schmidt WJ. Effects of MPEP on locomotion, sensitization and conditioned reward induced by cocaine or morphine. Neuropharmacology. 2004;47:973–984. doi: 10.1016/j.neuropharm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Herzig V, Schmidt WJ. Anti-craving drugs acamprosate and naloxone do not reduce expression of morphine conditioned place preference in isolated and group-housed rats. Neurosci Lett. 2005;374:119–123. doi: 10.1016/j.neulet.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Houdouin F, Cespuglio R, Jouvet M. Effects induced by the electrical stimulation of the nucleus raphe dorsalis upon hypothalamic release of 5-hydroxyindole compounds and sleep parameters in the rat. Brain Res. 1991;565:48–56. doi: 10.1016/0006-8993(91)91735-j. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jolas T, Aghajanian GK. Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat in vitro. Brain Res. 1997;755:229–245. doi: 10.1016/s0006-8993(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Jolas T, Nestler EJ, Aghajanian GK. Chronic morphine increases GABA tone on serotonergic neurons of the dorsal raphe nucleus: association with an up-regulation of the cyclic AMP pathway. Neuroscience. 2000;95:433–443. doi: 10.1016/s0306-4522(99)00436-4. [DOI] [PubMed] [Google Scholar]
- Kalyuzhny AE, Wessendorf MW. CNS GABA neurons express the mu-opioid receptor: immunocytochemical studies. Neuroreport. 1997;8:3367–3372. doi: 10.1097/00001756-199710200-00035. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1995;682:189–196. doi: 10.1016/0006-8993(95)00349-u. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Lucki I. The effect of repeated exposure to forced swimming on extracellular levels of 5-hydroxytryptamine in the rat. Stress. 1998;2:251–263. doi: 10.3109/10253899809167289. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy CM, Beck SG. Swim stress differentially blocks CRF receptor mediated responses in dorsal raphe nucleus. Psychoneuroendocrinology. 2010;35:1321–1332. doi: 10.1016/j.psyneuen.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Coen K, Li Z, Shaham Y. Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement of alcohol seeking in rats. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Li Z, Fletcher PJ. Intra-median raphe nucleus (MRN) infusions of muscimol, a GABA-A receptor agonist, reinstate alcohol seeking in rats: role of impulsivity and reward. Psychopharmacology (Berl) 2008;195:605–615. doi: 10.1007/s00213-007-0943-4. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Doya K. Activation of dorsal raphe serotonin neurons is necessary for waiting for delayed rewards. J Neurosci. 2012;32:10451–10457. doi: 10.1523/JNEUROSCI.0915-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol. 2007;18:791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci. 2009;123:382–396. doi: 10.1037/a0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edn. San Diego: Academic Press; 1998. [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Price ML, Kirby LG, Valentino RJ, Lucki I. Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptation produced by repeated swimming. Psychopharmacology (Berl) 2002;162:406–414. doi: 10.1007/s00213-002-1114-2. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompre PP, Boye S. Localization of reward-relevant neurons in the pontine tegmentum: a moveable electrode mapping study. Brain Res. 1989;496:295–302. doi: 10.1016/0006-8993(89)91076-7. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H, Le Moal M, Cardo B. Intracranial self-stimulation from the dorsal raphe nucleus of the rat: effects of the injection of para-chlorophenylalanine and of alpha-methylparatyrosine. Behav Biol. 1976;16:353–364. doi: 10.1016/s0091-6773(76)91486-3. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub DR, Lunden JW, Cathel AM, Dolben EL, Kirby LG. Morphine history sensitizes postsynaptic GABA receptors on dorsal raphe serotonin neurons in a stress-induced relapse model in rats. Psychoneuroendocrinology. 2012;37:859–870. doi: 10.1016/j.psyneuen.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36(Suppl 3):S207–S214. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Kwa C, Debold JF, Miczek KA. GABA(A) receptors in the dorsal raphe nucleus of mice: escalation of aggression after alcohol consumption. Psychopharmacology (Berl) 2010a;211:467–477. doi: 10.1007/s00213-010-1920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABA(B) receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J Neurosci. 2010b;30:11771–11780. doi: 10.1523/JNEUROSCI.1814-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. GABAergic and glutamatergic afferents in the dorsal raphe nucleus mediate morphine-induced increases in serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther. 2002;303:704–710. doi: 10.1124/jpet.102.038133. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z. Neural Circuit in the Dorsal Raphe Nucleus Responsible for Cannabinoid-Mediated Increases in 5-HT Efflux in the Nucleus Accumbens of the Rat Brain. ISRN Pharmacol. 2012;2012:276902. doi: 10.5402/2012/276902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Alteration in regulation of serotonin release in rat dorsal raphe nucleus after prolonged exposure to morphine. J Pharmacol Exp Ther. 1998;286:481–488. [PubMed] [Google Scholar]
- Tomkins DM, Fletcher PJ. Evidence that GABA(A) but not GABA(B) receptor activation in the dorsal raphe nucleus modulates ethanol intake in Wistar rats. Behav Pharmacol. 1996;7:85–93. [PubMed] [Google Scholar]
- Tomkins DM, Sellers EM, Fletcher PJ. Effect of dorsal raphe injections of the GABAA agonist, muscimol, on ethanol intake and measures of intoxication in Wistar rats. Alcohol Alcohol Suppl. 1994a;2:551–558. [PubMed] [Google Scholar]
- Tomkins DM, Sellers EM, Fletcher PJ. Median and dorsal raphe injections of the 5-HT1A agonist, 8-OH-DPAT, and the GABAA agonist, muscimol, increase voluntary ethanol intake in Wistar rats. Neuropharmacology. 1994b;33:349–358. doi: 10.1016/0028-3908(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Lucki I, Van Bockstaele E. Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- Zangrossi H, Jr, Viana MB, Zanoveli J, Bueno C, Nogueira RL, Graeff FG. Serotonergic regulation of inhibitory avoidance and one-way escape in the rat elevated T-maze. Neurosci Biobehav Rev. 2001;25:637–645. doi: 10.1016/s0149-7634(01)00047-1. [DOI] [PubMed] [Google Scholar]