Abstract
Purpose
A Preliminary report of clinical and treatment factors associated with toxicity in men receiving high dose radiation (RT) on a phase III dose escalation trial.
Methods and Materials
Trial was initiated with 3 dimensional RT (3DCRT) and amended after 1 year to allow intensity modulated RT (IMRT). Patients treated with 3DCRT received 55.8Gy to a planning target volume that included the prostate and seminal vesicles then 23.4Gy to prostate only. IMRT patients were treated to the prostate and proximal seminal vesicles to 79.2Gy. CTC v2.0 and RTOG/EORTC late morbidity scores were used for acute and late effects.
Results
748 of 763 patients randomized to the 79.2 Gy arm of RTOG 0126 were eligible and evaluable. 491 and 257 were treated with 3DCRT and IMRT, respectively. For both bladder and rectum, the volumes receiving 65, 70, and 75Gy were significantly lower with IMRT (all p<0.0001).
For G2+ acute GI/GU toxicity, both univariate and multivariate analyses show a statistically significant decrease in G2+ acute collective GI/GU toxicity for IMRT. There are no significant differences with 3DCRT or IMRT for acute or late, G2+ or 3+ GU toxicities. Univariate analysis shows a statistically significant decrease in late G2+ GI toxicity for IMRT (p=0.039). On multivariate analysis, IMRT shows a 26% reduction in G2+ late GI toxicity (p=0.099). Acute G3+ toxicity was associated with late G3+ toxicity (p=0.005). With DVH data in the multivariate analysis, RT modality is not significant whereas white race (p=0.001) and rectal V70 >=15% are associated with G2+ rectal toxicity (p=0.034).
Conclusions
IMRT is associated with a significant reduction in acute G2+ GI/GU toxicity. There is a trend for a clinically meaningful reduction in late G2+ GI toxicity with IMRT. The occurrence of acute GI toxicity and large (>15%) volumes of rectum >70Gy are associated with late rectal toxicity.
Keywords: GI/GU Toxicity, IMRT, 3DCRT
Introduction
A patient’s treatment choice to manage localized prostate cancer depends on multiple factors, many of which are unrelated to the likelihood of long term disease control.(1) Techniques to minimize the risk of treatment-related toxicity have been introduced but they have not been formally evaluated in the context of prospective clinical trials. This is especially relevant with external beam radiation therapy where dose escalation trials have demonstrated improvement in biochemical disease control of prostate cancer while variably being associated with higher rates of toxicity.
Several single institution series have reported a reduction in late toxicity with the introduction of IMRT compared to 3DCRT, even with dose escalation.(2-6) However, there are no reports of a contemporary cohort of patients treated to similar doses that compare toxicity between these two modalities. This report describes the toxicity outcomes of patients enrolled on the high dose arm of a Radiation Therapy Oncology Group (RTOG) prospective phase III trial of conventional dose versus dose escalated radiation therapy which allowed either IMRT or 3DCRT.
Materials and Methods
Study design
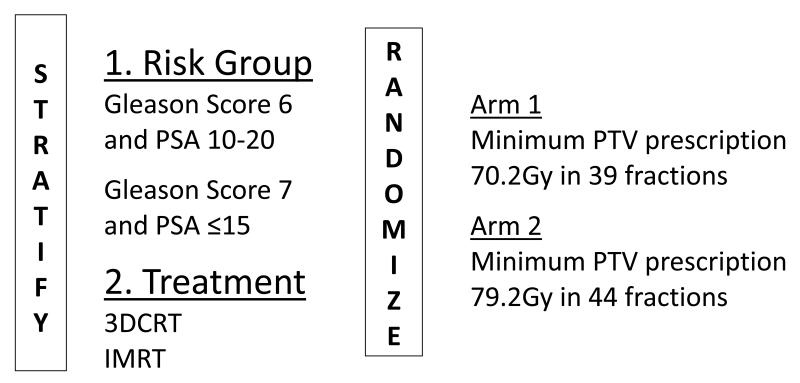
RTOG 0126 is a phase III trial that compares conventional dose (70.2Gy) radiation therapy to dose escalated (79.2Gy) conformal radiation therapy for the management of early stage intermediate risk prostate cancer. The primary objective of the trial is to determine whether an improvement in overall survival can be achieved with dose escalation. In September 2003 the trial (Figure 1) was amended to allow IMRT; treatment modality was added as a stratification variable in order to help avoid treatment arm modality imbalances.
Figure 1.
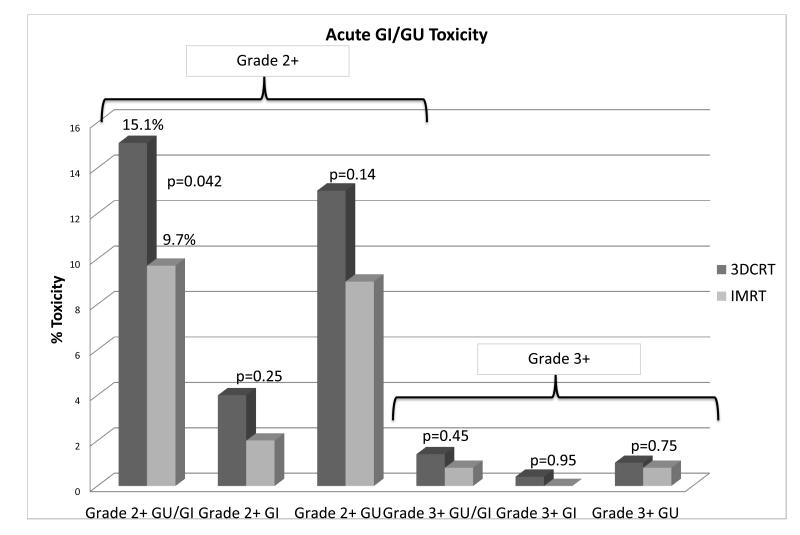
The incidences in grade 2 or greater and grade 3 or greater acute GI or GU, acute GI and acute GU toxicity by radiation modality
Statistical considerations
This is a preliminary analysis of patients treated on the high dose arm of the trial to evaluate potential associations between toxicity and radiation therapy modality. The Common Toxicity Criteria v2.0 (CTC v2.0) and RTOG/EORTC late morbidity scoring systems were used to prospectively collect toxicity data. Acute toxicities were those experienced within 90 days of the start of treatment and late toxicities occurred more than 90 days from the start of treatment. Univariate acute toxicity modality comparisons were done using the chi-square test. Multivariate comparisons were done using logistic regression. (7) Cumulative incidence methods (8) were used to estimate rates of late toxicity and univariate comparisons were done using the Gray’s test. Multivariate analyses for late toxicity were done using the Fine-Gray method.(9)
Patient population
Patients had prostate adenocarcinoma diagnosed within 180 days of registration. Intermediate risk disease with a clinical stage T1b-T2b, Gleason score of 2-6 and PSA ≥ 10 ng/ml but < 20 ng/ml or a Gleason score of 7 with a PSA < 15 ng/ml was eligible. Patients required no evidence of metastases, and no prior prostatectomy, pelvic irradiation, androgen deprivation therapy, 5-α reductase inhibitors, or chemotherapy.
Target Volumes and Organs at Risk
For patients receiving 3DCRT the clinical target volume (CTV) included the prostate and entire seminal vesicles for the first 55.8Gy followed by a boost to the prostate only to a total of 79.2Gy. Because IMRT would have required two separate plans to deliver treatment in a similarly phased schedule the decision was made to modify the high dose target volume for IMRT cases to include the prostate and the proximal 1cm of seminal vesicle tissue identified on the planning CT scan for the entire 79.2Gy. This CTV modification was based upon data demonstrating 93% of 344 prostatectomy specimens had no cancer beyond the first 1cm in the seminal vesicle tissue.(10) All CTVs were required to have a Planning Target Volume (PTV) margin of 0.5 to 1.0 cm surrounding them to account for organ motion or setup uncertainties. The bladder, rectum penile bulb and bilateral femora were defined previously described in other RTOG trials.(11, 12) The bladder was contoured from its base to the dome, and the rectum from the anus (at the level of the ischial tuberosities) for a length of 15 cm or to the rectosigmoid flexure. Both these organs were contoured as solid structures.
Quality Assurance
All treatment plans were submitted digitally to the Image-guided Therapy QA Center (St. Louis, MO) for central review. All centers using IMRT had to successfully irradiate an anthropomorphic phantom from the Radiological Physics Center to demonstrate ability to comply with treatment planning constraints.
Treatment plans were scored as following: No variation (total coverage); Prescription isodose surface covers ≥ 98% of the respective PTVs. Minor variation (marginal coverage); Prescription isodose surface coverage between ≥ 95% to <98% of the PTV. Major variation (suboptimal coverage); Prescription isodose surface coverage < 95% of the PTV, or less than 100% of CTV. The dose heterogeneity in the treatment plans was scored as following: Maximum dose to ≤ 2% of the PTV volume should not exceed the prescription dose by more than 7% (no variation: ≤ 7%; minor variation: > 7 to ≤ 10%; major variation: > 10%). This maximum dose volume of the PTV must not be shared by an “Organ at Risk”. The protocol offered dose guidelines to the bladder, rectum and penile bulb based upon prior RTOG published experience.(12-14)
Results
Patient characteristics
Of the 1532 patients enrolled in the trial from March 2002 to August 2008, 748 of the 763 cases randomized to the high dose arm are eligible and evaluable for this toxicity analysis. Fifteen cases were excluded from this analysis due to withdrawal of consent (n=5), histologic diagnosis established more than 180 days prior to study entry (n=7), ineligible Gleason score, PSA or T-stage combination (n=2) or pre-entry labs or imaging missing or out of protocol range (n=1). Table e.1 lists the pretreatment characteristics. Patients treated at U.S. institutions were significantly more likely to have been treated with IMRT. This finding may explain the racial imbalance and the preponderance of T1 stage in the IMRT group as US patients may have been more likely to be diagnosed by PSA screening. There were no significant differences in baseline urinary incontinence, urgency, frequency or sexual impotence. The median follow up for the 3DCRT patients is 4.6 (0.1-8.1) years and for the IMRT patients 3.5 (0.1-6.3) years.
Radiation therapy plan review
The CTVs were defined per protocol or with acceptable variation in 87.2% of the 3DCRT cases and 85.2% of the IMRT cases. The target volumes for 3DCRT cases allowed for a volume reduction from prostate and seminal vesicles to prostate only after 55.8Gy. Seventy-five percent of 3DCRT cases underwent a volume reduction. The average size and standard deviation of the high dose prescription volume for 3DCRT cases was 134.8 ± 48.8 ml. IMRT cases had a single target volume that consisted of the prostate and the proximal 1cm of seminal vesicle tissue. The average size of the high dose prescription volume for IMRT cases was larger than the 3DCRT cases,160.2 ± 61.2 ml. This larger volume is located predominantly at the anterior rectal wall and bladder trigone. The organs at risk were defined according to protocol or with acceptable variation in 94.5% of the 3DCRT and 90.3% of the IMRT cases. The median dose to 98% of the PTV volume (D98) was 80.0Gy and 79.2Gy for the 3DCRT and IMRT cases, respectively. None of the differences in target volume or normal organ definition were statistically significant.
Dosimetric comparison
The median % of the bladder receiving at least xGy (pVx) for pV65, pV70 and pV75 were 25.3%, 22.2%, and 17.7% for 3DCRT and 19.7%, 16.6% and 13.1% for IMRT. The median rectum pV65, pV70 and pV75 were 27.4%, 21.7%, and 15.8% for 3DCRT and 23.0%, 18.2% and 13.0% for IMRT. All the differences in organ at risk radiation doses between RT modalities were statistically significant (all p<0.0001).
Acute GI/GU Toxicity
Patients treated with 3DCRT experienced a 15.1% rate of grade 2 or worse acute GI and/or GU toxicity compared to 9.7% rate in patients treated with IMRT (p=0.042). A multivariate analysis that included radiation modality, age and race confirmed radiation modality and age were associated with lower rates of grade 2 or greater acute GI and/or GU toxicity (Table 1). Figure 2 shows the differences in combined acute GI/GU toxicity but individually the differences in either GI or GU effects alone were not significantly different. There were no differences in acute grade 3 or worse GI or GU toxicities. There were no significant differences in acute grade 2 or greater or grade 3 or greater genitourinary toxicity rates. The most common acute side effects were diarrhea, proctitis, dysuria, urinary frequency, and urinary retention.
Table 1.
Grade 2+ Acute GU/GI Toxicity, Multivariate analysis
| Stratified variables | Variable categories |
Observed risk | 95% Confidence Interval |
p-Value |
|---|---|---|---|---|
| RT Method | 3DCRT 79.2Gy | RL | (0.379, 0.999) | 0.049 |
| IMRT | 0.615 | |||
| Age | ≤70 y | RL | (0.361, 0.861) | 0.008 |
| >70 | 0.558 | |||
| Race | White | RL | (0.487,1.519) | 0.604 |
| Non-white | 0.860 |
RL=reference level
Figure 2.
Actuarial time to the development of grade 2 or greater and grade 3 or greater late GI toxicity by radiation modality.
Late GI/GU Toxicity
IMRT was associated with a significant reduction in late GI toxicity. Patients treated with 3DCRT had a 22.0% cumulative incidence at 3 years of grade 2 or greater toxicity compared to only 15.1% at 3 years for patients treated with IMRT (p=0.039). IMRT was associated with a reduction in the cumulative incidence at 3 years of grade 3 or greater late GI toxicity (5.1% vs 2.6%) although this trend was not statistically significant (p=0.09). Figure 3 illustrates the improvement in time to late GI toxicity associated with IMRT compared to 3DCRT. All reported toxicities are listed in Table e.2. There were no significant differences in time to late grade 2 or greater or grade 3 or greater GU toxicities.
Figure 3.
Actuarial time to the development of grade 2 or greater late GI or GU toxicity by race.
IMRT was associated with a 27% reduction in time to grade 2 or greater late GI toxicity in a multivariate analysis that included radiation modality, age and race although this difference was not statistically significant (p=0.077). Non-white race was associated with a significant reduction in the cumulative incidence of grade 2 or greater GI toxicity (p=0.001). The cumulative incidence at 3 years of grade 2 or greater GI toxicity was 24% for whites compared to 9% for non-whites, (p=0.0001, Figure e.1). There were no significant GU toxicity differences related to race. There was an association between experiencing an acute effect and developing a late toxicity. Patients who had experienced acute grade 2 or greater GI toxicity were significantly more likely to have grade 3 or worse late toxicity. This association of acute toxicity and the development of late toxicity was independent of the racial differences described above. The most common late effects were radiation proctitis and rectal bleeding.
Partial Volume Effects
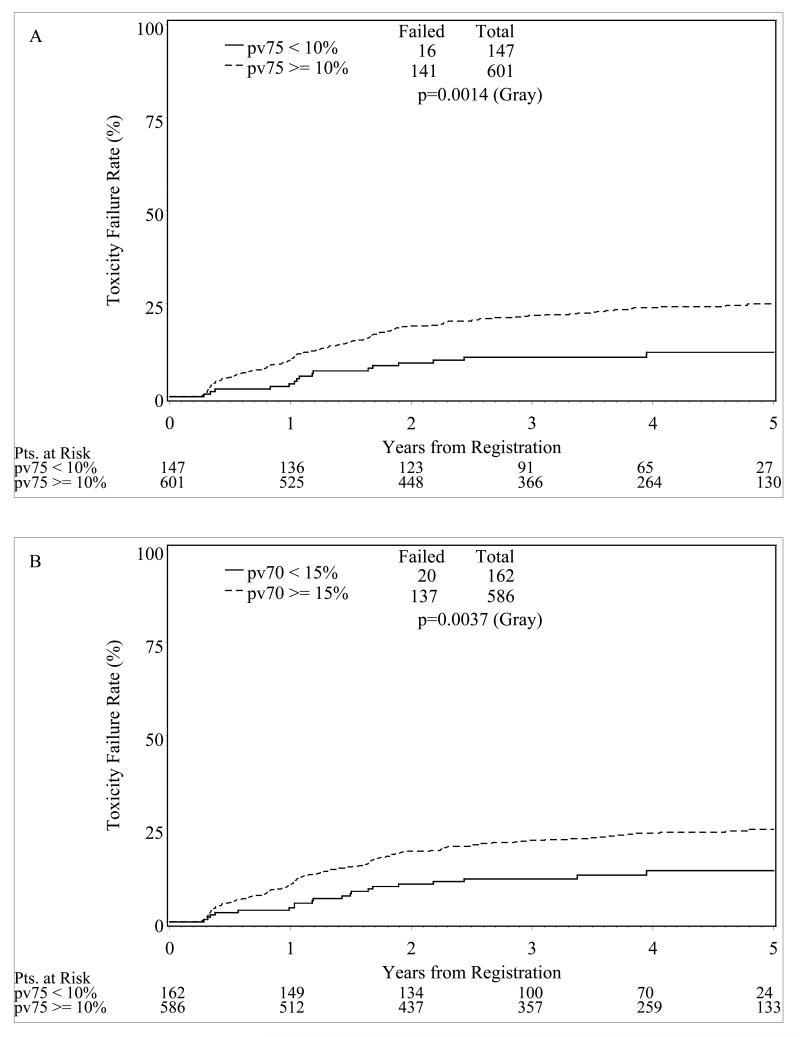
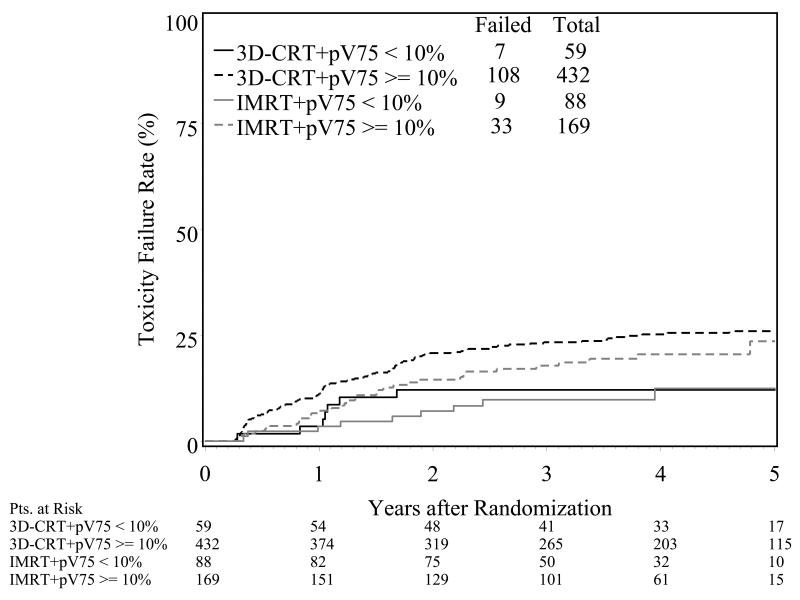
Small volumes of the rectum exceeding high threshold radiation doses were associated with a nearly two-fold risk of late grade 2 or greater toxicity. If more than 10% or 15% of the rectum exceeded 75Gy or 70Gy, respectively, patients had a significantly greater risk of late GI toxicity (Figure 4). When both modality and the dose thresholds were included in the GI toxicity analysis, there is a still a separation between the 3D and IMRT arms at each dose constraint level with a larger, but not statistically significant, separation for the > 10% dose constraint groups (Figure 5).
Figure 4.
Actuarial time to the development of grade 2 or greater late GI toxicity by dose volume metrics; pV70 <15% or pV75<10%
Figure 5.
Actuarial time to the development of grade 2 or greater late GI toxicity by pV75 and radiation modality.
Discussion
Dose Escalation
Over the past decade modern radiation therapy planning and delivery techniques have allowed safe escalation of radiation dose for early stage prostate cancer.(15, 16) Several clinical trials have been conducted to test the value of dose escalated radiation therapy.(17-20) In these studies, a 9 Gy to 10 Gy increase in radiation dose was associated with an improvement in biochemical disease-free survival. In the M.D. Anderson and Medical Research Council trials, the increase in radiation dose was also associated with an improvement in clinical disease-free survival.(18, 19) However, none of these trials has demonstrated nor was powered to detect a difference in overall survival. RTOG 0126 was designed to determine whether dose escalation will lead to a survival advantage and is currently in follow-up for the primary endpoint of overall survival.
In all the published dose escalation trials, there have been statistically significant increases in late rectal toxicity reported in the high dose radiation arms.(17-20) The Dutch trial reported a two-fold increase in the incidence of late rectal bleeding or fecal incontinence in the high dose arm.(17) The Proton Radiation Oncology Group trial showed an increased rate of acute grade 2 or worse toxicity for the patients receiving high dose. (20) The Proton group also reported a 24% incidence of late grade 2 or greater GI toxicity in the high dose arm compared to only 13% in the standard dose arm (p=0.09).(20) Dearnaley reported a 47% increase in grade 2 or greater late GI toxicity with a modest dose escalation with 3D CRT from 64Gy to 74Gy. (18) None of the trials has shown any significant differences in acute or late genitourinary toxicity.
With the exception of the proton trial, each of these previous studies utilized 3DCRT or in the case of the M.D. Anderson trial conventional radiotherapy with a 3D CRT boost. All the studies used uniform techniques in both dose arms and IMRT was not available or allowed in them. Dosimetric analyses reported from some of these trials have demonstrated an association of dose to the rectum and the development of late grade 2 or greater GI or rectal toxicity.(21, 22) In the MD Anderson trial when at least 25% of the rectum was treated to more than 70 Gy, the grade 2 or greater complication rate 6 years following therapy was significantly increased from 16% to 46%.(22) In a report by Peeters, the incidence of rectal bleeding increased from 1% to 9% when the volume of the anorectal wall exceeding 65Gy increased from 19% to 43%. (21) In the current study, dosimetric analyses of patients treated to 79.2Gy demonstrates an association for significant improvement in rectal, bladder and penile bulb dosimetry with IMRT compared to 3DCRT. IMRT is more likely to achieve the dose constraints on the rectum than is 3DCRT and it would be expected to lead to a lower rate of late rectal toxicity.
In this study, IMRT was associated with a significantly lower rate of grade 2 or greater acute GI or GU toxicity. While acute effects are reversible, there have been reports of an association between acute toxicity and the development of subsequent late complications. From the Dutch phase III trial, Peeters reported that prior acute GI toxicity was a significant factor for persistent stool frequency (≥6 stools/day).(21) In a review of their institutional experience, Vargas reported that any acute tenesmus or diarrhea was associated with chronic rectal toxicity.(23) Zelefsky also reported an association of acute GI toxicity and late grade 2 or greater rectal toxicity.(24) In a prospective trial testing the use of sucralfate to prevent late GI toxicity, O’Brien reported that acute symptoms predicted for late radiation proctitis.(25) Patients with moderate to severe pain were three times more likely to experience late rectal toxicity. Our data demonstrates an association of grade 3 or greater late GI effects with previous acute grade 2 or worse GI toxicity. Whether patients who develop acute effects are predisposed to develop late effects or if there is a direct relationship of acute injury and subsequent inflammation or late injury is unknown.
IMRT has been reported to be associated with a lower rate of late rectal toxicity compared to 3DCRT in patients receiving definitive radiation therapy for localized prostate cancer. In the prospective series of dose escalation at Memorial Sloan Kettering, Zelefsky reported a reduction in grade 2 or worse late rectal toxicity following the introduction of IMRT.(24) The 10 year incidence of grade >2 GI toxicity for patients receiving 70.2Gy, 75.6Gy and 81Gy was 7%, 18%, and 5%, respectively. The reduction in the rate of late toxicity was attributed to the use of IMRT in high dose group. While other single institutional studies have also reported a reduction in toxicity with the use of IMRT compared to 3DCRT, they have been complicated by using either different radiation doses or different treatment volumes between the comparison groups.(4-6) In an observational cohort study using 2002 to 2004 claims data from the SEER Medicare database, Bekelman reported reductions in composite bowel complications and proctitis/hemorrhage related to the use of IMRT compared to 3DCRT.(26) In a propensity score adjusted analysis of SEER data from 2000 to 2009, Sheets confirmed a reduction in gastrointestinal toxicity associated with IMRT compared to conformal radiation therapy.(27) This current analysis of the high dose arm of the RTOG 0126 phase III trial confirms the association of lower rates of late rectal toxicity with IMRT.
Like other dose escalation trials there did not appear to be any difference in late genitourinary toxicity by radiation technique.(24) This may be related to the fact that with both 3DCRT and IMRT the bladder neck and prostate urethra are unavoidably part of the target volume. Furthermore, variable bladder filling makes the development of models of partial organ irradiation complex. Finally, the expression of late bladder toxicity typically is years later than with rectal toxicity and our follow-up is too short to identify any latent differences.
A strength of the current study is the relatively uniform treatment patients received on the trial. On average, IMRT patients had larger high dose target volumes than 3DCRT patients. This was related to the allowance of a target volume reduction on the 3DCRT cases. While the magnitude of the volume reduction is small, it is specifically at the anterior rectal wall and base of the bladder. This may impact the expression of GI and GU toxicities. Toxicity endpoints were reported and collected consistently, irrespective of treatment modality. Many of the patients treated on this trial received treatment during the era that IMRT was being adopted in many clinics and normal tissue dose constraint guidelines were not as clearly established as they are today. (28) Whether current practice would yield similar or better outcomes is worthy of further investigation.
As this trial did not randomize patients to the different treatment techniques, the conclusions are not definitive. Furthermore, the treatment target volumes used were different from 3DCRT to IMRT. In the preceding dose escalation trial, RTOG 9406, patients with intermediate risk disease received treatment to the prostate and seminal vesicles to a dose of 55.8Gy following which the prostate gland was boosted to 79.2Gy. (15) Because IMRT techniques require two separate treatment plans for this target volume approach, the investigators chose to treat the prostate and proximal 1cm of seminal vesicle tissue to the study dose. This clinical target volume of prostate and proximal seminal vesicle was supported by a pathological analysis demonstrating that 1cm of seminal vesicle tissue encompasses 93% of cancer extension from the prostate gland. (10) This results in the IMRT patients having a slightly larger high dose clinical target volume than the 3DCRT patients. It is expected that the larger volume to the total prescription dose would bias for more toxicity in the IMRT group, however excess toxicity was not observed.
The evaluation of rectal volume and dose parameters confirms the association of partial rectal volumes exceeding defined threshold doses being associated with complications. In this study, if less than 15% of the rectum exceeded 70Gy (pV70<15%) or less than 10% exceeded 75Gy (pV75<10%) there was a significant reduction in grade ≥2 rectal late effects. Lower dose thresholds in the 40Gy to 60Gy range did not predict for rectal toxicity. This finding is consistent with the QUANTEC review that supported high dose limits were more important than lower doses to larger volumes. (28)
An unexpected finding was the association of significantly less late rectal toxicity in non-white patients. There was no association of race with acute toxicity. Whether this observation reflects a different biology and response to radiation therapy or a bias in patient or physician reporting requires further analysis.
Conclusions
The use of IMRT in the high dose treatment of men with localized prostate cancer was associated with significantly lower doses of radiation delivered to the rectum and bladder. Lower incidence of acute GI or GU toxicity is associated with the use of IMRT. IMRT is also associated with a lower cumulative incidence of late grade 2 or worse rectal toxicity. When planning radiation therapy, keeping the volume of rectum exceeding 70Gy or 75Gy to less than 15% and 10%, respectively, is associated with lower rates of GI toxicity.
Supplementary Material
Summary.
An analysis of the high dose arm of the randomized RTOG dose escalation trial for prostate cancer shows that compared to 3DCRT, IMRT reduces the volumes of bladder and rectum irradiated. IMRT is associated with lower rates of acute and late toxicity. Acute side effects and large volumes of rectum irradiated to high doses are associated with late rectal toxicity.
Acknowledgements
Supported by Radiation Oncology Institute, RTOG U10 CA21661, CCOP U10 CA37422, and ATC U24 CA 81647 grants from NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none
REFERENCES
- 1.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 2.Jani AB, Su A, Correa D, et al. Comparison of late gastrointestinal and genitourinary toxicity of prostate cancer patients undergoing intensity-modulated versus conventional radiotherapy using localized fields. Prostate Cancer Prostatic Dis. 2007;10:82–86. doi: 10.1038/sj.pcan.4500910. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Chan H, Hunt M, et al. Long-term Ourcome of High Dose Intensity Modulated Radiation therapy for Patients with Clinically Localized Prostate Cancer. J Urol. 2006;127:1415–1419. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Sanguineti G, Cavey ML, Endres EJ, et al. Does treatment of the pelvic nodes with IMRT increase late rectal toxicity over conformal prostate-only radiotherapy to 76 Gy? Strahlenther Onkol. 2006;182:543–549. doi: 10.1007/s00066-006-1586-9. [DOI] [PubMed] [Google Scholar]
- 5.Shu HK, Lee TT, Vigneauly E, et al. Toxicity following high-dose three-dimensional conformal and intensity-modulated radiation therapy for clinically localized prostate cancer. Urology. 2001;57:102–107. doi: 10.1016/s0090-4295(00)00890-6. [DOI] [PubMed] [Google Scholar]
- 6.Vora SA, Wong WW, Schild SE, et al. Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:1053–1058. doi: 10.1016/j.ijrobp.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 7.Agresti A. Categorical Data Analysis. Wiley; New York: 1990. [Google Scholar]
- 8.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annual Statistics. 1988;16:1141–1143. [Google Scholar]
- 9.Fine GR. A proportional hazards model for the sub distribution of a competing risk. J Am Statist Assoc. 1999;94:496–509. [Google Scholar]
- 10.Kestin L, Goldstein N, Vicini F, et al. Treatment of prostate cancer with radiotherapy: should the entire seminal vesicles be included in the clinical target volume? International Journal of Radiation Oncology, Biology, Physics. 2002;54:686–697. doi: 10.1016/s0360-3016(02)03011-0. [DOI] [PubMed] [Google Scholar]
- 11.Gay HA, Barthold HJ, O’Meara E, et al. Pelvic Normal Tissue Contouring Guidelines for Radiation Therapy: A Radiation therapy Oncology Group Consensus Panel Atlas. International Journal of Radiation Oncology, Biology, Physics. 2013;83(3):3353–3362. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michalski JM, Purdy JA, Winter K, et al. Preliminary report of toxicity following 3D radiation therapy for prostate cancer on 3DOG/RTOG 9406. International Journal of Radiation Oncology, Biology, Physics. 2000;46:391–402. doi: 10.1016/s0360-3016(99)00443-5. [DOI] [PubMed] [Google Scholar]
- 13.Ryu JK, Winter K, Michalski JM. Preliminary report of toxicity following 3D conformal radiation therapy (3DCRT) for prostate cancer on 3DOG/RTOG 9406, level III (79.2 Gy) Int J Radiat Oncol Biol Phys. 2001;51:136–137. [Google Scholar]
- 14.Roach M, Winter K, Michalski JM, et al. Penile bulb dose and impotence after three-dimensional conformal radiotherapy for prostate cancer on RTOG 9406: findings from a prospective, multi-institutional, phase I/II dose-escalation study. International Journal of Radiation Oncology, Biology, Physics. 2004;60:1351–1356. doi: 10.1016/j.ijrobp.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Michalski JM, Roach Iii M, Merrick G, et al. ACR Appropriateness Criteria ® on External Beam Radiation Therapy Treatment Planning for Clinically Localized Prostate Cancer. Expert Panel on Radiation Oncology-Prostate. International Journal of Radiation Oncology Biology Physics. 2009;74:667–672. doi: 10.1016/j.ijrobp.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 16.Zelefsky MJ, Cowen D, Fuks Z, et al. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer. 1999;85:2460–2468. doi: 10.1002/(sici)1097-0142(19990601)85:11<2460::aid-cncr23>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Al-Mamgani A, van Putten WL, Heemsbergen WD, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:980–988. doi: 10.1016/j.ijrobp.2008.02.073. [DOI] [PubMed] [Google Scholar]
- 18.Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8:475–487. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 19.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 20.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 21.Peeters ST, Lebesque JV, Heemsbergen WD, et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;64:1151–1161. doi: 10.1016/j.ijrobp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 23.Vargas C, Martinez A, Kestin LL, et al. Dose-volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1297–1308. doi: 10.1016/j.ijrobp.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 24.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien PC, Franklin CI, Poulsen MG, et al. Acute symptoms, not rectally administered sucralfate, predict for late radiation proctitis: longer term follow-up of a phase III trial--Trans-Tasman Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2002;54:442–449. doi: 10.1016/s0360-3016(02)02931-0. [DOI] [PubMed] [Google Scholar]
- 26.Bekelman JE, Mitra N, Efstathiou J, et al. Outcomes after intensity-modulated versus conformal radiotherapy in older men with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:e325–334. doi: 10.1016/j.ijrobp.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalski JM, Gay H, Jackson A, et al. Radiation Dose-Volume Effects in Radiation-Induced Rectal Injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.