Abstract
Regulatory T cells (Tregs) have been widely recognized as crucial players in controlling immune responses. Because their major role is to ensure that the immune system is not over reactive, Tregs have been the focus of multiple research studies including those investigating transplantation tolerance, autoimmunity and cancer treatment. On their surface Tregs constitutively express CD25, a high affinity receptor for the cytokine interleukin-2 (IL-2). The reagents constructed in this study were generated by genetically linking porcine IL-2 to the truncated diphtheria toxin (DT390). This reagent functions by first binding to the cell surface via the porcine IL-2/porcine CD25 interaction then the DT390 domain facilitates internalization followed by inhibition of protein synthesis resulting in cell death. Four versions of the porcine IL-2 fusion toxin were designed in an interest to find the most effective isoform: 1) monovalent glycosylated porcine IL-2 fusion toxin (Gly); 2) monovalent non-N-glycosylated porcine IL-2 fusion toxin (NonGly); 3) bivalent glycosylated porcine IL-2 fusion toxin (Bi-Gly); 4) bivalent non-N-glycosylated porcine IL-2 fusion toxin (Bi-NonGly). Using a porcine CD25+ B cell lymphoma cell line (LCL13271) in vitro analysis of the fusion toxins’ ability to inhibit protein synthesis demonstrated that the Bi-NonGly fusion toxin is the most efficient reagent. These in vitro results are consistent with binding affinity as the Bi-NonGly fusion toxin binds strongest to CD25 on the same LCL13271 cells. The Bi-Gly fusion toxin significantly prolonged the survival (p=0.028) of tumor-bearing NOD/SCID IL-2 receptor γ−/− (NSG) mice injected with LCL13271 cells compared with untreated controls. This recombinant protein has great potential to function as a useful tool for in vivo depletion of porcine CD25+ cells for studying immune regulation.
Keywords: Porcine IL-2, fusion toxin, diphtheria toxin, regulatory T cell, Pichia pastoris expression
1. Introduction
Antigen-specific immune responses such as those targeted against tumors are suppressed by Tregs characterized by CD4+CD25highFoxP3+ expression. Treg depletion combined with tumor vaccination is a potentially promising approach to improve cancer treatment. The United States Federal Drug Administration approved truncated diphtheria toxin based human IL-2 fusion toxin (ONTAK) has been shown to deplete Tregs in both pre-clinical and clinical settings thereby facilitating improved cancer treatment (Morse et al., 2008; Mahnke et al., 2007; Litzinger et al., 2007; Gritzapis et al., 2012). While it is effective in depleting Tregs during cancer treatment, ONTAK also creates unwanted side effects as it has been shown to completely deplete NK cells for a prolonged period in a cynomolgus monkey model (Yamada et al., 2012). Natural killer (NK) cells are a very important component of the innate immune system as their functions include fighting pathogenic infections and cancer (Salagianni et al., 2011).
IL-2 binds to its cell surface receptor with notably strong affinity. The IL-2 receptor is a trimer composed of three subunits, α-β-γ. The α-subunit of this receptor, also known as CD25, is constitutively expressed on Tregs and has very high affinity for IL-2. There are species differences between human and porcine IL-2 which affect CD25 binding and subsequent target cell proliferation and differentiation (Zhang et al., 2006; our unpublished data).
The truncated diphtheria toxin DT390 has been used to build recombinant immunotoxins (Woo et al., 2002; Kim et al., 2007; Wang et al., 2011). DT390 lacks the cell-surface binding domain and consists of the catalytic and translocation domains of the diphtheria toxin. In this study each of the glycosylated and non-N-glycosylated porcine IL-2 proteins were linked to DT390 through genetic engineering yielding porcine IL-2 fusion toxins. The ability of these reagents to deplete target cells was assessed using an in vitro assay which monitored the inhibition of protein synthesis. Binding specificity and affinity to the target cells was analyzed by flow cytometry. In vivo target cell depletion was assessed using a porcine CD25+ B-cell lymphoma (LCL13271) NOD/SCID IL-2 receptor γ−/− (NSG) mouse model.
Massachusetts General Hospital (MGH) major histocompatability complex (MHC)-defined miniature swine provide unique preclinical large animal model available for studying immune regulation and the induction of tolerance following transplantation. The purpose of this study was to build a DT390 based porcine IL-2 fusion toxin capable of effectively depleting porcine CD25+ cells with the ultimate goal of studying transplantation tolerance induction using the MGH MHC-defined swine model.
2. Materials and methods
2.1. Plasmid Construction
As shown in Figure 1, porcine IL-2 fusion toxins were built to contain two moieties using the codon-optimized nucleotide sequences; the first is DT390 (Woo et al., 2002) and the second is porcine IL-2. A strategy previously employed to construct A-dm-DT390biscFv (2-6-15) (Wang et al., 2011) was applied to build these porcine IL-2 fusion toxins. The biscFv (2-6-15) moiety was replaced with the codon-optimized glycosylated or non-N-glycosylated porcine IL-2 (Figure 2). A linker made up of three tandem chains each containing four glycine residues and a serine (G4S)3 was used to connect two porcine IL-2 proteins for building the bi-porcine IL-2 fusion toxins. Six histidines (6x His tag) were added to the C-terminus of each construct to facilitate later purification. The codon-optimized glycosylated porcine IL-2 DNA was synthesized by GenScript (Piscataway, NJ) and the codon-optimized non-N-Glycosylated porcine IL-2 DNA was generated by site-directed mutagenesis with sense PCR primer pIL2-N91A For and anti-sense PCR primer pIL2-N91A Rev (Agilent technologies). To construct DT390-pIL-2-Gly or DT390-pIL-2-Non-N-Gly, the codon-optimized glycosylated or non-N-glycosylated porcine IL-2 DNA (Figure 2) was amplified using PCR primers pIL2-X1 carrying XhoI and NcoI site + pIL2-E1 carrying an EcoRI site then cloned into pwPICZalpha (Peraino et al., 2012) between XhoI and EcoRI sites for sequencing confirmation. The insert was then cut out with NcoI + EcoRI and cloned into pwPICZalpha-DT390 (Wang et al., 2011) between NcoI and EcoRI sites yielding the final construct DT390-pIL-2-Gly or DT390-pIL-2-Non-N-Gly in pwPICZalpha. To construct DT390-bi-pIL-2-Gly or DT390-bi-pIL-2-Non-N-Gly, the first porcine IL-2-Gly or porcine IL-2-Non-N-Gly was amplified using PCR primers pIL2-X1 carrying XhoI and NcoI sites + pIL2-Bam1 carrying BamHI and EcoRI sites then cloned into pwPICZalpha between XhoI and EcoRI sites for sequencing confirmation. The insert was subsequently cut out with NcoI + BamHI as insert I. The second porcine IL-2-Gly or porcine IL-2-Non-N-Gly was PCR amplified using pIL2-Bam2 carrying XhoI and BamHI sites + pIL-2-E1 carrying an EcoRI site then cloned into pwPICZalpha between XhoI and EcoRI sites for sequencing confirmation. The insert was then cut out with BamHI + EcoRI as insert II. The insert I carrying NcoI and BamHI sites + insert II carrying BamHI and EcoRI sites (NcoI-pIL-2-BamHI/BamHI-pIL-2-EcoRI) were together cloned into pwPICZalpha-DT390 between NcoI and EcoRI yielding the final construct DT390-bi-pIL-2-Gly or DT390-bi-pIL-2-Non-N-Gly in pwPICZalpha. All PCR primers that were used are listed in Table 1.
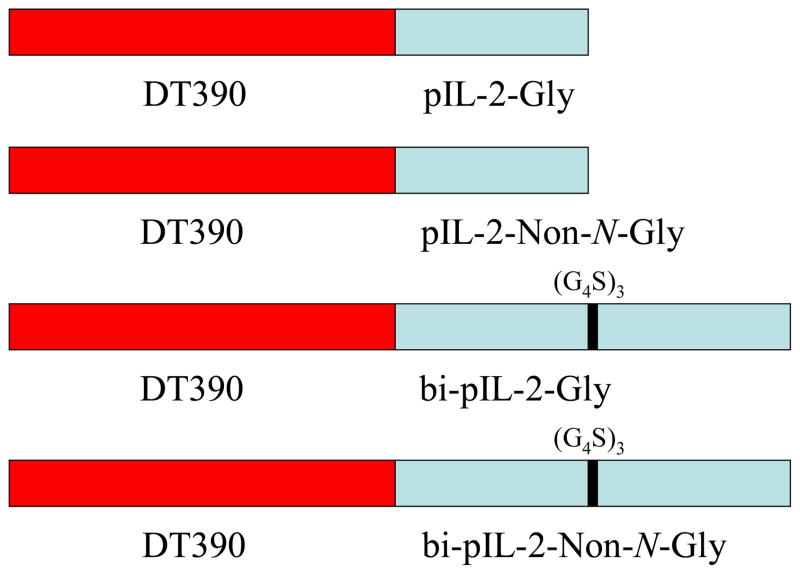
Figure 1.
Schematic Representation of the Four Porcine IL-2 Fusion Toxins
Figure 2.
Codon-optimized glycosylated and non-N-glycosylated porcine IL-2 DNA sequence. The aspapagine at position 91 (unique N-linked glycosylation site) was replaced with alanine (N91A, AAC → GCT) for non-N-glycosylated porcine IL-2. The * denotes the same nucleotide sequence between porcine IL-2-Gly and porcine IL-2-Non-N-Gly.
Table 1.
PCR primers used in this study
| pIl2-X1 5′ CCG CTC GAG CCA TGG GCT CCA ACT TCT TCC TCT ACT 3′ |
| pIL2-Bam1 5′ CCG GAA TTC GGA TCC ACC ACC ACC AGA ACC ACC ACC ACC AGT CAA AGT AGA GTA AAT AGA TTG 3′ |
| pIL2-Bam2 5′ CCG CTC GAG GGA TCC GGT GGT GGT GGT TCT GCT CCA ACT TCT TCC TCT ACT 3′ |
| pIl2-E1 5′ CCG GAA TTC TTA GTG GTG GTG GTG GTG GTG AGT CAA AGT AGA GTA AAT AGA TTG 3′ |
| pIL2-N91A For 5′ AAG GAG TCT ATG AAC AAC ATT GCT GTT ACT GTT TTG GAG TTG AAG 3′ |
| pIL2-N91A Rev 5′ CTT CAA CTC CAA AAC AGT AAC AGC AAT GTT GTT CAT AGA CTC CTT 3′ |
Protein expression and purification in Pichia pastoris were performed as previously described with the following modifications (Wang et al., 2011; Peraino et al., 2012). A Ni-Sepharose fast flow resin (GE healthcare) was used for the purification. Porcine IL-2 fusion toxins were eluted using 40 mM imidazole. Western blot analysis, FACS analysis, FACS competition/blocking analysis and KD determination were all performed as previously described (Peraino et al., 2012) using LCL13271 cells (Cho et al., 2007). The Ontak®-like monovalent human IL-2 fusion toxin (DT390-hIL-2) used as a control for our in vitro assay was constructed, expressed and purified exactly same as the monovalent porcine IL-2 fusion toxin. The DT390 alone and the non-N-glycosylated porcine IL-2 alone (pIL-2-Non-N-Gly) were used as controls for our in vitro assay. These products were also expressed and purified in the yeast Pichia Pastoris system.
2.2. Protein Synthesis Inhibition Assay
Porcine IL-2 fusion toxins (DT390-pIL-2-Gly, DT390-pIL-2-Non-N-Gly, DT390-bi-pIL-2-Gly and DT390-bi-pIL-2-Non-N-Gly) were diluted in 1x leucine-free RPMI 1640 media supplemented with 12% fetal bovine serum, 10 mM hepes (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid), 1x nonessential amino acids, 1mM sodium pyruvate, 2mM glutamine, and 2.5 × 10−5M 2-mercaptoethanol. Target porcine CD25+ LCL13271 cells were washed twice by centrifugation at 1000 rpm, 20°C for 5 minutes in 50 mL of leucine-free RPMI 1640 media described above. Cells were then diluted to 5.0 × 105 cells/mL in leucine-free RPMI 1640 media and 100 μL of cells was added to each well (three wells per fusion toxin dilution) in a 96-well flat bottom plate (Corning) to achieve a final cell concentration of 5.0 × 104 cells/well. Ten microliters of fusion toxin dilutions were added to each well containing LCL13271 cells. Six wells contained only cells, three of these remained without fusion toxin and served as the negative control and the other three were reserved for the positive control which was added later. Plates were then incubated at 37°C with 5% CO2 for 18 hours. Cyclohexamide (Sigma) was diluted 1:8 and 10 μL of this dilution was added to each of three wells containing cells only then incubated for the last 15 min. of the 18 hr. incubation. Cells were pulsed with 1 μCi/well of 3H-Leucine then incubated at 37°C with 5% CO2 for 1 hour. Cells were harvested onto filter mats (Perkin-Elmer) using a cell harvester (Harvester 96® Mach II). The filters were allowed to dry at room temperature overnight then Cpm was measured on a microbeta counter.
2.3. In vitro Cell Proliferation Inhibition Assay
Porcine IL-2 fusion toxins (DT390-pIL-2-Gly, DT390-pIL-2-Non-N-Gly, DT390-bi-pIL-2-Gly and DT390-bi-pIL-2-Non-N-Gly) were diluted in 1x RPMI 1640 media supplemented with 12% fetal bovine serum, 10 mM hepes (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid), 1x nonessential amino acids, 1mM sodium pyruvate, 2mM glutamine, and 2.5 × 10−5M 2-mercaptoethanol. Target porcine CD25+ LCL13271 cells were washed twice by centrifugation at 1000 rpm, 20°C for 5 minutes in 50 mL of supplemented RPMI 1640 media described above. Cells were then diluted to 5.0 × 105 cells/mL in supplemented RPMI 1640 media and 100 μL of cells was added to each well (three wells per fusion toxin dilution) in a 96-well flat bottom plate (Corning) to achieve a final cell concentration of 5.0 × 104 cells/well. Ten microliters of fusion toxin dilutions were added to each well containing LCL13271 cells. Six wells contained only cells, three of these remained without fusion toxin and served as the negative control and the other three were reserved for the positive control which was added later. Plates were then incubated at 37°C with 5% CO2 for 24 hours. Cyclohexamide (Sigma) was diluted 1:8 and 10 μL of this dilution was added to each of three wells containing cells only then incubated for the last 2 hr. of the 24 hr. incubation. Cells were pulsed with 1 μCi/well of 3H-Thymidine then incubated at 37°C with 5% CO2 for 24 hours. Cells were harvested onto filter mats (Perkin-Elmer) using a cell harvester (Harvester 96® Mach II). The filters were allowed to dry at room temperature overnight then CPM was measured on a microbeta counter.
2.4. In Vitro Depletion of Porcine CD25+ LCL13271 Tumor Cells
Porcine CD25+ B cell lymphoma LCL13271cells were counted using trypan blue. The optimal volume of cells to achieve a final concentration of 5.0 × 105 cells/mL was aliquoted into a 50 mL conical tube and cells were washed twice in 50 mL of the culture media (same as that which is described for the cellular proliferation inhibition assay above) by centrifugation at 1000 rpm, 20° C for 5 minutes. LCL13271 cells were then diluted to 5.0 × 105 cells/mL in the culture media and 100 μL of cells (5.0 × 104 cells) was added to each well in a 96-well flat bottom plate (Corning). Porcine IL-2 fusion toxins were serially diluted in the same culture media and then 10 μL of the fusion toxin was added to each appropriate well containing LCL13271 cells. Each fusion toxin dilution was analyzed in triplicate. Plates were incubated at 37° C, 5% CO2 and flow cytometry was performed after 24 and 48 hour incubations staining with an anti-porcine CD25 mAb (clone #231-3B2). Cells without fusion toxin served as negative control and cells with cyclohexamide (1:8) served as a positive control. All staining and FACS analysis were performed as described (Peraino et al., 2012)
2.5. Isolation of Porcine Peripheral Blood Mononuclear Cells (PBMC)
Fifteen to twenty milliliters of heparinized porcine whole blood was diluted to 40 mL with Hanks Balanced Salt Solution (HBSS, Invitrogen) containing calcium and magnesium then underplayed with 10 mL of histopaque 1077 (Sigma). The mixture was centrifuged at 2000 rpm for 30 min. at room temperature. The buffy layer was pulled off with a pipet and aliquoted into a clean conical tube then diluted to 50 mL with HBSS and centrifuged at 1600 rpm, 4° C for 10 minutes. The supernatant was discarded and the cell pellet was re-suspended before lysing the remaining red cells with 5–6 mL of ACK lysing buffer (BioWhittacker) for 2 min. at room temperature. Lysed cells were diluted to 50 mL with HBSS and centrifuged at 1200 rpm, 4° C for 10 minutes. Supernatants were discarded and the cells were re-suspended in culture media (described above in the cellular proliferation inhibition assay section) and stored at 4° C for 0–2 days prior to use in in vitro assays.
2.6 Binding Analysis of the Porcine IL-2 Fusion Toxins to Porcine CD25 on PBMC and LCL13271 porcine tumor cells in vitro
Flow cytometry staining procedures and analysis were performed as previously described (Peraino et al 2012) using both the LCL13271 porcine tumor cells and porcine PBMC to determine the binding affinity of the porcine IL-2 fusion toxins for porcine CD25 in vitro.
2.7. In vivo Functional Analysis
A breeding pair of NSG mice were purchased from Jackson laboratories and bred in our rodent barrier facilities for use in this study. All animal care procedures and experiments were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care (SRAC). MGH is an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) recognized research institution.
All NSG mice were given injections of 10 million porcine CD25+ tumor cells (LCL13271) IV via the tail vein. Six mice were injected with 50 μg/kg of porcine IL-2 fusion toxin (Bi-Gly version) on day 0 and the drug was administered IP twice a day for 4 days and then once a day every 3 days for 9 days. Controls (n=13) received the tumor cells without the fusion toxin and an additional two mice were given the tumor cells and treated with the drug vehicle (PBS). Injected animals were then observed daily for signs and symptoms of illness and scored biweekly based on several parameters (Schenk et al manuscript in preparation): respiratory effort (0–3), weight loss/gain (0–2), fur integrity (0–3), provoked (0–3) and non-provoked activity (0–1), posture (0–3), abdominal distention (0–3), abdominal palpation (0–3) and body condition score (0–3). The highest score in each category represents the worst possible condition for that parameter. The highest possible score on the scoring system is a 24. Mice were humanely euthanized and necropsy was performed after a score of 12 or higher was achieved or when an animal lost more than 15% of its pre-injection body weight. The liver was harvested from each mouse at the time of euthanasia and fixed in 10% neutral buffered formalin before routine histologic processing and paraffin embedding. Five micron sections were stained by hematoxylin & eosin for light microscopy and evaluated by a pathologist using an Olympus BX35 microscope and DP25 camera (EAF).
3. Results
3.1. Expression and Purification of the Porcine IL-2 Fusion Toxins
Four versions of the porcine IL-2 fusion toxin were constructed in an effort to develop the most effective reagent: 1) Gly = monovalent glycosylated porcine IL-2 (DT390-pIL-2-Gly); 2) NonGly = monovalent non-N-glycosylated porcine IL-2 (DT390-pIL-2-Non-N-Gly); 3) Bi-Gly = glycosylated bivalent porcine IL-2 (DT390-bi-pIL-2-Gly) and 4) Bi-NonGly = non-N-glycosylated bivalent porcine IL-2 (DT390-bi-pIL-2-Non-N-Gly). The bivalent isoforms were joined by a (G4S)3 linker. Figure 1 shows a schematic representation of the fusion toxins.
Each porcine IL-2 fusion toxin contains two domains; 1) the truncated diphtheria toxin DT390 and 2) porcine IL-2. The codon-optimized porcine IL-2 DNA (Figure 2) was cloned into the C-terminus of DT390 between NcoI and EcoRI in the DT390-containing yeast Pichia pastoris expression vector pwPICZalpha-A-dmDT390 (Wang et al., 2011). To facilitate the later purification we added a 6xHis tag to the C-terminus of each fusion toxin.
The porcine IL-2 fusion toxins were expressed in yeast Pichia pastoris using shaker flasks as described in Experimental Procedures. Western blot analysis confirmed the expression using mouse anti-6xHis monoclonal antibody (4A12E4, Invitrogen, data not shown). The secreted porcine IL-2 fusion toxin in the supernatant was purified using Ni-Sepharose fast flow resin (Figure 3). The final purification yield was ~30 mg per liter of the original harvested supernatant for the monovalent porcine IL-2 fusion toxins and ~15 mg per liter for the bivalent porcine IL-2 fusion toxins.
Figure 3.
SDS PAGE Gel (4–12% NuPAGE) Analysis of the Four Porcine IL-2 Fusion Toxins. Lane 1: Protein marker; Lane 2: DT390-pIL-2-Gly (58.7 kDa); Lane 3: DT390-pIL-2-Non-N-Gly (58.7 kDa); Lane 4: DT390-bi-pIL-2-Gly (75 kDa); Lane 5: DT390-bi-pIL-2-Non-N-Gly (75 kDa). The weak bands in lanes 2–5 at the lower positions are the break-down products between the diphtheria toxin (DT) A chain and the DT B chain which are linked by disulfide-bonds. The weak bands in lanes 2–5 at ~21 kDa are the DT A chains; the weak band in lane 2 at ~38 kDa is DT B chain-pIL-2-Gly; the weak band in lane 3 at ~33 kDa is DT B chain-pIL-2-Non-N-Gly; the weak band in lane 4 at ~54 kDa is DT B-chain-bi-pIL-2-Gly; the weak band in lane 5 at ~49 kDa is DT-B chain-bi-pIL-2-Non-N-Gly.
3.2. In Vitro Protein Synthesis Inhibition Analysis of the Porcine IL-2 Fusion Toxins
Figure 4 shows that all four porcine IL-2 fusion toxins are capable of inhibiting protein synthesis in vitro in porcine CD25+ LCL13271 cells. The same analysis also demonstrated that the bivalent isoform is more potent than the monovalent counterpart and the Bi-NonGly fusion toxin (DT390-bi-pIL-2-Non-N-Gly) is the most effective reagent. In addition, porcine IL-2-Non-N-Gly, DT390 alone and the Ontak®-like monovalent human IL-2 fusion toxin (DT390-hIL-2) were also included as controls in this protein synthesis inhibition assay. As shown in Figure 4, all of the four porcine IL-2 fusion toxins are more efficient than the Ontak®-like monovalent human IL-2 fusion toxin (DT390-hIL-2) on porcine CD25+ LCL13271 cells. These results confirm significant species specificity and demonstrate that the porcine IL-2 fusion toxins are most optimal for use in pre-clinical swine models.
Figure 4.
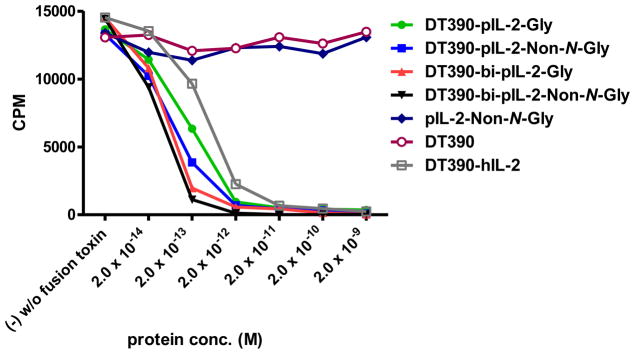
In vitro Protein Synthesis Inhibition Analysis of the Four Porcine IL-2 Fusion Toxins Using LCL13271 Cells: 1) DT390-pIL-2-Gly (green line); 2) DT390-pIL-2-Non-N-Gly (blue line); 3) DT390-bi-pIL-2-Gly (red line); 4) DT390-bi-pIL-2-Non-N-Gly (black line); 5) pIL-2-Non-N-Gly alone (navy blue); 6) DT390 alone (pink line); 7) Ontak®-like monovalent human IL-2 fusion toxin (DT390-hIL-2) (brown line). Y-axis: cpm value by incorporating the tritium-labeled leucine. X-axis: plated IL-2 fusion toxin concentration. Cyclohexmide (1:8) was used as a positive control. The negative control contained cells only without fusion toxin. Data are representative of three individual assays.
3.3. In Vitro Cell Proliferation Inhibition Analysis of the Porcine IL-2 Fusion Toxins
In order to further examine the potency of the four porcine IL-2 fusion toxins, cell proliferation inhibition analysis was performed. As shown in Figure 5, the data of the cell proliferation inhibition analysis is consistent with that of the protein synthesis inhibition analysis previously described (Figure 4). All four porcine IL-2 fusion toxins are capable of inhibiting cell proliferation in vitro using the same porcine CD25+ cell line, LCL13271 and the Bi-NonGly fusion toxin (DT390-bi-pIL-2-Non-N-Gly) is the most potent isoform. Porcine IL-2-Non-N-Gly, DT390 alone and the Ontak®-like monovalent human IL-2 fusion toxin (DT390-hIL-2) were also included as controls.
Figure 5.
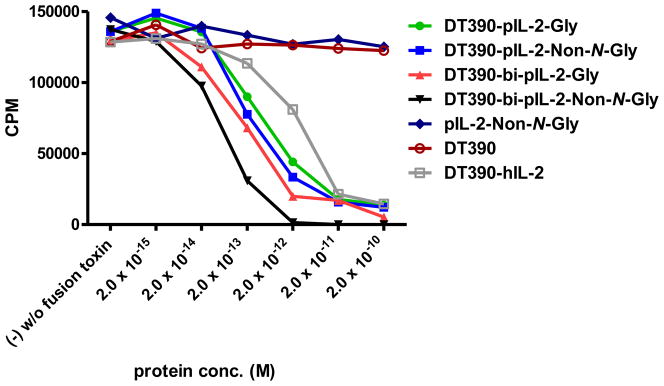
In vitro Cell Proliferation Inhibition Analysis of the Four Porcine IL-2 Fusion Toxins Using LCL13271 Cells: 1) DT390-pIL-2-Gly (green line); 2) DT390-pIL-2-Non-N-Gly (blue line); 3) DT390-bi-pIL-2-Gly (red line); 4) DT390-bi-pIL-2-Non-N-Gly (black line); 5) pIL-2-Non-N-Gly alone (navy blue); 6) DT390 alone (pink); 7) Ontak®-like monovalent human IL-2 fusion toxin (DT390-hIL-2) (brown).Y-axis: cpm value by incorporating the tritium-labeled thymidine. X-axis: plated IL-2 fusion toxin concentration. Cyclohexmide (1:8) was used as a positive control. The negative control contained cells only without fusion toxin. Data are representative of three individual assays.
3.4. Porcine IL-2 Fusion Toxins Deplete Porcine CD25+ Tumor Cells in Vitro
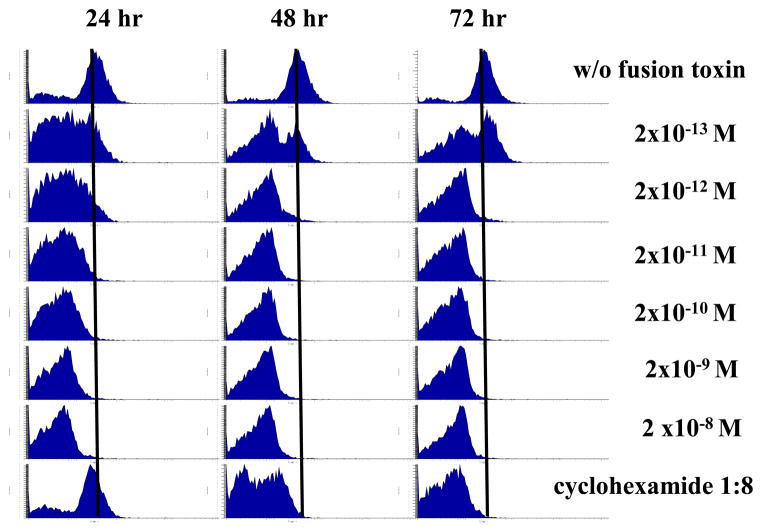
As shown in Figure 6, porcine CD25+ B-cell lymphoma cells (LCL13271) were incubated with the indicated concentrations of Bi-NonGly (DT390-bi-pIL-2-Non-N-Gly). The ability of the fusion toxins to deplete LCL13271 target cells via binding to CD25 was analyzed by flow cytometry using anti-porcine CD25 mAb at 24 h (Figure 6, left panel), 48 h (Figure 6, middle panel) and 72 h (Figure 6, right panel). After 24 h, the Bi-NonGly caused significant reduction of porcine CD25+ tumor cells in a dose dependent manner. After 48 and 72 h the depletion of target cells notably increased. Cyclohexamide (1:8) was used as positive control. The Bi-NonGly is more potent than the positive control.
Figure 6.
Bivalent porcine IL-2 fusion toxin depletes porcine CD25+ LCL13271 cells in vitro. Porcine CD25+ B-cell lymphoma cells (LCL13271) were incubated with the indicated concentrations of the Bi-NonGly fusion toxin (DT390-bi-pIL-2-Non-N-Gly). Reduction in CD25+ cells following treatment with the fusion toxin was measured by flow cytometry using anti-porcine CD25 mAb (Clone 231-3B2) at 24 h (left panel), 48 h (middle panel) and 72 h (right panel). Cyclohexamide (1:8) was used as positive control. Data are representative of multiple individual experiments.
3.5. KD Analysis of the Porcine IL-2 Fusion Toxins Binding to Porcine CD25
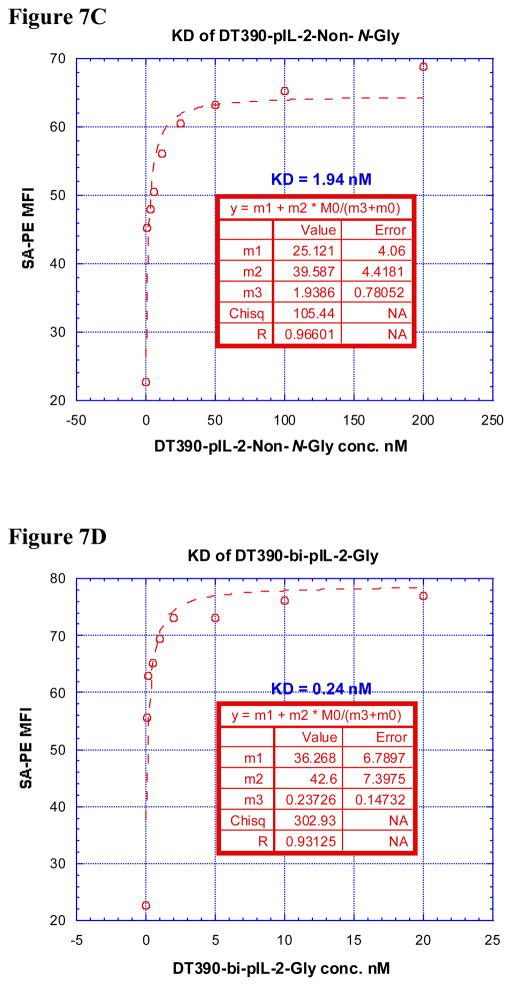
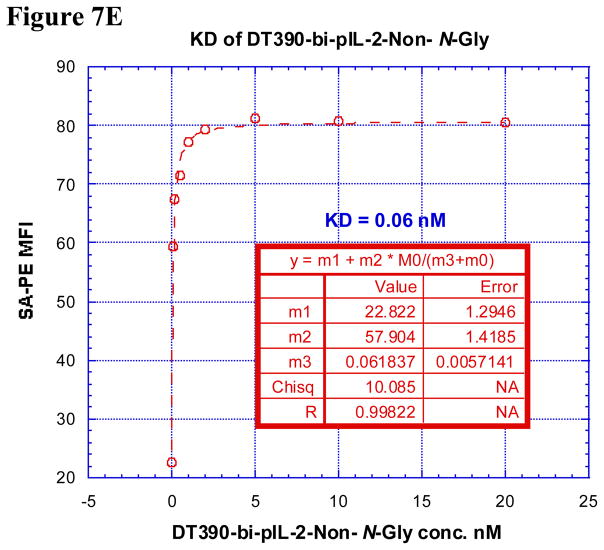
In order for these porcine IL-2 fusion toxins to be functional, they must first bind to the cell of interest via CD25 then internalize before inhibiting protein synthesis. Therefore, it was necessary to analyze the ability of these reagents to bind to CD25 on LCL13271 cells and determine if this strength in binding correlated with potency of in vitro protein synthesis inhibition. All four porcine IL-2 fusion toxins had relatively low dissociation constants (KD) (all ≤ 5.1 nM, Figure 7A–E) suggesting, that each of these fusion proteins has strong affinity for CD25 on LCL13271 cells. The Bi-NonGly fusion toxin has an extremely low KD value, 0.06 nM. This isoform was also by far the most potent reagent for inhibiting protein synthesis in vitro, thus these results show a definite correlation between binding and subsequent impeding of protein synthesis.
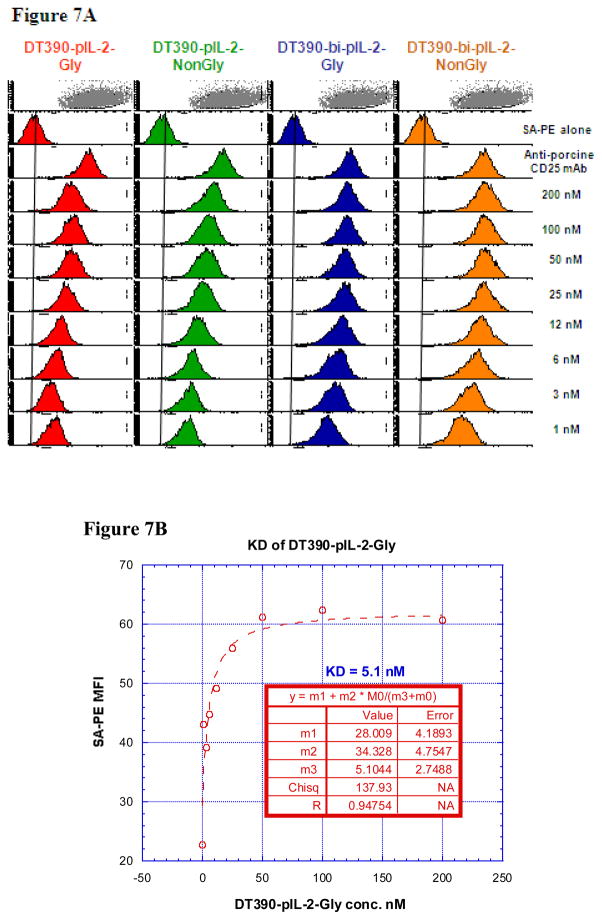
Figure 7.
A) Binding analysis using flow cytometry of biotinylated DT390-pIL-2-Gly (left panel), DT390-pIL-2-Non-N-Gly (middle left panel), DT390-bi-pIL-2-Gly (middle right panel) and DT390-bi-pIL-2-Non-N-Gly (right panel) to porcine CD25+ LCL 13271 cells (B-cell lymphoma cell line). Cells incubated with only the secondary reagent (PE-conjugated streptavidin) served as a negative control and biotinylated anti-porcine CD25 mAb (Clone 231-3B2) was used as a positive control. The data are representative of multiple individual experiments. B–E) KD Determination Using Flow Cytometry and Nonlinear Least Squares Fit. MFI was plotted over a wide range of concentrations of biotinylated B) DT390-pIL-2-Gly, C) DT390-pIL-2-Non-N-Gly, D) DT390-bi-pIL-2-Gly and E) DT390-bi-pIL-2-Non-N-Gly. The accompanying least-squares fits and parameters are shown based on the hyperbolic equation y = m1 + m2 * m0/(m3 + m0) where y = MFI at the given biotinylated porcine IL-2 fusion toxin concentration, m0 = biotinylated porcine IL-2 fusion toxin concentration, m1 = MFI of zero biotinylated porcine IL-2 fusion toxin control, m2 = MFI at saturation and m3 = KD. The inset table in B) shows a fitted KD of 5.1 nM for DT390-pIL-2-Gly. The inset table in C) shows a fitted KD of 1.94 nM for DT390-pIL-2-Non-N-Gly. The inset table in D) shows a fitted KD of 0.24 nM for DT390-bi-pIL-2-Gly. The inset table in E) shows a fitted KD of 0.06 nM for DT390-bi-pIL-2-Non-N-Gly.
The binding specificity of porcine IL-2 fusion toxins were also analyzed using blocking/competition of porcine CD25 mAb (clone #231-3B2) to porcine CD25+ LCL 13271 cells by flow cytometry. As expected, the blocking/competition assay demonstrated that all of the porcine IL-2 fusion toxins successfully blocked the binding of porcine CD25 mAb to LCL13271 cells and the Bi-NonGly construct is the best (data not shown).
3.6. Porcine IL-2 fusion toxins’ binding affinity within porcine PBMC in vitro
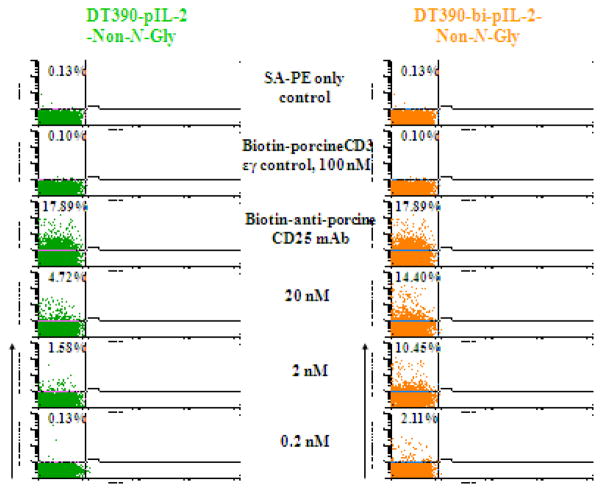
In order to examine the binding affinity of the porcine IL-2 fusion toxins to the porcine CD25, we analyzed their ability to bind to CD25+ cells within porcine PBMC. Isolated porcine PBMC were stained with biotinylated porcine IL-2 fusion toxins and analyzed by flow cytometry. As shown in Figure 8, both NonGly and Bi-NonGly fusion toxins bound to the porcine PBMC in a dose dependent manner with the Bi-NonGly isoform binding stronger than its monovalent counterpart. A biotinylated anti-porcine CD25 mAb (clone# 231-3B2) was included as positive control and biotinylated porcine CD3 εγ (Peraino et al., 2012b) as a control for any background as a result of protein biotinylation.
Figure 8.
Analysis of the porcine IL-2 fusion toxins’ ability to bind to CD25+ porcine PBMC in vitro. Porcine PBMC were stained with biotinylated porcine IL-2 fusion toxins and analyzed by flow cytometry. Biotinylated anti-porcine CD25 mAb (clone 231-3B2) was included as positive control and biotinylated porcine CD3 εγ (Peraino et al., 2012b) was utilized as a control for background due to biotinylation. Data are representative of multiple individual assays.
3.7. In vivo Functional Analysis of the Porcine IL-2 Fusion Toxin Using a Tumor-Bearing NSG Mouse Model
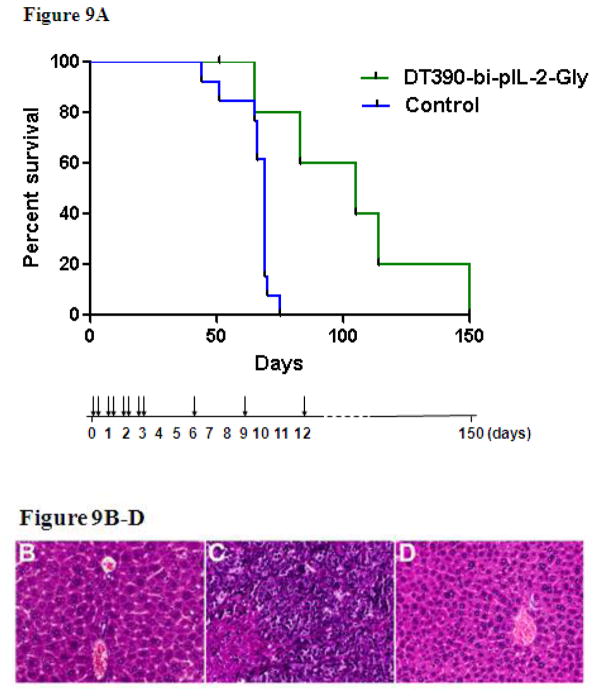
A porcine CD25+ tumor (LCL13271)-bearing NSG mouse model developed in our lab (Schenk et al., manuscript in preparation) was used to assess the in vivo function of the porcine IL-2 Bi-Gly fusion toxin. NSG mice injected with LCL13271 tumor cells and the Bi-Gly fusion toxin demonstrated prolonged survival in comparison to the untreated mice from a median of 69 days to 105 days (p= 0.028) (Figure 9A). Mice that received the Bi-Gly fusion toxin alone did not show any evidence of toxicity at the 50 μg/kg dose. All animals that were injected with LCL13271 cells succumbed to tumors, demonstrated by gross pathology and histopathology (data not shown). Overall, prolonged survival was observed in mice that were treated with the porcine IL-2 Bi-Gly fusion toxin. Further studies will be needed in order to modulate the tumor burden/fusion toxin dose.
Figure 9. In vivo Functional Analysis of the Porcine IL-2 Fusion Toxin.
A) NSG mice were injected with porcine CD25+ B-cell lymphoma cells (LCL13271). The experimental group (n=6) treated with DT390-bi-pIL-2-Gly (green) had a median survival time of 105 days (p=0.028) compared to 69 days in untreated controls that received no fusion toxin (n=13) (blue). The drug administration schedule was shown using vertical arrows. B) Liver from an NSG mouse injected with DT390-bi-IL-2-Gly (U11 H1200 MX24064) shows unremarkable hepatic parenchyma with no increase in hepatocyte necrosis or inflammation. C) Liver from a mouse injected with LCL13271 tumor cells (U10 H1267 MX24065) shows replacement of liver parenchyma by sheets of malignant cells with condensed chromatin, prominent nucleoli, an elevated nuclear:cytoplasmic ratio, and geographic necrosis. D) Liver from a mouse injected with both LCL13271 tumor cells and DT390-bi-IL-2-Gly (U8 1226.2 MX24069) shows normal hepatic parenchyma with no evidence of tumor. (H&E, 400x)
Liver pathology analysis was performed for the following three representative NSG mice. Animal #H1200 was injected only with porcine IL-2 Bi-Gly fusion toxin at 27 weeks of age as a control to determine the toxicity of the porcine IL-2 fusion toxins on host mouse cells. This animal was sacrificed at age 49 weeks and pathology review revealed no effect of the fusion toxin on the host NSG mouse liver (Figure 9B). Animal #1267 was injected only with LCL13271 porcine tumor cells at age 10 weeks to serve as an untreated control. This animal succumbed to tumors and was sacrificed at 17 weeks of age. Pathology review showed extensive replacement of the liver parenchyma by a partially necrotic malignant tumor (Figure 9C). Animal #H1226 was injected with both LCL13271 porcine tumor cells and porcine IL-2 Bi-Gly fusion toxin on the same day at age 19 weeks. This animal was sacrificed at 26 weeks of age no malignant cells were identified in examined sections of the liver (Figure 9D). This pathology analysis demonstrated that the porcine IL-2 Bi-Gly fusion toxin effectively depleted the porcine CD25+ tumor cells in vivo, as the untreated control animal developed visible malignancy (Figure 9C) following the tumor cell injection.
4. Discussion
Denileukin diftitox (DAB389IL2; ONTAK) is a US FDA approved immunotoxin drug which is similar to our DT390-based porcine IL-2 fusion toxin. It contains a monovalent human IL-2 and was expressed in E.coli. The most effective porcine IL-2 fusion toxin described in this study was generated by linking two tandem porcine IL-2 proteins to DT390. Adding a second porcine IL-2 to this fusion toxin significantly improved the reagent’s ability to bind to CD25 on LCL13271 target cells and subsequently inhibits protein synthesis. These results demonstrate that linking two IL-2 proteins together is a novel strategy which can be applied to build an improved DT390-based human IL-2 fusion toxin for pharmaceutical use. Additionally, because these porcine IL-2 fusion toxins were expressed in yeast Pichia pastoris their production can be easily scaled up for industry and does not carry the risk of endotoxin as when using an E.coli expression system. Based on the success of these experiments, the strategy of linking two tandem IL-2 proteins to DT390 is currently being applied to generate both mouse and human bi-IL-2 fusion toxins as a mouse construct will drive in vivo analysis forward and a human fusion toxin carries clinical relevance.
Each of the four DT390-based porcine IL-2 fusion toxins generated in this study proved to be functional in binding strongly and specifically to porcine CD25 and thereafter inhibiting protein synthesis in target cells. The Bi-NonGly fusion toxin was by far the most functional reagent; however, at the time of in vivo analysis only the Bi-Gly isoform was available. Still, the Bi-Gly fusion toxin significantly prolonged the survival of tumor bearing NSG mice. Consequently, as its function was better in vitro (Figure 4–6), the Bi-NonGly fusion toxin should also be more functional in vivo. It will be necessary to add a non-binding control (DT390 alone) in our future in vivo studies.
These results directly demonstrate that the DT390 based bi-porcine IL-2 fusion toxin is capable of depleting porcine CD25+ expressing tumor cells in vivo. Therefore, this reagent can be used to further study species specific CD25+ tumor treatment in a miniature swine model.
Acknowledgments
The work was supported in part by Dana Farber/Harvard Cancer Center Core development grant and by NIH grant R01AI084657 (CAH). We would like to thank Angimmune LLC for kindly providing the diphtheria toxin resistant yeast Pichia pastoris strain and the codon-optimized DT390 DNA. We would like to thank Drs. Lucia Madariaga and Sabastian Michel for intensive manuscript review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cho PS, Lo DP, Wikiel KJ, Rowland HC, Coburn RC, McMorrow IM, Goodrich JG, Arn JS, Billiter RA, Houser SL, Shimizu A, Yang YG, Sachs DH, Huang CA. Establishment of transplantable porcine tumor cell lines derived from MHC-inbred miniature swine. Blood. 2007;110:3996. doi: 10.1182/blood-2007-02-074450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritzapis AD, Voutsas IF, Baxevanis CN. Ontak reduces the immunosuppressive tumor environment and enhances successful therapeutic vaccination in HER-2/neu-tolerant mice. Cancer Immunol Immunother. 2012;61:397. doi: 10.1007/s00262-011-1113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GB, Wang Z, Liu YY, Stavrou S, Mathias A, Goodwin KJ, Thomas JM, Neville DM., Jr A fold-back single-chain diabody format enhances the bioactivity of an anti-monkey CD3 recombinant diphtheria toxin-based immunotoxin. Protein Eng Des Sel. 2007;20:425. doi: 10.1093/protein/gzm040. [DOI] [PubMed] [Google Scholar]
- Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110:3192. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnke K, Schönfeld K, Fondel S, Ring S, Karakhanova S, Wiedemeyer K, Bedke T, Johnson TS, Storn V, Schallenberg S, Enk AH. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120:2723. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraino J, Zhang H, Hermanrud CE, Li G, Sachs DH, Huang CA, Wang Z. Expression and purification of soluble porcine CTLA-4 in yeast Pichia pastoris. Protein Expr Purif. 2012;82:270. doi: 10.1016/j.pep.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraino JS, Hermanrud CE, Springett L, Zhang H, Li G, Srinivasan S, Gusha A, Sachs DH, Huang CA, Wang Z. Expression and characterization of recombinant soluble porcine CD3 ectodomain molecules: mapping the epitope of an anti-porcine CD3 monoclonal antibody 898H2-6-15. Cell Immunol. 2012;276:162. doi: 10.1016/j.cellimm.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salagianni M, Lekka E, Moustaki A, Iliopoulou EG, Baxevanis CN, Papamichail M, Perez SA. NK cell adoptive transfer combined with Ontak-mediated regulatory T cell elimination induces effective adaptive antitumor immune responses. J Immunol. 2011;186:3327. doi: 10.4049/jimmunol.1000652. [DOI] [PubMed] [Google Scholar]
- Wang Z, Duran-Struuck R, Crepeau R, Matar A, Hanekamp I, Srinivasan S, Neville DM, Sachs DH, Huang CA. Development of a Diphtheria Toxin Based Antiporcine CD3 Recombinant Immunotoxin. Bioconjug Chem. 2011;22:2014. doi: 10.1021/bc200230h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JH, Liu YY, Mathias A, Stavrou S, Wang Z, Thompson J, Neville DM., Jr Gene optimization is necessary to express a bivalent anti-human anti-T cell immunotoxin in Pichia pastoris. Protein Expr Purif. 2002;25:270. doi: 10.1016/s1046-5928(02)00009-8. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Aoyama A, Tocco G, Boskovic S, Nadazdin O, Alessandrini A, Madsen JC, Cosimi AB, Benichou G, Kawai T. Differential Effects of Denileukin Diftitox IL-2 Immunotoxin on NK and Regulatory T Cells in Nonhuman Primates. J Immunol. 2012;188:6063. doi: 10.4049/jimmunol.1200656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Ge B, Xia X, Liu J, Sun L, Rao E, Zhao Y. Compatibility of porcine and human interleukin 2: implications for xenotransplantation. Xenotransplantation. 2006;13:423–32. doi: 10.1111/j.1399-3089.2006.00329.x. [DOI] [PubMed] [Google Scholar]