Summary
The calcium-activated small conductance potassium channel, SK3, plays an essential role in the regulation of dopamine neuron activity patterns. Here we demonstrate that expression of a human disease-related SK3 mutation (hSK3Δ) in dopamine neurons of mice disrupts the balance between tonic and phasic dopamine neuron activity. Expression of hSK3Δ suppressed endogenous SK currents, reducing coupling between SK channels and NMDA receptors (NMDARs) and increasing permissiveness for burst firing. Consistent with enhanced excitability of dopamine neurons, hSK3Δ increased evoked calcium signals in dopamine neurons in vivo and potentiated evoked dopamine release. Specific expression of hSK3Δ led to deficits in attention and sensory gating and heightened sensitivity to a psychomimetic drug. Sensory-motor alterations and psychomimetic sensitivity were recapitulated in a mouse model of transient, reversible dopamine neuron activation. These results demonstrate the cell-autonomous effects of a human ion channel mutation on dopamine neuron physiology and the impact of activity pattern disruption on behavior.
Introduction
Dopamine neurons of the ventral midbrain fire in distinct tonic and phasic patterns (Bunney et al., 1973, Grace and Bunney, 1984a, b), providing essential signals to cortical and striatal circuits responsible for various forms of motivation, learning, salience processing, and attention (Schultz, 2007, Bromberg-Martin et al., 2010). Convergent glutamate, GABA, and acetylcholine neurotransmitter systems, as well as multiple voltage-gated and calcium-activated ion channels coordinately regulate action potential firing in dopamine neurons (Shepard and Bunney, 1988, Nedergaard et al., 1993, Overton and Clark, 1997, Wolfart et al., 2001, Wolfart and Roeper, 2002, Tepper and Lee, 2007). Mutations within several ion channels known to regulate dopamine neuron physiology have been linked to mental illnesses, including schizophrenia and bipolar disorder (Liao and Soong, 2010, Askland et al., 2012), yet little is known about how specific channel mutations impact dopamine neuron activity.
KCNN3 (SK3) shows regionally restricted expression in the brain (Kohler et al., 1996) and is highly enriched in dopamine neurons (Sarpal et al., 2004), where expression is proportional to the regularity of pacemaker action potential firing (Wolfart et al., 2001). Suppression of SK-mediated currents by the selective channel blocker apamin or the negative modulator NS8539 attenuates the refractory after-hyperpolarization (AHP) phase of the action potential and increases spike firing irregularity in slice (Shepard and Bunney, 1991, Wolfart et al., 2001, Bond et al., 2005, Ji et al., 2009). Pharmacalogical inhibition of SK currents in vivo facilitates a transition from tonic to burst firing (Waroux et al., 2005, Ji and Shepard, 2006, Herrik et al., 2010) and promotes enhanced accumulation of dopamine metabolites (Steketee and Kalivas, 1990), consistent with elevated dopamine release.
An increase in the ratio of phasic-to-tonic dopamine signals has been proposed as an underlying contributor to the disregulation of cortico-striatal information gating associated with schizophrenia (Grace, 1991). The specific behavioral impact of altering these ratios through a cell-autonomous manipulation of dopamine neuron activity patterns is not known. Intriguingly, a spontaneous mutation in KCNN3 (hSK3Δ) was identified in a patient with schizophrenia (Bowen et al., 2001) and was later demonstrated to dominantly suppress SK-mediated currents in cell culture (Miller et al., 2001). The extent to which this mutation influences dopamine neuron firing patterns is not known, but could provide key insight into the effects of activity pattern disruption on specific dimensions of behavior associated with mental illness.
hSK3Δ is a frame-shift mutation in exon 1 of KCNN3, resulting in a premature stop codon and expression of only the first 283 amino acids of the protein, prior to the first transmembrane domain (Figure 1A; Bowen et al., 2001, Miller et al., 2001). To elucidate the potential impact of hSK3Δ on dopamine physiology and behavior, we selectively expressed hSK3Δ in dopamine neurons of the ventral tegmental area (VTA). This mutation suppressed endogenous SK-mediated currents, altered spike firing patterns ex vivo and in vivo, potentiated NMDAR-mediated currents, increased evoked calcium signals, and amplified dopamine release. Behaviorally, altered dopamine physiology associated with hSK3Δ expression disrupted sensory gating and heightened sensitivity to a psychomimetic. These behaviors were recapitulated using an independent mouse model of transient, reversible enhancement of dopamine neuron excitability. Together, these results reveal the influence of a disease-related KCNN3 mutation on dopamine neuron physiology and support the hypothesis that dopamine neuron activity pattern disregulation is a contributing factor to specific dimensions of behavioral disruption.
Figure 1. Conditional expression of hSK3Δ in DA neurons inhibits SK channels.
(A) Top: DNA sequence of KCNN3 and corresponding amino acid sequence of hSK3; 4 bases deleted in the hSK3Δ mutant are in red. Bottom: DNA and corresponding amino acid sequence of hSK3Δ; three amino acid substitution and premature stop codon are in bold. (B) Schematic of Cre-dependent AAV-FLEx-hSK3ΔGFP vector. (C) Immunohistochemistry of coronal VTA slices from Slc6aCre/+ mice injected with AAV-FLEx-hSK3ΔGFP (top) or AAV-FLEx-hSK3ΔNLS-GFP (bottom). Left: anti-GFP; scale: 40 μm (inset: high magnification; scale: 10 μm). Center: anti-tyrosine hydroxylase (TH), a dopamine neuron marker. (D) Example tail current trace (top) elicited by 500-ms voltage step (bottom). Scale: 200 pA, 250 ms. (E) Average tail currents from DA neurons expressing GFP (n=33), hSK3ΔGFP (n=30), or hSK3ΔNLS-GFP (n=26), or in the presence of apamin (n=24). Shaded area=SEM. Scale: 20 pA, 100 ms. (F) Tail current amplitudes (one-way ANOVA: F(3,109)=29.83, p<0.0001, Tukey's multiple comparison test:***p<0.001 GFP vs. all other groups; #p<0.01 hSK3Δ and hSK3ΔNLS vs. apamin). (G) Tail current charge transfer (area under the curve; one-way ANOVA: F(3,109)=24.42, p<0.0001,Tukey's multiple comparison test:: ***p < 0.001). For all figures bars represent mean ± SEM. See also Figure S1.
Results
Conditional Expression of hSK3Δ Suppresses SK-mediated Currents
To selectively express hSK3Δ in dopamine neurons, we generated a Cre-dependent adeno-associated viral vector (AAV-FLEx-hSK3ΔGFP; Figure 1B). Injection of AAV-FLEx-hSK3ΔGFP into the ventral-medial midbrain of mice expressing Cre recombinase under control of the endogenous dopamine transporter locus (Slc6a3Cre/+; Zhuang et al., 2005) resulted in highly specific expression, largely restricted to the VTA (Figures 1C and S1). hSK3ΔGFP protein localizes to dopamine neuron processes, similar to endogenous SK3 (Wolfart et al., 2001). A portion of the protein is also trafficked to the nucleus, due to unmasking of two canonical nuclear localization sequences (NLS; Figures 1C and S1), as reported in cell culture (Miller et al., 2001). To eliminate the possibility that nuclear localization is responsible for any effects on cell physiology, we generated a second construct in which the NLSs were removed (AAV-FLEx-hSK3ΔNLS-GFP; Figure S1). This truncation redistributed the protein to the soma and maintained localization to processes (Figure 1C).
To determine whether hSK3Δ suppresses endogenous SK currents, we evoked SK-mediated tail currents in dopamine neurons in an acute VTA slice preparation (Figure 1D). hSK3Δ reduced these currents regardless of the presence of the NLS, but was not as robust as inhibition by apamin (Kohler et al., 1996; Figures 1E–G).
Decreased AHP Amplitude and Increased Firing Irregularity by hSK3Δ
To determine whether expression of hSK3ΔGFP in dopamine neurons alters action potential waveforms, as described for pharmacological suppression of SK currents with apamin (Shepard and Bunney, 1991, Wolfart et al., 2001, Ji et al., 2009), we recorded spontaneous action potential firing in slice. In agreement with reduced SK currents, hSK3Δ significantly reduced AHP amplitudes (Figures 2A and 2B). Other action potential properties, such as peak and threshold voltage, were not different from controls (Figure S2).
Figure 2. hSK3Δ alters action potential firing in slice.
(A) Average action potential waveforms. Scale: 10 mV, 25 ms. Inset: expanded view of AHP. Scale: 5 mV, 25 ms. (B) AHP amplitude (GFP n=15, hSK3ΔGFP n=10, hSK3ΔNLS-GFP n=11, apamin n=10; one-way ANOVA: F(3,42)=6.112, p<0.01, Tukey's multiple comparison test: *p<0.05, **p<0.01). (C) Example action potential traces. Scale: 20 mV, 1 s. (D) Irregularity of action potential firing, measured by the CV-ISI (n=same as in (B); one-way ANOVA: F(3,42) = 4.625, p<0.01, Tukey's multiple comparison test: *p<0.05). See also Figure S2.
Consistent with a role for SK channels in regulating pacemaker precision, pharmacological inhibition of SK channels increases irregularity of action potential firing in slice (Wolfart et al., 2001, Ji et al., 2009). Similar to apamin, expression of either hSK3ΔGFP or hSK3ΔNLS-GFP increased spike-timing irregularity, measured by the coefficient of variation of the interspike interval (CV-ISI; Figures 2C and 2D); however, overall spike frequency was unchanged (Figure S2). Together, these results demonstrate that hSK3Δ is a dominant-negative mutation that suppresses SK channel function in dopamine neurons.
Disruption of Dopamine Neuron Activity Pattern Regulation in vivo by hSK3Δ
Suppression of SK channels by apamin or the negative modulator NS8593 alters activity patterns in dopamine neurons in vivo, with tonically firing neurons becoming irregular (as observed in slice), and irregular neurons becoming bursty (Waroux et al., 2005, Ji and Shepard, 2006, Herrik et al., 2010). The reciprocal holds for the positive SK channel modulator NS309 (Herrik et al., 2010). To establish the impact of hSK3Δ on dopamine neuron activity patterns in vivo, we monitored spontaneous activity using chronic tetrode recordings in freely moving mice (Figure S3). Putative dopamine neurons were identified based on firing rate and sensitivity to autoreceptor activation by the D2-selective agonist quinpirole (Figure S3; Zweifel et al., 2009). The proportion of dopamine neurons firing in a tonic, bursty, or irregular pattern were characterized based on their ISI distributions (Figure 3A; Herrik et al., 2010). Relative to controls, hSK3Δ-expressing mice exhibited a greater proportion of bursty cells and a reduced proportion of tonically active neurons, with little effect on the proportion of cells firing an intermediate (irregular) pattern (Bursty: GFP 43% vs. hSK3Δ 58%; Tonic: GFP 33% vs. hSK3Δ 17%; Irregular: GFP 24% vs. hSK3Δ 22%; Chi squared, p<0.05; Figure 3A).
Figure 3. hSK3Δ increases dopamine neuron burst firing in vivo.
(A) Example ISI histograms from putative dopamine neurons recorded in vivo, demonstrating distributions consistent with tonic, irregular, and burst activity. Inset: percentage of cells classified as bursty (black), irregular (gray), or tonic (white); *Chi squared analysis: p<0.05. For all panels: GFP: n=74 cells/7 mice, hSK3ΔGFP: n=90 cells/5 mice. (B) Average normalized ISI histograms from putative dopamine neurons (2-way repeated measures (RM) ANOVA: virus × ISI, F(299,48600)=4.3, p<0.0001; Bonferroni post hoc analysis: ****p<0.0001 for ISIs 5–23 ms). (C) Rate of burst epochs, (D) percentage of spikes fired in bursts, (E) inter-spike interval of the first two spikes in a burst, and (F) overall firing rate of putative dopamine neurons recorded in vivo (Student's t test; *p<0.05, **p<0.01, ****p<0.0001). (G) Overall firing rate and burst set rate were correlated in both groups (R square: GFP = 0.691, hSK3ΔGFP = 0.740); the slope of the correlation is significantly steeper in hSK3ΔGFP-expressing neurons (GFP = 0.100±0.0079, hSK3Δ = 0.125±0.0079; p<0.01). See also Figure S3 and Table S1.
Redistribution of the proportion of neurons within spike pattern categories was reflected as a significant increase in the high frequency range of the average population ISI distribution (Figure 3B). Consistent with an increase in the number of bursty cells, we also observed a significant increase in the frequency of burst events and the percentage of spikes fired in burst in putative dopamine neurons from hSK3ΔGFP mice relative to GFP controls (Figure 3C and D). Additionally, within bursts the ISI of the first two spikes was decreased (Figure 3E) and the ISI of the second two spikes trended toward decrease (Figure S3), indicative of heightened firing rate during burst initiation. In agreement with the increased number of burst events and a higher frequency of spikes at burst onset, overall firing rate was increased by hSK3ΔGFP (Figure 3F) and was more steeply correlated with burst set rate when compared to controls (Figure 3G). Other burst parameters, including spikes per burst and burst duration, were unaltered, and the frequency of spikes not associated with a burst was unchanged (Figure S3). Analysis of neurons recorded on the same tetrode as putative dopamine neurons, but outside the bounds of dopamine neuron classification, did not show significant differences in firing rate or burst properties (Table S1, see supplementary methods).
To characterize the burst properties of putative dopamine neurons described above, we used a conventional method in which burst onset is marked by two spikes with an ISI of ≤ 80 ms and offset is marked by a subsequent ISI ≥ 160 ms (Grace and Bunney, 1984a). Due to the frequency-dependent nature of this method there is the potential for spurious detection of burst activity in cells with higher firing rates; therefore, we performed additional analysis using a modified method of burst detection. We set the threshold for burst initiation to three spikes occurring within the mean of the ISI, allowing us to assess transient rate increases relative to the overall firing rate of the cell. Similar to conventional burst detection, this method also detected a significant increase in burst events and a decrease in the ISI of the first spikes within a burst in hSK3ΔGFP-expressing mice compared to controls (Figures S3). Other burst properties were largely similar to those detected with the conventional method (Figure S3).
SK3 Co-localizes with NMDAR and hSK3Δ Augments NMDAR-Mediated Currents
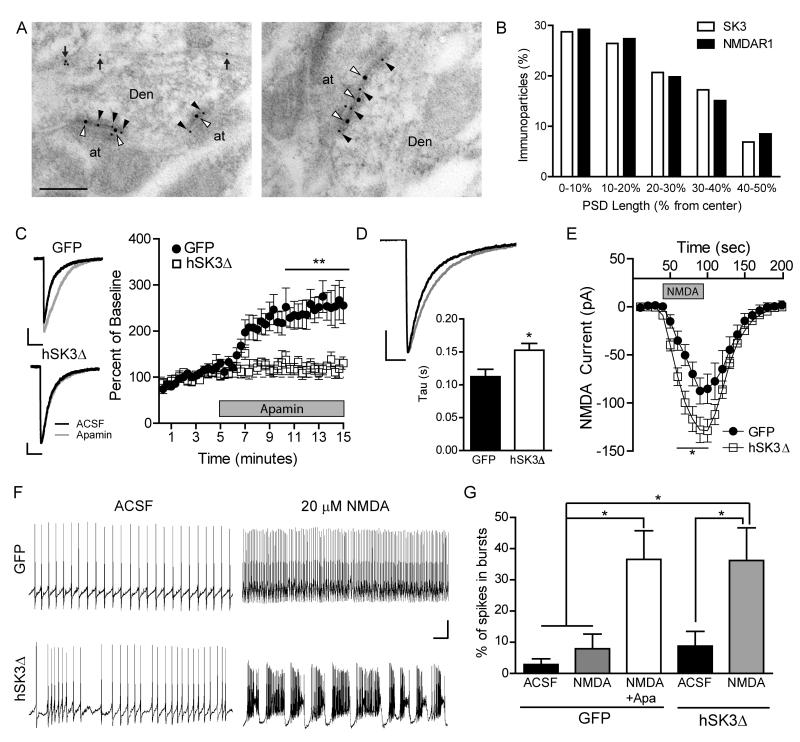
Increased burst firing and elevated firing rate of putative dopamine neurons is consistent with suppression of SK currents enhancing the excitability of dopamine neurons (Ji et al., 2009). The mechanism of this enhanced excitability is not well understood, but could involve modulation of glutamatergic synaptic transmission. It is known that SK channel blockade facilitates NMDA-evoked burst firing of dopamine neurons in slice (Seutin et al., 1993, Johnson and Seutin, 1997). Additionally, in other brain regions SK2 channels co-localize with NMDARs in the post-synaptic density (PSD) where they form a calcium-mediated feedback loop (Faber et al., 2005, Ngo-Anh et al., 2005, Lin et al., 2008); thus hSK3Δ may enhance excitability of dopamine neurons by potentiating NMDAR-mediated EPSCs. To determine whether SK3 co-localizes with NMDARs in dopamine neurons we performed immuno-electron microscopy in the VTA of wild-type mice. Immunogold labeling for the NMDAR subunit NR1 and for SK3 revealed a close juxtaposition of the two channels within the PSD (Figure 4A). Quantification of the distribution of gold particles within the PSD revealed similar profiles of both NR1 and SK3 (Figure 4B).
Figure 4. Inhibition of SK channels by hSK3Δ enhances NMDA-mediated currents and facilitates bursting in slice.
(A) Double immunogold labelling for SK3 and NR1 in two representative asymmetric VTA synapses. SK3 (10 nm gold) was detected along extrasynaptic (arrows) and synaptic (black arrowheads) plasma membrane of dopamine neuron dendritic shafts (Den). Co-localization of SK3 and NR1 (20 nm gold, white arrowheads) was observed in the PSD of dendritic shafts establishing synapses with axon terminals (at). Scale: 0.2 μm. (B) Quantitative analysis showing tangential distribution of SK3 and NR1 across the PSD. SK3: 87 immunoparticles/39 synapses; NR1: 106 immunoparticles/33 synapses. (C) Left: example NMDA EPSCs recorded in the absence (black) or presence (gray) of apamin. Scale: 25 pA, 200 ms. Right: Average percent change in current charge transfer of NMDA EPSC during bath application of apamin (GFP: n=8 cells, hSK3ΔGFP n=7 cells; 2-way RM ANOVA, virus × time, F(89,1157)=6.6, p<0.001; Bonferroni post hoc analysis: **p<0. 01). (D) Average NMDA EPSCs (normalized to peak amplitude) recorded from GFP (black) or hSK3ΔGFP (gray) neurons, and average decay times (tau), fit by a 1-phase exponential (Student's t test: *p<0.05). (E) Current evoked by bath application of 10 μM NMDA (GFP: n=8 cells, hSK3ΔGFP n=13 cells; 2-way RM ANOVA, virus × time, F19,361)=3.19, p<0.0001; Bonferroni post hoc analysis: *p<0. 05). (F) Example action potential traces before or after addition of 20 μM NMDA (Scale: 20 mV, 5 s). (G) Percentage of spikes in bursts before or after addition of NMDA or NMDA+Apamin (GFP: n=10 cells, hSK3ΔGFP n=10 cells; one-way ANOVA, F(4,44)=6.514, p<0.001; Tukey's Multiple Comparison Test: *p<0. 05). See also Figure S4.
To test whether suppression of SK channels alters NMDAR-mediated currents in dopamine neurons, we monitored evoked EPSCs in acute slices before and after addition of apamin. Cells were recorded in a zero magnesium solution to relieve the blockade of NMDARs, and NMDAR and AMPAR currents were isolated by bath application of CNQX or AP5, respectively. In GFP control neurons, SK channel blockade with apamin increased amplitude and charge transfer of NMDAR EPSCs without affecting AMPAR currents (Figures 4C and S4). The effect of apamin on NMDAR EPSCs was largely occluded in neurons expressing hSK3ΔGFP (Figures 4C and S4). Consistent with this observation, NMDAR EPSCs from hSK3ΔGFP-expressing cells had a slower decay time than did EPSCs from control neurons (Figure 4D). Moreover, bath application of NMDA evoked larger currents in hSK3ΔGFP-expressing dopamine neurons relative to controls (Figure 4E).
NMDAR activation facilitates burst firing of dopamine neurons and phasic dopamine release in vivo (Chergui et al., 1993, Tong et al., 1996, Sombers et al., 2009, Zweifel et al., 2009, Wang et al., 2011). Dopamine neurons do not typically exhibit spontaneous burst activity in slice (Shepard and Bunney, 1991, Overton and Clark, 1997, Wolfart et al., 2001, Wolfart and Roeper, 2002, Hopf et al., 2007). However, bath application of NMDA can occasionally lead to burst firing in dopamine neurons (Johnson et al., 1992, Johnson and Wu, 2004), which is enhanced by pharmacological suppression of SK currents (Seutin et al., 1993, Johnson and Seutin, 1997). To determine the extent to which hSK3Δ facilitates NMDAR-mediated burst firing in slice, we recorded spontaneous action potentials in GFP- and hSK3ΔGFP-expressing neurons following bath application of NMDA (20 μM). NMDA application in control slices increased firing rate, but rarely evoked burst firing (1 out of 10 cells). Addition of apamin subsequent to NMDA induced bursting in 44% of cells (4/9). By contrast, 60% of hSK3ΔGFP neurons (6/10) exhibited burst firing in the presence of NMDA alone (Figure 4F, Chi squared GFP vs. hSK3Δ p<0.05). Quantification revealed NMDA plus apamin, but not NMDA alone, increased the percentage of spikes fired in bursts in GFP neurons (Figure 4G). NMDA alone was sufficient to increase the percentage of burst spikes in hSK3ΔGFP neurons (Figure 4G).
Potentiation of Evoked Calcium and Dopamine Release by hSK3Δ
Calcium influx through NMDA receptors and other voltage- and ligand-gated channels plays an important role in generating patterns of dopamine neuron activity (Tong et al., 1996, Amini et al., 1999, Wolfart and Roeper, 2002, Zhang et al., 2005), and direct injection of calcium into dopamine neurons can generate burst spiking (Grace and Bunney, 1984a). To ascertain the impact of reduced SK currents on calcium dynamics, we directly imaged calcium transients in vivo utilizing fiber-optic fluorescence microscopy (Vincent et al., 2006) in combination with the genetically encoded calcium indicator GCaMP3 (Tian et al., 2009). GCaMP3 and an HA-tagged hSK3Δ (hSK3ΔHA) were conditionally co-expressed in dopamine neurons, with greater than 93% of GCaMP-positive neurons co-expressing hSK3ΔHA (Figure S5). GCaMP3 fluorescence was monitored in anesthetized mice during stimulation of the pedunculopontine tegmental nucleus (PPTg), an afferent population known to facilitate dopamine neuron activation and phasic dopamine release (Lokwan et al., 1999, Floresco et al., 2003, Geisler et al., 2007). Increasing PPTg stimulus intensity significantly enhanced calcium signal detection in GCaMP3-expressing control mice (Figures 5A–C and S5). In contrast, SK3ΔHA expression resulted in potentiated calcium signals that were significantly elevated compared to controls and relatively insensitive to stimulus intensity (Figures 5B–C and S5).
Figure 5. hSK3Δ increases evoked calcium signals and dopamine release.
(A) Pseudocolor image obtained with fiber-optic probe of VTA dopamine neurons expressing GCaMP3. Scale: 40 μm. Right (top to bottom): single neuron before, during, and after PPTg stimulation. Scale: 10 μm. (B) Average changes in GCaMP3 fluorescence intensity in the VTA following PPTg stimulation at indicated stimulus intensities (stimulus onset at time 0; control: n=26 cells/3 mice; hSK3ΔHA: n=14 cells/3 mice). (C) Quantification of area under the curve of the fluorescence signal (2-way RM ANOVA, virus × time, F(3,114)=9.74, p<0.001; Bonferroni post hoc analysis: ****p<0.0001). (D) Example pseudocolor plot depicting changes in redox currents in the nucelus accumbens evoked by PPTg stimulation as a function of applied potential over time. Right, example voltammograms of stimulated DA release in mice expressing GFP (black) or hSK3ΔGFP (red). (E) Average DA oxidation currents following PPTg stimulation at indicated stimulus durations (400 μA stimulus intensity; stimulus onset at time 0; stimulus artifacts removed for ease of viewing; n=3 mice per group). (F) Peak DA oxidation currents (2-way RM ANOVA, virus × duration, F(6,24)=3.77, p<0.01; Bonferroni post hoc analysis: ***p<0.001, **p<0.01, *p<0.05). See also Figure S5.
Enhancing excitability of dopamine neurons and increasing evoked calcium is predicted to elevate neurotransmitter release (Steketee and Kalivas, 1990, Sombers et al., 2009). To test this prediction we measured dopamine oxidation currents in the nucleus accumbens using fast-scan cyclic voltammetry in anesthetized mice during PPTg stimulation, as described (Zweifel et al., 2009, Clark et al., 2010). Because 400 μA PPTg stimulation elicited robust calcium signals in both control and hSK3ΔHA-expressing mice, we measured dopamine release using this stimulus intensity at decreasing stimulus durations. Similar to evoked calcium signals, dopamine release following PPTg stimulation was significantly elevated in hSK3ΔGFP-expressing mice compared to GFP controls (Figures 5D–F and S5); this response attenuated in both groups with decreasing stimulus duration. Direct simulation of the medial forebrain bundle (MFB), which contains projections to and from midbrain dopamine neurons (Yeomans, 1989), evoked robust dopamine release that did not differ between groups (Figure S5).
Disruption of Dopamine Neuron Activity Pattern Regulation Impairs Attention and Sensory Gating
Dopamine is important for the modulation of cortico-striatal circuits involved in gating behavioral responses to sensory information (Swerdlow et al., 1994, Grace, 2000). Whether disease-related mutations that alter dopamine neuron activity patterns disrupt these processes is unknown. To address this question, we assayed attention gating using a Pavlovian attention task in mice expressing either hSK3ΔGFP or GFP in dopamine neurons (Figure 6A). Mice were trained in a dark, sound-attenuated chamber to discriminate between auditory cues that were either highly predictive (reward on 100% of trials; CSHigh) or rarely predictive (reward on 12% of trials; CSLow) of food pellet delivery. Repeated days of conditioning resulted in cue discrimination and rapid head entry to the reward delivery port following presentation of the CSHigh, but not the CSLow (Figures 6B–C and S6). Behavior during this initial conditioning phase was not different between groups. We next monitored the ability of mice to attend to an overt, unexpected sensory stimulus (flashing chamber illumination) coincident with CSHigh delivery (Figure 6A). Control mice attended to the unexpected stimulus during early trials, as evidenced by increased latency to retrieve the reward during the flashing light trials compared to during interspersed normal CSHigh trials. This response attenuated with repeated presentations as the stimulus became less salient (Figures 6D and S6). In contrast, hSK3ΔGFP-expressing mice failed to attend to the overt sensory stimulus, either during early or late trials (Figures 6D and S6).
Figure 6. hSK3Δ alters sensory gating.
(A) Schematic of Pavlovian attention paradigm. After 7 days of Pavlovian training mice undergo one session that includes normal CShigh trials alternating pseudorandomly with novel stimulus trials (flashing light during CShigh delivery). (B) Cue discrimination during Pavlovian training. Cue discrimination score = (head entry/min during CShigh) – (head entry/min during CSlow). (GFP: n=15, hSK3ΔGFP: n=16; 2-way RM ANOVA, significant effect of day F(6,174)=9.58, p<0.0001; Bonferroni post hoc analysis comparing to day 1: *p<0.05, ***p<0.001.) (C) Average latency to head entry into food hopper following CS delivery during training. (D) Difference in pellet retrieval time between novel stimulus and normal trials at the beginning (first 5 trials of each type) and end (last 5 trials of each type) of the test session. (2-way RM ANOVA, significant effect of genotype, F(1,29)= 3.91, *p < 0.05, Bonferroni post hoc analysis: p<0.05). (E) Percent inhibition in startle response at various prepulse volumes (GFP: n=10, hSK3ΔGFP: n=9; 2-way RM ANOVA, virus × prepulse intensity, F(2,34)=3.66, p<0.05, Bonferroni post hoc analysis: ***p<0.001). (F) Percent inhibition in startle response following pretreatment with haloperidol. (G) TPV1-DA mice are on a TRPV1 knockout background and express TRPV1 only in dopamine neurons. Capsaicin (from chili peppers) increases burst firing in these neurons. (H) Prepulse inhibition at 75 dB in TRPV1-DA mice after capsaicin injection, with or without haloperidol pretreatment (n=14, 1-way RM ANOVA: F(3,13)=7.659, p<0.001, Bonferroni post hoc analysis: **p<0.01 capsaicin vs. all other groups). See also Figure S6.
The bright flashing light used as the overt sensory stimulus could be anxiogenic in mice. Therefore, we tested whether hSK3Δ-expressing mice are less anxious than GFP controls, which could explain the lack of impact of the light on the latency of reward retrieval. We detected no significant differences between groups in either an open field or elevated plus maze task (Figure S6).
Failure to attend to an unexpected sensory stimulus in hSK3Δ expressing mice is consistent with a deficit in sensory gating. To confirm this hypothesis, we tested mice in an acoustic pre-pulse inhibition (PPI) paradigm, a standard reflexive measure of sensory gating used in both humans and rodents that is sensitive to alterations in dopamine (Swerdlow et al., 1994, Ralph-Williams et al., 2002). We observed significantly reduced PPI in hSK3ΔGFP-expressing mice compared to GFP controls (Figure 6E). Consistent with a disruption of the dopamine system, this effect was reversed by acute treatment with the antipsychotic dopamine D2 receptor antagonist haloperidol (Figure 6F). No differences were detected in baseline startle responses between groups (Figure S6).
To establish whether disruption of sensory gating caused by expression of hSK3Δ is related to changes in dopamine neuron activity, we used a genetic mouse line in which activation of dopamine neurons can be induced rapidly and reversibly (Guler et al., 2012). In this model, TRPV1, a calcium-permeable cation channel, is expressed exclusively in dopamine neurons (TRPV1-DA mice; Figure 6G), and moderate doses of the TRPV1 agonist capsaicin, a component of chili peppers, increase dopamine burst firing (Guler et al., 2012). The effects of capsaicin are transient, lasting only 15–20 minutes (Guler et al., 2012); therefore, we assessed PPI in TRPV1-DA mice with an abbreviated protocol using only one prepulse volume (75 dB). Injection of capsaicin immediately prior to testing reduced PPI compared to vehicle injections in the same animals (Figure 6H). As in hSK3ΔGFP-expressing mice, this reduction was blocked by haloperidol (Figure 6H).
Expression of hSK3Δ Potentiates Psychomotor Activation
Disruption of sensory-motor gating in hSK3Δ-expressing mice is consistent with an alteration in cortico-striatal dopamine signaling (Swerdlow et al., 1994). In addition to impairing PPI, enhanced dopamine release can also potentiate behavioral responses to psychomimetic drugs, such as MK-801 (Gainetdinov et al., 2001), in part through a glutamate- and dopamine-dependent cortico-meso feedback loop (Moghaddam et al., 1997). To establish whether altered dopamine activity and potentiated dopamine release associated with hSK3Δ expression alters sensitivity to a psychomimetic, we monitored locomotor responses in hSK3ΔGFP- or GFP-expressing mice before and after administration of low doses of MK-801. Systemic administration of MK-801 significantly enhanced locomotion in hSK3ΔGFP mice relative to GFP controls. This effect was blocked by pre-treatment with haloperidol (Figures 7A–B). Consistent with the dopamine-independent nature of high doses of MK-801 (Chartoff et al., 2005), we did not observe a significant effect of hSK3ΔGFP expression on locomotion at 0.5 mg/kg MK-801 (Figure S7). Heightened sensitivity to low doses of MK-801 was not associated with gross changes in basal locomotor activity (Figure S7).
Figure 7. hSK3Δ increases psychomotor activation.
(A) Locomotor response to 0.2 mg/kg MK-801 (n=14 in each group; distance traveled in 5 min bins; 2-way RM ANOVA, genotype × time, F(72,1248)=3.90, Bonferroni post hoc analysis: **p<0.01 for hSK3ΔGFP vs. all other groups at t = 30–50 min). (B) Total distance traveled in 90 minutes following injection of MK-801 or saline, with and without haloperidol (2-way RM ANOVA, genotype × dose, F(6,108)=4.98, p<0.001, Bonferroni post hoc analysis: ***p<0.01). (C) Locomotor response to injections of MK-801 and capsaicin in TRPV1-DA mice (n=15; distance traveled in 5 min bins; 2-way RM ANOVA: F(205,3444)=5.01, Bonferroni post hoc analysis: **p<0.01 for Capsaicin+MK-801 vs. all other treatments at t = 30–60 min). (D) Total distance travelled in 20 minutes following capsaicin injection (1-way RM ANOVA: F(4,14)=25.06, p<0.0001, Bonferroni post hoc analysis: ****p<0.01 MK+Cap vs. all other groups, #p<0.05 Halo+MK+Cap vs. Cap and MK). See also Figure S7.
We next assessed MK-801 sensitivity in TRPV1-DA mice by adjusting the timing of drug injections such that the peak activities of capsaicin and MK-801 would coincide. Treatment with either capsaicin or MK-801 induced a small increase in activity, though not significantly different from vehicle. Treatment with both drugs led to a synergistic increase in locomotor activity, which was blocked by haloperidol (Figures 7C–D). Thus, enhanced phasic dopamine, whether induced chronically by suppression of SK3 or acutely by exogenous activation, profoundly disrupts sensory-motor processes.
Discussion
Here we have shown that cell-selective suppression of SK currents in dopamine neurons, mediated by expression of a mutant form of the human KCNN3 gene, alters activity pattern regulation. We further demonstrate that SK channel suppression enhances excitability permissive for burst firing through augmentation of NMDAR excitatory synaptic currents. Finally, our results reveal how disruption of dopamine activity pattern regulation by a disease-related ion channel mutation impacts specific dimensions of behavior.
Suppression of SK currents by hSK3Δ potentiated evoked calcium signals in dopamine neurons in vivo, consistent with both increased neuronal excitability and attenuation of a SK-mediated negative feedback loop on calcium influx (Ngo-Anh et al., 2005). Direct infusion of calcium into dopamine neurons enhances burst activation and reduced calcium dampens burst activity (Grace and Bunney, 1984a), suggesting a key role for calcium in regulating dopamine neuron activity patterns. It is well established that activation of the calcium-permeable NMDAR facilitates burst activation of dopamine neurons and phasic dopamine release in vivo (Tong et al., 1996, Sombers et al., 2009, Zweifel et al., 2009, Wang et al., 2011). The interaction between NMDAR and SK channels in facilitating burst firing in an acute slice preparation is also well documented (Seutin et al., 1993, Johnson and Seutin, 1997, Hopf et al., 2007). By demonstrating co-localization of SK3 and NMDAR in the PSD and the influence of SK on NMDAR EPSCs in dopamine neurons, we established a mechanism whereby coupling between SK and NMDAR can influence neuronal excitability and regulate permissiveness for burst spike firing. The inverse relationship between the magnitude of SK channel currents and NMDAR EPSCs is consistent with those previously reported for SK2 channels and NMDARs in the hippocampus and amygdala (Faber et al., 2005, Ngo-Anh et al., 2005, Lin et al., 2008), thus illustrating a common feature of neuronal excitability coupling between SK and NMDAR.
Major contributors to the regulation of dopamine neuron physiology are voltage-gated calcium channels (CaV). Direct coupling between SK3 and CaV3 in dopamine neurons has been demonstrated; with inhibition of Cav3 suppressing the AHP and inducing spike firing irregularity and burst firing (Wolfart and Roeper, 2002). Furthermore, burst firing associated with suppression of SK channels by apamin is blocked by Cav1-selective antagonist nifedipine (Shepard and Stump, 1999). In addition to localizing to the PSD with NMDARs, we also observed SK3 extrasynaptically, consistent with previous reports of SK3 in both the soma and dendrites of dopamine neurons (Deignan et al., 2012). It is likely that extrasynaptic SK3 channels are those associated with CaV channels, which also show a range of cellular compartmentalization (Catterall, 2011). Thus, differential localization of SK3 likely reflects distinct roles for the ion channel in regulation of dopamine neuron activity through coupling with different calcium-permeable ion channels.
Our data support a model in which glutamate activates postsynaptic AMPARs and NMDARs to facilitate membrane depolarization and recruitment of CaV channels. The juxtaposition of SK channels with CaV and NMDARs allows for rapid activation of SKs upon calcium influx, forming a negative feedback loop to shunt depolarizing currents (Ngo-Anh et al., 2005). Suppression of SK channels by hSK3Δ removes this feedback loop, allowing for elevated calcium influx, increased excitability, increased permissiveness for burst activation, and enhanced dopamine release.
Schizophrenia is a developmental disorder resulting from altered cortical and subcortical circuit function, which frequently intersects with the midbrain dopamine system (Grace, 2000, Winterer and Weinberger, 2004). Indeed, dopamine has been linked to psychosis since the discovery of dopamine receptors as a central target of antipsychotics (Seeman and Lee, 1975, Creese et al., 1976). Expression of hSK3Δ in adult dopamine neurons does not represent a model of schizophrenia, but instead our data demonstrate how disregulation of dopamine neuron activity patterns on a time-scale of weeks (hSK3Δ expression) or even minutes (TRPV1 activation) is sufficient to disrupt behavioral processes dependent on cortico-striatal networks.
Preattentive sensory gating is dependent upon cortico-striatal circuits that are modulated by dopamine and disrupted in patients with schizophrenia and related disorders (Swerdlow et al., 1994). We observed impairment in gating of attention away from a previously defined stimulus towards an overt sensory stimulus, as well as an impairment of reflexive auditory PPI. These findings support a model in which an imbalance in dopamine neuron activity patterns disrupts gating of cortical information to the nucleus accumbens (Grace 2000), a major target of the VTA. In addition to reflecting a deficit in gating information relating to an unexpected stimulus, failure to attend to a competing stimulus could also reflect an alteration in salience processing resulting in obsessive fixation on the goal-directed behavior. Indeed, obsessions in psychosis have been described for decades (Gordon, 1926) and alterations in dopamine-dependent regulation of salience processes have been proposed as a major contributor to psychotic behavior (Kapur, 2003). Further exploration into the role of altered dopamine neuron activity patterns in the processing of salient information in the prefrontal cortex and nucleus accumbens will shed further light on this subject.
We did not observe gross deficits in cognitive function in mice expressing hSK3Δ in dopamine neurons, as appetitive cue discrimination learning was unaltered. These results are not consistent with anhedonia, cognitive deficits, and spurious salience assignment to irrelevant stimuli associated with schizophrenia (Weinberger and Gallhofer, 1997, Heinz and Schlagenhauf, 2010), further highlighting the selective nature of disrupting dopamine neuron activity in adult mice. It is possible that the alterations in dopamine activity caused by hSK3Δ are not sufficient to induce these behaviors, or that these behavioral manifestations are a reflection of altered dopamine signaling during development (Moore et al., 2006, Lodge and Grace, 2007). Alternatively, alterations in dopamine neuron activity may precipitate these behaviors only in the context of altered cortical glutamate or GABA function.
Systemic administration of psychomimetic drugs such as ketamine, phencyclidine (PCP), and MK-801 increase firing rates in dopamine neurons (Zhang et al., 1992, French et al., 1993), evoke hallucinations and delusions when administered to healthy subjects (Malhotra et al., 1996, Lahti et al., 2001), and intensify positive symptoms in schizophrenics (Malhotra et al., 1997, Lahti et al., 2001). In mice these drugs elevate locomotor activity, with animal models of psychosis showing increased sensitivity to these locomotor-inducing effects (Miyakawa et al., 2003, Zuckerman and Weiner, 2005). We observed increased sensitivity to MK-801 in mice expressing hSK3Δ. This result is consistent with increased dopamine release in striatum and prefrontal cortex (Imperato et al., 1990, Miller and Abercrombie, 1996) mediated by a cortico-meso positive feedback loop (Moghaddam et al., 1997). Our results are also consistent with the ability of dopamine-selective antagonists to block hyperactivity associated with psychomimetic administration (Ouagazzal et al., 1993), and with elevated synaptic dopamine increasing psychomimetic sensitivity (Gainetdinov et al., 2001). Based on these findings it will be interesting to determine whether subtle cognitive impairments associated with other mouse models of cortical dysfunction can also be exacerbated by dopamine activity pattern disregulation.
Schizophrenia is a complex developmental disorder, frequently resulting from multiple common alleles that are influenced by environment factors (Gottesman et al., 1982). In some cases schizophrenia can result from a single rare, but highly penetrant mutation (McClellan et al., 2007). hSK3Δ is a rare mutation and does not represent a common cause of the disorder (Bowen et al., 2001). Nonetheless, exploring of the effects of rare mutations, such as hSK3Δ, on neural function provides considerable information that could point to common cellular and circuit level alterations underlying specific dimensions of the disease.
The extent to which KCNN3 is involved in schizophrenia is currently debated (Chandy et al., 1998, Cardno et al., 1999, Brzustowicz et al., 2000, Glatt et al., 2003, Grube et al., 2011). Interestingly, in addition to being expressed in the ventral midbrain, KCNN3 is also highly expressed in the striatum and thalamus (Kohler et al., 1996), two additional brain regions broadly linked to schizophrenia (Grace, 2000, Lisman, 2012). Further exploration of the impact of hSK3Δ expression in these brain regions will help to define how disease-related ion channel mutations may alter activity patterns or circuit function independently of dopamine neurons.
It is important to note that reduced SK function is not the only route to altered dopamine firing patterns. This can also be achieved through developmental alterations in cortico-striatal feedback loops to the VTA (Grace, 1991), reduced GABAergic transmission (Parker et al., 2011), or potentially through hypo-NMDA receptor-mediated suppression of GABAergic tone (Moghaddam et al., 1997). Our results suggest that therapeutics targeted towards normalization of dopamine activity patterns are likely to prove more effective and have fewer side effects than current antipsychotics, which chronically suppress dopamine receptor signaling.
Experimental Procedures
Animals
All experiments were approved by the University of Washington Animal Care and Use Committee. Slc6a3Cre/+ (DAT-Cre) mice were as described (Zhuang et al., 2005). TRPV1-DA mice were as described (Guler et al., 2012). Male and female mice were used in this study. For details on viral injections, see supplemental methods.
Immunohistochemistry
Primary antibodies used were against TH (monoclonal, 1:1000, Millipore), GFP (polyclonal, 1:1000, Invitrogen), and HA (monoclonal, 1:1000, ABM). Secondary antibodies (donkey anti rabbit or mouse) were conjugated to DyLight488 or CY3 (1:200, Jackson Immunolabs).
Slice electrophysiology
Dopamine neurons were identified by fluoresence. For electrophysiology solutions and additional information see supplemental methods.
Tail Currents
Neurons were held at −70 mV in voltage clamp mode and tail currents were evoked with a 500 ms depolarization to 0 mV. Apamin (300 nM, Tocris) was bath-applied to a subset of neurons to block SK3 channels.
Action Potentials
Recordings were made in current clamp mode; frequency, CV-ISI, and average waveforms were taken from a 2.5 min recording window.
Evoked EPSCs
Neurons were held at −60 mV in ACSF with 0 mM Mg2+ containing picrotoxin (100 μM, Ascent Scientific). A concentric bipolar stimulating electrode was placed rostral to the VTA and stimuli were evoked at 0.1 Hz. AMPA currents were isolated by bath application of AP5 (100 μM, Tocris) and NMDA currents were isolated in separate cells by bath application of CNQX (10 μM, Tocris).
Bath NMDA
Neurons were held at −60 mV in ACSF with 0 mM Mg2+ containing picrotoxin (100 μM), tetrodotoxin (500 nM, Ascent Scientific) and CNQX (10 μM). After 5 minutes of baseline recording, 10 μM NMDA was bath applied for 1 minute. Points are an average of 300 msec each.
NMDA-induced bursting
Neurons were recorded in current clamp mode in normal ACSF. 20 μM NMDA was added to the bath and burst analysis was performed on a 2.5 minute window beginning approximately 3 minutes after addition of NMDA.
In vivo electrophysiology
Electrophysiology in freely moving mice was performed using microdrives fabricated in house. Microdrive implantation and data acquisition were as described (Zweifel et al., 2009). Clustered waveforms were analyzed using MATLAB software (Mathworks) with conventional burst detection parameters (≤80 ms ISI burst onset, ≥160 ms ISI burst offset; Grace and Bunney, 1984a). Alternative burst detection was based on the following criteria: ≥3 spikes within a time frame of 1/Firing Rate (Hz) for burst onset and diminished spiking to 1/Firing Rate (Hz) for burst offset. Assignment to ISI categories was performed independently by two researchers, both blinded to virus type. Also see supplemental methods.
Calcium imaging
The fiber-optic probe (S-300B fiber-optic, Mauna Kea Technologies) was lowered into the ventral midbrain until fluorescence was detected. GCaMP3 signals were acquired using a CellVizio 488 imaging system (Mauna Kea Technologies). A 0.23 mm diameter stainless steel bipolar stimulating electrode (Plastics One) was used with a stimulus isolator (Iso-Flex, AMPI). The stimulating electrode was placed above the PPTg and lowered until evoked calcium signals were detected using 400 μA stimulation. Fluorescence signals were acquired for 10 s in response to stimulus intensities of decreasing amplitude (400, 300, 200, 100 μA; 60 Hz, 1 s duration) beginning 3 s after imaging acquisition started. Also see supplemental methods.
Voltammetry
FSCV was performed using carbon-fiber microelectrodes encased by fused-silica capillary tubing (Polymicro Technologies) (Clark et al., 2010). A Ag/AgCl reference electrode was placed in the hemisphere contralateral to the carbon fiber microelectrode. The stimulating electrode (as above) was placed above the PPTg and lowered until dopamine release was observed. PPTg stimulation and data acquisition were preformed as described (Zweifel et al., 2009). Also see supplemental methods.
Behavioral testing
Pavlovian conditioning
Mice were food restricted and maintained at 85% of ad libitum body weight. Each session consisted of 50 trials: 25 CShigh trials randomly interspersed (variable 60 s ITI) with 25 CSlow trials. During CShigh trials a 10 s auditory tone terminated with pellet delivery 100% of the time. During CSlow trials a different frequency tone terminated with pellet delivery 12% of the time. Assignment of each frequency tone to CShigh or CSlow was counterbalanced across groups. Head entries into the food hopper were quantified by infrared beam breaks. Mice received 1 session/day for 7 days.
Attention assay
Mice were given one session of 30 trials: 15 normal CShigh trials randomly interspersed with 15 novel stimulus trials. During novel stimulus trials the CShigh tone was accompanied by a flashing house light, located on the side of the chamber opposite the food hopper. All trials terminated with pellet delivery. Latency to head entry (to retrieve food pellet) following each trial was recorded; attention index = (average latency to retreive pellet after novel stimulus trials) – (average latency to retreive pellet after normal trials).
Startle response and prepulse inhibition
These assays were performed as described (Zweifel et al., 2009). 30 min prior to PPI testing mice were injected (i.p.) with saline or haloperidol (volume of injection was 0.01 ml/g body weight, for a final dose of 0.2 mg/kg). For TRPV1-DA mice, the protocol was abbreviated to account for the transient effects of capsaicin. Also see supplemental methods.
Locomotion
Locomotor activity was measured as described (Zweifel et al., 2009). For MK-801-induced activity, animals were habituated to the chambers and to i.p. injections for two days. On experimental days animals were placed in the chambers for 90 min before i.p. injection of saline or haloperidol (0.2 mg/kg) followed 30 min later with an injection of saline or MK-801; activity was monitored for 90 min following the second injection. Each drug treatment day was followed by at least two days of no treatment; all animals received all drug treatment conditions. For DAT-TRPV1 animals the protocol was identical, with the exception of an additional injection (vehicle or 5.6 mg/kg capsaicin) 20 min following the MK-801 injection. Only the 0.2 mg/kg dose of MK-801 was tested in these animals.
Statistics
Statistical analyses were performed using Prism (GraphPad).
Supplementary Material
Acknowledgments
The authors thank Drs. Erik Carlson, Bryan Gore, Richard Palmiter, Matthew Carter, and John Adelman for thoughtful discussion of the manuscript; Drs. Richard Palmiter, and Matthew Carter for reagents; Drs. Julia Lemos and Mathew Wanat for technical assistance with slice preparation; Drs. Paul Phillips, Stephan Sandberg, and Ingo Willuhn for technical assistance with FSCV; Cerise Knakal, Heather Lee, Timothy Lee, and Timothy Locke for technical assistance. This work was supported by: National Science Foundation: DGE0718124 (CAS); Life Sciences Research Fellowships and the Howard Hughes Medical Institute (ADG); Spanish Ministry of Education and Science: BFU-2012-38348 and CONSOLIDER: CSD2008-00005 (RL); National Institutes of Health: 5T32DA727817 (MES), KO5DA020570 (CC), and P30MH089887 and 1R01MH094536 (LSZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amini B, Clark JW, Jr., Canavier CC. Calcium dynamics underlying pacemaker-like and burst firing oscillations in midbrain dopaminergic neurons: a computational study. J Neurophysiol. 1999;82:2249–2261. doi: 10.1152/jn.1999.82.5.2249. [DOI] [PubMed] [Google Scholar]
- Askland K, Read C, O'Connell C, Moore JH. Ion channels and schizophrenia: a gene set-based analytic approach to GWAS data for biological hypothesis testing. Hum Genet. 2012;131:373–391. doi: 10.1007/s00439-011-1082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CT, Maylie J, Adelman JP. SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol. 2005;15:305–311. doi: 10.1016/j.conb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Bowen T, Williams N, Norton N, Spurlock G, Wittekindt OH, Morris-Rosendahl DJ, Williams H, Brzustowicz L, Hoogendoorn B, Zammit S, Jones G, Sanders RD, Jones LA, McCarthy G, Jones S, Bassett A, Cardno AG, Owen MJ, O'Donovan MC. Mutation screening of the KCNN3 gene reveals a rare frameshift mutation. Mol Psychiatry. 2001;6:259–260. doi: 10.1038/sj.mp.4000128. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzustowicz LM, Hodgkinson KA, Chow EW, Honer WG, Bassett AS. Location of a major susceptibility locus for familial schizophrenia on chromosome 1q21–q22. Science. 2000;288:678–682. doi: 10.1126/science.288.5466.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- Cardno AG, Bowen T, Guy CA, Jones LA, McCarthy G, Williams NM, Murphy KC, Spurlock G, Gray M, Sanders RD, Craddock N, McGuffin P, Owen MJ, O'Donovan MC. CAG repeat length in the hKCa3 gene and symptom dimensions in schizophrenia. Biol Psychiatry. 1999;45:1592–1596. doi: 10.1016/s0006-3223(99)00033-5. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy KG, Fantino E, Wittekindt O, Kalman K, Tong LL, Ho TH, Gutman GA, Crocq MA, Ganguli R, Nimgaonkar V, Morris-Rosendahl DJ, Gargus JJ. Isolation of a novel potassium channel gene hSKCa3 containing a polymorphic CAG repeat: a candidate for schizophrenia and bipolar disorder? Mol Psychiatry. 1998;3:32–37. doi: 10.1038/sj.mp.4000353. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Heusner CL, Palmiter RD. Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology. 2005;30:1324–1333. doi: 10.1038/sj.npp.1300678. [DOI] [PubMed] [Google Scholar]
- Chergui K, Charlety PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur J Neurosci. 1993;5:137–144. doi: 10.1111/j.1460-9568.1993.tb00479.x. [DOI] [PubMed] [Google Scholar]
- Clark JJ, Sandberg SG, Wanat MJ, Gan JO, Horne EA, Hart AS, Akers CA, Parker JG, Willuhn I, Martinez V, Evans SB, Stella N, Phillips PE. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat Methods. 2010;7:126–129. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Deignan J, Lujan R, Bond C, Riegel A, Watanabe M, Williams JT, Maylie J, Adelman JP. SK2 and SK3 expression differentially affect firing frequency and precision in dopamine neurons. Neuroscience. 2012;217:67–76. doi: 10.1016/j.neuroscience.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- French ED, Mura A, Wang T. MK-801, phencyclidine (PCP), and PCP-like drugs increase burst firing in rat A10 dopamine neurons: comparison to competitive NMDA antagonists. Synapse. 1993;13:108–116. doi: 10.1002/syn.890130203. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Mohn AR, Bohn LM, Caron MG. Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc Natl Acad Sci USA. 2001;98:11047–11054. doi: 10.1073/pnas.191353298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Faraone SV, Tsuang MT. CAG-repeat length in exon 1 of KCNN3 does not influence risk for schizophrenia or bipolar disorder: a meta-analysis of association studies. Am J Med Genet B Neuropsychiatr Genet. 2003;121B:14–20. doi: 10.1002/ajmg.b.20048. [DOI] [PubMed] [Google Scholar]
- Gordon A. Obsessions in their relation to psychosis. American Journal of Psychiatry. 1926;5:647–659. [Google Scholar]
- Gottesman II, Shields J, Hanson DR. Schizophrenia, the epigenetic puzzle. Cambridge University Press; Cambridge ; New York: 1982. [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31:330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984a;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984b;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube S, Gerchen MF, Adamcio B, Pardo LA, Martin S, Malzahn D, Papiol S, Begemann M, Ribbe K, Friedrichs H, Radyushkin KA, Muller M, Benseler F, Riggert J, Falkai P, Bickeboller H, Nave KA, Brose N, Stuhmer W, Ehrenreich H. A CAG repeat polymorphism of KCNN3 predicts SK3 channel function and cognitive performance in schizophrenia. EMBO Mol Med. 2011;3:309–319. doi: 10.1002/emmm.201100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Rainwater A, Parker JG, Jones GL, Argilli E, Arenkiel BR, Ehlers MD, Bonci A, Zweifel LS, Palmiter RD. Transient activation of specific neurons in mice by selective expression of the capsaicin receptor. Nat Commun. 2012;3:746. doi: 10.1038/ncomms1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrik KF, Christophersen P, Shepard PD. Pharmacological modulation of the gating properties of small conductance Ca2+-activated K+ channels alters the firing pattern of dopamine neurons in vivo. J Neurophysiol. 2010;104:1726–1735. doi: 10.1152/jn.01126.2009. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol. 2007;98:2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- Imperato A, Scrocco MG, Bacchi S, Angelucci L. NMDA receptors and in vivo dopamine release in the nucleus accumbens and caudatus. Eur J Pharmacol. 1990;187:555–556. doi: 10.1016/0014-2999(90)90387-l. [DOI] [PubMed] [Google Scholar]
- Ji H, Hougaard C, Herrik KF, Strobaek D, Christophersen P, Shepard PD. Tuning the excitability of midbrain dopamine neurons by modulating the Ca2+ sensitivity of SK channels. Eur J Neurosci. 2009;29:1883–1895. doi: 10.1111/j.1460-9568.2009.06735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD. SK Ca2+-activated K+ channel ligands alter the firing pattern of dopamine-containing neurons in vivo. Neuroscience. 2006;140:623–633. doi: 10.1016/j.neuroscience.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Seutin V. Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2+-activated K+ currents. Neurosci Lett. 1997;231:13–16. doi: 10.1016/s0304-3940(97)00508-9. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Seutin V, North RA. Burst firing in dopamine neurons induced by N-methyl-D-aspartate: role of electrogenic sodium pump. Science. 1992;258:665–667. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Wu YN. Multiple mechanisms underlie burst firing in rat midbrain dopamine neurons in vitro. Brain Res. 2004;1019:293–296. doi: 10.1016/j.brainres.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Liao P, Soong TW. CaV1.2 channelopathies: from arrhythmias to autism, bipolar disorder, and immunodeficiency. Pflugers Arch. 2010;460:353–359. doi: 10.1007/s00424-009-0753-0. [DOI] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11:170–177. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol. 2012;22:537–544. doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokwan SJ, Overton PG, Berry MS, Clark D. Stimulation of the pedunculopontine tegmental nucleus in the rat produces burst firing in A9 dopaminergic neurons. Neuroscience. 1999;92:245–254. doi: 10.1016/s0306-4522(98)00748-9. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- McClellan JM, Susser E, King MC. Schizophrenia: a common disease caused by multiple rare alleles. Br J Psychiatry. 2007;190:194–199. doi: 10.1192/bjp.bp.106.025585. [DOI] [PubMed] [Google Scholar]
- Miller DW, Abercrombie ED. Effects of MK-801 on spontaneous and amphetamine-stimulated dopamine release in striatum measured with in vivo microdialysis in awake rats. Brain Res Bull. 1996;40:57–62. doi: 10.1016/0361-9230(95)02144-2. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Rauer H, Tomita H, Gargus JJ, Gutman GA, Cahalan MD, Chandy KG. Nuclear localization and dominant-negative suppression by a mutant SKCa3 N-terminal channel fragment identified in a patient with schizophrenia. J Biol Chem. 2001;276:27753–27756. doi: 10.1074/jbc.C100221200. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Leiter LM, Gerber DJ, Gainetdinov RR, Sotnikova TD, Zeng H, Caron MG, Tonegawa S. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100:8987–8992. doi: 10.1073/pnas.1432926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard S, Flatman JA, Engberg I. Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. J Physiol. 1993;466:727–747. [PMC free article] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Ouagazzal A, Nieoullon A, Amalric M. Effects of dopamine D1 and D2 receptor blockade on MK-801-induced hyperlocomotion in rats. Psychopharmacology (Berl) 1993;111:427–434. doi: 10.1007/BF02253532. [DOI] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Parker JG, Wanat MJ, Soden ME, Ahmad K, Zweifel LS, Bamford NS, Palmiter RD. Attenuating GABAA Receptor Signaling in Dopamine Neurons Selectively Enhances Reward Learning and Alters Risk Preference in Mice. J Neurosci. 2011;31:17103–17112. doi: 10.1523/JNEUROSCI.1715-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice. J Neurosci. 2002;22:9604–9611. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal D, Koenig JI, Adelman JP, Brady D, Prendeville LC, Shepard PD. Regional distribution of SK3 mRNA-containing neurons in the adult and adolescent rat ventral midbrain and their relationship to dopamine-containing cells. Synapse. 2004;53:104–113. doi: 10.1002/syn.20042. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- Seutin V, Johnson SW, North RA. Apamin increases NMDA-induced burst-firing of rat mesencephalic dopamine neurons. Brain Res. 1993;630:341–344. doi: 10.1016/0006-8993(93)90675-d. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Bunney BS. Effects of apamin on the discharge properties of putative dopamine-containing neurons in vitro. Brain Res. 1988;463:380–384. doi: 10.1016/0006-8993(88)90414-3. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Bunney BS. Repetitive firing properties of putative dopamine-containing neurons in vitro: regulation by an apamin-sensitive Ca(2+)-activated K+ conductance. Exp Brain Res. 1991;86:141–150. doi: 10.1007/BF00231048. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Stump D. Nifedipine blocks apamin-induced bursting activity in nigral dopamine-containing neurons. Brain Res. 1999;817:104–109. doi: 10.1016/s0006-8993(98)01231-1. [DOI] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Effect of microinjections of apamin into the A10 dopamine region of rats: a behavioral and neurochemical analysis. J Pharmacol Exp Ther. 1990;254:711–719. [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Lee CR. GABAergic control of substantia nigra dopaminergic neurons. Prog Brain Res. 2007;160:189–208. doi: 10.1016/S0079-6123(06)60011-3. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Antagonism of NMDA receptors but not AMPA/kainate receptors blocks bursting in dopaminergic neurons induced by electrical stimulation of the prefrontal cortex. J Neural Transm. 1996;103:889–904. doi: 10.1007/BF01291780. [DOI] [PubMed] [Google Scholar]
- Vincent P, Maskos U, Charvet I, Bourgeais L, Stoppini L, Leresche N, Changeux JP, Lambert R, Meda P, Paupardin-Tritsch D. Live imaging of neural structure and function by fibred fluorescence microscopy. EMBO Rep. 2006;7:1154–1161. doi: 10.1038/sj.embor.7400801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Li F, Wang D, Xie K, Shen X, Tsien JZ. NMDA receptors in dopaminergic neurons are crucial for habit learning. Neuron. 2011;72:1055–1066. doi: 10.1016/j.neuron.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waroux O, Massotte L, Alleva L, Graulich A, Thomas E, Liegeois JF, Scuvee-Moreau J, Seutin V. SK channels control the firing pattern of midbrain dopaminergic neurons in vivo. Eur J Neurosci. 2005;22:3111–3121. doi: 10.1111/j.1460-9568.2005.04484.x. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Gallhofer B. Cognitive function in schizophrenia. Int Clin Psychopharmacol. 1997;12(Suppl 4):S29–36. doi: 10.1097/00004850-199709004-00006. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci. 2001;21:3443–3456. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci. 2002;22:3404–3413. doi: 10.1523/JNEUROSCI.22-09-03404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS. Two substrates for medial forebrain bundle self-stimulation: myelinated axons and dopamine axons. Neurosci Biobehav Rev. 1989;13:91–98. doi: 10.1016/s0149-7634(89)80016-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chiodo LA, Freeman AS. Electrophysiological effects of MK-801 on rat nigrostriatal and mesoaccumbal dopaminergic neurons. Brain Res. 1992;590:153–163. doi: 10.1016/0006-8993(92)91091-r. [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu Y, Chen X. Carbachol induces burst firing of dopamine cells in the ventral tegmental area by promoting calcium entry through L-type channels in the rat. J Physiol. 2005;568:469–481. doi: 10.1113/jphysiol.2005.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods. 2005;143:27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.