Abstract
Cardiovascular disease (CVD) is less common in premenopausal women than men of the same age or postmenopausal women, suggesting vascular benefits of estrogen. Estrogen activates estrogen receptors ERα, ERβ and GPR30 in endothelium and vascular smooth muscle (VSM), which trigger downstream signaling pathways and lead to genomic and non-genomic vascular effects such as vasodilation, decreased VSM contraction and growth and reduced vascular remodeling. However, randomized clinical trials (RCTs), such as the Women’s Health Initiative (WHI) and Heart and Estrogen/progestin Replacement Study (HERS), have shown little vascular benefits and even adverse events with menopausal hormone therapy (MHT), likely due to factors related to the MHT used, ER profile, and RCT design. Some MHT forms, dose, combinations or route of administration may have inadequate vascular effects. Age-related changes in ER amount, distribution, integrity and post-ER signaling could alter the vascular response to MHT. The subject’s age, preexisting CVD, and hormone environment could also reduce the effects of MHT. Further evaluation of natural and synthetic estrogens, phytoestrogens, and selective estrogen-receptor modulators (SERMs), and the design of appropriate MHT combinations, dose, route and 'timing' could improve the effectiveness of conventional MHT and provide alternative therapies in the peri-menopausal period. Targeting ER using specific ER agonists, localized MHT delivery, and activation of specific post-ER signaling pathways could counter age-related changes in ER. Examination of the hormone environment and conditions associated with hormone imbalance such as polycystic ovary syndrome may reveal the causes of abnormal hormone-receptor interactions. Consideration of these factors in new RCTs such as the Kronos Early Estrogen Prevention Study (KEEPS) could enhance the vascular benefits of estrogen in postmenopausal CVD.
Keywords: sex hormones, progesterone, testosterone, endothelium, vascular smooth muscle, extracellular matrix, hypertension
INTRODUCTION
Cardiovascular disease (CVD), such as coronary heart disease (CHD) and hypertension, is less common in premenopausal women (Pre-MW) than in men of the same age, suggesting vascular benefits of estrogen [1, 2]. Also, the risk of CVD increases with age in postmenopausal women (Post-MW) compared with Pre-MW, partly due to decreased plasma estrogen levels. Estrogen is the predominant sex hormone in women, affecting the development and function of the female reproductive system. Estrogen is commonly used as a contraceptive, and as a component of menopausal hormone therapy (MHT) for hot flushes, night sweats and vaginal dryness [3].
Earlier observational studies, such as the Nurses’ Health Study (NHS) in the mid 1970s, suggested that estrogen therapy in Post-MW reduced the risk of CVD by 35 to 50% [1]. Also, a meta-analysis of observational studies showed ~33% reduction in fatal CVD among MHT users compared with nonusers [3]. Experimental studies supported vascular benefits of estrogen. Acute administration of estrogen in female or male patients improves vasodilator responses and ameliorates myocardial ischemia [4]. Also, acute administration of estrogen in dogs and isolated rat and rabbit hearts lowers coronary vascular resistance and enhances coronary blood flow [5]. Estrogen modulates vascular function by targeting estrogen receptor (ER) in endothelial cells (ECs) and vascular smooth muscle (VSM) [1, 2]. Estrogen also enhances the release of vasodilator substances such as nitric oxide (NO) and prostacyclin (PGI2), and decreases the production and effects of vasoconstrictors such as endothelin (ET-1) and angiotensin II (AngII) [1, 2].
However, randomized clinical trials (RCTs) such as the Women’s Health Initiative (WHI) and Heart and Estrogen/progestin Replacement Study (HERS) showed no cardiovascular benefits and even increased risk for cerebrovascular events with MHT [6, 7]. These discrepant findings have prompted investigators to examine the causes of failure of MHT to elicit vascular benefits in RCTs. The purpose of this review is to discuss how factors related to the MHT used, vascular ER, and the design of RCTs could have led to the paradoxical cardiovascular outcomes of MHT in RCTs. A brief description of the cardiovascular outcomes of initial observational studies, small clinical trials and major RCTs of MHT will first be outlined. Factors related to biosynthesis and levels of natural estrogens, and alternative estrogenic compounds including phytoestrogens and selective estrogen receptor modulators (SERMs) will then be discussed. ER subtypes, variants, polymorphisms, tissue distribution, and age-related changes as well as post-ER signaling pathways in ECs, VSM and extracellular matrix (ECM) and the ensuing genomic and nongenomic vascular effects will be described. Factors related to the design of RCTs, including subjects' age, pre-existing CVD, the time of starting MHT, the hormone environment and interaction with other hormones such as progesterone (P4) and testosterone (T) will then be discussed. The review will conclude with how lessons learned from previous RCTs could help in the design of more directed RCTs, and how examination of age-related changes in vascular ER and signaling mechanisms could maximize the effects of conventional MHT and alternative estrogenic compounds in postmenopausal CVD.
MHT Clinical Trials
Earlier observational studies in Post-MW showed reduced risk of a cardiovascular event in women using unopposed oral estrogen [8]. Also, a meta-analysis of these studies showed that MHT use was associated with a one-third reduction in fatal CVD. A study in 337 women undergoing elective coronary angioplasty showed that MHT users had fewer cardiovascular events (12% vs. 35%) and better survival (93% vs. 75%) than nonusers [9]. Similarly, a small clinical trial showed less progression in carotid artery intima–media thickness in early Post-MW randomized to estrogen than those randomized to placebo [10]. However, questions were raised regarding the vascular benefits of MHT in these observational studies and small clinical trials partly because they were not well-randomized, women who chose to take MHT might have been healthier than those who did not (“healthy user” bias), and results were difficult to compare because different MHT preparations were used for different periods of time. Importantly, some observational studies suggest that short-term use of MHT may be associated with increased risk of CVD. NHS, a prospective observational cohort study of secondary prevention of CHD which included 2,489 women with a previous MI or documented atherosclerosis, showed that the risk of recurrent major coronary events increased among short-term MHT users, although the risk decreased among long-term users [11]. Also, major RCTs failed to show tangible cardiovascular benefits of MHT [12] (Table 1). HERS showed no difference in secondary CVD outcome (nonfatal MI and CVD death), even though MHT reduced LDL-c and increased HDL-c [12]. Also, a 52% increase in adverse CVD events was found in the MHT group during the first year of treatment, and no protective effects were observed after an additional 2.7 years of follow-up [6]. WHI showed that neither CEE alone nor CEE+MPA decreased CVD, and was stopped because evidence of harm for breast cancer, along with some increase in CHD, stroke and pulmonary embolism outweighed the benefits for bone fractures and colon cancer [7].
Table 1.
Representative MHT clinical trials and their CVD outcome
| Clinical Trial |
Type | Subjects Number, Condition |
Mean follow-up Period |
MHT Type | MHT Route |
Outcome | Ref |
|---|---|---|---|---|---|---|---|
| PEPI | Multi-center, RDBPC | 875 45–64 yr Healthy | 3 yr (1991–1994) | CEE 0.625 mg CEE+progestin or placebo | Oral | CEE or CEE+progestin improves lipoproteins and lowers fibrinogen levels. | [112] |
| HERS | RDBPC | 2763 44–79 yr Average 67 yr with CVD | 4.1 yr (1993–1997) | CEE 0.625 mg +MPA 2.5 mg or placebo | Oral | No overall reduction in CVD events in women with established coronary disease. High risk of CVD in first year | [12] |
| HERS II | RDBPC Follow-up | 2321 Average 67 yr | 6.8 (2.7 yr added to HERS) (1993–1999) | CEE+MPA | Oral | MHT did not reduce risk of cardiovascular events in women with CHD | [6] |
| HERS - UA (Uric Acid) | RDBPC | 2763 44–79 yr | 4.1 yr (1993–1997) | CEE+MPA | Oral | CEE+MPA lowered serum UA levels slightly, but neither baseline UA nor change in UA affected CHD risk. | [113] |
| WHI | RDBPC | 16608 50–79 yr mean 63 Healthy with uterus 10739 Healthy without uterus |
5.2 yr (1993–1998) | CEE+MPA or placebo CEE or placebo | Oral | Overall health risks exceeded benefits. Overall doubling of VTE events in CEE+MPA arm. 30% to 40% increased risk of stroke. | [7] |
| WEST | RDBPC | 664 Mean 71 yr | Mean 2.8 yr (Dec. 1993 - May 1998) | E2 or placebo | Oral | No significant difference in incidence of stroke or death. | [17] |
| PHOREA | RDBPC | 321 >55 yr | 48 wk (1995–1996) | E2+ gestodene | Oral | Studied if MHT slows atherosclerosis measured by intima-media thickness in carotid. No benefit of MHT. | [114] |
| ERA | Three-arm, RDBPC | 309 Mean 65.8 yr with coronary disease | 3.2 yr (1996–1999) | CEE CEE+MPA or placebo | Oral | Angiography detected no difference in progression of coronary atherosclerosis despite increased HDL and decreased LDL | [54] |
| WISDOM | Multicenter, RDBPC | 5692 50–69 yr Mean 62.8 | Median 1 yr (1999- 2002) | CEE CEE+MPA or placebo | Oral | MHT increases CVD and VTE risk when started many years after menopause. | [115] |
| WAVE | RDBPC | 423 >55 yr With 15–75% coronary stenosis | 2.8 yr (July 1999 -January 2002) | CEE+MPA Vitamin E, C or placebo | Oral | No cardiovascular benefit of MHT or Vitamin C, E | [116] |
| ESTHER | Multicenter, case-control study | 881 271-case 610-control 45–70 yr | 6 yr (1999–2006) | Current MHT users classified by route of estrogen and type of progestogen | Oral vs Trans-dermal | Oral but not transdermal estrogen increases VTE risk. Norpregnanes may be thrombogenic. Micronized P4 and pregnanes are safe with respect to thrombotic risk. | [117] |
| RUTH | Multi-center, RDBPC | 10,101 Mean 67.5 yr | 5.6 yr (2000–2005) | Raloxifene daily or placebo | Oral | Raloxifene did not affect risk of CVD. Benefits of raloxifene in reducing risk of invasive breast cancer and vertebral fracture should be weighed against risk of VTE and stroke. | [31] |
| EPAT | RDBPC | 222 Mean 57 yr | 2 yr (2001–2003) | E2 | Oral | Average rate of progression of subclinical atherosclerosis slower in healthy Post-MW taking E2 | [118] |
| KEEPS | DBRCT of secondary prevention | 720 42–58 yr 6–36 month postmeno pause | 5yr (2005–2010) | CEE 0.45mg/d +E2 50µg/d/wk +Micronized P4 200mg/d, 12d/m | Oral Trans-dermal Oral | The study will examine whether MHT prevents progression of carotid intima thickness and accrual of coronary calcium. | [111] |
| ELITE | DBRCT | 643 <6 yr or >10 yr Postmeno pausal | 2005–2012 | E2 1mg/day ±P4 gel 4% or placebo | Oral Vaginal | The study will examine progression of carotid intima thickness, accrual of coronary calcium and lesions, and neurocognitive function and | [19] |
PEPI, Postmenopausal Estrogen/Progestin Interventions; HERS, Heart and Estrogen/progestin Replacement Study; WHI, Women’s Health Initiative; WEST, Women’s Estrogen for Stroke Trial; PHOREA, Postmenopausal Hormone REplacement against Atherosclerosis; ERA, Estrogen Replacement and Atherosclerosis; WISDOM, Women's International Study of long Duration Oestrogen after Menopause; WAVE, Women's Angiographic Vitamin and Estrogen trial; ESTHER, EStrogen and THromboEmbolism Risk study; RUTH, Raloxifene Use for The Heart; EPAT, Estrogen in the Prevention of Atherosclerosis Trial; KEEPS, Kronos Early Estrogen Prevention Study; ELITE, Early versus Late Intervention Trial with Estradiol; CEE, conjugated equine estrogen; CVD, cardiovascular disease; CHD, coronary heart disease; MPA, medroxyprogesterone acetate; RCT, randomized clinical trial; RDBPC, randomized double-blinded placebo-controlled; UA, uric acid; VTE, venous thrombo-embolism
Clinical studies also raised concerns about possible relationship between MHT and venous thrombo-embolism (VTE). VTE is uncommon before menopause, but its incidence increases with age after menopause and reaches 1 per 100 over age 75 years. Observational studies suggested that MHT use may increase the risk of VTE [13]. Also, WHI showed an overall doubling of VTE events with MHT in the CEE+MPA arm [14], although the effect of MHT on VTE events was not significant in the CEE-alone arm (Table 1). A recent large cohort case-control study of 23,505 Post-MW with VTE matched with 231,562 controls revealed that the risk of VTE increased with use of oral estrogen and oral estrogen+progestogen, with the risk being highest in the first year after initiation of treatment but decreased in subsequent years. There was no increase in VTE risk with the use of transdermal estrogen, even in patients with pre-existing thrombophilia [15].
Another concern with MHT use is the risk of stroke. NHS showed that MHT increased the risk of stroke by 35% [11]. WHI showed a 30% to 40% increased risk of stroke for women given CEE+MPA or CEE alone [16]. The Women’s Estrogen for Stroke Trial (WEST) examined the effect of MHT on ischemic stroke and transient ischemic attacks (TIA) among Post-MW randomized to either E2 or placebo, with stroke and death as primary endpoint, and TIA and nonfatal MI as secondary endpoints [17]. The study showed no net benefit for the primary endpoint, and suggested that E2 therapy might worsen the injury caused by recurrent cerebral ischemia. During the first 6 months, there were 3 fatal strokes and 18 nonfatal strokes in the group randomized to E2, compared with 1 fatal stroke and 8 nonfatal strokes in the placebo group. Women receiving E2 were more likely than those receiving placebo to die from a stroke, and nonfatal events in the E2 group were associated with greater neurologic and functional deficits. The study also suggested the possibility of increased risk of stroke early after the initiation of E2 therapy. The mechanism by which exogenous E2 may exacerbate the injury caused by stroke or precipitate stroke is unclear. Although E2 may reduce some vascular risk factors, it may have direct effects on neurons and increase sensitivity to ischemia by modulating the excitatory effects of glutamate or the inhibitory effects of γ-aminobutyric acid, or it may promote proinflammatory effects [17].
Factors Responsible for the Divergent Outcomes of MHT in CVD
The lack of vascular benefit of MHT in RCTs may be explained by several factors including the type, dose and route of administration of MHT, age-related changes in ER, the subjects’ age and timing of MHT, and MHT interaction with other sex hormones.
Type of MHT
The outcome of MHT in Post-MW could be affected by the type of estrogen used. Endogenous natural estrogens include estrone (E1), estradiol (E2) and estriol (E3). Biosynthesis of natural estrogens starts with cholesterol binding to lipoprotein receptors on steroidogenic cells, which is then taken up and transferred to the steroid synthesis sites with the aid of sterol carrier protein-2. The ovaries are the main source of circulating E2 in Pre-MW. Most E1 and E3 are formed in the liver from E2 or in peripheral tissues from androstenedione. The sources and plasma levels of estrogens change with age. In Post-MW, circulating androstenedione, T and E1 are the major precursors of estrogen production in peripheral tissues [18, 19]. Steroidogenic enzymes are localized in the endoplasmic reticulum of adipose stroma and other tissues. Polymorphisms in the genes coding for these steroidogenic enzymes may affect estrogen production, and evaluation of these polymorphisms may help to design a more individualized MHT in Post-MW [20].
Most circulating E2 and natural estrogens are bound strongly but reversibly to sex hormone-binding globulin (SHBG). About 2–3% of natural estrogens are unbound and active and because of their small size and lipophilic nature they distribute rapidly and extensively. E2 half-life is ~3 hours and exists in a dynamic metabolic inter-conversion state with E1 and E3. Natural estrogens are readily metabolized and excreted in urine and feces. Estrogen metabolic pathways include oxidation (hydroxylation) by cytochrome P450s (CYPs), glucuronidation by UDP-glucuronosyltransferase, sulfation by sulfotransferase, and O-methylation by catechol O-methyltransferase (COMT) [19]. Estrogen metabolism occurs mainly in the liver, where most CYPs are expressed. E2 metabolism varies with the stage of menstrual cycle, menopausal status, ethnic background and gene polymorphisms. Also, drugs and cigarette smoke may affect estrogen metabolizing enzymes [18, 19]. Changes in E2 metabolism with aging may alter its effects on the vasculature.
CEE is a common form of MHT derived from urine of pregnant mares. In 1942, premarin was the first CEE preparation approved by the FDA for treatment of menopausal symptoms. CEE is available in oral, transdermal, parenteral or topical preparations. CEE contains saturated estrogens e.g. E1, 17β-E2 and 17α-E2, and unsaturated estrogens e.g. equilin, 17β-dihydroequilin, 17α-dihydroequilin, equilenin, 17β-dihydroequilenin, and 17α-dihydroequilenin. Unconjugated estrogens (e.g. equilin) are absorbed more rapidly than conjugated estrogens (e.g. equilin sulfate), but they are soon conjugated in the liver (first pass effect) and circulate together with E1-sulfate as a hormonally inert estrogen reservoir. Tissue enzymes such as sulfatases and 17β-hydroxysteroid dehydrogenases secreted by the bowel bacterial flora and intestinal mucosa activate these inert estrogens to E1 and E2. These estrogens along with glucuronide and sulfate conjugates are excreted in urine. Oral CEE increase flow-mediated vasodilation in healthy Post-MW [21]. However, Post-MW with hysterectomy and treated with CEE showed increased risk of carotid arterial events, lower extremity arterial events, and abdominal aortic aneurysm [22]. The untoward vascular effects of CEE could be related to the fact that E2 levels decrease after menopause, while E1 levels remain unchanged. In effect, the term CEE may obscure the fact that it does not contain E2. CEE and E2 have different chemical structures, pharmacological properties, metabolic products, and ER binding affinity, selectivity, and agonistic properties [1]. Because both ER-dependent and ER-independent mechanisms play a role in mediating the cardiovascular actions of E2, CEE may not mimic the cardiovascular effects of E2. In monkeys, CEE show no effect on intimal hyperplasia after balloon injury [23]. In human aortic VSM cells (VSMCs), some components of CEE such as E1, E3 and E1-sulfate are less potent than E2 in inhibiting mitogen-induced VSMC growth and MAPK activity [1]. Thus estrogens other than E2 may be less effective in CVD, contributing to the lack of vascular protective actions of MHT.
Because of low bioavailability of oral estrogen and their first-pass metabolism, chemical alterations in natural estrogens have been utilized to produce semisynthetic estrogens with greater oral effectiveness. Ethinyl substitution at C17 position produces ethinyl-E2 and minimizes first-pass hepatic metabolism. Synthetic estrogens such as diethylstilbestrol, esterified estrogen, ethinyl-E2, E2-benzoate, cypionate, and valerate may provide cardiovascular benefits. In Post-MW, treatment with esterified estrogen shows lower risk of ischemic stroke and MI, and no increase in VTE risk compared to CEE [13]. Also, treatment of ovariectomized (OVX) hypertensive rats with E2-valerate increases serum vascular endothelial growth factor (VEGF).
There has been growing interest in phytoestrogens, which are plant-derived compounds that contain a phenolic ring, bind ERs and produce estrogenic effects. Food containing phytoestrogens is consumed in large quantities in cultures with lower rate of menopausal symptoms, osteoporosis, cancer and CVD such as Japan, China and South East Asia. Phytoestrogens are found in a variety of foods particularly soybeans, red clover, wheat grains, and peas. Phytoestogens are classified into flavonoids (flavones, flavonols and flavanones), isoflavonoids (isoflavones and coumestanes), stillbenes and lignans (Table 2). Soy beans contain three primary isoflavones in glycoside form – genistin, daidzin and glycitin, and digestion leads to cleavage of the sugar moiety and release of the respective aglycones genistein, daidzein and glycitein. Stillbenes include resveratrol which is found in red wine and peanuts, but only its trans form has estrogenic activity. Lignans include secoisolariciresinol and matairesinol, which are converted to enterodiol and enterolactone by intestinal microflora. Dietary sources of lignans include flaxseed, whole grain bread, vegetables and tea [24]. Phytoestrogens bind ER with weak affinity (10−4 to 10−2 of E2), and isoflavones bind with greater affinity to ERβ than ERα. However, phytoestrogens can be found in blood at levels up to 10,000 times that of steroidal estrogens [25]. Isoflavones activate eNOS, induce vasodilatation and may have anti-atherogenic and anti-thrombotic effects [25].
Table 2.
Vascular estrogen receptor distribution, function, signaling, agonists and antagonists
| ERα | ERβ | GPR30 | |
|---|---|---|---|
| Molecular Weight | 66–70 kDa | 53–59 kDa | 38 kDa |
| Vascularz Distribution | |||
| Endothelium | ++ | ++ | + |
| VSM | ++ | ++ | + |
| Cellular Distribution | |||
| Plasma Membrane | ++ | ++ | ++ |
| Nucleus | ++ | ++ | + (Envelope) |
| Cytosol | ++ | ++ | + |
| Endop Reticulum | + | + | ++ |
| Golgi | + | + | |
| Mitochondria | + | + | |
| Function | Transcription regulation, vascular relaxation, inhibition of VSM contraction, growth & proliferation | Transcription regulation, vascular relaxation, inhibition of VSM contraction, growth & proliferation | Vascular relaxation |
| Signaling Pathway Activate | cSrc/PI3K/Akt, Src/MAPK, NO-cGMP, PGI2-cAMP | cSrc/PI3K/Akt, Src/MAPK, NO-cGMP, PGI2-cAMP | AC-cAMP, PI3K EGFR/Erk1/2 |
| Inhibit | Ca2+ influx, MLC kinase, PKC Rho-kinase, MAPK | Ca2+ influx, MLC kinase, PKC Rho-kinase, MAPK | |
| Agonists Natural | Estradiol, Estrone, Estriol Conjugated equine estrogen | Estradiol, Estrone, Estriol Conjugated equine estrogen | Estradiol |
| Synthetic | Chlorotrianisene, Dienestrol, Diethylstilbestrol, Ethinylestradiol, Fosfestrol, Mestranol, Polyestradiol phosphate, S-S tetrahydrochrysene Estradiol cypionate, valerate | Chlorotrianisene, Dienestrol, Diethylstilbestrol, Ethinylestradiol, Fosfestrol, Mestranol, Polyestradiol phosphate, S-S tetrahydrochrysene Estradiol cypionate, valerate | -Kepone, ICI 182,780 Bisphenol A, Nonylphenol |
| Phytoestrogens Isoflavones | Daidzein | Genistein, Daidzein, Biochanin A | Genistein |
| Flavonols | Quercetin | ||
| Flavanones | Naringenin, Taxifolin | Naringenin, Taxifolin | |
| Coumestans | Coumestrol, 4′ methoxycoumestrol | Coumestrol, 4′ methoxycoumestrol | |
| Lignans | Enterodiol, Enterolactone, Secoisolariciresinol, Matairesinol | Enterodiol, Enterolactone, Secoisolariciresinol, Matairesinol | |
| Stilbenes | Resveratrol | Resveratrol | |
| SERMS (partial agonists) | Raloxifene,Tamoxifen, Idoxifene, Toremifene, Bazedoxifene | Raloxifene,Tamoxifen, Idoxifene, Toremifene, Bazedoxifene | Raloxifene, Tamoxifen |
| Specific Agonists | PPT, 16 α-LE2, ERA-45 | DPN, 8β-VE2, Androstene 3,5 diene, DCW234, ERB-041, Monoaryl-Substituted Salicylaldoximes, WAY-202196, WAY-214156 | G1 |
| Antagonists | ICI 182,780, Clomifene, Glyceollin | ICI 182,780, Clomifene | Pertussis Toxin |
| Specific Antagonists | MPP | RR-tetrahydrochrysene | G15 |
Although phytoestrogens could exert estrogenic effects, they have not been tested in well-designed RCTs. Soybean foods, soybean protein extract and red-clover extract have limited effects on menopausal symptoms, while soybean isoflavone extracts reduce hot flushes, suggesting that the benefits are subtle and not in all individuals, and highlighting the need of adequately-powered RCTs. Also, while phytoestrogens affinity to ERs is weaker than E2, their stability and longer duration in the body raises concerns of potential toxicity. A meta-analysis of randomized placebo controlled trials to evaluate the effects of oral isoflavone on endothelial function in Post-MW as measured by flow mediated dilatation (FMD) showed improved flow in women with low baseline but not high baseline FMD [26]. Also, a meta-analysis of 38 controlled studies of soy consumption showed positive effect on lipid profile e.g. decreased LDL-c and triglycerides and increased HDL-c [27]. However, a 12 month double-blind RCT comparing the effects of soy protein containing 99 mg isoflavones/day with milk protein in 202 Post-MW aged 60–75 years did not find any effect on blood pressure, body weight or endothelial function [28].
Selective estrogen receptor modulators (SERMs) such as raloxifene, tamoxifen, toremifene, and idoxifene are non-steroidal molecules that bind with high affinity to ERs, but have distinct effects depending on the drug’s structure and specific tissue. SERMs have a wide range of activity from purely estrogenic, purely anti-estrogenic, to partial estrogenic in some tissues and anti-estrogenic or no activity in other tissues. The SERMs' agonist/antagonist activity and tissue selectivity may be related to the ratio of co-activator/co-repressor proteins in different cell types, and the ER conformation induced by drug binding. This in turn determines how strongly the SERM/ER complex recruits co-activators, resulting in agonsim, relative to co-repressors, resulting in antagonism. An ideal SERM is expected to act as an ER agonist on the cardiovascular system, bone, vagina and bladder, and as an ER antagonist on the breast and endometrium. Raloxifene is an ER agonist in bone and serum lipids, but ER antagonist in endometrial and breast tissue. In rat renal and pulmonary artery and rabbit and porcine coronary artery, raloxifene causes endothelium-independent inhibition of VSM contraction and Ca2+ influx [29]. Raloxifene induces IGF-I and COX-2 expression and recruitment of co-activator complexes and histone acetylation at both the IGF-I and COX-2 gene promoter in human umbilical vein ECs (HUVECs), but reduces these parameters and serum-induced COX-2 expression in human aortic smooth muscle cells (HASMCs) [30], supporting that SERMs have different effects in different tissues.
SERMs reduce the levels of total cholesterol, LDL-c, triglycerides, fibrinogen and cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TMF-α), suggesting that they could reduce CHD in Post-MW. The Raloxifene Use for The Heart (RUTH) evaluated the long-term efficacy and safety of raloxifene in prevention of CHD and breast cancer in Post-MW with CVD or at increased risk for CVD and showed that raloxifene treatment for a median of 5.6 years reduced the risk of invasive breast cancer but did not affect the risk of primary coronary events and increased the risk of fatal stroke and VTE [31] (Table 1). As in WHI, the advanced age of the participants could have affected the estrogenic response. Also, the Multiple Outcomes of Raloxifene Evaluation (MORE) study showed that raloxifene therapy for 4 years did not affect the risk of cardiovascular events in the overall cohort, but reduced the risk of cardiovascular events in a subset of women with increased cardiovascular risk and multiple risk factors. The risk of stroke was unchanged and the risk of VTE was increased over the first 4 years of MORE, and 4 years’ extension [32]. Although currently available SERMs show limited cardiovascular benefits, as new SERMs are developed they may be used as MHT to decrease the risk of CVD in Post-MW.
27-hydroxycholesterol (27HC), a cholesterol metabolite elevated in hypercholesterolemia and found in atherosclerotic lesions, is a competitive ER antagonist that acts as endogenous SERM. 27HC shows similar affinity for ERα and ERβ, but stronger inhibition of E2-induced transcription through ERβ than ERα [33]. In Pre-MW, the amount of 27HC is lower than E2, leading to preserved ER function and vascular protection. During menopause and in hypercholesterolemia, 27HC levels are higher than E2, and thus reduce ER function and vascular protection, and may partly explain why MHT fails to show cardiovascular benefits in Post-MW [33].
Dose of MHT
The adverse effects of MHT observed in RCTs may be related to the relatively high dose of estrogens used. Estrogens are highly lipophilic compounds and their circulating levels may not reflect their vascular tissue level. Estrogen regulates fat mass, adipose deposition and differentiation, and adipocyte metabolism, and estrogen deficiency in Post-MW could result in increases in adipose tissue and visceral fat. Adipose tissue has P450 aromatase activity, which converts androstenedione into E1, the main estrogen in Post-MW. Conversion of androstenedione into E1 increases with age and obesity. Also, adipose tissue secretes cytokines such as IL-6 and TNFα, and since obesity is more common in Post-MW, the increased cytokines from excess adipose tissue could affect vascular ER and their signaling mechanisms. Estrogens are also extensively bound to plasma proteins particularly globulin, and the hormone pharmacokinetics and volume of distribution may change in Post-MW particularly those with liver or kidney disease. Thus, an estrogen dose that may seem normal in Pre-MW could produce superphysiological plasma levels in Post-MW, and lead to estrogenic side-effects [19]. These observations have suggested that the benefits of MHT may be maintained with a dose lower than previously used. Women’s Health, Osteoporosis, Progestin, Estrogen (Women’s HOPE) trial showed efficacy and improved benefit/risk ratio of lower-dose CEE/MPA (0.45/1.5 mg and 0.3/1.5 mg) for prevention of bone loss, relief of vaginal atrophy and vasomotor symptoms, and reduction in endometrial bleeding [34].
The effect of estrogen on lipids and lipoproteins may also depend on the dose used. In the HOPE trial, the reduction of LDL-c and increased HDL-c were less with lower doses of CEE, but the beneficial effects were maintained and the reduction in protein S activity and increase in triglycerides were less pronounced [35]. Also, a study comparing low dose CEE 0.3 mg/MPA 2.5mg and standard-dose CEE 0.625 mg/MPA 2.5 mg showed similar increase in maximal forearm blood flow, and decrease in serum LDL-c and malondialdehyde)-modified LDL in both groups [36].
The vascular effects of estrogen may in part reflect its effect on matrix metalloproteinases (MMPs). At low doses, E2 may inhibit MMPs and attenuate collagen deposition, whereas high E2 doses may activate MMPs and promote vascular lesion formation and plaque destabilization. In cultured human coronary artery and umbilical artery VSMCs increasing E2 from physiological to supraphysiological levels causes dose-dependent increases in MMP-2 levels in culture media, supporting different effects of low and high E2 doses on vascular integrity [37]. Thus, the dose of estrogen could influence its vascular effects and the relatively high doses of MHT may explain some of the adverse effects in RCTs.
Route of Administration of MHT
The route of administration could be a factor in successful MHT. Estrogen can be administered orally, as percutaneous gel, transdermal patch or subcutaneous implant. Transdermal E2 avoids exaggerated peaks in plasma estrogen levels that occur during oral administration, undergoes a rate of conversion to E1 that is similar to that during the menstrual cycle, and may have similar vascular benefits and less adverse effects than oral preparations [38]. Oral E2 increases C-reactive protein (CRP) levels and MMPs expression in the vascular wall [39]. In healthy Post-MW, transdermal E2 increases brachial artery flow-mediated vasodilation similar to oral E2, but does not increase CRP levels [40]. Also, a study in Post-MW showed that oral E2/P4 increased the risk of breast cancer, while oral unopposed E2 and transdermal E2/P4 did not [41]. Transdermal MHT may show less risk for VTE than oral MHT, but this needs to be confirmed.
Because CEE is given orally and E2 is often administered transdermally the differences in outcome between CEE and E2 may be partly caused by the route of administration. Most observational studies such NHS tested almost entirely the effects of oral MHT and showed that MHT use is associated with ~40% reduction in CHD [11]. One epidemiological study reported a decreased risk of CHD with transdermal MHT, and no overall effect of oral MHT [42]. However, most epidemiological data suggest that the route of administration of MHT has no impact on the risk of breast cancer and hip fracture, and the results regarding the risk of CHD and colorectal cancer are inconsistent. Also, few MHT studies evaluated the risk of stroke and diabetes to allow meaningful conclusions. Thus, the route of administration of MHT should be an active area of research to identify treatment modalities that would have the least adverse effects.
Factors Related to Estrogen Receptor (ER)
Estrogens bind ERs with high affinity and specificity. Two nuclear ERs have been cloned: ERα and ERβ. ERα knock-out (KO), ERβ KO, and ERα/ERβ double KO mice survive, suggesting that life is possible without ERs, but the reproductive functions are impaired [43]. ERα is expressed in the uterus, vagina, ovaries, mammary gland and hypothalamus. ERβ is highly expressed in the ovaries, with smaller amount in lung, brain and bone. Also, ERα and ERβ have distinct roles in the immune, skeletal, central nervous system and cardiovascular system [44, 45]. ERα and ERβ have been localized in ECs and VSM [46]. While most plasma membrane ERα and ERβ form homodimers in response to E2, a small portion of the ER pool forms ERα/ERβ heterodimers [47]. Vascular ERs expression varies with gender and the vascular bed studied. ERα and ERβ are expressed in human VSM from coronary artery, iliac artery, aorta, and saphenous vein, and the expression of ERβ is greater in females [48]. In rats, ERα is found mainly in uterine artery, while ERβ is more abundant in ECs and VSM of the aorta, tail and uterine arteries [49].
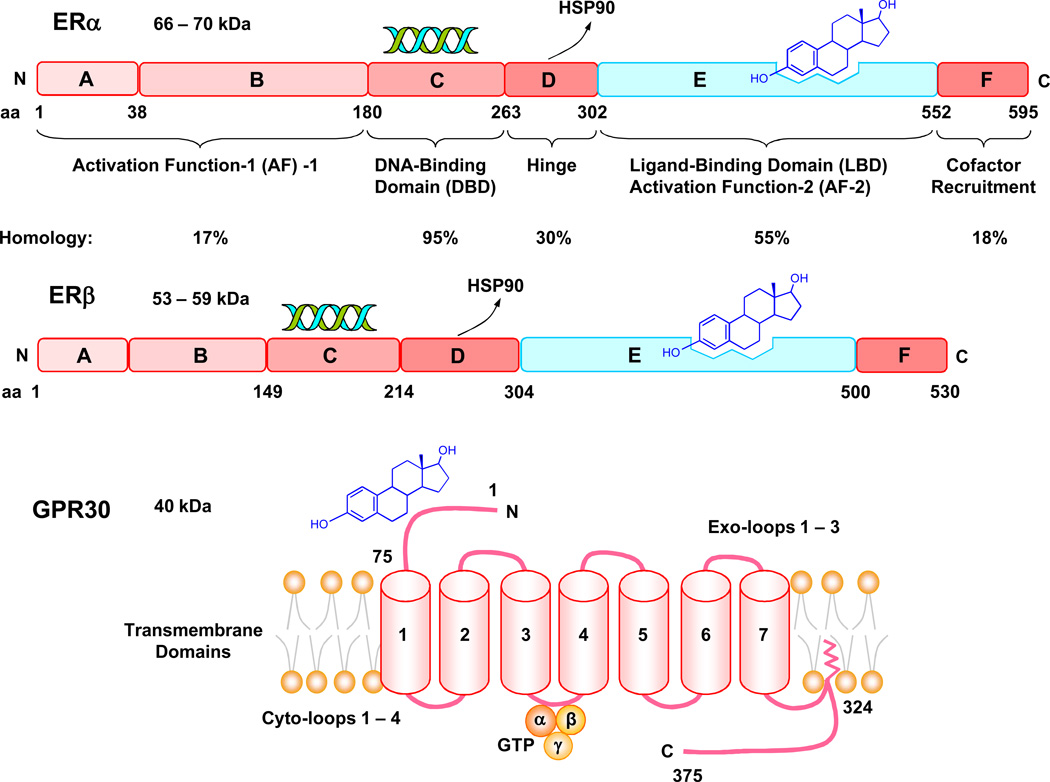
ER genes are located on separate chromosomes; ESR1 is on chromosome 6q(25.1) and encodes ERα, while ESR2 is on chromosome 14q(23–24.1) and encodes ERβ [50]. Like other members of the nuclear receptor superfamily, one ER gene may result in the expression of multiple ER proteins and diverse responses. The mechanisms of this diversity include epigenetic changes and methylation of the encoding genes, alternative RNA splicing leading to multiple mRNA isoforms of each receptor, and multiple sites for initiation of translation of ER mRNA. Human ERα and ERβ have 44% homology in amino acid sequence and share the domain structure common to members of the nuclear superfamily i.e. A/B, C, D, E and F domains [51] (Fig. 1).
Fig. 1.
Structure of ERα, ERβ, and GPR30. ERα and ERβ share a common structure with five functional domains A/B, C, D, E and F. The A/B region is involved in protein-protein interaction and transcriptional activation of target-genes. The A/B region harbors activation function-1 (AF-1), located toward the N-terminal end, that is ligand-independent and shows promoter and cell-specific activity. Human ERα and ERβ share <20% amino acid identity in A/B region, suggesting that it may contribute to ER-specific actions on target genes. The central C-domain is the DNA-binding domain (DBD), which contains 4 cysteines arranged in 2 zinc fingers, and is involved in DNA binding and receptor dimerization. It is highly conserved between ERα and ERβ and shares 95% amino acid identity, suggesting that both ERs recognize similar DNA sequences and hence regulate many of the same target genes. D-domain is not well-conserved between ERα and ERβ (30%) and works as a flexible hinge between DBD and ligand-binding domain (LBD). D- domain also functions in promoting the association of ER with heat shock protein 90 (HSP90) and nuclear localization of ER. E-domain is the LBD, and ERα and ERβ share ~55% amino acid identity in this region. LBD contains a ligand-dependent AF-2, and is important for ligand binding and receptor dimerization. F-domain has <20% amino acid homology between ERα and ERβ, includes co-factor recruitment regions, but its function is unclear. Full transcriptional activation by ERs is mediated by synergism between AF-1 and AF-2. AF-1 and AF-2 are also required for ligand-independent receptor functions, including growth factor activation by AF-1 and cAMP activation by AF-2. GPR30 is a G protein-coupled receptor that has estrogenic activity and shares little homology with the classical ERs. GPR30 has an extracellular N terminal, 7 transmembrane α helices, 3 exo-loops involved in ligand binding, 3 or 4 cyto-loops involved in G protein binding, and a C terminal linked to the membrane through lipid addition, and also involved in G protein binding.
Polymorphisms in ER genes may account for the differences in ER function and the outcome of MHT. Two common polymorphisms of ERα at positions c.454–397 T>C (PvuII) and c.454–351 A>G (XbaI) may be associated with the severity of CHD in Post-MW [52]. Post-MW with the ERα IVS1–397 polymorphism C/C genotype (recessive) have greater HDL and apolipoprotein A-1 levels and greater forearm blood flow, brachial artery diameter, and endothelium-dependent dilation compared to the dominant phenotype [53]. In Post-MW with CHD and being treated with estrogen alone or estrogen+progestin, a 2 times greater increase in HDL levels was seen in women with the ERα IVSI-401 polymorphism C/C genotype compared to those without the polymorphism [54].
Although estrogen levels decrease with aging, little is known about age-related changes in ERs. ER expression is not different in aorta of aging and adult OVX spontaneously hypertensive rats (SHR) [55]. However, gender and age-related differences in ER expression have been shown in other tissues. ERα is detected in the retina of young females but not men or Post-MW. ERβ mRNA is decreased in specific regions of the brain of old compared with young and middle-aged female rats, and the changes in expression are not altered by estrogen treatment. Aging may also affect the subcellular distribution of ERα in cholinergic neurons of transgenic and wild-type mice, and is associated with translocation of ERα from the nucleus to cytoplasm [56].
Splice variants or isoforms of ER have been described. Most ERα variants differ in their 5’-untranslated region, not in the coding sequence. ERα isoforms have not been identified in tissues, and their biological function is unclear, but they are good research tools as they heterodimerize with the full-length ERα and repress AF-1-mediated activity. They may also localize in plasma membrane and thereby help to elucidate the non-genomic ER signaling mechanisms. Multiple ERβ isoforms exist as a result of either alternative splicing of the last coding exon 8, deletion of one or more coding exons, or alternative usage of untranslated exons in the 5’ region. Among them, 5 full-length transcripts ERβ1–5 have been reported in human [45]. Whether ER isoforms are distributed differently in the vasculature or change with aging is an important area for investigation.
Regulation of gene expression could involve epigenetic modification and methylation of the CpG island, a cytosine and guanine rich area in the gene promoter region, resulting in inactivation of gene transcription. ERβ expression is regulated by methylation of CpG islands in the ERβ gene promoter, and may be an epigenetic mechanism in age-related atherosclerosis and vascular aging [57]. Methylation of ER genes also increases with passage of cultured ECs and VSM and may explain the decreased ER responsiveness and vascular effects of estrogen with aging [57].
The ligand binding sites of ERα and ERβ are nearly identical; only two residues are different: Leu384/Met421 in ERα correspond to Met336/Ile373 in ERβ. Also, the ligand binding cavity of ERβ is ~20% smaller than ERα and this may affect the selective affinity of ligands to ERs [51]. E2 binding with ER typically involves non-covalent H-bonding and hydrophobic interactions. For H-bonding, the phenolic ring is more important than any other structure. With regard to hydrophobic interactions, the volume of the ER binding pocket is ~twice the size of E2, leading to large unoccupied cavities opposite C7α and C11β positions of E2 [58]. These cavities allow steric groups of certain sizes to fit and could be essential for binding of estrogenic compounds such as diethylstilbesterol. Tamoxifen has almost identical binding affinity for ERα and ERβ, although its binding affinity is only 3–4% of E2 and 7–10% of the ER antagonist ICI-182,780. The binding affinity of raloxifene is similar for ERβ as tamoxifen, 16-fold higher for ERα than tamoxifen, and comparable with ICI-182,780. Metabolites such as E1–3-sulfate and E2–3-sulfate have little affinity for ERα and ERβ, while 16α-OH-E1 has higher affinity than E1. The phytoestrogen genistein has high affinity for ERβ, almost identical to E2, and its affinity for ERα is only 6% of that for ERβ. Coumestrol has high affinity for ERα and slightly higher affinity for ERβ. Daidzein has weak affinity for ERα and ERβ, but higher affinity for ERβ than ERα. Further elucidation of the estrogen-ER interactions should permit the design of selective estrogenic drugs for aging women.
Recently, a novel 7-transmembrane G protein-coupled receptor, termed GPR30 has been identified [53] (Fig. 1). GPR30 is structurally unrelated to ERα or ERβ, binds E2 with high affinity, and mediates some of its non-genomic effects [59]. GPR30 was cloned in 1998 and is located on chromosome 7p22. GPR30 is widely distributed in the brain and peripheral tissues and may play a role in vasculature [60]. GPR30 is expressed in human mammary artery and saphenous vein [61]. The distribution of GPR30 varies in different cell types, and has been localized in the endoplasmic reticulum [59] and plasma membrane [62]. Activation of GPR30 results in intracellular Ca2+ mobilization and synthesis of phosphatidylinositol 3,4,5-trisphosphate in the nucleus. GPR30 may also function in conjunction with ERα to assemble a complex for rapid estrogen signaling. The ER antagonists tamoxifen and ICI 187,280 may act as GPR30 agonists [62]. However, the role of GPR30 as an ER has been questioned. In ER-positive, GPR30-positive MCF-7 cells, nongenomic E2 responses were blocked by ICI 187,280 and were dependent on ER, while silencing of GPR30 had no effect on E2-induced cAMP elevation or ERK activation [63]. Thus, although GPR30 has been found in the vasculature, its functional role needs further investigation.
Selective ER Agonists and Antagonists
Selective ER agonists have been developed. Triarylpyrazoles such as propylpyrazole trisphenol (PPT) are 400-fold more potent on ERα than ERβ. Studies with selective ER agonists suggest that ERα may mediate most of the vascular actions of estrogen. PPT increases flow-mediated relaxation in small mesenteric arteries from female, but not male mice [64]. Diarylpropionitrile (DPN) is a potent ERβ agonist with a 30- to 70-fold selectivity over ERα [41]. DPN causes rapid NO-dependent vasodilation. G1 is a selective GPR30 agonist. Specific ER antagonists may be useful in preventing the undesirable effects of E2 such as breast and uterine cancer. ICI 164,384, the first estrogen antagonist described, blocks the uterotrophic effects of E2 or tamoxifen in rats. ICI 182,780 (fulvestrant) is a more potent E2 antagonist approved for treatment of advanced breast cancer in Post-MW. ICI 164,384 and ICI 182,780 block E2 activity via inhibition of both AF-1 and AF-2 action [65]. By introducing basic side chain substituents such as those found in tamoxifen, selective ERα antagonists, such as methyl-piperidino-pyrazole (MPP) have been developed. Further research is needed to develop specific estrogenic compounds with high ER and cardiovascular selectivity in Post-MW.
Post-ER Signaling Pathways
The decrease in vascular actions of estrogen with aging could be due to downregulation of post-ER signaling pathways. Vascular effects of estrogen include alteration of serum lipid, coagulation and fibrinolysis, antioxidant properties, and release of vasoactive substances such as NO and prostaglandins, and these effects involve genomic and non-genomic pathways [60].
In the absence of estrogen, ER exists as an inactivated monomer bound with heat shock protein-90 (HSP90). Upon binding to estrogen, ER undergoes conformational changes that result in dissociation of HSP90 and formation of a homo- or heterodimer with high affinity for estrogen and DNA. Estrogen-ER mediated genomic pathways involves nuclear translocation, binding to specific estrogen response elements (ERE) and regulation of target gene expression. Estrogen affects genes regulating vascular tone, and those involved in the response to vascular injury and atherosclerosis [46]. Estrogen increases gene expression of NO synthase and prostacyclin synthase. Ligand-bound ER can bind directly to ERE in the promoters of target genes or interact with other transcription factor complexes like Fos/Jun (AP-1-responsive elements) or SP-1 (GC-rich SP-1 motifs) and influence transcription of genes whose promoters do not harbor ERE. Ligand-independent pathways may also activate ERs. Growth factor signaling leads to activation of kinases that may phosphorylate and activate ERs or associated co-regulators in the absence of ligand and contribute to hormone-independent growth of some tumors.
Non-genomic effects are rapid responses that occur too quickly to be mediated by gene transcription, are independent of protein synthesis, and typically involve modulation of membrane bound and cytoplasmic regulatory proteins. In several cell types, ERs associate with plasma membrane caveolae to trigger G protein-coupled second messengers and intracellular pathways including MAPK, PI3K/Akt, and ion fluxes. E2 induces the phosphorylation of p38 and p42/44 MAPK (ERK-1/2) and proliferation and migration of porcine aortic
Estrogen and ECs
E2 induces phosphorylation and activation of MAPK, and proliferation of ECs via cytosolic and nuclear ERs. Estrogen also affects vascular reactivity through direct effects on ECs [60]. HUVECs express both ERα and ERβ [46]. Endothelium-dependent vascular relaxation is greater in female than male SHR. Selective ERα agonists improve EC dysfunction in blood vessels of OVX female SHR [66]. Also, E2-induced vascular relaxation and NO production are greater in mice expressing only ERα [67]. E2 administration in OVX female mice causes rapid dilation of elastic and muscular arteries, as a result of ER-mediated NO production. E2 causes rapid activation of MAPK/ERK and PI3K, and inhibiting either kinase prevents E2-induced vasodilatation. E2-induced kinase activation and vasodilation are absent in ERα and ERβ double-KO mice [68]. Small arteries isolated from healthy Post-MW not receiving MHT show impaired morphology and EC function, and these impairments are improved in vessels treated with E2 [69]. Estrogen-induced endothelium-dependent vasodilation involves modulation of synthesis, release and bioactivity of relaxing factors such as NO, PGI2 and endothelium-derived hyperpolarizing factor (EDHF), as well as contracting factors such as endothelin-1 (ET-1) and thromboxane A2 (TXA2) [19].
NO is a powerful vasodilator produced from the transformation of L-arginine to L-citrulline by NO synthase (NOS). E2 promotes vasodilation by increasing eNOS transcription via genomic pathways as well as eNOS activity and NO production via non-genomic pathways (Fig. 2). Estrogen increases eNOS mRNA expression in cultured ECs. Endothelial NO production is greater in arteries of females than males, and the inhibitory effect of the NOS inhibitor L-NAME on acetylcholine (ACh)-induced relaxation is more pronounced in mesenteric arteries of female than male rats. In basilar artery from OVX female rabbit, E2 increases the response of VSM to NO. The expression of eNOS in the uterine vasculature increases during pregnancy, supporting a role of NO in E2-induced vascular relaxation. Estrogen has antioxidant properties, decreases the expression of NADPH oxidase and generation of superoxide and peroxynitrite, and increases NO bioavailability. Superoxide levels are less in the aorta of female than male rats [70]. In OVX female rats, the increase in blood pressure is associated with low plasma antioxidant levels and increased plasma lipoperoxides and vascular free radicals, and E2 replacement prevents these effects [71]. ER has been localized in mitochondria and E2 decreases mitochondrial superoxide production and oxidative stress, suggesting that ER mediates effects on not only nuclear but also mitochondrial-encoded genes [60].
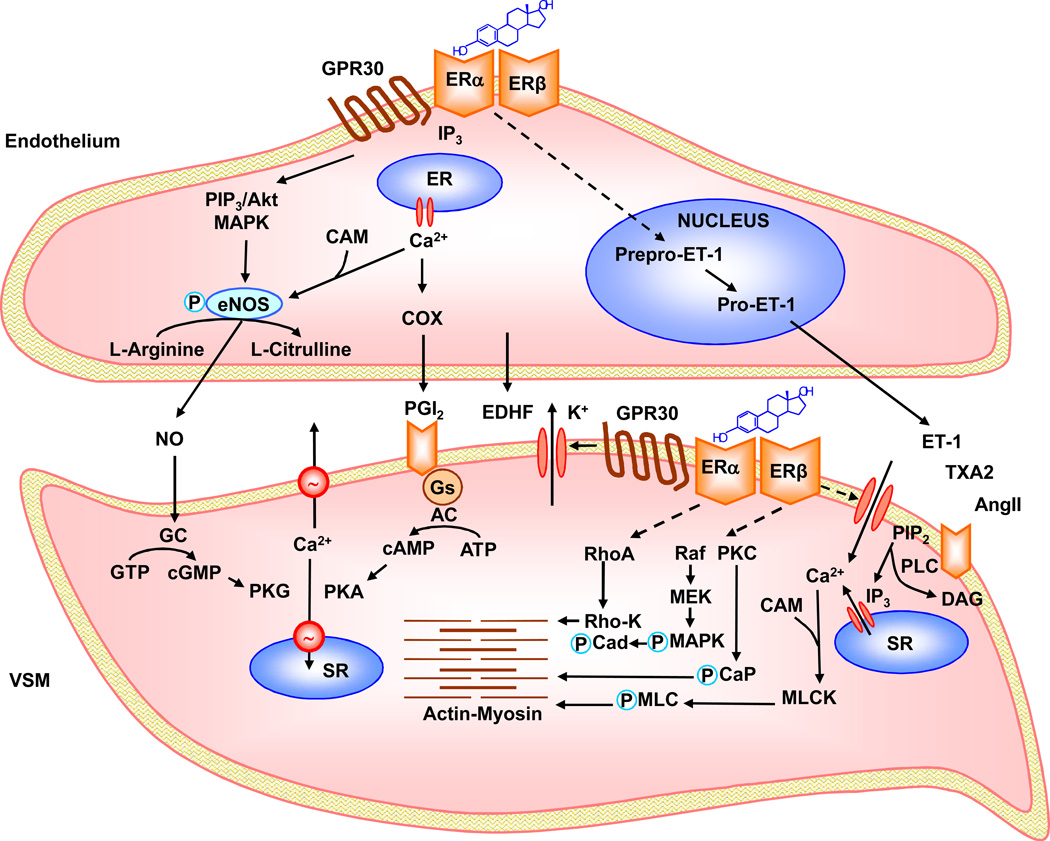
Fig. 2.
Estrogen-induced ER-mediated pathways of vascular relaxation. Estrogens such 17β-estradiol (E2) binds to endothelial ERα, ERβ or GPR30 and activates phospholipase C (PLC), leading to the generation of inositol 1,4,5- triphosphate (IP3) and diacylglycerol (DAG). IP3 causes the release of Ca2+ from the endoplasmic reticulum (ER). Ca2+ forms a complex with calmodulin (CAM), which causes initial activation of eNOS. E2 also activates phosphatidylinositol 3-kinase (PI3K), which transforms phosphatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol 3,4,5-trisphosphate (PIP3), and activates Akt. ER-mediated activation of Akt or MAPK causes phosphorylation and full activation of eNOS. Fully activated eNOS transforms L-arginine to L-citrulline and produces NO, which diffuses through ECs and activates guanylate cyclase in VSM leading to increased cGMP and stimulation of cGMP-dependent protein kinase (PKG). PKG decreases [Ca2+]i by stimulating Ca2+ extrusion pumps in the plasma membrane and Ca2+ uptake pumps in SR and/or decreases the sensitivity of the contractile myofilaments to [Ca2+]i and thereby promotes VSM relaxation. E2 also activates COX to produce prostacyclin (PGI2) which activates cAMP-dependent pathway, protein kinase A (PKA), and promotes relaxation pathways similar to cGMP/PKG. E2 also induces the release of EDHF which activates K+ channels and causes hyperpolarization and relaxation of VSM. E2 could also decrease endothelial ET-1 expression and production via genomic pathway. In VSM, agonists such as ET-1, TXA2 and AngII activate specific VSM receptors, stimulate PLC, and increase the production of IP3 and DAG. IP3 stimulates Ca2+ release from the sarcoplasmic reticulum. Agonists also stimulate Ca2+ entry through Ca2+ channels. Ca2+ binds CAM, activates myosin light chain kinase (MLCK), causes MLC phosphorylation, and initiates actin-myocin interaction and VSM contraction. DAG activates PKC, which in turn phosphorylates calponin (CaP) and/or activate a protein kinase cascade involving Raf, MAPK kinase (MEK) and MAPK, leading to phosphorylation of caldesmon (CaD) and increased myofilament force sensitivity to Ca2+. E2 binds to VSM ERs, leading to inhibition of agonist-activated mechanisms of VSM contraction. E2 activates K+ channels, leading to membrane hyperpolarization and inhibition of Ca2+ entry through Ca2+ channels. E2 may also inhibit PKC, MAPK or the RhoA/Rho-kinase (Rho-K) [Ca2+]i sensitization pathway. Dashed lines indicate inhibition.
E2 may increase the release of PGI2, a potent inhibitor of platelet aggregation and strong vasodilator. PGI2 is produced from arachidonic acid by cyclooxygenase-1 (COX-1) and COX-2. In arteries from OVX female monkeys with induced atherosclerosis, the amount of PGI2 is inversely related to plaque size, and arteries treated with E2 show increased PGI2 production [72]. E2 stimulates urinary excretion of COX-2-derived PGI2 metabolites in ERβ but not ERα KO mice, supporting a role for ERα in PGI2 production [73]. The COX inhibitor indomethacin partly inhibits endothelium-dependent vascular relaxation, and gender differences in indomethacin-sensitive vascular relaxation is attributed to differences in COX products. However, COX modulation by E2 may be tissue specific. Indomethacin does not affect E2-induced relaxation in coronary artery. E2 causes upregulation of COX-2 in human uterine microvascular ECs, but not dermal microvascular ECs. Cultured ECs show a more positive relation between estrogen, COX and PGI2. E2 causes upregulation of COX-1 expression and PGI2 synthesis in ECs. E2 also causes rapid ER mediated PGI2 synthesis in ovine fetal pulmonary artery ECs via a Ca2+-dependent, but MAPK-independent pathway [74]. In HUVECs, PGI2 production is stimulated by serum from Post-MW receiving a mixture of phytoestrogens, and raloxifene increases PGI2 synthesis by modifying the expression/activity of COX-1 and −2.
In some blood vessels such as small mesenteric arteries incubated in the presence of NOS and COX inhibitors, ECs release EDHF. E2 increases the production of EDHF, which activates K+ channels, causes hyperpolarization, and in turn inhibits Ca2+ influx and causes VSM relaxation [2]. ACh-induced hyperpolarization and relaxation of mesenteric arteries are greater in intact female than intact male or OVX female rats, and the differences are eliminated by K+ channel blockers. ACh-induced vascular hyperpolarization and relaxation are improved in E2-replaced OVX females, confirming that estrogen-deficient states attenuate vascular relaxation by EDHF [75]. Phytoestrogens may also induce vascular relaxation through production of EDHF.
In addition to vasodilators, ECs release ET-1. ET-1 activates endothelial ETB1 receptors, which mediate the release of relaxing factors. ET-1 also stimulates ETA and ETB2 receptors in VSM to cause vasoconstriction. ET-1 induces greater contraction in mesenteric arteries of male than female DOCA-salt hypertensive rats. ET-1 and ETB2 receptor mRNA are increased in mesenteric arteries of OVX females, and E2 replacement reverses these increases. The ETB agonist IRL-1620 induces greater vasoconstriction in mesenteric arteries of OVX compared with intact OVX females, and E2 replacement decreases IRL-1620-induced vasoconstriction in OVX females, suggesting that E2 attenuates ET-1 and ETB2 receptor expression and their vascular response [76]. E2 also modulates ET-1 expression in human ECs. E2 treatment of ECs inhibits ET-1 production in response to serum, TNF-α, transforming growth factor β1, and AngII [1]. ET-1 release is less in ECs of female than male SHR. Also, trauma hemorrhage increases ET-1 induced vasoconstriction, and treatment with E2 and ERβ agonist DPN counteracts the vasoconstriction [77].
Other endothelium-derived vasoconstrictors include AngII and TXA2. The Angiotensin Converting Enzyme (ACE) Insertion/Deletion(I/D) polymorphism appears to be involved in EC dysfunction in Post-MW [78]. Also, basal release of TXA2 from platelets is greater in raloxifene- compared to E2-treated OVX pigs. Raloxifene treatment, compared to E2, increases the production of contractile and proaggregatory prostanoids from venous ECs and platelets, suggesting a greater thrombotic risk with SERMs compared with natural estrogen.
Although no significant changes in vascular ER expression were observed in aging compared with adult OVX SHR, possible age-related reduction in the estrogen/ER binding or the post-ER signaling pathways of endothelium-dependent vasodilation may occur.
Estrogen and Mechanisms of VSM Contraction
Estrogen causes relaxation of endothelium-denuded vessels, suggesting direct effects on VSM contraction mechanisms [2]. VSM contraction is triggered by Ca2+ release from the sarcoplasmic reticulum and Ca2+ entry from the extracellular space. Activation of myosin light chain (MLC) kinase, Rho-kinase and MAPK as well as inhibition of MLC phosphatase also contribute to VSM contraction. Activation of protein kinase C (PKC) also increases the myofilament force sensitivity to [Ca2+]i and thereby maintains vascular contraction (Fig. 2).
The α-adrenergic agonist phenylephrine (Phe) causes contraction that is greater in the aorta of male than female rats. Phe contraction is not different in intact and castrated males, but greater in OVX than intact female rats, suggesting a role of estrogen in the reduced vascular contraction in females [19]. In aortic strips incubated in Ca2+-free solution Phe or caffeine induces a transient contraction that is not different between intact and gonadectomized male and female rats, suggesting no sex differences in the Ca2+ release mechanism. Membrane depolarization by high KCl stimulates Ca2+ influx through voltage-gated Ca2+ channels. KCl-induced vascular contraction and Phe and KCl-induced Ca2+ influx are less in intact female than male rats or OVX female rats, suggesting an effect of E2 on the expression/permeability of VSM Ca2+ channels [19]. In endothelium-denuded porcine coronary artery and isolated coronary VSMCs, E2 inhibits PGF2α-and KCl-induced contraction and Ca2+ influx, suggesting inhibitory effects on Ca2+ entry mechanisms. E2 also attenuates voltage-dependent Ca2+ current in A7r5 VSMC line [79].
PKC is a family of Ca2+-dependent and Ca2+-independent isoforms, expressed in VSM of various vascular beds. During cell activation, PKC translocation to the cell surface may trigger a cascade of protein kinases that ultimately interact with the contractile myofilaments and cause VSM contraction. The gender-related decrease in VSM contraction in female rats compared with males is associated with reduction in the expression/activity of vascular α-, δ-, and ζ-PKC. E2 replacement in OVX females reduces Phe and phorbol ester induced contraction and PKC activity, suggesting that the sex differences in VSM contraction and PKC activity are caused by E2 [19].
Rho-kinase inhibits MLC phosphatase and enhances VSM sensitivity to [Ca2+]i, and is upregulated in CVD and coronary vasospasm. E2 may inhibit Rho-Kinase expression/activity. Rho-kinase expression may involve a PKC/NF-κB pathway that is inhibited by E2. Also, the vasodilator response to the Rho-kinase inhibitor Y-27632 is reduced in OVX female and male rats, and restored in E2-replaced OVX females [80]. E2 also decreases Rho-kinase mRNA expression in cultured human coronary VSMCs.
Estrogen and Extracellular Matrix (ECM)
The actin cytoskeleton forms the cell backbone, and its spatial organization is crucial for cell migration. ERα interacts with Gα13 to induce activation of RhoA/Rho-kinase and phosphorylation of the actin-regulatory protein moesin, leading to remodeling of the actin cytoskeleton and EC migration [81]. Vascular remodeling occurs during all stages of atherosclerosis, and MMPs are involved in these processes. Increased MMP activity occurs in cancer, arthritis and CVD. Also, MMP-induced ECM degradation within the atherosclerotic plaque may be involved in plaque instability and cardiovascular events. Changes in the levels of MMP-2, −9 and −10 in women receiving MHT may contribute to the risk of cardiovascular events and cancer [82].
MHT and Subject’s Age
Age of participating women may have played a role in the divergent MHT data in observational studies and RCTs. The menopausal and cardiovascular changes in aging women are partly due to decreased plasma estrogen levels to <100 pmol/L. Age-related changes in ER amount, distribution or affinity may also contribute to menopausal and cardiovascular symptoms [19]. DNA methylation may play a role in age-related disease. Methylation of the cytosine and guanine rich CpG island in the promoter region of ERα gene causes its downregulation in vascular tissue and may play a role in atherogenesis and aging of the vascular system [57]. Also, human coronary atherosclerotic tissues show higher methylation levels than normal arterial and venous tissues [83].
The vascular effects of estrogen could also be modified by age-related vascular remodeling, endothelial dysfunction, enhanced VSMC growth, wall thickness and media to lumen ratio. and vascular plaques, and increased production of inflammatory cytokines. With age, NO production decreases and ET-1 production increases. This favors a procoagulant state that promotes VSM growth. The reduced vascular effects of estrogen after menopause could also be due to age-related changes in the blood vessel architecture, and the mechanical and structural properties of the vessel wall. Elastin fibers determine the mechanical strength of the vessels at lower pressures and collagen fibers bear most of the strength at higher pressures. During vascular aging, there is progressive arterial stiffening and arteriosclerosis, due to quantitatively less elastin and more collagen, and qualitative changes in the content of the vessel wall. In larger elastic arteries, aging is associated with an increase in collagen content, covalent cross-linking of collagen, elastin fracture, reduction in the elastin content, and calcification.
Early loss of endogenous E2 is associated with CVD. Autopsy studies show increased CVD in young oophorectomized women [8]. The Framingham Study shows that women entering menopause early naturally or surgically are more likely to develop CVD than age-matched Pre-MW. NHS showed increased risk of CVD in women with bilateral oophorectomy not receiving MHT. Although it is difficult to separate vascular changes due to aging from those due to menopause, comparisons between men and age-matched Post-MW and between Pre-MW and Post-MW suggest that endogenous E2 delays CVD. In Pre-MW, endogenous E2 may counteract age-related vascular remodeling, endothelial dysfunction and VSMC proliferation, in addition to decreasing cholesterol level and vascular plaques [1], and these benefits are lost in Post-MW.
MHT and Pre-existing CVD
One criticism of HERS was that the participants had CVD at baseline. Also, while WHI was designed as a primary prevention RCT in “healthy” women, 36% of the women assigned MHT had hypertension, 49% were current or past smokers, 4.4% being treated for diabetes, 34% were obese, 13% being treated for hypercholesterolemia, suggesting an active atherosclerotic process. Atherosclerosis is an inflammatory process involving EC dysfunction and excess deposition of oxidized lipids, that progresses through lifetime and is enhanced by smoking, dyslipidemia, diabetes, and hypertension. Atherosclerosis leads to thick stiff arterial wall, calcification and plaque formation, which changes the mechanical properties of the arteries and increases the risk of cardiovascular events. Arteries of Post-MW have some degree of atherosclerosis that could mechanically impede the vasodilator effects of estrogen.
In women at different stages of life the presence or absence of endogenous estrogens affects cardiovascular function, and these effects may be closely related to the stage of atherosclerosis. Atherosclerotic lesions evolve from an initial accumulation of foam cells in the endothelium leading to a fatty streak. This is followed by accumulation of fatty deposits including cholesterol, creating a true atheroma. Once an atheroma is formed, collagen in the fibrous cap stabilizes the plaque and prevents it from rupturing. MMPs, which are produced by inflammatory cells in the atheroma, degrade collagen, leading to disruption of the fibrous cap, rupture of the plaque, and release of a thrombus that may occlude the artery. E2 reduces the development of early atherosclerotic lesions by affecting lipid metabolism and reducing lipid deposits in the endothelium. However, once an atheroma is established, E2 increases MMPs expression, causing disruption of the fibrous cap and rupture of the plaque. If the capsule ruptures, E2 could become thrombogenic, leading to clot formation and arterial occlusion. Thus, E2 inhibits early development of atherosclerosis, but may increase the risk of cardiovascular events once atherosclerosis has been established.
Earlier clinical studies have shown that intravenous administration of E2 may cause direct vasodilatation in healthy women and in women with atherosclerotic disease [84]. However, data from the Cardiovascular Health Study, a longitudinal study of cardiovascular risk factors in 1,636 women older than 65 years of age, showed an association between MHT and flow-mediated vasodilation only in healthy Post-MW. MHT had no effect on brachial artery blood flow in women older than 80 years or women with CVD or multiple cardiovascular risk factors [54].
Thus, the pre-existing cardiovascular condition and age-related vascular disease could affect the outcome of MHT in aging Post-MW. This may be partly related to the socioeconomic status. In general, women who take MHT are more educated, wealthier, have healthier lifestyle, and fewer cardiovascular risk factors. A meta-analysis showed that the previously observed reduced risk for CHD among MHT users was lost when the statistical analysis included socioeconomic status [1].
Timing of MHT
The discrepancy in the results of observational studies and RCTs may be related to the time of initiation of MHT. The onset of atherosclerosis may be delayed by estrogen, while the more advanced atherosclerotic lesions may not be influenced by MHT, or may become more unstable [46]. Because atherosclerosis is age-dependent, a delay in MHT by a few years may influence outcome. E2 administration improves flow-mediated vasodilatation to a greater extent in women within 5 years than those more than 5 years after menopause [85], supporting sensitivity to E2 in younger women with healthy endothelium. Time since menopause, rather than type of menopause, natural or surgical, is a major factor in subclinical atherosclerosis.In women 35 years of age, only fatty streaks and minimal atherosclerotic plaques occur in coronary arteries. Plaques become evident in women around perimenopause (40–50 years age), and show steep increase after 50 years of age (after menopause), and these lesions develop complications at ≥65 years of age [1]. Menopause is also associated with endothelial dysfunction, decreased endothelium-dependent relaxation and flow-mediated dilation, and intimal thickening, and a significant increase in intima-media thickening occurs 5–8 years after menopause [1]. Also, compared with Pre-MW, Post-MW have greater levels of primary cardiovascular risk factors such as total cholesterol, LDL cholesterol, and apolipoprotein B. Progression of vascular disease may also depend on age at menopause and status of subclinical atherosclerosis. In women who underwent bilateral oophorectomy, intimal thickening increases with years since menopause and reaches significance 15 years after menopause [86]. Age-dependent progression of carotid intimal thickening in Post-MW is reduced by MHT [87], supporting a role of estrogen in regulating vascular intimal thickening. Hypertension may accelerate atherosclerosis after menopause. Post-MW ≥60 years old make up the majority of hypertensive patients and are more likely to develop CVD. In Post-MW the synthesis/activity of hypertensive factors is increased, and blood pressure-lowering factors decrease [88]. Thus age-related changes in plasma estrogen levels and vascular ERs are major factors in the progression of atherosclerosis and vascular dysfunction, and could affect the cardiovascular effects of MHT in aging women.
Data from NHS suggest that the time when MHT is initiated influences cardiovascular benefit. Women who participated in NHS were 30 to 55 years of age, and ~80% of participants initiated MHT within 2 years of menopause [11]. In contrast with the observational NHS, RCTs enrolled older women who initiated MHT ~10 years after menopause. Women in HERS were on average 67 years of age and had been postmenopausal for many years at the time of enrollment. In HERS, the time from menopause to randomization was 23 years compared to 13 years in the Estrogen in the Prevention of Atherosclerosis Trial (EPAT) [10]. Similar to HERS, the participants in WHI were older (50–79 years) with only 10% of the participants between 50 and 54 years and 20% between 54 and 59 years. Even younger healthy participants (aged 50 to 59) in WHI had been menopausal ~6 years before enrollment, which may be sufficient to reduce the vascular benefits of MHT [1].
Studies in nonhuman primates showed that the vascular protective effects of MHT were greater if MHT started before the onset of atherosclerosis, suggesting that MHT may promote primary prevention. In OVX cynomolgus monkeys fed an atherosclerotic diet, early CEE administration simultaneously with the atherosclerotic diet caused a 70% reduction in coronary artery plaque size relative to placebo. A delay in hormone therapy until after the development of moderate atherosclerosis resulted in only 50% protection. In primates who received CEE after being on atherosclerotic diet for 2 years, no protection was observed [89]. Similarly, in OVX female rats with carotid artery balloon injury, administration of E2 resulted in inhibition of intimal hyperplasia, neointima formation and VSMC proliferation when given on the day of injury, 15 minutes before, and continuing 3 days after injury, but did not show beneficial effects when administered 7 days after balloon injury [90].
Thus the timing of initiation of MHT with respect to the onset of menopause may have potential benefits in preventing or delaying the progression of atherosclerosis and CVD. Around the time of menopause, women still have relatively healthy arteries, allowing a “window” for MHT to produce cardiovascular benefit. However, arterial disease increases as a function of time after menopause, and diseased arteries become less responsive to the beneficial effects of estrogen.
MHT and Interaction with Other Sex Hormones
Other sex hormones may modify the vascular actions of estrogen, or have direct effects on the vasculature. Studies suggest that the relationship between circulating free E2, free T, and sex hormone-binding globulin (SHBG) may be more predictive of changes in carotid intimal thickening than the levels of any of these hormones alone. Also, conversion of T to E2 may contribute to the regulation of circulation in men, and administration of an aromatase inhibitor to young men results in a decrease in endothelial vasodilator function. Additionally, circulating levels of estrogen and androgens may not reflect those at the tissue level, as both aromatase and 5-α-reductase are found in various tissues including blood vessels [91]. Steroid hormone metabolism is a complex process that allows inter-conversion of sex hormones precursors and metabolites. With the growing use of steroid metabolism inhibitors, including aromatase and 5-α-reductase inhibitors, e.g. in women with history of breast cancer, cardiovascular side effects are predicted [19].
Role of Progesterone (P4)
P4 is a steroid hormone produced by the gonads and adrenal cortex, and by the placenta during pregnancy. P4 receptors have been identified in ECs and VSM of human, mouse, rat, rabbit and non-human primates. Similar to E2, P4 has anti-atherosclerotic effects, decreases LDL-c, and increases HDL-c. P4 causes endothelium-dependent pulmonary vasodilation and stimulates eNOS expression, and NO-mediated relaxation in rat aorta and ovine uterine artery [92]. P4 also causes nongenomic activation of COX and increases PGI2 production. Combined estrogen/progestin decrease ET-1 production in cultured bovine aortic ECs. P4 inhibits VSM proliferation/migration and facilitates the inhibitory effects of E2. P4 also causes rapid relaxation of agonist- or KCl-induced contraction in endothelium-denuded porcine coronary artery [19].
However, P4 produces less vasorelaxation than E2, and may even antagonize the vasoprotective effects of E2. P4 counteracts the stimulatory effects of E2 on NO production and vascular relaxation in canine and porcine coronary artery [93]. P4 antagonizes the antioxidant effect of E2, and enhances NADPH oxidase activity and production of ROS in OVX mice [94]. P4 also promotes vasoconstriction by upregulating vascular AT1R [95]. P4 may also diminish E2-induced anti-inflammatory effects and its attenuation of ischemic brain injury.
P4 may alter the vascular effects of E2 in MHT. MPA antagonizes the inhibitory effects of CEE on coronary atherosclerosis, and may attenuate E2-induced increase of nitrate/nitrite and NO production in Post-MW [96]. A study comparing the effect of sequential E2/progestin treatment cycle, two weeks with E2 alone followed by two weeks with E2/norethisterone acetate (NETA), in Post-MW showed increased excretion of cGMP with both forms of administration [97]. The same study compared the effect of oral vs. transdermal sequential E2 and E2/NETA on the PGI2 to TXA2 ratio and found an increase in PGI2 metabolite during the E2 phase, and an increase of TXA2 metabolite during combined E2/NETA phase with both oral and transdermal administration, but the differences were not significant possibly due to inter-individual variations [97]. Another study showed an increase in TXA2 metabolites with oral but transdermal E2/MPA after 12 months [98]. In vitro experiments showed that the addition of MPA or NETA to E2 on EC cultures from human female coronary arteries enhances E2-induced reduction of MMP-1 and may therefore affect plaque stability. However, CEE alone or CEE+MPA increased MMP-9 levels after 4 weeks in Post-MW with established CHD [96]. These complex interactions of E2 and P4 on the vasculature highlight the need to determine the benefits vs. risk of combined E2/P4 in postmenopausal CVD.
Drospirenone (DRSP), derived from 17α-spironolactone, has the same affinity to P4 receptor and similar pharmacodynamics as natural P4. DRSP is used as oral contraceptive in combination with ethinyl-E2 and as MHT in combination with E2. It has greater anti-mineralocorticoid effects that counteract the salt-retaining actions of E2 and more anti-androgenic effects than P4. Some clinical trials have shown that E2/DRSP lowers BP in hypertensive Post-MW, particularly when administered in combination with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers [99]. Angeliq, an MHT composed of DRSP 2 mg/E2 1 mg, reduces carotid intima–media thickness and climacteric complaints including vertigo/dizziness in Post-MW likely due to its anti-androgenic and anti-mineralocorticoid effects, respectively [100].
In women with intact uterus, estrogens are given in combination with a progestin in order to reduce the risk of endometrial cancer. The negative findings of HERS and one arm of WHI may have been caused by concomitant use of MPA, as evidenced by increased stroke risk in women taking CEE+MPA vs. women never using MHT. Also, in Postmenopausal Estrogen/Progestin Interventions (PEPI) trial, CEE caused beneficial effects on LDL and HDL levels that were attenuated by MPA [1]. However, other studies did not find any attenuation of CEE-induced dilatation by MPA or micronized P4 [101]. Also, in one arm of WHI in women taking CEE alone, no protective effects were observed even though lipids were favorably changed. Moreover, NHS showed a similar risk reduction for CHD among women taking CEE alone or CEE+MPA [11].
In cynomolgus monkeys, chronic E2 or E2+P4 had similar anti-atherosclerotic effects. In contrast, loss of protective effects were observed in monkeys administered CEE+MPA as compared to CEE alone [102]. Also, studies on postmenopausal cynomolgus monkeys suggested that MPA abrogates the vascular benefits of estrogen [103]. In these studies ACh caused vasoconstrictor responses in estrogen-deprived monkeys not receiving MHT; however, a vasodilatory response was observed in monkeys treated with estrogen alone, and the beneficial effect of estrogen was reduced by 50% by co-administration of MPA [103]. In a rat model, MPA abrogated the ability of E2 to attenuate balloon injury-induced intimal thickening [104]. On the other hand, studies in rabbits indicated that the protective actions of CEE or E2 on atherosclerosis were not prevented by MPA or other progestins [1]. Thus, whether MPA is responsible for the lack of vascular protective actions of MHT in RCTs needs to be further examined, and the termination of the estrogen alone arm of the WHI study suggests that factors other than MPA may be involved [1].
Role of Testosterone (T)
In addition to E2 and P4, androgens may play a role in determining the cardiovascular risk in Post-MW. Androgens are sex steroids that maintain masculine secondary sexual characteristics. In women, androgens are important for maintaining bone mass, secondary sex characteristics and libido [19]. Androgens are produced in the testis, adrenal glands and ovaries. The adrenal steroid dehydroepiandrosterone (DHEA) and its sulfate ester (DHEAS) are the most abundant steroids in human circulation. DHEA and androstenedione do not have significant biological activity, but are converted to T. T is converted to dihydrotestosterone (DHT), with higher binding affinity to androgen receptor. T is also aromatized mainly in adipose tissue to E2.
During the normal female reproductive years ~25% of circulating T originates from the ovaries, 25% from the adrenal glands and 50% from conversion of peripheral androstenedione. Serum T levels are markedly lower in women than in men. While androgen levels like E2, are predicted to decrease with age [88], serum T levels range from 1.0 to 1.5 nmol/L in Pre-MW, and decrease to 0.3–0.5 nmol/L in Post-MW [105]. However, serial measurements of sex hormones in Post-MW for 10 years after cessation of cycling showed age-related increase in serum T and androstenedione and decrease in E2 and DHT [106]. In cross-sectional studies of women in the Rancho Bernardo cohort, serum T decreased immediately after menopause, but then increased with age, reaching Pre-MW levels at 70–79 years of age. In women with surgical menopause serum T levels did not increase with age, but were 40–50% lower than in women with natural menopause, suggesting that natural postmenopause is a relatively hyperandrogenic state [88].
Because men have higher BP and develop CVD at an earlier age than women, it is thought that androgens promote CVD in men. However, serum T levels are lower in men with chronic CVD than in healthy age-matched men. Although this suggests that androgens may not mediate CVD, decreased androgen synthesis may be a protective compensatory mechanism in response to CVD. Experimental studies have shown that adult male SHR have higher BP than females, castration of males reduces BP to levels observed in females, and T treatment of OVX females increases BP in a dose-dependent manner, suggesting that increases in androgen/E2 ratio in Post-MW may promote pro-hypertensive mechanisms [88].
While it is often thought that T may have unfavorable cardiovascular effects, some studies suggest beneficial effects. A cross-sectional population-based study of 6440 perimenopausal women aged 50–59 years showed that T was positively associated with low LDL and high HDL levels in all women, and women with CVD had lower serum T levels [107]. In healthy Post-MW, plasma levels of T and DHEA-S positively correlate with brachial artery flow-mediated vasodilation, suggesting protective effects on endothelium [108]. In men, low circulating T is positively correlated with risk factors for CHD [109]. T-induced protection could involve anti-atherogenic effects on lipid and lipoprotein metabolism and reduction of atherogenic serum lipid profile [180]. T stimulates hepatic lipase and lipoprotein lipase activity and reduces serum triglycerides. Interestingly, in men with established CVD, short-term intracoronary administration of T increases coronary blood flow within minutes and is not antagonized by androgen receptor blockers. Also, acute intra-coronary or intravenous infusion of T in men rapidly improves myocardial ischemia [4].
Experimental studies also show that T may produce vasodilation independently of E2. In porcine coronary artery, T causes relaxation and decreases VSM [Ca2+]i [19]. Similarly, in porcine coronary artery treatment with DHT, which cannot be aromatized to estrogen, causes vasodilation. These data suggest that T could be protective against CVD in Post-MW, and make it important to further investigate the effects of T on the cardiovascular system.
Hormone imbalance conditions may serve as models to identify the effects of changes in hormone environment on cardiovascular function. Polycystic Ovary Syndrome (PCOS) is characterized by polycystic ovaries, menstrual abnormalities (oligomenorrhoea/amenorrhoea), hirsutism, anovulatory infertility, elevated androgens (hyperandrogenemia) and estrogens, as well as obesity, insulin resistance, and lipid abnormalities. In PCOS, both insulin and leutinizing hormone stimulate androgen production from ovarian theca cells, leading to elevated levels of T and androstenedione. Because most women with PCOS are severely overweight or obese elevated androgen levels may contribute to increased E1 levels through increased conversion of androgens to E1 in peripheral adipose tissue by aromatase enzyme. E2 levels also increase in PCOS due to increased local conversion of E1 to E2 at peripheral and target tissues, and increased biologically available circulating E2 because of decreased SHBG. Women with PCOS have low HDL-C (68%), elevated BMI (67%), high BP (45%), hypertriglyceridemia (35%), and high fasting serum glucose (4%), and increased risk of CVD. While these criteria suggest an association between PCOS and metabolic syndrome, the limited studies conducted in women with PCOS yielded conflicting data regarding cardiovascular events. Large prospective cohort studies such as the Framingham study or NHS, which focused on hard outcomes such as heart disease or cancer, did not identify hyperandrogenemia and anovulation as a separate risk phenotype. A retrospective study from the United Kingdom involving 786 PCOS women diagnosed between 1930 and 1979 failed to establish increased cardiovascular mortality or morbidity [110]. Hence, despite the existence of risk factors for CVD such as diabetes, hypertension and dyslipidemia in PCOS and the impact of PCOS on surrogate outcomes for CVD, a clear effect of PCOS on cardiovascular events has not been established. Importantly, since Post-MW and women with PCOS have similar sex hormone profiles, namely the lack of P4, E1 being the major estrogen, and relative increase in androgens further studies in women with PCOS and animal models of PCOS could provide insights into the role of other sex hormones in affecting the actions of estrogen on vascular ERs.
Lessons Learned and Recent MHT Clinical trials
MHT is used to treat vasomotor menopausal symptoms such as hot flushes and night sweats, but its vascular benefits have been questioned. Although RCTs have shown increased risk of cardiovascular events in Post-MW receiving MHT, the final chapter on estrogen therapy has not yet been written. The failure of vascular benefits of MHT to materialize in RCTs is likely related to age-related changes in ER amount, distribution, integrity, and downstream signaling mechanisms as well as structural changes in the vasculature. These changes may be attributed to age-related ER gene mutations or methylation. Further research is needed to characterize the ER subtypes, distribution, and function in various blood vessels. Characterization of currently available estrogens and the exploration of novel estrogenic compounds would provide more effective and specific ER modulators. Subtype-specific ER agonists such as PPT, DPN and G1 coupled with specific targeting or drug-delivery techniques (drug-eluting stents, perivascular gel) could be useful in modulating vascular ER activity in a specific blood vessel without affecting other vessels in the systemic circulation. SERMs could be selective in targeting vascular ERs while having little undesirable effects on breast cancer. Natural hormones that avoid first-pass metabolism in the liver could increase MHT bioavailability. Phytoestrogens may provide a more natural MHT than synthetic compounds. Lower doses of estrogen (0.3 mg) displayed comparable protective effects as normal doses (0.625 mg) including decreased LDL-c, lipoproteins and coronary atherosclerosis. Also, transdermal estrogens could enhance vascular relaxation, and reduce platelet aggregation and vascular inflammation with less incidence of VTE. Study of diseases associated with abnormal estrogen production or metabolism such as PCOS may provide insights into the sex hormone environment and ER regulation in Post-MW. Also, the benefits/risks of combined sex hormone therapy and modulators of P4 and androgen receptors should be examined.
An important factor is the time of initiation of MHT [10]. In most observational studies, MHT started in the perimenopausal period for the relief of menopausal symptoms. The mean age of menopause in the United States is 51 years, which corresponds to the age of women in NHS who initiated MHT as part of the usual clinical practice for menopausal symptoms in the perimenopause [111]. This age and time of MHT initiation contrasts with that of participants in WHI who were on average 63 years of age at MHT initiation, ~12 years postmenopause. Because atherosclerosis progresses gradually with age, a significant number of women in WHI likely had subclinical atherosclerosis at randomization, and may account for the increased CVD events early in WHI. Also, in HERS, participants were on average 67 years of age with preexisting CHD disease, and there was an increase in CVD events in the first two years of the trial. Although continued use of MHT in HERS was associated with a reversal of this trend, the major conclusion was that women with preexisting CHD were not candidates for preventive therapy with MHT [111].
The timing of MHT was considered in two recent RCTs; The Kronos Early Estrogen Prevention Study (KEEPS) and Early versus Late Intervention Trial with Estradiol (ELITE) [111] (Table 1). KEEPS is a placebo-controlled double-blinded prospective RCT to evaluate the effects of MHT on progression of atherosclerosis as defined by carotid intima–media thickness (CIMT) and coronary arterial calcification (CAC) in early menopausal women who more closely match the age of initiation of MHT in observational studies. Women were randomized to daily low dose oral CEE or weekly transdermal E2, both in combination with cyclic oral micronized P4 or placebo for 12 days/month. Participants were 42 to 58 years of age and 6 to 36 months postmenopause with plasma follicle-stimulating hormone level ≥35 ng/mL and/or E2 levels <40 pg/mL; women post-hysterectomy excluded [111]. Thus, women in KEEPS are younger, healthier, reported more menopausal symptoms, and have fewer cardiovascular risk factors than women in WHI. One limitation of KEEPS is that participants are largely Caucasian and results may not be applicable to diverse ethnic populations. ELITE will randomize 643 women (504 initially proposed), < 6 years or > 10 years postmenopausal, to receive oral E2 for 2 to 5 years, and as in KEEPS, the primary endpoint is change in CIMT. ELITE will test for differences between early and late initiation of MHT, and KEEPS will examine differences between low-dose oral CEE and low-dose transdermal E2. The results from these two studies will help clarify the benefits of MHT timing, and the differences between oral E2 and CEE, and between oral and transdermal E2 [19].
Further research into vascular ERs and alternatives to traditional MHT such as selective ER agonists, SERMs and phytoestrogens, as well as the appropriate route of administration, dose, and timing could provide better approaches to increase the benefits of MHT in CVD. As newer forms of MHT become available, and if used at the right dose, route of administration and timing depending on the subjects’ age and preexisting cardiovascular condition, the vascular effects of estrogen could be translated into beneficial outcomes of MHT in post-menopausal CVD
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from National Heart, Lung, and Blood Institute (HL-65998, HL-98724, HL-111775) and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD-60702).
List of Abbreviations
- Akt
protein kinase B
- AngII
angiotensin II
- CEE
conjugated equine estrogen
- CRP
C-reactive protein
- CVD
cardiovascular disease
- E2
17β-estradiol
- EC
endothelial cell
- ECM
extracellular matrix
- ELITE
Early versus Late Intervention Trial with Estradiol
- eNOS
endothelial nitric oxide synthase
- ER
estrogen receptor
- FMD
flow mediated dilation
- GPR30
G protein-coupled receptor 30
- HSP90
heat shock protein-90
- HERS
Heart and Estrogen/progestin Replacement Study
- 27HC
27-hydroxycholesterol
- IL-6
interleukin-6
- KEEPS
Kronos Early Estrogen Prevention Study
- MAPK
mitogen-activated protein kinase
- MHT
menopausal hormone therapy
- MI
myocardial infarction
- MMP
matrix metalloproteinase
- MPA
medroxyprogesterone acetate
- NO
nitric oxide
- NHS
Nurses’ Health Study
- OVX
ovariectomized
- P4
progesterone
- PCOS
polycystic ovary syndrome
- PI3K
phosphatidylinositol 3-kinase
- Pre-MW
premenopausal women
- Post-MW
postmenopausal women
- RCT
randomized clinical trial
- SHR
spontaneously hypertensive rat
- T
testosterone
- TMF-α
tumor necrosis factor-α
- TXA2
thromboxane A2
- VSM
vascular smooth muscle
- VSMC
VSM cell
- VTE
venous thrombo-embolism
- WHI
Women’s Health Initiative
REFERENCES
- 1.Dubey RK, Imthurn B, Zacharia LC, Jackson EK. Hormone replacement therapy and cardiovascular disease: what went wrong and where do we go from here? Hypertension. 2004;44:789–795. doi: 10.1161/01.HYP.0000145988.95551.28. [DOI] [PubMed] [Google Scholar]
- 2.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 3.Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118(Suppl 12B):64–73. doi: 10.1016/j.amjmed.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 4.Rosano GM, Leonardo F, Pagnotta P, Pelliccia F, Panina G, Cerquetani E, et al. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–1670. doi: 10.1161/01.cir.99.13.1666. [DOI] [PubMed] [Google Scholar]
- 5.Eckstein N, Nadler E, Barnea O, Shavit G, Ayalon D. Acute effects of 17 beta-estradiol on the rat heart. Am J Obstet Gynecol. 1994;171:844–848. doi: 10.1016/0002-9378(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 6.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991;265:1861–1867. [PubMed] [Google Scholar]
- 9.O'Keefe JH, Jr, Kim SC, Hall RR, Cochran VC, Lawhorn SL, McCallister BD. Estrogen replacement therapy after coronary angioplasty in women. J Am Coll Cardiol. 1997;29:1–5. doi: 10.1016/s0735-1097(96)00443-3. [DOI] [PubMed] [Google Scholar]
- 10.Hodis HN, Mack WJ, Azen SP, Lobo RA, Shoupe D, Mahrer PR, et al. Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. N Engl J Med. 2003;349:535–545. doi: 10.1056/NEJMoa030830. [DOI] [PubMed] [Google Scholar]
- 11.Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the nurses' health study a prospective, observational study. Ann Intern Med. 2001;135:1–8. doi: 10.7326/0003-4819-135-1-200107030-00003. [DOI] [PubMed] [Google Scholar]
- 12.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 13.Smith NL, Heckbert SR, Lemaitre RN, Reiner AP, Lumley T, Weiss NS, et al. Esterified estrogens and conjugated equine estrogens and the risk of venous thrombosis. JAMA. 2004;292:1581–1587. doi: 10.1001/jama.292.13.1581. [DOI] [PubMed] [Google Scholar]
- 14.Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292:1573–1580. doi: 10.1001/jama.292.13.1573. [DOI] [PubMed] [Google Scholar]
- 15.Renoux C, Dell'Aniello S, Suissa S. Hormone replacement therapy and the risk of venous thromboembolism: a population-based study. J Thromb Haemost. 2010;8:979–986. doi: 10.1111/j.1538-7836.2010.03839.x. [DOI] [PubMed] [Google Scholar]
- 16.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, et al. Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 17.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 18.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 19.Smiley DA, Khalil RA. Estrogenic compounds, estrogen receptors and vascular cell signaling in the aging blood vessels. Curr Med Chem. 2009;16:1863–1887. doi: 10.2174/092986709788186093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feigelson HS, McKean-Cowdin R, Pike MC, Coetzee GA, Kolonel LN, Nomura AM, et al. Cytochrome P450c17alpha gene (CYP17) polymorphism predicts use of hormone replacement therapy. Cancer Res. 1999;59:3908–3910. [PubMed] [Google Scholar]
- 21.de Kleijn MJ, Wilmink HW, Bots ML, Bak AA, van der Schouw YT, Planellas J, et al. Hormone replacement therapy endothelial function Results of a randomized controlled trial in healthy postmenopausal women. Atherosclerosis. 2001;159:357–365. doi: 10.1016/s0021-9150(01)00507-x. [DOI] [PubMed] [Google Scholar]
- 22.Hsia J, Criqui MH, Herrington DM, Manson JE, Wu L, Heckbert SR, et al. Conjugated equine estrogens and peripheral arterial disease risk: the Women's Health Initiative. Am Heart J. 2006;152:170–176. doi: 10.1016/j.ahj.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Geary RL, Adams MR, Benjamin ME, Williams JK. Conjugated equine estrogens inhibit progression of atherosclerosis but have no effect on intimal hyperplasia or arterial remodeling induced by balloon catheter injury in monkeys. J Am Coll Cardiol. 1998;31:1158–1164. doi: 10.1016/s0735-1097(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 24.Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65:995–1016. doi: 10.1016/j.phytochem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Baber R. Phytoestrogens and post reproductive health. Maturitas. 2010;66:344–349. doi: 10.1016/j.maturitas.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Li SH, Liu XX, Bai YY, Wang XJ, Sun K, Chen JZ, et al. Effect of oral isoflavone supplementation on vascular endothelial function in postmenopausal women: a meta-analysis of randomized placebo-controlled trials. Am J Clin Nutr. 2010;91:480–486. doi: 10.3945/ajcn.2009.28203. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333:276–282. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 28.Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, Lampe JW, van der Schouw YT. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am J Clin Nutr. 2005;81:189–195. doi: 10.1093/ajcn/81.1.189. [DOI] [PubMed] [Google Scholar]
- 29.Leung HS, Seto SW, Kwan YW, Leung FP, Au AL, Yung LM, et al. Endothelium-independent relaxation to raloxifene in porcine coronary artery. Eur J Pharmacol. 2007;555:178–184. doi: 10.1016/j.ejphar.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Kawagoe J, Ohmichi M, Tsutsumi S, Ohta T, Takahashi K, Kurachi H. Mechanism of the divergent effects of estrogen on the cell proliferation of human umbilical endothelial versus aortic smooth muscle cells. Endocrinology. 2007;148:6092–6099. doi: 10.1210/en.2007-0188. [DOI] [PubMed] [Google Scholar]
- 31.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 32.Barrett-Connor E, Grady D, Sashegyi A, Anderson PW, Cox DA, Hoszowski K, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2002;287:847–857. doi: 10.1001/jama.287.7.847. [DOI] [PubMed] [Google Scholar]
- 33.Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 34.Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate: two-year substudy results. Fertil Steril. 2003;80:1234–1240. doi: 10.1016/s0015-0282(03)01167-1. [DOI] [PubMed] [Google Scholar]
- 35.Lobo RA, Bush T, Carr BR, Pickar JH. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on plasma lipids and lipoproteins, coagulation factors, and carbohydrate metabolism. Fertil Steril. 2001;76:13–24. doi: 10.1016/s0015-0282(01)01829-5. [DOI] [PubMed] [Google Scholar]
- 36.Sanada M, Higashi Y, Nakagawa K, Tsuda M, Kodama I, Kimura M, et al. A comparison of low-dose and standard-dose oral estrogen on forearm endothelial function in early postmenopausal women. J Clin Endocrinol Metab. 2003;88:1303–1309. doi: 10.1210/jc.2002-021147. [DOI] [PubMed] [Google Scholar]
- 37.Wingrove CS, Garr E, Godsland IF, Stevenson JC. 17beta-oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim Biophys Acta. 1998;1406:169–174. doi: 10.1016/s0925-4439(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 38.Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev. 2006;27:575–605. doi: 10.1210/er.2005-0020. [DOI] [PubMed] [Google Scholar]
- 39.Gouva L, Tsatsoulis A. The role of estrogens in cardiovascular disease in the aftermath of clinical trials. Hormones (Athens) 2004;3:171–183. doi: 10.14310/horm.2002.11124. [DOI] [PubMed] [Google Scholar]
- 40.Ho JY, Chen MJ, Sheu WH, Yi YC, Tsai AC, Guu HF, et al. Differential effects of oral conjugated equine estrogen and transdermal estrogen on atherosclerotic vascular disease risk markers and endothelial function in healthy postmenopausal women. Hum Reprod. 2006;21:2715–2720. doi: 10.1093/humrep/del245. [DOI] [PubMed] [Google Scholar]
- 41.Opatrny L, Dell'Aniello S, Assouline S, Suissa S. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169–75. doi: 10.1111/j.1471-0528.2007.01520.x. discussion 75. [DOI] [PubMed] [Google Scholar]
- 42.Lokkegaard E, Andreasen AH, Jacobsen RK, Nielsen LH, Agger C, Lidegaard O. Hormone therapy and risk of myocardial infarction: a national register study. Eur Heart J. 2008;29:2660–2668. doi: 10.1093/eurheartj/ehn408. [DOI] [PubMed] [Google Scholar]
- 43.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris HA. Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol. 2007;21:1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- 45.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 46.Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol. 2002;90:3F–6F. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- 47.Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7:497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hodges YK, Tung L, Yan XD, Graham JD, Horwitz KB, Horwitz LD. Estrogen receptors alpha and beta: prevalence of estrogen receptor beta mRNA in human vascular smooth muscle and transcriptional effects. Circulation. 2000;101:1792–1798. doi: 10.1161/01.cir.101.15.1792. [DOI] [PubMed] [Google Scholar]
- 49.Andersson C, Lydrup ML, Ferno M, Idvall I, Gustafsson J, Nilsson BO. Immunocytochemical demonstration of oestrogen receptor beta in blood vessels of the female rat. J Endocrinol. 2001;169:241–247. doi: 10.1677/joe.0.1690241. [DOI] [PubMed] [Google Scholar]
- 50.Zhu BT, Han GZ, Shim JY, Wen Y, Jiang XR. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology. 2006;147:4132–4150. doi: 10.1210/en.2006-0113. [DOI] [PubMed] [Google Scholar]
- 51.Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen receptor beta: an overview and update. Nucl Recept Signal. 2008;6 doi: 10.1621/nrs.06003. e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alevizaki M, Saltiki K, Cimponeriu A, Kanakakis I, Xita N, Alevizaki CC, et al. Severity of cardiovascular disease in postmenopausal women: associations with common estrogen receptor alpha polymorphic variants. Eur J Endocrinol. 2007;156:489–496. doi: 10.1530/EJE-06-0685. [DOI] [PubMed] [Google Scholar]
- 53.Emre A, Sahin S, Erzik C, Nurkalem Z, Oz D, Cirakoglu B, et al. Effect of hormone replacement therapy on plasma lipoproteins and apolipoproteins, endothelial function and myocardial perfusion in postmenopausal women with estrogen receptor-alpha IVS1-397 C/C genotype and established coronary artery disease. Cardiology. 2006;106:44–50. doi: 10.1159/000092598. [DOI] [PubMed] [Google Scholar]
- 54.Herrington DM, Howard TD, Hawkins GA, Reboussin DM, Xu J, Zheng SL, et al. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N Engl J Med. 2002;346:967–974. doi: 10.1056/NEJMoa012952. [DOI] [PubMed] [Google Scholar]
- 55.Wynne FL, Payne JA, Cain AE, Reckelhoff JF, Khalil RA. Age-related reduction in estrogen receptor-mediated mechanisms of vascular relaxation in female spontaneously hypertensive rats. Hypertension. 2004;43:405–412. doi: 10.1161/01.HYP.0000111833.82664.0c. [DOI] [PubMed] [Google Scholar]
- 56.Kalesnykas G, Roschier U, Puolivali J, Wang J, Miettinen R. The effect of aging on the subcellular distribution of estrogen receptor-alpha in the cholinergic neurons of transgenic and wild-type mice. Eur J Neurosci. 2005;21:1437–1442. doi: 10.1111/j.1460-9568.2005.03953.x. [DOI] [PubMed] [Google Scholar]
- 57.Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, et al. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res. 1999;43:985–991. doi: 10.1016/s0008-6363(99)00153-4. [DOI] [PubMed] [Google Scholar]
- 58.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 59.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 60.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, et al. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49:1358–1363. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- 62.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, et al. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 63.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, et al. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 64.Douglas G, Cruz MN, Poston L, Gustafsson JA, Kublickiene K. Functional characterization and sex differences in small mesenteric arteries of the estrogen receptor-beta knockout mouse. Am J Physiol Regul Integr Comp Physiol. 2008;294:R112–R120. doi: 10.1152/ajpregu.00421.2007. [DOI] [PubMed] [Google Scholar]
- 65.Pike AC, Brzozowski AM, Walton J, Hubbard RE, Thorsell AG, Li YL, et al. Structural insights into the mode of action of a pure antiestrogen. Structure. 2001;9:145–153. doi: 10.1016/s0969-2126(01)00568-8. [DOI] [PubMed] [Google Scholar]
- 66.Widder J, Pelzer T, von Poser-Klein C, Hu K, Jazbutyte V, Fritzemeier KH, et al. Improvement of endothelial dysfunction by selective estrogen receptor-alpha stimulation in ovariectomized SHR. Hypertension. 2003;42:991–996. doi: 10.1161/01.HYP.0000098661.37637.89. [DOI] [PubMed] [Google Scholar]
- 67.Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, et al. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, estrogen receptor. Circ Res. 2002;90:413–419. doi: 10.1161/hh0402.105096. [DOI] [PubMed] [Google Scholar]
- 68.Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J Biol Chem. 2005;280:19704–197010. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- 69.Kublickiene K, Svedas E, Landgren BM, Crisby M, Nahar N, Nisell H, et al. Small artery endothelial dysfunction in postmenopausal women: in vitro function, morphology, and modification by estrogen and selective estrogen receptor modulators. J Clin Endocrinol Metab. 2005;90:6113–6122. doi: 10.1210/jc.2005-0419. [DOI] [PubMed] [Google Scholar]
- 70.Brandes RP, Mugge A. Gender differences in the generation of superoxide anions in the rat aorta. Life Sci. 1997;60:391–396. doi: 10.1016/s0024-3205(96)00663-7. [DOI] [PubMed] [Google Scholar]
- 71.Hernandez I, Delgado JL, Diaz J, Quesada T, Teruel MJ, Llanos MC, et al. 17beta-estradiol prevents oxidative stress and decreases blood pressure in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1599–R1605. doi: 10.1152/ajpregu.2000.279.5.R1599. [DOI] [PubMed] [Google Scholar]
- 72.O'Sullivan MG, Goodrich JA, Adams MR. Increased prostacyclin synthesis by atherosclerotic arteries from estrogen-treated monkeys. Life Sci. 2001;69:395–401. doi: 10.1016/s0024-3205(01)01131-6. [DOI] [PubMed] [Google Scholar]
- 73.Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, et al. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306:1954–1957. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 74.Sherman TS, Chambliss KL, Gibson LL, Pace MC, Mendelsohn ME, Pfister SL, et al. Estrogen acutely activates prostacyclin synthesis in ovine fetal pulmonary artery endothelium. Am J Respir Cell Mol Biol. 2002;26:610–616. doi: 10.1165/ajrcmb.26.5.4528. [DOI] [PubMed] [Google Scholar]
- 75.Nawate S, Fukao M, Sakuma I, Soma T, Nagai K, Takikawa O, et al. Reciprocal changes in endothelium-derived hyperpolarizing factor- and nitric oxide-system in the mesenteric artery of adult female rats following ovariectomy. Br J Pharmacol. 2005;144:178–189. doi: 10.1038/sj.bjp.0706091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.David FL, Carvalho MH, Cobra AL, Nigro D, Fortes ZB, Reboucas NA, et al. Ovarian hormones modulate endothelin-1 vascular reactivity and mRNA expression in DOCA-salt hypertensive rats. Hypertension. 2001;38:692–696. doi: 10.1161/01.hyp.38.3.692. [DOI] [PubMed] [Google Scholar]
- 77.Ba ZF, Lu A, Shimizu T, Szalay L, Schwacha MG, Rue LW, 3rd, et al. 17beta-Estradiol modulates vasoconstriction induced by endothelin-1 following trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2007;292:H245–H250. doi: 10.1152/ajpheart.00809.2006. [DOI] [PubMed] [Google Scholar]
- 78.Methot J, Hamelin BA, Arsenault M, Bogaty P, Plante S, Poirier P. The ACE-DD genotype is associated with endothelial dysfunction in postmenopausal women. Menopause. 2006;13:959–966. doi: 10.1097/01.gme.0000243576.09065.93. [DOI] [PubMed] [Google Scholar]
- 79.Zhang F, Ram JL, Standley PR, Sowers JR. 17 beta-Estradiol attenuates voltage-dependent Ca2+ currents in A7r5 vascular smooth muscle cell line. Am J Physiol. 1994;266:C975–C980. doi: 10.1152/ajpcell.1994.266.4.C975. [DOI] [PubMed] [Google Scholar]
- 80.Chrissobolis S, Budzyn K, Marley PD, Sobey CG. Evidence that estrogen suppresses rho-kinase function in the cerebral circulation in vivo. Stroke. 2004;35:2200–2205. doi: 10.1161/01.STR.0000136951.85586.c8. [DOI] [PubMed] [Google Scholar]
- 81.Simoncini T, Scorticati C, Mannella P, Fadiel A, Giretti MS, Fu XD, et al. Estrogen receptor alpha interacts with Galpha13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol. 2006;20:1756–1771. doi: 10.1210/me.2005-0259. [DOI] [PubMed] [Google Scholar]
- 82.Lewandowski KC, Komorowski J, Mikhalidis DP, Bienkiewicz M, Tan BK, O'Callaghan CJ, et al. Effects of hormone replacement therapy type and route of administration on plasma matrix metalloproteinases and their tissue inhibitors in postmenopausal women. J Clin Endocrinol Metab. 2006;91:3123–3130. doi: 10.1210/jc.2005-2789. [DOI] [PubMed] [Google Scholar]
- 83.Kim J, Kim JY, Song KS, Lee YH, Seo JS, Jelinek J, et al. Epigenetic changes in estrogen receptor beta gene in atherosclerotic cardiovascular tissues and in-vitro vascular senescence. Biochim Biophys Acta. 2007;1772:72–80. doi: 10.1016/j.bbadis.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 84.Collins P, Rosano GM, Sarrel PM, Ulrich L, Adamopoulos S, Beale CM, et al. 17 beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease. Circulation. 1995;92:24–30. doi: 10.1161/01.cir.92.1.24. [DOI] [PubMed] [Google Scholar]
- 85.Vitale C, Mercuro G, Cerquetani E, Marazzi G, Patrizi R, Pelliccia F, et al. Time since menopause influences the acute and chronic effect of estrogens on endothelial function. Arterioscler Thromb Vasc Biol. 2008;28:348–352. doi: 10.1161/ATVBAHA.107.158634. [DOI] [PubMed] [Google Scholar]
- 86.Mack WJ, Slater CC, Xiang M, Shoupe D, Lobo RA, Hodis HN. Elevated subclinical atherosclerosis associated with oophorectomy is related to time since menopause rather than type of menopause. Fertil Steril. 2004;82:391–397. doi: 10.1016/j.fertnstert.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 87.McGrath BP, Liang YL, Teede H, Shiel LM, Cameron JD, Dart A. Age-related deterioration in arterial structure and function in postmenopausal women: impact of hormone replacement therapy. Arterioscler Thromb Vasc Biol. 1998;18:1149–1156. doi: 10.1161/01.atv.18.7.1149. [DOI] [PubMed] [Google Scholar]
- 88.Reckelhoff JF, Yanes LL, Iliescu R, Fortepiani LA, Granger JP. Testosterone supplementation in aging men and women: possible impact on cardiovascular-renal disease. Am J Physiol Renal Physiol. 2005;289:F941–F948. doi: 10.1152/ajprenal.00034.2005. [DOI] [PubMed] [Google Scholar]
- 89.Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53:605–619. doi: 10.1016/s0008-6363(01)00466-7. [DOI] [PubMed] [Google Scholar]
- 90.Mori T, Durand J, Chen Y, Thompson JA, Bakir S, Oparil S. Effects of short-term estrogen treatment on the neointimal response to balloon injury of rat carotid artery. Am J Cardiol. 2000;85:1276–1279. doi: 10.1016/s0002-9149(00)00748-7. [DOI] [PubMed] [Google Scholar]
- 91.Gonzales RJ, Ansar S, Duckles SP, Krause DN. Androgenic/estrogenic balance in the male rat cerebral circulation: metabolic enzymes and sex steroid receptors. J Cereb Blood Flow Metab. 2007;27:1841–1852. doi: 10.1038/sj.jcbfm.9600483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Selles J, Polini N, Alvarez C, Massheimer V. Progesterone and 17 beta-estradiol acutely stimulate nitric oxide synthase activity in rat aorta and inhibit platelet aggregation. Life Sci. 2001;69:815–827. doi: 10.1016/s0024-3205(01)01174-2. [DOI] [PubMed] [Google Scholar]
- 93.Cox MW, Fu W, Chai H, Paladugu R, Lin PH, Lumsden AB, et al. Effects of progesterone and estrogen on endothelial dysfunction in porcine coronary arteries. J Surg Res. 2005;124:104–111. doi: 10.1016/j.jss.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 94.Wassmann K, Wassmann S, Nickenig G. Progesterone antagonizes the vasoprotective effect of estrogen on antioxidant enzyme expression and function. Circ Res. 2005;97:1046–1054. doi: 10.1161/01.RES.0000188212.57180.55. [DOI] [PubMed] [Google Scholar]
- 95.Nickenig G, Strehlow K, Wassmann S, Baumer AT, Albory K, Sauer H, et al. Differential effects of estrogen and progesterone on AT(1) receptor gene expression in vascular smooth muscle cells. Circulation. 2000;102:1828–1833. doi: 10.1161/01.cir.102.15.1828. [DOI] [PubMed] [Google Scholar]
- 96.Mueck AO, Seeger H. Progestogens and target tissues: vascular systems. Maturitas. 2009;62:356–361. doi: 10.1016/j.maturitas.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 97.Seeger H, Mueck AO, Teichmann AT, Lippert TH. Effect of sequential estrogen/progestin treatment on biochemical vasoactive markers in postmenopausal women comparing oral and transdermal application. Clin Exp Obstet Gynecol. 2000;27:17–20. [PubMed] [Google Scholar]
- 98.Viinikka L, Orpana A, Puolakka J, Pyorala T, Ylikorkala O. Different effects of oral and transdermal hormonal replacement on prostacyclin and thromboxane A2. Obstet Gynecol. 1997;89:104–107. doi: 10.1016/s0029-7844(96)00379-1. [DOI] [PubMed] [Google Scholar]
- 99.Preston RA, White WB, Pitt B, Bakris G, Norris PM, Hanes V. Effects of drospirenone/17-beta estradiol on blood pressure and potassium balance in hypertensive postmenopausal women. Am J Hypertens. 2005;18:797–804. doi: 10.1016/j.amjhyper.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 100.Coksuer H, Koplay M, Oghan F, Coksuer C, Keskin N, Ozveren O. Effects of estradiol-drospirenone hormone treatment on carotid artery intima-media thickness and vertigo/dizziness in postmenopausal women. Arch Gynecol Obstet. 2011;283:1045–1051. doi: 10.1007/s00404-010-1487-0. [DOI] [PubMed] [Google Scholar]
- 101.Koh KK, Jin DK, Yang SH, Lee SK, Hwang HY, Kang MH, et al. Vascular effects of synthetic or natural progestagen combined with conjugated equine estrogen in healthy postmenopausal women. Circulation. 2001;103:1961–1966. doi: 10.1161/01.cir.103.15.1961. [DOI] [PubMed] [Google Scholar]
- 102.Clarkson TB, Anthony MS, Wagner JD. A comparison of tibolone and conjugated equine estrogens effects on coronary artery atherosclerosis and bone density of postmenopausal monkeys. J Clin Endocrinol Metab. 2001;86:5396–5404. doi: 10.1210/jcem.86.11.8021. [DOI] [PubMed] [Google Scholar]
- 103.Williams JK, Anthony MS, Honore EK, Herrington DM, Morgan TM, Register TC, et al. Regression of atherosclerosis in female monkeys. Arterioscler Thromb Vasc Biol. 1995;15:827–836. doi: 10.1161/01.atv.15.7.827. [DOI] [PubMed] [Google Scholar]
- 104.Levine RL, Chen SJ, Durand J, Chen YF, Oparil S. Medroxyprogesterone attenuates estrogen-mediated inhibition of neointima formation after balloon injury of the rat carotid artery. Circulation. 1996;94:2221–2227. doi: 10.1161/01.cir.94.9.2221. [DOI] [PubMed] [Google Scholar]
- 105.Serock MR, Wells AK, Khalil RA. Modulators of vascular sex hormone receptors and their effects in estrogen-deficiency states associated with menopause. Recent Pat Cardiovasc Drug Discov. 2008;3:165–186. doi: 10.2174/157489008786263970. [DOI] [PubMed] [Google Scholar]
- 106.Jiroutek MR, Chen MH, Johnston CC, Longcope C. Changes in reproductive hormones and sex hormone-binding globulin in a group of postmenopausal women measured over 10 years. Menopause. 1998;5:90–94. [PubMed] [Google Scholar]
- 107.Khatibi A, Agardh CD, Shakir YA, Nerbrand C, Nyberg P, Lidfeldt J, et al. Could androgens protect middle-aged women from cardiovascular events? A population-based study of Swedish women: The Women's Health in the Lund Area (WHILA) Study. Climacteric. 2007;10:386–392. doi: 10.1080/13697130701377265. [DOI] [PubMed] [Google Scholar]
- 108.Montalcini T, Gorgone G, Gazzaruso C, Sesti G, Perticone F, Pujia A. Endogenous testosterone and endothelial function in postmenopausal women. Coron Artery Dis. 2007;18:9–13. doi: 10.1097/01.mca.0000236290.79306.d1. [DOI] [PubMed] [Google Scholar]
- 109.English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: A randomized, double-blind, placebo-controlled study. Circulation. 2000;102:1906–1911. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 110.Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf) 2000;52:595–600. doi: 10.1046/j.1365-2265.2000.01000.x. [DOI] [PubMed] [Google Scholar]
- 111.Miller VM, Black DM, Brinton EA, Budoff MJ, Cedars MI, Hodis HN, et al. Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) J Cardiovasc Transl Res. 2009;2:228–239. doi: 10.1007/s12265-009-9104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stefanick ML, Legault C, Tracy RP, Howard G, Kessler CM, Lucas DL, et al. Distribution and correlates of plasma fibrinogen in middle-aged women Initial findings of the Postmenopausal Estrogen/Progestin Interventions (PEPI) study. Arterioscler Thromb Vasc Biol. 1995;15:2085–2093. doi: 10.1161/01.atv.15.12.2085. [DOI] [PubMed] [Google Scholar]
- 113.Simon JA, Lin F, Vittinghoff E, Bittner V. The relation of postmenopausal hormone therapy to serum uric acid and the risk of coronary heart disease events: the Heart and Estrogen-Progestin Replacement Study (HERS) Ann Epidemiol. 2006;16:138–145. doi: 10.1016/j.annepidem.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 114.Angerer P, Stork S, Kothny W, Schmitt P, von Schacky C. Effect of oral postmenopausal hormone replacement on progression of atherosclerosis : a randomized, controlled trial. Arterioscler Thromb Vasc Biol. 2001;21:262–268. doi: 10.1161/01.atv.21.2.262. [DOI] [PubMed] [Google Scholar]
- 115.Vickers MR, MacLennan AH, Lawton B, Ford D, Martin J, Meredith SK, et al. Main morbidities recorded in the women's international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal women. BMJ. 2007;335:239. doi: 10.1136/bmj.39266.425069.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR, Rogers WJ, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288:2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 117.Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Levesque H, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–805. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- 118.Hwang J, Mack WJ, Xiang M, Sevanian A, Lobo RA, Hodis HN. Long-term effect of estrogen replacement on plasma nitric oxide levels: results from the estrogen in the prevention of atherosclerosis trial (EPAT) Atherosclerosis. 2005;181:375–380. doi: 10.1016/j.atherosclerosis.2004.12.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.