Abstract
Rationale
The common val158met polymorphism in the catechol-O-methyltransferase (COMT) gene has been associated with nicotine dependence, alterations in executive cognitive function, and abstinence-induced working memory deficits in smokers.
Objectives
We sought to replicate the association of the COMT val allele with abstinence-induced alterations in working memory-related activity in task-positive (executive control) and task-negative (default mode network) regions.
Methods
Forty smokers (20 val/val and 20 met/met) performed an N-back task while undergoing blood oxygen level-dependent (BOLD) fMRI on two separate occasions: following 72 hours of confirmed abstinence and during smoking as usual. An independent sample of 48 smokers who completed the identical N-back task during fMRI in smoking versus abstinence for another study was used as a validation sample.
Results
Contrary to expectations, genotype by session interactions on BOLD signal in executive control regions (dorsolateral prefrontal cortex (DLPFC) and dorsal cingulate/medial prefrontal cortex (MF/CG)) revealed significant abstinence-induced reductions in the met/met group, but not the val/val group. Results also revealed that val/val smokers may exhibit less suppression of activation in task-negative regions such as the posterior cingulate cortex during abstinence (versus smoking). These patterns were confirmed in the validation sample and in the whole-brain analysis, though the regions differed from the apriori ROIs (e.g., precuneus, insula).
Conclusions
The COMT val158met polymorphism was associated with abstinence-related working memory deficits in two independent samples of smokers. However, inconsistencies compared to prior findings and across methods (ROI vs. whole-brain analysis) highlight the challenges inherent in reproducing results of imaging genetic studies in addiction.
Keywords: Smoking, nicotine, COMT, genetic, fMRI, cognition, working memory
Cognitive impairment is a core feature of the nicotine withdrawal syndrome (Hughes, 2007) and a target for the development of new medications to treat tobacco dependence (Brady et al., 2011; Lerman et al., 2007; Sofuoglu, 2010). The mild cognitive impairments observed in abstaining smokers using objective performance assessments include deficits in sustained attention (Myers et al., 2005), working memory (Jacobsen et al., 2005; Mendrek et al., 2006), and behavioral control (Ashare and Hawk, 2012; Harrison et al., 2009). Nearly 50% of smokers report cognitive symptoms during a quit attempt (Hughes, 2007; Ward et al., 2001), and these deficits, particularly in working memory, are predictive of smoking relapse (Culhane et al., 2008; Dolan et al., 2004; Krishnan-Sarin et al., 2007; Patterson et al., 2010). Moreover, nicotine re-exposure and efficacious medications for nicotine dependence reverse abstinence-induced cognitive impairments in animals and humans smokers (Davis et al., 2005; Myers et al., 2008; Patterson et al., 2009; Portugal and Gould, 2007; Raybuck et al., 2008). Thus, a better understanding of the underlying mechanism of cognitive deficits during abstinence may guide treatment development efforts (Lerman et al., 2008; Sofuoglu, 2010).
Recent work has begun to investigate the genetic and neural substrates that underlie the cognitive symptoms associated with smoking abstinence. Prefrontal dopamine function is a plausible target because of its role in executive cognitive function and in partly mediating the addictive properties of nicotine (Goldberg and Weinberger, 2004; Nestler, 2005). Accordingly, individual differences in catechol-O-methyltransferase (COMT) levels, the enzyme that regulates dopamine in the prefrontal cortex, are associated with addiction and cognitive function (Mier et al., 2010; Tammimaki and Mannisto, 2010). The human COMT gene has G>A transition in exon 3 that results in a substitution of methionine (met) for valine (val) at codon 158 (val158met; rs#4680). The val allele is associated with an approximately 40% reduction in enzyme activity (Tunbridge, 2010; Yavich et al., 2007) and this reduction may lead to decreased prefrontal dopamine levels (Chen et al., 2004). Indeed, smokers homozygous for the high activity COMT val allele exhibit increased smoking-induced dopamine release, an effect that may contribute to enhanced rewarding effects of nicotine (Brody et al., 2006). Several studies have independently validated the relationship of the val allele with nicotine dependence and smoking relapse (Colilla et al., 2005; Johnstone et al., 2007; Munafo et al., 2008; Nedic et al., 2010), though others have found no association between COMT genotype and smoking (Mutschler et al., 2013). Furthermore, COMT val carriers exhibit poorer performance on measures of working memory and sustained attention (Caldu et al., 2007; Liu et al., 2008; Tan et al., 2007; Winterer and Goldman, 2003). Although evidence for the association of the COMT genotype with cognitive function is mixed (Barnett et al., 2008; Mier et al., 2010), variability in participants’ smoking status may contribute to inconsistencies in prior research (Loughead et al., 2009).
Recent evidence suggests that smokers with val/val genotypes may be more sensitive to the effects of an abstinence challenge on working memory and prefrontal brain function than carriers of the met allele (Loughead et al., 2009). In our prior study, the val/val group exhibited a decrease in working memory performance and blood oxygen level-dependent (BOLD) fMRI signal during abstinence, compared to smoking as usual; no such effects were observed for met allele carriers (val/met or met/met). However, because this previous study was relatively small (only 10 val/val smokers), we sought to validate the prior findings in a larger sample.
In addition to task-positive regions activated during working memory tasks, the deactivation of regions within the “default mode network” (DMN) may also play an important role in the ability to sustain engagement with externally-focused tasks, including working memory (Anticevic et al., 2012). Recent evidence suggests that nicotine deprivation in smokers may result in less suppression of activation in task-negative regions including the posterior cingulate cortex (PCC) and to a lesser extent in the ventromedial PFC (vmPFC) (Falcone et al., 2013). Some propose that compared to met allele carriers, individuals with the val/val genotype may exhibit less suppression of task-negative regions during resting states and during cognitive task performance (Dang et al., 2013; Pomarol-Clotet et al., 2010) whereas, others have found greater de-activation in task-negative regions among val/val homozygotes (Stokes et al., 2011). However, no study that we know of has explored the association of the COMT genotype with working memory-related brain activity in both task-positive and task-negative brain regions.
The goal of the present study was to examine the neural correlates of abstinence-induced cognitive deficits in working memory in chronic smokers prescreened for the COMT val158met allele. In the primary study, 40 smokers (20 val/val and 20 met/met) performed a visual N-back task while undergoing BOLD fMRI on two separate occasions in counterbalanced order: following a 3-day (72 hours) period of mandatory abstinence and while smoking as usual. We predicted that, during abstinence, compared to smoking as usual, smokers with the high risk val/val genotype would exhibit poorer task performance and reduced BOLD signal in task-positive regions including bilateral dorsolateral prefrontal cortex (DLPFC) and dorsal cingulate/medial prefrontal cortex (MF/CG), compared to smokers with met/met genotypes. In addition, we tested the hypothesis that smoking abstinence would result in less suppression of task-negative regions (e.g., vmPFC and PCC) among smokers with the val/val genotype, compared to those with the met/met genotype. In an exploratory analysis, we sought to replicate the current findings using an independent sample of 48 smokers (27 met allele carriers and 21 val/val) who completed a similar paradigm of working memory-related fMRI BOLD signal change after 24 hours abstinence compared to smoking (Falcone et al., 2013).
METHODS
Primary Study
Participants
Potential participants of European ancestry were recruited through mass media. Eligible smokers were between the ages of 18 and 65 who smoked at least 10 cigarettes/day for at least 6 months. To ensure balance between the two genotype groups (met/met or val/val), smokers were selected prospectively based on genotyping for the COMT val158met polymorphism [rs4680, Assay on Demand (c_25746809_50) from Applied Biosystems, Inc. (Foster City)]. Persons with a history of DSM-IV Axis I psychiatric or substance disorders (except nicotine) and those taking psychotropic medications (e.g., monoamine oxidase inhibitors, benzodiazepines, antidepressants, antipsychotics) were excluded. Other exclusion criteria included: current use of chewing tobacco, snuff, or smoking cessation products; pregnancy, planned pregnancy or breastfeeding during the study period; history of brain injury; left-handedness; presence of fMRI contraindicated material in the body; low or borderline intelligence (<90 score on Shipley’s IQ test); and any impairment that would prevent cognitive task performance. Of the 40 participants who completed the study, four participants were excluded (technical error (n=2) and mean relative motion >0.3mm (n=2)), resulting in a final sample of 36 (17 val/val and 19 met/met). To reduce potential bias due to ethnic admixture, all participants were of European ancestry. On average, participants were 36.4 years old (SD=13.3), had an average Shipley IQ score of 109.2 (SD=6.8), smoked 16.2 cigarettes per day (SD=5.2), and were moderately nicotine dependent (mean FTND=4.34, SD=2.01).
Procedures
All procedures were approved by the University of Pennsylvania Institutional Review Board and all participants provided written informed consent. This fMRI study included two blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) sessions, compared within subjects, in counterbalanced order: (1) smoking as usual and (2) 72 hours after monitored abstinence. Participants completed a physical examination including a urine drug screen, breath alcohol test, and pregnancy test. The presence of psychiatric or substance abuse disorders was assessed using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). The Shipley Institute of Living Scale (Zachary, 2000) and Fagerström Test for Nicotine Dependence (Heatherton et al., 1991) were also administered.
Three days before the abstinent scanning session, participants received a 20-minute counseling call to prepare them for the 72-hour abstinence period. At 48 hours before the abstinent scan, participants were reminded via telephone to remain abstinent. Twenty-four hours prior to the scanning session, participants provided a breath carbon monoxide (CO) sample to verify compliance with the abstinence condition (i.e., <10ppm). Sessions were scheduled to occur at the same time of day (+/- 3 hours) 1-3 weeks apart, and subjects were instructed to refrain from alcohol or other drugs for at least 24 hours before the session. On the scanning session days, those with a positive drug screen, a breath alcohol test >0.01, or a breath carbon monoxide (CO) test >9ppm (abstinent session only) were excluded. Participants completed the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes et al., 1984) and Questionnaire of Smoking Urges (QSU-Brief; Cox et al., 2001). Following a short practice session to allow participants to become familiar with the task and response device, participants were escorted to the radiology clinic for the fMRI scan. On the smoking as usual day, participants smoked immediately before initiating the scanning protocol to standardize exposure (~20-30 minutes prior to completing the N-back).
Task Design
Working memory function was assessed in both the original sample and the replication sample using a visual N-back paradigm (Ragland et al., 2002) used in our prior research (Loughead et al., 2010; Loughead et al., 2009). The N-back task presents complex geometric figures (fractals) for 500 ms, followed by an interstimulus interval of 2500 ms under four conditions: 0-back, 1-back, 2-back, and 3-back. In the 0-back condition, participants respond with a button press to a specified target fractal; for the 1-back condition, participants respond if the current fractal was identical to the previous one; for the 2-back condition, if the current fractal was identical to the item presented two trials back; etc. No response was required for nontargets. Each condition was presented three times in 20-trial blocks (25% targets; 60 s). Blocks were presented in order of increasing memory load for the first set, after which conditions were presented pseudo-randomly; visual instructions (9 s) preceded each block to indicate the upcoming condition. The task began with a 48 s baseline rest period (fixation point) of which the first 24 s was discarded to ensure the MRI signal reached steady state. Equivalent N-back tasks with unique stimuli were used for the two sessions; version order was counterbalanced.
Image Acquisition
Blood oxygenation level dependent (BOLD) fMRI was acquired with a Siemens Trio 3T (Erlangen, Germany) system using a whole-brain, single-shot gradient-echo (GE) echoplanar sequence with the following parameters: TR/TE=3000/30 ms, FOV=448×448 mm, matrix=64×64, flip angle=90°, slices=48, slice thickness/gap=3.4 mm/0mm and effective voxel resolution=3.4 × 3.4 × 3.4. RF transmission utilized a quadrature body-coil and reception used an 8-channel head coil. After BOLD fMRI, a 5-minute magnetization-prepared, rapid acquisition gradient echo T1-weighted image (MPRAGE, TR=1810 ms, TE=3.51 ms, FOV =180×240 mm, matrix=256×192, 160 slices, TI=1100 ms, flip angle=9°, effective voxel resolution of 1 × 1 × 1mm) was acquired for anatomic overlays of functional data and to aid spatial normalization to a standard atlas.
Image Preprocessing
BOLD time series data were preprocessed and analyzed by standard procedures using fMRI Expert Analysis Tool (FEAT version 5.98) of FSL (FMRIB’s Software Library, Oxford, UK). Single subject preprocessing included nonbrain removal using BET (Smith, 2002), slice time correction, motion correction to the median image using MCFLIRT (Jenkinson et al., 2002), high pass temporal filtering (100 s), spatial smoothing using a Gaussian kernel (6 mm full-width at half-maximum, isotropic) and mean-based intensity normalization of all volumes using the same multiplicative factor. The median functional volume was coregistered to the anatomical T1-weighted structural volume and then transformed into standard anatomical space (T1 MNI template) using FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001). Transformation parameters were later applied to all statistical contrast maps for group-level analyses.
Image Quality Assessment
All images were carefully examined for artifacts, acquisition problems and preprocessing errors. Image quality assessment procedures assessed temporal signal-to-noise ratio (tSNR) of both smoking and abstinence sessions for artifacts and poor quality data. To assess excessive head motion, mean relative volume-to-volume displacement for each session was also evaluated. Subjects with mean tSNR > 30 (equivalent to more than 2SD) and/or mean relative motion > 0.3 were excluded from the analysis. Two additional subjects were excluded due to a technical error, leaving a final sample of 36 subjects (17 val/val and 19 met/met).
Subject-level Image Analysis
Subject-level statistical analyses were carried out voxelwise using FILM (FMRIB’s Improved General Linear Model) with local autocorrelation correction (Woolrich et al., 2001). Four condition events (0-back, 1-back, 2-back, and 3-back) were modeled using a canonical hemodynamic response function. The instruction period and six motion correction parameters were included as nuisance covariates and the three rest periods (fixation point) were treated as the baseline. Image analysis was completed for each individual in subject space, and resulting contrast maps were spatially normalized as described above.
Region of Interest Image Analysis
To characterize the group (val/val versus met/met) by session (smoking, abstinent) effects, mean percent signal change was extracted from a priori regions of interest (ROIs) in task-positive (right and left DLPFC and MF/CG) and task-negative regions (vmPFC and PCC). ROI masks were functionally defined using the replication sample (n=63; described below) studied under comparable abstinence conditions (Falcone et al., 2013). ROI masks were then registered into native subject space using methods described above. Finally, mean percent signal change was calculated per subject for the four load conditions separately for each ROI. These values were exported for further analysis using standard statistical software and procedures described below.
Exploratory Whole-brain Image Analysis
To characterize whole-brain genotype by session effects, an exploratory whole brain genotype (val/val vs. met/met) by session (abstinent vs. smoking) by memory load (0-, 1-, 2-, and 3-back) repeated measures ANOVA was performed. Resulting Z (Gaussianised F) statistic image of the interaction was thresholded using a whole-brain family-wise error (FWE) correction of p<0.05 (equivalent to z>4.69) (Beckmann and Smith, 2004). For clarity only clusters greater than 100 contiguous voxels are reported. Anatomic assignment of all clusters was based on the max z-score within the cluster using the Talairach Daemon Database and confirmed by visual inspection. Mean scaled beta coefficients (percent BOLD signal change) from each significant cluster in the interaction map for genotype by session were extracted for graphic examination and further statistical testing.
Data Analysis
Mean percent BOLD signal change was examined using random effects maximum likelihood regression (Stata xt-reg; Stata Corporation, College Station, TX, USA). Models included terms for the main effects of genotype (val/val vs. met/met), session (abstinent vs. smoking as usual), back level (0, 1, 2, and 3), and relevant covariates (age, sex, Shipley IQ score, and baseline FTND score). Because interactions with back level were not significant, only the genotype × session interaction is reported and back level was included as a covariate in all models. Behavioral performance measures (accuracy and reaction time) were tested as described above. Correlations between BOLD signal and behavioral performance were examined using models of behavioral performance, including percent BOLD signal change as a predictor (controlling for session, back level, and relevant covariates). Alpha levels were adjusted to p=0.007 for all models using a Bonferroni correction to account for the two performance and five ROI models.
Replication Sample
Participants and Procedures
Details regarding participants and procedures for the replication sample have been previously described (Falcone, et al., 2013). For the replication analysis, COMT val158met genotype information was available for 48 smokers (3 met/met, 24 val/met, 21 val/val). Because of the small number of met/met homozygotes, this group was combined with heterozygotes for the purpose of analysis (27 met carriers, 21 val/val). Both cohorts were similar except that the replication cohort consisted of treatment-seeking smokers, was abstinent for 24-hours instead of 72-hours, and included individuals of all ethnic backgrounds. The N-back task, fMRI scanning procedures, and data analyses were identical to those used in the primary study (for additional details see Falcone et al., 2013).
RESULTS
Participants
Demographic and smoking characteristics by genotype for the primary study are presented in Table 1. All smokers in the primary study self-reported Caucasian race. Except for a significantly lower Shipley Institute of Living Scale (IQ) score for the val/val group compared to the met/met group (p=0.02), there were no significant differences by genotype. Shipley score was included as a covariate in all models. As expected, CO levels during the abstinent session (2.5ppm, SD=1.4) were significantly lower than during the smoking as usual session (23.6ppm, SD=10.5, p<0.0001), but this did not vary by genotype.
Table 1.
Demographic and smoking characteristics across genotype.
| Measure | Val/Val (n=17) | Met/Met (n=19) | p-value |
|---|---|---|---|
| Sex, % female | 44 | 50 | 0.73 |
| Age | 37.4 (15) | 35.5 (12) | 0.88 |
| Nicotine dependence | 4.1 (2.4) | 4.5 (1.6) | 0.50 |
| Baseline cigarettes per day | 15.6 (4.5) | 16.7 (6.1) | 0.55 |
| Cigarettes smoked prior to smoking session | 15.3 (3.6) | 16.7 (6.2) | 0.58 |
| Shipley Institute of Living Scale | 111.6 (4.3) | 106.5 (8.0) | 0.02 |
| CO during smoking session (ppm) | 19.8 (9.8) | 25.8 (10.5) | 0.09 |
| CO during abstinent session (ppm) | 2.7 (1.6) | 2.4 (1.3) | 0.50 |
Note. Values are mean (standard deviation). p-values are unadjusted for multiple comparison; ppm=parts per million
Subjective Measures
Craving and withdrawal scores were higher during the abstinent session (means=36.6 and 11.8, SDs=17.1 and 9.4, respectively) compared to the smoking as usual session (means=23.6 and 2.5, SDs=10.1 and 2.7, respectively; p<0.0001). However, there were no significant main or interacting effects of genotype for craving or withdrawal (all ps>0.48).
Genotype Associations with Behavioral Performance
For median correct reaction time (RT) on the N-back task, neither the genotype × session interaction (β=59.3, CI: 4.82 to 113.8, p=0.03) nor the main effect of genotype (β=71.5, CI: -8.1 to 151.1, p=0.078) survived Bonferroni correction. There were no significant main or interacting effects with genotype on true positives (ps>0.07). For both measures, performance decreased with increasing memory load (ps<0.0001).
Region of Interest Analysis
In task-positive regions, there were significant genotype by session interactions on BOLD signal change in MF/CG (β=0.23, CI: 0.12 to 0.34, p=4.7×10-5), and right and left DLPFC (β=0.25, CI: 0.13 to 0.37, p=4.1×10-5 and β=0.26, CI: 0.13 to 0.38, p=5.8×10-5, respectively) (Figure 1a-c). Contrary to expectations, in all three task-positive regions, abstinence resulted in significantly less activation compared to smoking as usual in the met/met group (p<0.0001), but not the val/val group (p>0.22).
Fig. 1.
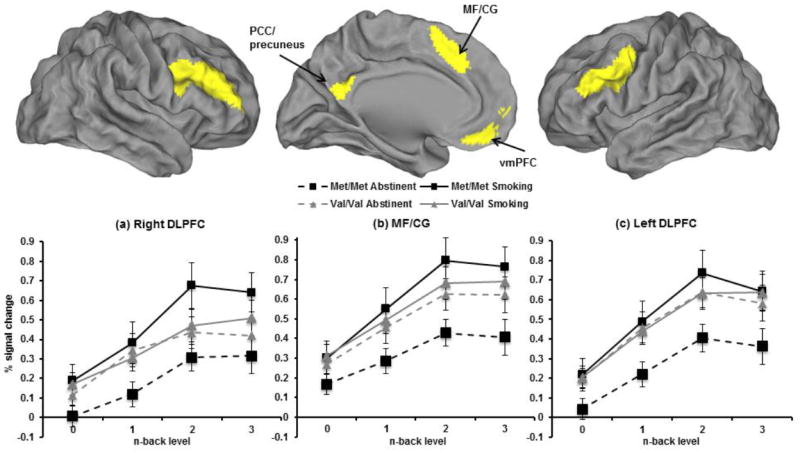
Colored regions represent functionally defined ROIs identified from the main effect of working memory load in a Fractal N-back task. Brain rendering performed with CARET (http://www.nitrc.org/projects/caret/). Line graphs represent BOLD % signal change during the N-back task by genotype, session, and back level. Genotype × session interaction in task-positive regions: (a) Right DLPFC (p=4.1×10-5), (b) bilateral MF/CG (p=4.7×10-5), and (c) left DLPFC (p=5.8×10-5) (Met/Met n=19; Val/Val n=17).
Although the genotype by session interaction in the PCC did not survive Bonferroni correction (β=0.19, CI: 0.03 to 0.34, p=0.018), there was a trend for the val/val group to exhibit less deactivation during task performance in the abstinent compared to smoking state, whereas the met/met group showed no change (Figure 2a). There were no main or interacting effects with genotype in the vmPFC (all ps>0.13) (Figure 2b).
Fig. 2.
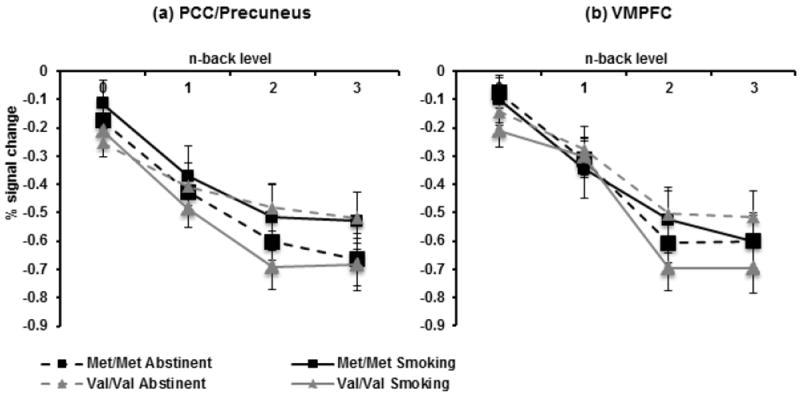
BOLD % signal change in task-negative regions during the N-back task by genotype, session, and back level (Met/Met n=19; Val/Val n=17). Genotype × session interaction in (a) PCC (p=0.018) (b) vmPFC (ps>0.13). See Figure 1 for ROI masks.
Exploratory Whole Brain Analysis
The genotype by session whole brain interaction resulted in significant clusters bilaterally in the lateral occipital/precuneus and insula; in the right fusiform gyrus, superior temporal gyrus, medial frontal gyrus, and cerebellum; and in the left precentral gyrus, cuneus, and cerebellum (Supplemental Table 1, Supplemental Figure 1). Examination of the percent signal change in these significant clusters showed that for most regions abstinence resulted in significantly less activation compared to smoking for the met/met group, but not the val/val group, a pattern similar to the apriori task positive ROI findings described above. The right cerebellum showed an opposite pattern of increased, and not reduced, activation for the met/met group during abstinence compared to smoking, with no significant difference in activation for the val/val group. The apriori ROIs did not survive the stringent whole brain correction but were observed at an uncorrected p=0.05 threshold.
BOLD-Behavior Correlations
None of the models using BOLD signal change to predict median correct RT or true positives survived Bonferroni correction (all ps>0.03).
Replication Analysis of Behavior and BOLD Signal Change
Except for a significantly lower Shipley IQ score (mean=103.4, SD=8.4, t(82)=3.3, p=0.001), the replication sample was comparable to the primary sample. On average, participants were 40.2 years old (SD=13.3), smoked 16.1 cigarettes per day (SD=5.0), and were moderately nicotine dependent (mean FTND=4.8, SD=1.8). There were no significant differences by genotype on age, Shipley, cigarettes per day, nicotine dependence, or CO levels during either session (ps>0.08). Likewise, there were no main or interacting effects of genotype on reaction time or true positives, ps>0.3.
In task-positive regions, we confirmed the significant genotype by session interactions on BOLD signal change in right and left DLPFC (βs=0.19, CIs: 0.07 to 0.32, ps=0.003) (Figure 3a-b). Similar to the primary study, in both task-positive regions, abstinence resulted in significantly less activation compared to smoking as usual in met carriers (p<0.0001), but not the val/val group (p>0.45). The interaction was not significant in MF/CG, p=0.5.
Fig. 3.
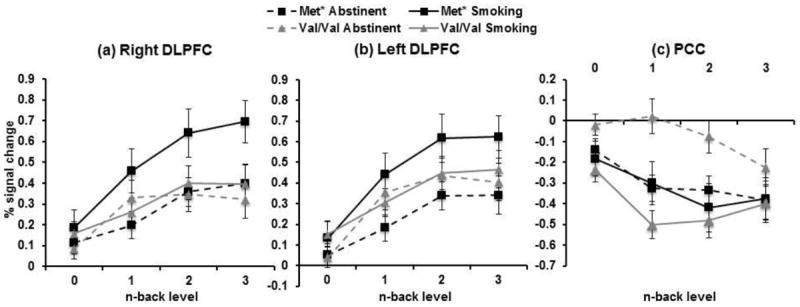
Line graphs represent BOLD % signal change during the N-back task by genotype, session, and back level in the validation sample (Met* n=27; Val/Val n=21). Genotype × session interactions: (a) right DLPFC (p=0.003), (b) left DLPFC (p=0.003), and (c) PCC (p=0.001).
In task-negative regions, we confirmed a significant genotype by session interaction in the PCC (β=0.31, CI: 0.12 to 0.49, p=0.001) (Figure 3c). Similar to the trend (p=0.018) in the primary study, the val/val group exhibited significantly less deactivation during task performance in the abstinent compared to smoking state (p<0.0001), whereas met carriers showed no change (p=0.71). There were no main or interacting effects with genotype in the vmPFC (all ps>0.1).
Discussion
We tested whether the val158met polymorphism of the COMT gene is associated with the effects of abstinence on working memory performance and brain activity in smokers. Contrary to our hypothesis, with respect to the task-positive regions, bilateral DLPFC and MF/CG, effects of abstinence were stronger in the met/met group. This finding was reproduced in an independent sample of smokers participating in a similar fMRI investigation. In addition to concordant data for genotype by session interactions on activity in task-positive regions, both the primary and replication samples provided support for the hypothesis that COMT genotype is associated with abstinence effects on brain activity within task-negative regions. Specifically, following 72 hours of abstinence, compared to smoking as usual, val/val homozygotes exhibited less suppression of activity in the PCC, whereas the met/met group did not. This pattern was confirmed in the replication sample.
Deactivation of task-negative regions is important for successful performance of externally-focused tasks (Anticevic et al., 2012) and decreased negative coupling between executive control and default networks is associated with nicotine withdrawal symptoms (Cole et al., 2010; Sutherland et al., 2012). Thus, the val/val group may experience more difficulty suppressing goal-irrelevant cognitive function while performing a working memory task during abstinence from smoking. This interpretation is consistent with the notion that decreased prefrontal dopamine levels may alter the balance between excitatory and inhibitory synaptic interactions, decrease the signal-to-noise ratio, and thus reduce cognitive stability (Winterer et al., 2006; Winterer and Weinberger, 2004). The failure to suppress activity within task-negative regions, combined with abstinence-induced decrements in performance in the val/val group, may contribute to smoking relapse. However, this conclusion is tempered by the fact that BOLD signal in PCC was unrelated to behavioral performance on the N-back task.
While the results of the two studies presented herein reveal concordant findings for COMT genotype associations with activation in both task-positive and task-negative regions, the findings for task-positive regions differ from our previous study (Loughead et al., 2009). Further, the whole-brain analysis identified several additional regions that demonstrated a genotype by session effect (e.g., bilateral precuneus, insula, medial frontal gyrus, left precentral gyrus, etc.), though the apriori regions did not survive correction. Nevertheless, all three studies used an identical within subject design and N-back task. Participants in both the prior study (Loughead et al., 2009) and the primary study reported here were prescreened for a single functional polymorphism for which we had a priori hypotheses. Although the sample size for the current study (20 per group) provided adequate power to detect differences based on prior observed effect sizes, the numbers remain small for studies of genetic association. Indeed, there is also conflicting evidence regarding the COMT genotype associations with cognition in prior studies of healthy subjects, with some studies finding cognitive benefits among met allele carriers (Goldberg and Weinberger, 2004; Mier et al., 2010; Tunbridge et al., 2006), whereas others find no genotype association (Barnett et al., 2008; Wardle et al., 2013). Furthermore, the current findings cannot be explained by the “inefficiency hypothesis,” which posits that cognitive performance may require greater effort (and brain activation) in the val allele carriers in an unchallenged state (Bertolino et al., 2006). Although this was a trend-level finding in our prior study (Loughead et al., 2009), the val/val group did not exhibit increased activation in task-positive regions during the smoking session compared to met allele carriers in either the primary or the validation sample. These discrepancies across studies may reflect variability in BOLD signal and working memory performance attributable to other unmeasured genetic or environmental influences that vary across these small study samples. Furthermore, there is increasing support for sex differences in the effect of the val158met polymorphism on cognitive function (Tunbridge and Harrison, 2011). Although our studies were not powered to detect genotype by sex interactions on abstinence-induced changes in brain function, this question could be addressed in future work. Other polymorphisms in COMT and other genes that regulate dopamine or other neurotransmitter levels may have important contributing effects (Berryhill et al., 2013).
To advance the science of nicotine addiction and its treatment, imaging genetic studies such as ours must address several methodological challenges. Although neuroimaging offers the advantage of detecting more subtle objectively measured phenotypes, the sample sizes tend to be small, due in part to the intensity and cost of these assessments. Studies that incorporate prospective genotyping of functional variants with an a priori hypothesis may reduce the likelihood of spurious findings, but replication is necessary. Additionally, a focus on variants identified in genome-wide association studies (GWAS) may provide a more powerful approach than selecting variants identified only in prior candidate gene studies (Tost et al, 2012). With data sharing initiatives underway, it may be feasible to build polygenic models of neuroimaging outcomes that more accurately represent biological function. Such knowledge could lay the foundation for incorporating genetics and imaging to explain individual variability in response to addiction treatment.
Supplementary Material
Acknowledgments
This research was supported by grants R03 DA027438 (CL), R01 DA026849 (CL) and pilot funding from UL1 RR024134 (RLA). The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
References
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Hawk LW., Jr Effects of smoking abstinence on impulsive behavior among smokers high and low in ADHD-like symptoms. Psychopharmacology (Berl) 2012;219:537–547. doi: 10.1007/s00213-011-2324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafo MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Wiener M, Stephens JA, Lohoff FW, Coslett HB. COMT and ANKK1-Taq-Ia Genetic Polymorphisms Influence Visual Working Memory. PLoS ONE. 2013;8:e55862. doi: 10.1371/journal.pone.0055862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Caforio G, Petruzzella V, Pizzuti A, Scarabino T, Nardini M, Weinberger DR, Dallapiccola B. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Gray KM, Tolliver BK. Cognitive enhancers in the treatment of substance use disorders: clinical evidence. Pharmacol Biochem Behav. 2011;99:285–294. doi: 10.1016/j.pbb.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, Zamora-Paja E, Farahi J, Saxena S, London ED, McCracken JT. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Archives of general psychiatry. 2006;63:808–816. doi: 10.1001/archpsyc.63.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldu X, Vendrell P, Bartres-Faz D, Clemente I, Bargallo N, Jurado MA, Serra-Grabulosa JM, Junque C. Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. NeuroImage. 2007;37:1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. American journal of human genetics. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Colilla S, Lerman C, Shields PG, Jepson C, Rukstalis M, Berlin J, DeMichele A, Bunin G, Strom BL, Rebbeck TR. Association of catechol-O-methyltransferase with smoking cessation in two independent studies of women. Pharmacogenetics and genomics. 2005;15:393. doi: 10.1097/01213011-200506000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Culhane MA, Schoenfeld DA, Barr RS, Cather C, Deckersbach T, Freudenreich O, Goff DC, Rigotti NA, Evins EA. Predictors of early abstinence in smokers with schizophrenia. The Journal of clinical psychiatry. 2008 doi: 10.4088/jcp.v69n1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang LC, O’Neil JP, Jagust WJ. Genetic effects on behavior are mediated by neurotransmitters and large-scale neural networks. Neuroimage. 2013;66C:203–214. doi: 10.1016/j.neuroimage.2012.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SL, Sacco KA, Termine A, Seyal AA, Dudas MM, Vessicchio JC, Wexler BE, George TP. Neuropsychological deficits are associated with smoking cessation treatment failure in patients with schizophrenia. Schizophrenia research. 2004;70:263–275. doi: 10.1016/j.schres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Falcone M, Wileyto EP, Ruparel K, Gerraty RT, Laprate L, Detre JA, Gur R, Loughead J, Lerman C. Age-related differences in working memory deficits during nicotine withdrawal. Addict Biol. 2013 doi: 10.1111/adb.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Harrison EL, Coppola S, McKee SA. Nicotine deprivation and trait impulsivity affect smokers’ performance on cognitive tasks of inhibition and attention. Experimental and Clinical Psychopharmacology. 2009;17:91–98. doi: 10.1037/a0015657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami D, Pickens R, Krahn D, Malin S, Luknic A. Effect of nicotine on the tobacco withdrawal syndrome. Psychopharmacology (Berl) 1984;83:82–87. doi: 10.1007/BF00427428. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Elliot KM, David SP, Murphy MF, Walton RT, Munafo MR. Association of COMT Val108/158Met genotype with smoking cessation in a nicotine replacement therapy randomized trial. Cancer Epidemiol Biomarkers Prev. 2007;16:1065–1069. doi: 10.1158/1055-9965.EPI-06-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Cavallo DA, Carroll KM, Potenza MN. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, Corrigall WA. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–762. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- Lerman C, Perkins KA, Gould T. National Cancer Institute, Phenotypes and Endophenotypes: Foundations for Genetic Studies of Nicotine Use and Dependence, Tobacco Control Monograph No 20. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. NIH Publication No. 08-6366; 2008. Nicotine dependence endophenotypes in chronic smokers. [Google Scholar]
- Liu ME, Hong CJ, Liou YJ, Tsai YL, Hsieh CH, Tsai SJ. Association study of a functional catechol-O-methyltransferase polymorphism and executive function in elderly males without dementia. Neuroscience letters. 2008;436:193–195. doi: 10.1016/j.neulet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, Ruparel K, Ray R, Gur RC, Lerman C. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatry. 2009;14:820–826. doi: 10.1038/mp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. 2010;15:918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Johnstone EC, Guo B, Murphy MF, Aveyard P. Association of COMT Val108/158Met genotype with smoking cessation. Pharmacogenet Genomics. 2008;18:121–128. doi: 10.1097/FPC.0b013e3282f44daa. [DOI] [PubMed] [Google Scholar]
- Mutschler J, Abbruzzese E, von der Goltz C, Dinter C, Mobascher A, Thiele H, Diaz-Lacava A, Dahmen N, Gallinat J, Majic T, Petrovsky N, Thuerauf N, Kornhuber J, Grunder G, Rademacher L, Brinkmeyer J, Wienker T, Wagner M, Winterer G, Kiefer F. Lack of Association of a Functional Catechol-O-Methyltransferase Gene Polymorphism With Risk of Tobacco Smoking: Results From a Multicenter Case-Control Study. Nicotine Tob Res. 2013 doi: 10.1093/ntr/nts334. [DOI] [PubMed] [Google Scholar]
- Myers CS, Richard CT, Etter JR, Moolchan ET, Heishman SJ. Nicotine nasal spray dose-dependently enhanced sustained attention as assessed by the continous performance task. Presented to the Society for Research on Nicotine and Tobacco; Prague. 2005. [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Nedic G, Nikolac M, Borovecki F, Hajnsek S, Muck-Seler D, Pivac N. Association study of a functional catechol-O-methyltransferase polymorphism and smoking in healthy Caucasian subjects. Neurosci Lett. 2010;473:216–219. doi: 10.1016/j.neulet.2010.02.050. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature neuroscience. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser A, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline Improves Mood and Cognition During Smoking Abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Fatjo-Vilas M, McKenna PJ, Monte GC, Sarro S, Ortiz-Gil J, Aguirre C, Gomar JJ, Guerrero A, Landin R, Capdevila A, Fananas L, Salvador R. COMT Val158Met polymorphism in relation to activation and de-activation in the prefrontal cortex: A study in patients with schizophrenia and healthy subjects. Neuroimage. 2010;53:899–907. doi: 10.1016/j.neuroimage.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Bupropion dose-dependently reverses nicotine withdrawal deficits in contextual fear conditioning. Pharmacology, biochemistry, and behavior. 2007;88:179–187. doi: 10.1016/j.pbb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, Chan R, Gur RE. Working memory for complex figures: An fMRI comparison of letter and fractal-n-back tasks. Neuropsychology. 2002;16:370. [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, Gould TJ. Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behavioral neuroscience. 2008;122:1166–1171. doi: 10.1037/a0012601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes PR, Rhodes RA, Grasby PM, Mehta MA. The effects of the COMT Val108/158Met polymorphism on BOLD activation during working memory, planning, and response inhibition: a role for the posterior cingulate cortex? Neuropsychopharmacology. 2011;36:763–771. doi: 10.1038/npp.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammimaki AE, Mannisto PT. Are genetic variants of COMT associated with addiction? Pharmacogenet Genomics. 2010;20:717–741. doi: 10.1097/FPC.0b013e328340bdf2. [DOI] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, Callicott JH. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 2007;27:13393–13401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM. The catechol-O-methyltransferase gene: its regulation and polymorphisms. Int Rev Neurobiol. 2010;95:7–27. doi: 10.1016/B978-0-12-381326-8.00002-8. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ. Importance of the COMT gene for sex differences in brain function and predisposition to psychiatric disorders. Current topics in behavioral neurosciences. 2011;8:119–140. doi: 10.1007/7854_2010_97. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Ward MM, Swan GE, Jack LM. Self-reported abstinence effects in the first month after smoking cessation. Addictive behaviors. 2001;26:311–327. doi: 10.1016/s0306-4603(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Wardle MC, de Wit H, Penton-Voak I, Lewis G, Munafo MR. Lack of Association Between COMT and Working Memory in a Population-Based Cohort of Healthy Young Adults. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Goldman D. Genetics of human prefrontal function. Brain research. 2003;43:134–163. doi: 10.1016/s0165-0173(03)00205-4. [DOI] [PubMed] [Google Scholar]
- Winterer G, Musso F, Vucurevic G, Stoeter P, Konrad A, Seker B, Gallinat J, Dahmen N, Weinberger DR. COMT genotype predicts BOLD signal and noise characteristics in prefrontal circuits. Neuroimage. 2006;32:1722–1732. doi: 10.1016/j.neuroimage.2006.05.058. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary RS. Shipley Institute of Living Scale - Revised Manual. Western Psychological Services 2000 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


