Abstract
Background & Aims
Chronic inflammation contributes to the pathogenesis of gastric tumorigenesis. The Aurora kinase A gene (AURKA) is frequently amplified and overexpressed in gastrointestinal cancers. We investigated the roles of AURKA in inflammation and gastric tumorigenesis.
Methods
We used quantitative real-time reverse transcription PCR, immunofluorescence, immunohistochemistry, luciferase reporter, immunoblot, co-immunoprecipitation, and in vitro kinase assays to analyze AGS and MKN28 gastric cancer cells. We also analyzed Tff1−/− mice, growth of tumor xenografts, and human tissues.
Results
We correlated increased expression of AURKA with increased levels of tumor necrosis factor-α and inflammation in the gastric mucosa of Tff1−/− mice (r = 0.62; P=.0001). MLN8237, an investigational small-molecule selective inhibitor of AURKA, reduced nuclear staining of NFκBp65 in human gastric cancer samples and mouse epithelial cells, suppressed NFκB reporter activity, and reduced the expression of NFκB target genes that regulate inflammation and cell survival. Inhibition of AURKA also reduced growth of xenograft tumors from human gastric cancer cells in mice and reversed the development of gastric tumors in Tff1−/− mice. AURKA was found to regulate NFκB activity by binding directly and phosphorylating IκBα in cells. Premalignant and malignant lesions from the gastric mucuosa of patients had increased levels of AURKA protein and nuclear NFκB, compared with healthy gastric tissue.
Conclusions
In analyses of gastric cancer cell lines, human tissue samples, and mouse models, we found AURKA to be upregulated during chronic inflammation to promote activation of NFκB and tumorigenesis. AURKA inhibitors might be developed as therapeutic agents for gastric cancer.
Keywords: stomach cancer, mouse model, gene regulation, TNFα
Introduction
Gastric adenocarcinoma remains the fourth most common malignancy worldwide and the second leading cause of cancer-related deaths.1,2 Although various chemotherapeutic drugs have been utilized, drug resistance has significantly attenuated the effectiveness of chemotherapy, leading to poor survival rates in patients with gastric cancer.3 Chronic inflammation and constitutive activation of NF-κB signaling are important factors in gastric tumorigenesis in mouse models and human disease. 4–6 In fact, activation of NF-κB signaling has been implicated in the tumorigenesis of gastric cancer. 5, 7 A complex signaling cascade leading to NF-κB activation is mediated by phosphorylation of IκB by IKKα and IKKβ. 8, 9 Phosphorylation of the IκBα protein induces its ubiquitination and subsequent degradation in the proteasome. Upon the degradation of IκB proteins, the IκB-bound NF-κB is released and translocated to the nucleus to drive expression of several pro-inflammatory and pro-survival target genes in cancer cells. 8, 10–13
Aurora Kinase A (AURKA) is a serine threonine kinase that plays an important role in mitosis 14 and ensuring correct spindle assembly in normal cells.15 Aberrant AURKA amplification and/or overexpression are frequent findings in adenocarcinomas of the breast, pancreas, ovary, colon, stomach, and esophagus. 14, 16–18 In Addition, Myc oncoproteins have been reported to transcriptionally up-regulate AURKA expression in tumors.19 The overexpression of AURKA in cancer cells mediates several oncogenic functions that involve activation of survival and growth signaling pathways such as PI3K/AKT and β-catenin. 20 Furthermore, AURKA overexpression plays an important role in attenuating both p53 and p73 tumor suppressor functions.16,21 Several studies have indicated that overexpression of AURKA contributes to chemotherapeutic drug resistance and tumor recurrence.22–24 These tumorigenic features of AURKA have triggered the development of several small molecule AURKA inhibitors. 14 MLN8237, also known as alisertib, is an investigational small molecule that selectively inhibits AURKA. This inhibitor has shown promising therapeutic efficacy in pre-clinical studies14, 25 and has been tested in multiple Phase I and Phase II clinical trials for advanced solid tumors and hematological malignancies. 25–27
In this study, we have established the association of AURKA overexpression with inflammation in gastric tumorigenesis using in vivo, ex-vivo and in vitro models. Collectively, the results indicate that AURKA directly binds and phosphorylates IκB, thereby playing a key role in regulating the NF-κB-mediated pro-inflammatory and pro-survival responses in gastric tumorigenesis.
Materials and Methods
Detailed methods for Cell culture and reagents, AURKA expression and plasmids, In vitro kinase assay and protein pull-down experiment, Western blotting, Immunoprecipitation, Immunofluorescence, Human tissue microarrays and immunohistochemistry, Luciferase reporter assay, clonogenic survival assay, AURKA silencing by Small interfering RNA (siRNA), RNA extraction and real-time RT-PCR, Ex vivo culture of Tff1-knockout mouse gastric primary epithelial cells, In vivo tumor xenograft model, and Mouse imaging can be found in the Supplementary Methods.
Tff1-knockout gastric cancer mouse model
The Tff1-knockout (Tff1−/−) and normal Tff1 wild-type mice28 were used in this study. Tff1-knockout (n=20) and wild-type (n=16) mice between 2 – 4 months of age were used to examine the association between AURKA expression and inflammation before the incidence of gastric neoplasms. At this age, the mice develop gastritis or gastritis with elongation of gastric glands. To evaluate the effects of AURKA inhibition using MLN8237 on gastric tumorigenesis, we utilized older Tff1-knockout mice (12 – 14 month). At this age these mice generally develop high grade dysplasia or gastric adenocarcinoma. For analysis of NF-κB targets, we utilized a short term treatment of the Tff1-knockout mice (n=10) with MLN8237 for 8 days and compared the results to non-treated group (n=10). For testing the therapeutic outcome of MLN8237, we treated an additional set of Tff1-knockout mice for 12 weeks (n=10) and compared the results to untreated mice of similar age (n=20). A group of six of these MLN8237 treated mice were subjected to [18F]FLT imaging as described in Supplementary Methods. At the end of the indicated treatments, all animals were sacrificed and stomach tissue samples were collected and examined by our pathologists for histological changes using H&E staining.
Statistical Analysis
Two t-test samples, Wilcoxon Rank Sum Test and linear regression modeling methods, were used to compare the statistical difference between two groups. Multivariate model, ANOVA and Kruskal-Wallis rank sum test were used to examine the associations between variables. The correlation between two parameters was determined by the Spearman correlation and kappa test. Data were expressed as mean ± standard deviation of 3 independent experiments. The correlation between AURKA and chronic inflammation scores, and between AURKA and TNF-α was determined by Spearman correlation. Statistical significance of the in vitro studies was analyzed by a Student’s t test and analysis of variance. The differences were considered statistically significant when the p value was ≤0.05.
Results
AURKA overexpression is associated with inflammation in the gastric mucosa of the Tff1-knockout gastric cancer mouse model
The development of gastritis was observed in all samples from Tff1-knockout mice irrespective of age and was detectable as early as 8 weeks of age, before the development of dysplastic lesions. Mild elongation of gastric glands was present at the age of 8 weeks. At the age of 16 weeks, approximately half of the Tff1-knockout mice develop low-grade dysplasia but none has high-grade dysplasia. At the age of 12 month, more than half of the mice have high-grade dysplasia with multifocal intraepithelial or intramucosal carcinomas which progress, in some cases, into the submucosa through gaps in the muscularis mucosa and form invasive adenocarcinomas 6.
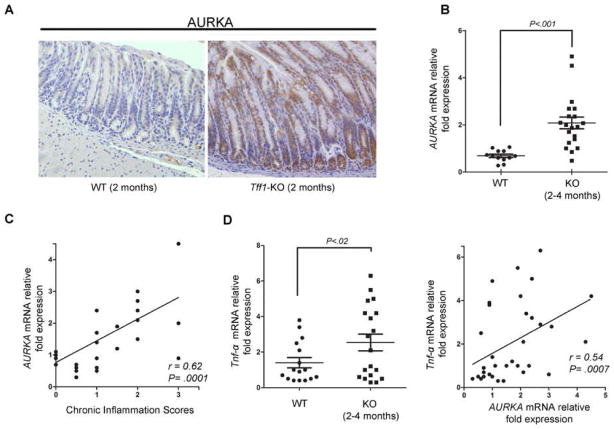
We investigated the role of AURKA in regulating inflammation and gastric tumorigenesis in Tff1-knockout gastric cancer mouse model that exhibited up-regulation of several pro-inflammatory genes.6 The IHC analyses of non-dysplastic lesions with gastritis from the Tff1-knockout mice stomach showed remarkably stronger AURKA staining than wild-type (Figure 1A). Using qRT-PCR analysis, we found that AURKA mRNA expression was significantly higher in the gastric mucosa of the Tff1-knockout mice than wild-type (p<0.001), at 2 and 4 months of age (Figure 1B). We next tested if AURKA expression was associated with inflammation scores in the Tff1-knockout mouse model. Indeed, histological evaluation suggested a direct correlation between chronic inflammation scores and AURKA overexpression in this model (coefficient r = 0.62; P = 0.0001) (Figure 1C). In corroboration of these results, we found that the pro-inflammatory Tnf-α mRNA expression was significantly higher in the Tff1-knockout mice than wild-type (p<0.02), at 2 and 4 months of age (Figure 1D, left panel). Additionally, we observed a direct correlation between Tnf-α and AURKA mRNA expression (coefficient r= 0.54; P= 0.0007) (Figure 1D, right panel). Collectively, these results suggested a strong association between aberrant AURKA overexpression and chronic inflammation.
Figure 1. AURKA expression levels directly correlate with inflammation in Tff1-knockout mouse model.
A) Immunohistochemical staining of Tff1-knockout mouse (KO) (2 months of age) stomach tissue shows increased AURKA protein levels as compared to wild-type mice (WT). B) Real-time RT-PCR analysis of Tff1-knockout mouse gastric tissue showed significant increase in AURKA expression levels at 2 and 4 months of age relative to wild-type (p<0.001). C) AURKA mRNA expression levels are significantly associated with inflammation scores in Tff1-knockout animals (p=0.0001). D) Pro-inflammatory cytokine Tnf-α mRNA expression was significantly up-regulated in Tff1-knockout mice at 2 and 4 months of age (p<0.02) (left panel), and directly correlated with increased AURKA mRNA levels (p=0.0007) (right panel).
AURKA regulates NF-κB activation in gastric cancer cell models
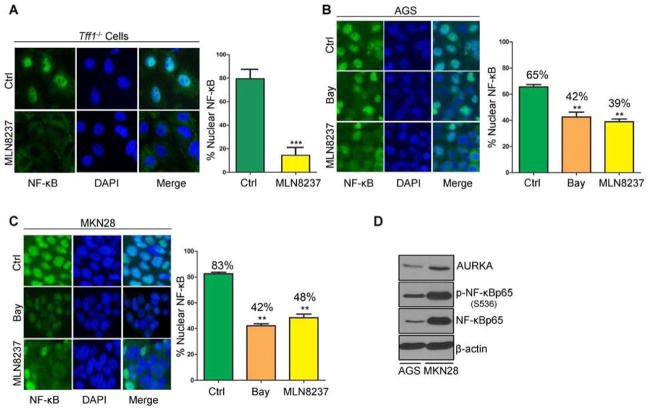
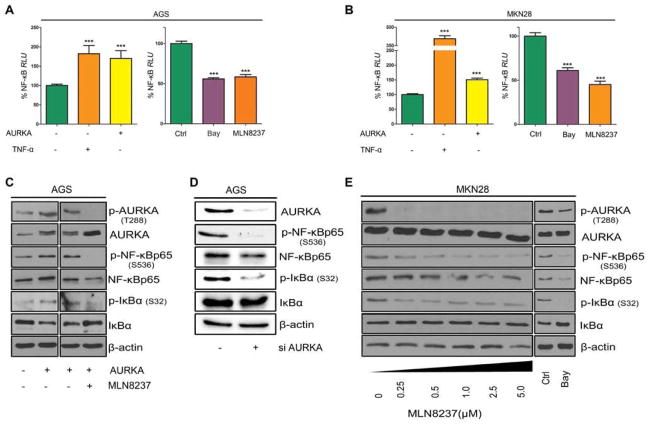
Constitutive activation of NF-κB signaling is an important step of tumorigenesis in human gastric cancer and in the Tff1-knockout gastric cancer mouse model.6 As shown in Figure 1A, gastric mucosa from these mice displayed aberrant overexpression of AURKA. To investigate whether pharmacological inhibition of AURKA could attenuate inflammation through suppression of NF-κB, we isolated gastric antrum mucosa cells of Tff1-knockout animals and treated them ex vivo with 0.5 μmol/L MLN8237 (selective AURKA inhibitor) for 3h. Immunofluorescence data showed that MLN8237 significantly reduced the levels of nuclear NF-κBp65 (p<0.001) (Figure 2A). Similarly, the in vitro results demonstrated that MLN8237 effectively inhibited the translocation of NF-κBp65 to the nucleus in both AGS and MKN28 cell lines (p<0.01, Figure 2B–C). Further, western blot analysis demonstrated higher levels of AURKA expression in MKN28 than in AGS cells (Figure 2D). In fact, MKN28 cells exhibited higher levels of AURKA and p-NF-κBp65 (S536), indicative of more activation of NF-κB, than AGS cells (Figure 2D). These results suggested a possible role for AURKA in regulating NF-κB activity in gastric cancer cells. We next utilized the NF-κB luciferase reporter, as a measure of NF-κB activity, following overexpression or inhibition of AURKA. The data indicated that overexpression of AURKA in both AGS and MKN28 cells significantly induced the NF-κB reporter (p<0.01 and p<0.001, respectively), indicative of activation of NF-κB (Figure 3A–B, left panels). A similar induction was achieved using TNF-α, a cytokine known to activate NF-κB signaling (Figure 3A–B, left panels). On the other hand, inhibition of AURKA with MLN8237 significantly reduced NF-κB reporter activity to levels similar to those achieved using the NF-κB inhibitor (Bay 11–7085) in both cell lines (Figure 3A–B, right panels). To confirm the role of AURKA in promoting NF-κB activation, we induced NF-κB with TNF-α in the presence or absence of MLN8237 in AGS and MKN28 cells. The reporter data showed that AURKA pharmacological inhibition completely blocked TNF-α-induced NF-κB activation in both cell lines (Supp. Figure 1A–B). To examine the molecular signaling of AURKA-dependent activation of NF-κB, we transiently overexpressed AURKA in AGS cells (Figure 3C). Indeed, overexpression of AURKA induced a remarkable increase in NF-κBp65 and p-NF-κBp65 (S536) protein levels (Figure 3C). One of the key steps in the activation of NF-κB depends on phosphorylation and degradation of IκBα. Western blot analysis indicated that overexpression of AURKA in AGS cells increased the p-IκBα (S32) and decreased total IκBα protein levels (Figure 3C). These molecular changes were reversed by MLN8237-dependent AURKA inhibition in AGS cells (Figure 3C). To further confirm that the down regulation of p-NF-κBp65 (S536) and p-IκBα (S32) was due to AURKA inhibition and not a result of MLN8237 off-target activity, we genetically knocked down AURKA in AGS cells using siRNA. Our data showed that AURKA knockdown significantly decreased both p-NF-κBp65 (S536) and p-IκBα (S32) protein levels (Figure 3D). Notably, there was no change in p-IKK α/β levels after AURKA knockdown (Supp. Figure 2), suggesting that NF-κB regulation by AURKA is mainly mediated by IκBα. We validated these results in MKN28 cells by inhibiting AURKA with MLN8237 at doses as low as 250 nmol/L (Figure 3E). The AURKA inhibition was associated with a remarkable decrease in p-IκBα (S32) combined with a reduction in the levels of p-NF-κBp65 (S536) (Figure 3E). Together, these data strongly suggest that AURKA activates NF-κB through IκBα regulation, whereby inhibition of AURKA could be effective in suppressing this critical pathway in gastric cancers.
Figure 2. AURKA regulates NF-κBp65 nuclear translocation ex vivo and in vitro.
A) Antrum epithelial cells were extracted from Tff1-knockout mice. Selectively cultured epithelial cells were treated with vehicle (ctrl) or MLN8237 (500 nmol/L) and subjected to immunofluorescence analysis of NF-κBp65. Original magnification is shown at ×100. Treatment with MLN8237 significantly reduced nuclear NF-κBp65–positive staining relative to vehicle-treated control (p<0.001). B–C) NF-κBp65 immunofluorescence in AGS (B) and MKN28 (C) cells after 30 min treatment with NF-κB inhibitor Bay 11-7085 (10 μmol/L) or AURKA inhibitor MLN8237 (500 nmol/L). Nuclear localization of NF-κBp65 is shown in green. DAPI (blue) was used as a nuclear counterstain. Graphs indicate the quantification of nuclear NF-κBp65–positive staining in at least 200 counted cells presented as a percentage. D) Western blot analysis of AURKA, p-NF-κBp65 (S536), and NF-κBp65 proteins in AGS and MKN28 cell lines suggesting a correlation between AURKA and p-NF-κBp65 (S536) protein levels.
Figure 3. AURKA activates NF-κB through regulation of IκBα.
A–B) Left panels show NF-κB Luciferase reporter assay in AGS (A) and MKN28 (B) cells infected with control or AURKA adenoviruses with or without TNF-α treatment (50 ng/ml). Right panels show NF-κB Luciferase reporter assay in AGS (A) and MKN28 (B) cells treated with either Bay κ11-7085 (10 μmol/L) or MLN8237 (500 nmol/L). The % relative luciferase units (RLU) results indicate that AURKA can enhance NF-κB luciferase reporter activity. C) Western blot analysis of the indicated proteins in AGS cells infected with control or AURKA adenoviruses and treated with vehicle or MLN8237 (5 μmol/L). D) Western blot analysis of the indicated proteins in AGS cells after knocking down AURKA with siRNA. E) Western blotting for the indicated proteins in MKN28 cells treated with the selected doses of MLN8237 or Bay 11-7085 (10 μmol/L). The results indicate that NF-κB activation by AURKA is mediated by IκBα.
AURKA directly interacts and phosphorylates IκBα
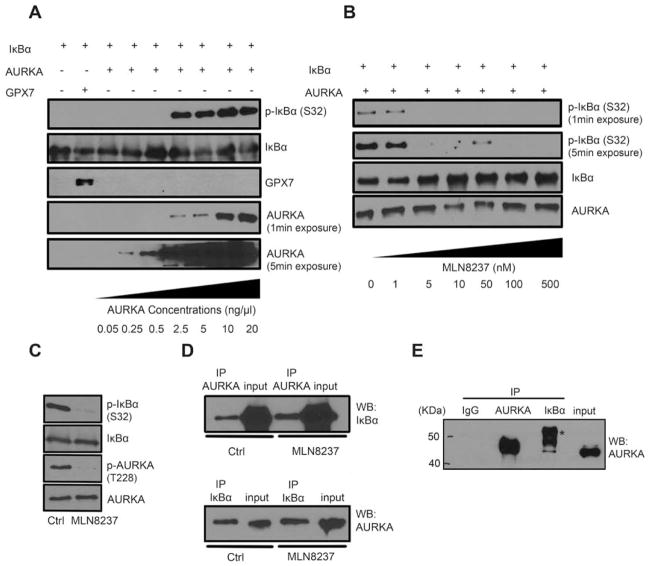
Based on our aforementioned results we decided to investigate whether AURKA directly binds and phosphorylates IκBα subunit or not. The results of the in vitro kinase assay, using purified human recombinant AURKA and IκBα proteins, confirmed that AURKA can directly phosphorylate IκBα (Ser32) at a concentration as low as 2.5 ng/μl (Figure 4A). Notably, this effect was not seen using glutathione peroxidase 7 (GPX7) recombinant protein (negative control). To confirm the ability of MLN8237 to interfere with IκBα phosphorylation, we performed an additional in vitro kinase assay with increasing concentrations of MLN8237 (1 to 500 nmol/L). The results indicated that MLN8237 can effectively block IκBα phosphorylation by AURKA starting at a concentration of 5 nmol/L (Figure 4B). To investigate if this phosphorylation was due to direct interaction and activity of AURKA, we re-performed the in vitro kinase assay with or without MLN8237 and evaluated phosphorylation of AURKA and IκBα by Western blot analysis (Figure 4C). In parallel, we pulled down the in vitro kinase assay products using antibodies against AURKA or IκBα. Western blot analysis indicated that AURKA directly binds IκBα (Figure 4D). Interestingly, although MLN8237 was able to block IκBα phosphorylation (Figure 4C), it did not affect the binding in our in vitro kinase assay (Figure 4D), suggesting that AURKA and IκBα interaction is independent of AURKA activity. To further confirm the AURKA-IκBα interaction, we pulled-down the endogenous AURKA and IκBα proteins in MKN28 cells. Protein analysis by Western Blot indicated the existence of AURKA and IκBα in the same complex (Figure 4E). Together, these data demonstrate that AURKA can directly interact and phosphorylate IκBα, revealing a novel mechanism for activation of the NF-κB pathway in gastric cancers that overexpress AURKA.
Figure 4. AURKA directly binds and phosphorylates IκBα in vitro.
A) The in vitro kinase assay with IκBα and increasing concentrations of AURKA recombinant proteins was performed. Assay products were analyzed by Western blotting for p-IκBα (S32), IκBα, and AURKA proteins. GPX7 protein was used as a negative control. B) The in vitro kinase assay for AURKA, IκBα, and increasing concentrations of MLN8237 was carried out, and the proteins were subjected to Western blot analysis. C) In vitro kinase assay for AURKA and IκBα with or without MLN8237 (500 nmol/L) was performed and followed by Western blotting for p-AURKA (T288), AURKA, p-IκBα (S32), and IκBα. D) Following the in vitro kinase assay (panel C), AURKA or IκBα pull-down products were subjected to Western blot analysis. The data indicate that AURKA directly binds and phosphorylates IκBα protein. E) Endogenous AURKA or IκBα pull-down proteins were subjected to Western blot analysis of AURKA in MKN28 cells. *, IgG heavy chain.
Inhibition of AURKA with MLN8237 reduces survival of gastric cancer cells
We next examined the biological impact of inhibiting AURKA with MLN8237 in gastric cancer cells. The long-term clonogenic survival assay (10 days) demonstrated a significant reduction in the number of viable cell colonies in both MKN28 and AGS cells following a single overnight treatment with increasing concentrations of MLN8237 (Supp. Figures 2A–D). This inhibitory effect was significant at doses starting at 250 nmol/L of MLN8237 (p<0.001). NF-κB regulates several key pro-survival molecules including XIAP in cancer cells. 7 Accordingly, Western blot and real-time PCR data showed that treatment with MLN8237 (500 nmol/L) for 48h reduced the XIAP protein (Supp. Figures 2B–E) and mRNA (Supp. Figures 2C–F) levels in MKN28 and AGS cells. We also examined the levels of cleaved caspase-3, a marker of apoptosis, in MKN28 and AGS cells in response to MLN8237. Our Western blot data showed that caspase-3 is cleaved in both cell lines (Supp. Figures 2B–E), indicative of activation of apoptotic cascade. These results clearly indicate that AURKA inhibition reduces survival of gastric cancer cells.
Pharmacological inhibition of AURKA induces antitumor activity in Tff1-knockout mouse and xenograft gastric tumor models
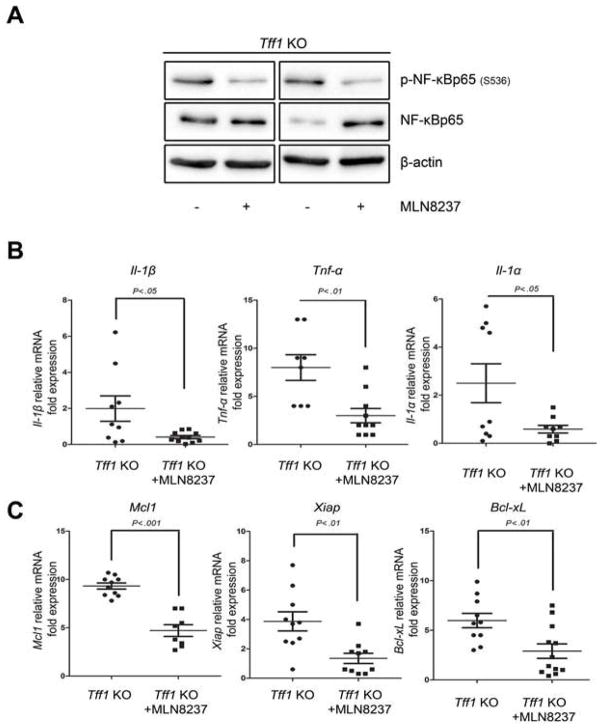
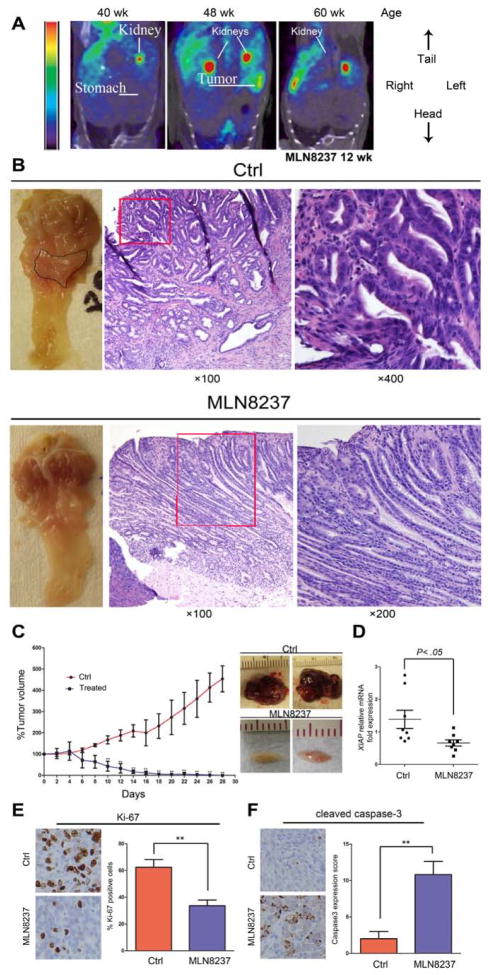
Because AURKA was overexpressed in gastric tissues of the Tff1-knockout mouse model (Figure 1A), we tested if AURKA inhibition could affect tumor growth and development in vivo. First, we treated tumor-bearing 12-month-old Tff1-knockout mice with MLN8237 daily for 8 days, and examined the effects of AURKA inhibition on the regulation of NF-κB signaling. Western Blot data showed that p-NF-kBp65 (S536) levels were down regulated in response to MLN8237 (Figure 5A). Additionally, qRT-PCR data indicated that pro-inflammatory (Tnf-α, Il-1α and Il-1β) and pro-survival (Bcl-xL, Mcl1 and Xiap) NF-κB downstream target genes were all significantly down-regulated in gastric tissues in response to MLN8237 (Figure 5B&C). Together, these data demonstrate direct in vivo validation of the therapeutic role of MLN8237 in gastric tumors that overexpress AURKA with constitutive activation of NF-κB. Next, we treated tumor-bearing 12-month-old Tff1-knockout mice with MLN8237 daily for 3 months, and examined gastric tumorigenesis. The [18F]FLT microPET imaging data showed that the gastric tumors were undetectable in response to MLN8237 (Figure 6A). This was validated upon mice euthanasia and visual examination where gastric tumors were either substantially reduced in size or completely disappeared in the MLN8237-treated animals (Figures 6B). The IHC data showed a significant decrease in Ki-67 expression, indicative of less proliferation activity, in 8-day or 12-week MLN8237-treated groups as compared to control animals (Supp. Figure 4A–B). Histology analysis indicated a significant decrease (p<0.01) in the percentage of high-grade dysplasia (HGD) and adenocarcinoma (AC) in the MLN8237- treated group versus control animals (Supp. Table 2). To further confirm that AURKA inhibition affects tumor growth, we employed a xenograft tumor model using AGS cells. Indeed, treatment with MLN8237 significantly reduced tumor volume (p<0.01) (Figure 6C), accompanied with a marked decrease in mRNA levels of the pro-survival NF-κB target gene XIAP (Figure 6D). In addition, immunohistochemistry analysis showed significant reduction in expression of the proliferation marker, Ki-67, (Figure 6E), and increased levels of cleaved caspase-3 (Figure 6F) in response to MLN8237 as compared to non-treated animals.
Figure 5. AURKA inhibition induces anti-inflammatory and anti-survival signaling in vivo.
A) Proteins extracted from 8-day MLN8237-treated Tff1-knockout mice or control animals were analyzed by Western Blot for p-NF-κBp65 (S536) and NF-κBp65 proteins. B) Real-time RT-PCR analysis demonstrated the expression levels of pro-inflammatory and pro-survival NF-κB target genes in the Tff1-knockout animals in control or in 8 days MLN8237 treated animals. AURKA inhibition significantly attenuated mRNA expression of these genes.
Figure 6. MLN8237 treatment reduces tumor growth in vivo.
A) A representative microPET coronal view of a mouse injected with [18F]FLT and imaged before and after the MLN8237 treatment. The tumors are invisible after 12 weeks post treatment. Because of the high uptake, kidneys showed typical [18F]FLT staining. B) Representative images of the antrum and H&E staining of gastric mucosa of Tff1-knockout mice. While tumors were visibly large in control (upper left panel), they became undetectable or small after 3 months of treatment (lower left panel). H&E staining indicated adenocarcinoma in control animals (upper middle and right panels) whereas MLN8237-treated animals exhibited low grade dysplasia (lower middle and right panels). C) AGS tumor xenografts were treated with MLN8237 (30 mg/kg) for 28 days. Tumor volume was dramatically reduced in the treated group as illustrated in the graph (p<0.01) (left panel). After collection of tumors at the end of experiment, representative tumors from the control or MLN8237 treated groups are shown (right panel). D) Real-time RT-PCR analysis of XIAP in xenograft tumors in control or ML8237- treated animals. E) Representative images of immunohistochemical analysis of Ki-67 protein expression in MLN8237-treated or control xenografts. F) Representative images of Immunohistochemical analysis of cleaved caspase-3 protein expression in MLN8237-treated or control xenografts.
Aberrant overexpression of AURKA in the multi-step progression of human gastric cancer
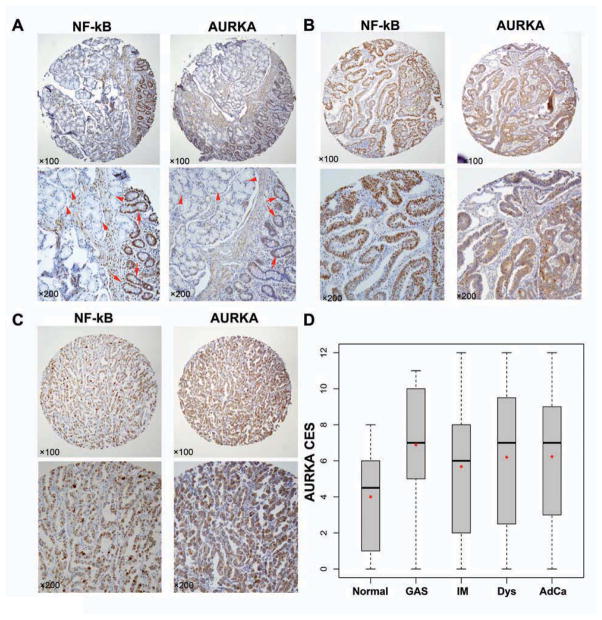
To evaluate the role of AURKA and NF-κB in human gastric tumorigenesis, we examined their expression levels in human gastric tissues at different stages of tumor development. The IHC data showed weak staining of AURKA and NF-κB in intestinal metaplasia (Figure 7A), and strong staining in moderately (Figure 7B) or poorly differentiated (Figure 7C) adenocarcinomas. A summary of the AURKA CES versus histology index parameters is shown in Figure 7D. The linear regression model was applied to check the relationship between AURKA (Figure 7D) or NF-κB (Supp. Figure 5) CES scores and 5 histology index levels (normal, gastritis, intestinal metaplasia, dysplasia, and adenocarcinoma). The analysis showed a significant association between the histology index and AURKA (p<0.001) or NF-κB (p<0.01). Results from multivariate model, ANOVA and Kruskal-Wallis rank sum test, did not show significant associations between the AURKA CES score and tumor size, grade or stage. Using the Kruskal-Wallis chi-squared test, there was a non-significant trend with lymph node metastasis (p = 0.10) and tumor stage (p = 0.13).
Figure 7. Expression of AURKA and NF-κB is associated with human gastric cancer development and progression.
A) IHC staining for AURKA and NF-κB on consecutive replicates of the same tissue sample. Normal gastric glands with negative AURKA and NF-κB immunostaining are indicated by arrow-heads whereas glands with intestinal metaplasia showing positive AURKA and NF-κB staining are indicated with arrows. B) IHC staining for AURKA and NF-κB on consecutive replicates of moderately differentiated gastric adenocarcinoma showing strong immunostaining for AURKA and NF-κB. C) The same as in panel B showing a case of poorly differentiated gastric adenocarcinoma. D) The graph summarizes the AURKA immunohistochemical staining results on gastric tissue microarrays. Horizontal bars indicate the median whereas red dots depict the mean. Normal, normal gastric glands; GAS, gastritis; IM, intestinal metaplasia; Dys, dysplasia; AdCa, adenocarcinoma. AURKA was significantly overexpressed in all stages of gastric tumorigenesis (p<0.001).
Discussion
Chronic inflammation and activation of NF-κB pathway are critical steps in gastric tumorigenesis in human and mouse models. 4–6 Our understanding of the role of overexpression of AURKA in inflammation and gastric cancer is limited in absence of a comprehensive evaluation of the histological progressive cascade of gastric tumorigenesis. Accordingly, we have performed a systematic study to define the histological, molecular and signaling components that could establish a link between AURKA overexpression and inflammation using in vitro and in vivo gastric cancer models.
Using the Tff1-knockout mouse model of gastric neoplasia, which exhibits constitutive activation of NF-κB in the gastric mucosa6, we observed aberrant overexpression of AURKA in gastric mucosa as early as eight weeks of age. The increased expression of AURKA was significantly associated with higher chronic inflammatory scores. The link to inflammation was further strengthened by demonstrating a direct correlation between increased expression of AURKA and TNF-α. Because of the constitutive activation of NF-κB in the gastric mucosa of this mouse model6; we examined the molecular impact of AURKA inhibition on NF-κB signaling. We showed that pharmacological inhibition of AURKA with the small molecule inhibitor (MLN8237) attenuated the nuclear localization of NF-κB in ex vivo experiments. These findings are the first to demonstrate a direct association between AURKA, chronic inflammation, and activation of NF-κB in gastric mucosa in vivo.
To gain insight into how AURKA regulates NF-κB, we employed in vitro gastric cancer cell models to identify the molecular components that link AURKA to NF-κB and inflammation. The overexpression of AURKA significantly increased phosphorylation and activation of NF-κB, an effect that was reversed upon inhibition of AURKA. Therefore, these data indicated that the in vitro models could recapitulate the in vivo mouse model. In the absence of cellular stimuli, NF-κB transcription factors are bound to inhibitory IκB proteins and are thereby sequestered in the cytoplasm. Two highly similar protein kinases, IKKα and IKKβ, can phosphorylate IκB, which is considered a convergence step for most signal transduction pathways that lead to activation of NF-κB. 8,29 Several reports showed that AKT phosphorylates the IKKα/β proteins30,31 and more significantly the IKKα kinase than IKKβ 32–34, leading to phosphorylation and degradation of IκB. Although we have previously reported that AURKA can regulate the PI3K/AKT in gastrointestinal cancers20, we could not detect significant changes in the phosphorylation of IKKα/β in response to inhibition or knockdown of AURKA. While an earlier study suggested that AURKA down-regulates IκBα indirectly through activation of the PI3K/AKT pathway35, our data demonstrate, for the first time, that AURKA directly binds and phosphorylates the IκBα subunit, leading to activation of NF-κB. Interestingly, inhibition of AURKA completely abrogated TNF-α-induced activation of NF-κB independent of IKKα/β. Our data strongly suggest that in addition to the direct regulation of IκBα, AURKA is a positive regulator of NF-κBp65 protein expression. Even though Inhibition of AURKA blocked phosphorylation of IκBα, it had no effect on the protein interaction between AURKA and IκBα. Hence, our data suggest that AURKA-induced activation of the NF-κB pathway is dependent on IκBα phosphorylation by AURKA. Phosphorylation of IκB proteins leads to their subsequent recognition by ubiquitinating enzymes and degradation. The proteasomal degradation of IκB proteins releases IκB-bound NF-κB, which translocates to the nucleus to drive expression of target genes. 8
We investigated the anti-tumor activity of AURKA in two in vivo models. AURKA inhibition significantly reduced tumor growth and cell proliferation, and increased apoptosis in a xenograft mouse model. Similarly, treatment for three months with MLN8237 led to complete disappearance or reduction in gastric tumors in Tff1-knockout mice. While AURKA inhibition is known to reduce cancer growth in in vitro and in vivo experimental models 16, 18, 36, our findings suggest that inhibition of AURKA can attenuate the tumorigenic progression through regulation of NF-κB; a finding that could have clinical implications in tumors with AURKA overexpression and constitutive activation of NF-κB. Several studies have shown that NF-κB plays a major role not only in tumor development and progression but also in mediating acquired resistance to chemotherapeutic agents.10,37 Conversely, other reports suggested that NF-κB has a tumor suppressor function as it is required for p53-dependent apoptosis.38 However, upon loss of the tumor suppressor genes function, NF-κB becomes a tumor promoter.39 While inhibition of NF-κB has been considered as an effective approach in circumventing therapeutic drug resistance 38, clinical trials have shown disappointing results possibly due to the involvement of NF-κB in regulating several fundamental physiological functions of normal cells such as immune response. 37, 40 Therefore, there is still a critical need to have selective inhibition of NF-κB that can target cancer cell-specific oncogenic functions with minimal toxic side effects on other normal physiological functions. In this regards, AURKA inhibition could offer a novel approach that is not limited to suppress AURKA oncogenic functions, but also interfere with NF-κB-dependent gastric tumorigenesis and cancer cell survival. Indeed, a one week inhibition of AURKA using MLN8237 in the Tff1-knockout mice exhibiting overexpression of AURKA and activation of NF-κB, down-regulated several NF-κB downstream pro-inflammatory and pro-survival genes.
The aforementioned data suggest a key role for AURKA in the development of gastric tumors in the Tff1-knockout mouse model, which might be applicable to human gastric cancer. Hence, we examined the protein expression pattern of AURKA in human gastric tissue samples. Indeed, our findings demonstrated a significant increase in AURKA expression level in histological stages as early as gastritis, a finding that persisted across intestinal metaplasia, dysplasia and adenocarcinomas. This suggests that AURKA overexpression is important in the early and advanced stages of tumor development, a finding that could have important clinical implications and better understanding of the biology of gastric cancer.
In summary, we have established an important link between AURKA and chronic inflammation, a key step in gastric tumorigenesis. The aberrant overexpression of AURKA could promote gastric tumor development by regulating NF-κB activity through direct binding and phosphorylation of IκBα. This finding highlights the importance of AURKA in gastric tumorigenesis and argues for testing the therapeutic potential of MLN8237 in clinical trials that include gastric cancer.
Supplementary Material
Acknowledgments
Grant Support: This study was supported by grants from the National Cancer Institute (R01CA131225), Vanderbilt SPORE in Gastrointestinal Cancer (P50 CA95103), Vanderbilt Ingram Cancer Center (P30 CA68485), the Vanderbilt Digestive Disease Research Center (DK058404), and the Department of Veterans Affairs. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, Department of Veterans Affairs, or Vanderbilt University.
Footnotes
Disclosure of Potential Conflicts of Interest: All authors indicated “no conflict of interest”.
Authors’ contribution:
Ahmed Katsha: Design of in vitro experiments and acquisition of data; analysis and interpretation of data; drafting of the manuscript; technical and material support
Mohammed Soutto: Design of in vivo experiments and acquisition of data; analysis and interpretation of data
Vikas Sehdev: assisted in in vitro experiments and interpretation of data
Dunfa Peng: Analysis of immunohistochemical data
M. Kay Washington: histopathology analysis of mouse and human tissues
M. Blanca Piazuelo: histopathology analysis of mouse and human tissues
Mohammed N Tantawy: performed in vivo imaging experiments
H. Charles Manning: interpretation and analysis of in vivo imaging data
Pengcheng Lu: statistical analysis
Yu Shyr: interpretation of statistical data
Jeffrey Ecsedy: consultation in experimental design using MLN8237 and assisted in manuscript draft
Abbes Belkhiri: analysis and interpretation of data; experimental troubleshooting; drafting of the manuscript; critical revision of the manuscript
Wael El-Rifai: study concept and design; obtained funding; study supervision; experimental troubleshooting; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 (Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Roder DM. The epidemiology of gastric cancer. Gastric Cancer. 2002;5 (Suppl 1):5–11. doi: 10.1007/s10120-002-0203-6. [DOI] [PubMed] [Google Scholar]
- 3.Becker JC, Domschke W, Pohle T. Medicinal prevention of gastrointestinal tumors: aspirin, Helicobacter and more? Internist (Berl) 2006;47:1229–30. 1232–4, 1236–8. doi: 10.1007/s00108-006-1731-7. [DOI] [PubMed] [Google Scholar]
- 4.Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008;99:836–42. doi: 10.1111/j.1349-7006.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–9. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soutto M, Belkhiri A, Piazuelo MB, Schneider BG, Peng D, Jiang A, Washington MK, Kokoye Y, Crowe SE, Zaika A, Correa P, Peek RM, Jr, El-Rifai W. Loss of TFF1 is associated with activation of NF-kappaB-mediated inflammation and gastric neoplasia in mice and humans. J Clin Invest. 2011;121:1753–67. doi: 10.1172/JCI43922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 9.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 10.Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–72. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 11.Galban S, Duckett CS. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ. 2010;17:54–60. doi: 10.1038/cdd.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grad JM, Zeng XR, Boise LH. Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr Opin Oncol. 2000;12:543–9. doi: 10.1097/00001622-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy. J Clin Invest. 2005;115:2625–32. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dar AA, Goff LW, Majid S, Berlin J, El-Rifai W. Aurora kinase inhibitors--rising stars in cancer therapeutics? Mol Cancer Ther. 2010;9:268–78. doi: 10.1158/1535-7163.MCT-09-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825–41. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 16.Dar AA, Belkhiri A, Ecsedy J, Zaika A, El-Rifai W. Aurora kinase A inhibition leads to p73-dependent apoptosis in p53-deficient cancer cells. Cancer Res. 2008;68:8998–9004. doi: 10.1158/0008-5472.CAN-08-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 18.Sehdev V, Peng D, Soutto M, Washington MK, Revetta F, Ecsedy J, Zaika A, Rau TT, Schneider-Stock R, Belkhiri A, El-Rifai W. The aurora kinase A inhibitor MLN8237 enhances cisplatin-induced cell death in esophageal adenocarcinoma cells. Mol Cancer Ther. 2012;11:763–74. doi: 10.1158/1535-7163.MCT-11-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.den Hollander J, Rimpi S, Doherty JR, Rudelius M, Buck A, Hoellein A, Kremer M, Graf N, Scheerer M, Hall MA, Goga A, von Bubnoff N, Duyster J, Peschel C, Cleveland JL, Nilsson JA, Keller U. Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood. 2010;116:1498–505. doi: 10.1182/blood-2009-11-251074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dar AA, Belkhiri A, El-Rifai W. The aurora kinase A regulates GSK-3beta in gastric cancer cells. Oncogene. 2009;28:866–75. doi: 10.1038/onc.2008.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katayama H, Sasai K, Kawai H, Yuan ZM, Bondaruk J, Suzuki F, Fujii S, Arlinghaus RB, Czerniak BA, Sen S. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, He L, Kruk P, Nicosia SV, Cheng JQ. Aurora-A induces cell survival and chemoresistance by activation of Akt through a p53-dependent manner in ovarian cancer cells. Int J Cancer. 2006;119:2304–12. doi: 10.1002/ijc.22154. [DOI] [PubMed] [Google Scholar]
- 23.Sumi K, Tago K, Kasahara T, Funakoshi-Tago M. Aurora kinase A critically contributes to the resistance to anti-cancer drug cisplatin in JAK2 V617F mutant-induced transformed cells. FEBS Lett. 2011;585:1884–90. doi: 10.1016/j.febslet.2011.04.068. [DOI] [PubMed] [Google Scholar]
- 24.Otto T, Horn S, Brockmann M, Eilers U, Schuttrumpf L, Popov N, Kenney AM, Schulte JH, Beijersbergen R, Christiansen H, Berwanger B, Eilers M. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. 2009;15:67–78. doi: 10.1016/j.ccr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Gorgun G, Calabrese E, Hideshima T, Ecsedy J, Perrone G, Mani M, Ikeda H, Bianchi G, Hu Y, Cirstea D, Santo L, Tai YT, Nahar S, Zheng M, Bandi M, Carrasco RD, Raje N, Munshi N, Richardson P, Anderson KC. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010;115:5202–13. doi: 10.1182/blood-2009-12-259523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly KR, Ecsedy J, Medina E, Mahalingam D, Padmanabhan S, Nawrocki ST, Giles FJ, Carew JS. The novel Aurora A kinase inhibitor MLN8237 is active in resistant chronic myeloid leukaemia and significantly increases the efficacy of nilotinib. J Cell Mol Med. 2011;15:2057–70. doi: 10.1111/j.1582-4934.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manfredi MG, Ecsedy JA, Chakravarty A, Silverman L, Zhang M, Hoar KM, Stroud SG, Chen W, Shinde V, Huck JJ, Wysong DR, Janowick DA, Hyer ML, Leroy PJ, Gershman RE, Silva MD, Germanos MS, Bolen JB, Claiborne CF, Sells TB. Characterization of Alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin Cancer Res. 2011;17:7614–24. doi: 10.1158/1078-0432.CCR-11-1536. [DOI] [PubMed] [Google Scholar]
- 28.Lefebvre O, Chenard MP, Masson R, Linares J, Dierich A, LeMeur M, Wendling C, Tomasetto C, Chambon P, Rio MC. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996;274:259–62. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- 29.Wagner M, Seitz U, Buck A, Neumaier B, Schultheiss S, Bangerter M, Bommer M, Leithauser F, Wawra E, Munzert G, Reske SN. 3′-[18F]fluoro-3′-deoxythymidine ([18F]-FLT) as positron emission tomography tracer for imaging proliferation in a murine B-Cell lymphoma model and in the human disease. Cancer Res. 2003;63:2681–7. [PubMed] [Google Scholar]
- 30.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–40. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 31.Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin AS, Jr, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20:1626–38. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 33.Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem. 2008;283:25900–12. doi: 10.1074/jbc.M707692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao JE, Yan M, Guan Z, Pan CB, Xia LP, Li CX, Wang LH, Long ZJ, Zhao Y, Li MW, Zheng FM, Xu J, Lin DJ, Liu Q. Aurora-A down-regulates IkappaBalpha via Akt activation and interacts with insulin-like growth factor-1 induced phosphatidylinositol 3-kinase pathway for cancer cell survival. Mol Cancer. 2009;8:95. doi: 10.1186/1476-4598-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sehdev V, Katsha A, Ecsedy J, Zaika A, Belkhiri A, El-Rifai W. The combination of alisertib, an investigational Aurora kinase A inhibitor, and docetaxel promotes cell death and reduces tumor growth in preclinical cell models of upper gastrointestinal adenocarcinomas. Cancer. 2012 doi: 10.1002/cncr.27801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan KM, Ernst MK, Rice NR, Vousden KH. Role of NF-kappaB in p53-mediated programmed cell death. Nature. 2000;404:892–7. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- 39.Perkins ND. NF-kappaB: tumor promoter or suppressor? Trends Cell Biol. 2004;14:64–9. doi: 10.1016/j.tcb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Rocha S, Campbell KJ, Perkins ND. p53- and Mdm2-independent repression of NF-kappa B transactivation by the ARF tumor suppressor. Mol Cell. 2003;12:15–25. doi: 10.1016/s1097-2765(03)00223-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.