Abstract
This was a naturalistic study of 23 abstinent cocaine-dependent patients and 38 controls who were studied using a paired-stimulus paradigm to elicit three mid-latency auditory evoked responses (MLAERs), namely, the P50, N100, and P200. Sensory gating was defined as the ratio of the S2 amplitude to the S1 amplitude. Psychosis-proneness was assessed using four Chapman psychosisproneness scales measuring perceptual aberration, magical ideation, social anhedonia, and physical anhedonia. Omnibus correlations based upon the entire sample revealed significant and differential relationships between the MLAER components and psychosis-proneness. Social Anhedonia scale scores accounted for the largest proportion of variance in the P50 gating ratio, while Perceptual Aberration scores accounted for the largest proportion of variance in P200 gating. Psychosis proneness and sensory gating appear to be associated. In particular, poorer P50 gating is related to higher scores on the Social Anhedonia scale in healthy controls and across mixed samples of cocaine dependent patients and controls. These findings hold significance for the further understanding of the relationship between deficient sensory gating ability and the propensity to developing psychotic symptoms in a vulnerable population like cocaine-dependent individuals.
1. INTRODUCTION
Sensory gating, the ability of the central nervous system to inhibit or attenuate incoming redundant stimuli, is proposed to be a major mechanism for the protection of the subject’s internal environment as well as their higher cortical centers from being flooded with irrelevant information (Venables, 1964). One of the most commonly studied measures of sensory gating is the attenuation of the amplitude of the P50 with stimulus repetition. The P50 is an early positive component of the mid-latency auditory evoked responses (MLAERs) occurring between 45 and 90 ms post-stimulus onset. While the P50 component of the MLAERS reflects the preattentive stage of information processing, early and later attentive phases of information processing are reflected by the N100 and P200 components, respectively. A growing literature reveals that the well-documented P50 sensory gating deficit of schizophrenia patients extents to these later phases of information processing (Smith et al., 2010; Turetsky et al., 2009). Schizophrenia patients display significantly decreased N100 gating (Boutros et al., 1999; Boutros et al., 2009; Boutros et al., 2004b; Clementz and Blumenfeld, 2001; Clementz et al., 2003) and P200 gating (Boutros et al., 2009; Boutros et al., 2004b) relative to healthy controls. Although relatively little is known regarding the clinical correlates of the sensory gating deficits in schizophrenia-spectrum patients, one hypothesis is that the sensory gating deficits may be related to psychosis-proneness. Schizotypal personality disordered patients display similar P40 deficits as schizophrenia patients (Cadenhead et al., 2000; Croft et al., 2001).
1.1 Sensory Gating in Non-Clinical Populations
To date, a number of studies have examined the relationship between schizotypy and sensory gating in nonclinical populations. However, the methodological shortcomings of much of this work limit the conclusions that can be drawn. Using a convenience sample of volunteers at a medical school, Croft et al. (Croft et al., 2001) observed that participants with higher schizotypal characteristics namely, perceptual experiences and magical ideation, exhibited poorer P50 suppression. When smoking status was assessed, Croft et al. (Croft et al., 2004) found a difference in patterns of associations between lighter and heavier smokers, whereby lighter smokers showed a positive association between positive schizotypy and poor P50 suppression. One criticism of both of these studies, however, is that schizotypy was assessed using the Personality Syndrome Questionnaire (Gruzelier et al., 1995), a questionnaire for which there are no published psychometric data available (Evans et al., 2007). In contrast to the Croft et al. (Croft et al., 2004; Croft et al., 2001) findings, Wang et al. (Wang et al., 2004) observed that negative schizotypy (i.e., the “withdrawn” dimension as measured by Raine’s (Raine, 1991) Schizotypal Personality Questionnaire) was associated with poor P50 suppression. The smoking status of the nonclinical volunteers in the Wang et al. (Wang et al., 2004) study was not provided. Furthermore, in the Wang et al. (Wang et al., 2004) study, the investigators used a shorter (e.g., 250 msec) than the usual 500 msec interstimulus interval; it is unclear whether this may have affected the findings. A later study conducted by Wan et al. (Wan et al., 2008) also used Raine’s Schizotypal Personality Questionnaire to assess schizotypy, and improved upon prior studies by allowing a comparison of smokers vs. nonsmokers in both high and low schizotypal groups. Unfortunately, the investigators failed to exclude participants on the basis of their family history, so that both the high and low schizotypal groups included participants who had first degree relatives with schizophrenia. This presented a major confound, given that P50 nonsuppression is also observed among first degree relatives of schizophrenia patients (Siegel et al., 1984; Waldo et al., 1988), rendering it difficult to interpret the study findings. It should also be noted that all of the aforementioned studies had rather modest sample sizes of generally young adults. Evans et al. (Evans et al., 2007) assessed the relationship between schizotypy, P50 and N100 suppression in a relatively large sample of healthy non-smoking undergraduates. Using the Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE; (Mason et al., 1995)), a psychometrically sound multidimensional measure of schizotypy, the investigators found a significant negative relationship between P50 suppression and cognitive disorganization. Their analyses did not reveal any significant relationships between N100 suppression and schizotypy. Overall, in healthy nonclinical populations, there appears to be a relationship between reduced P50 suppression and psychometric indicators of schizotypy, though the precise nature of the relationship is far from clear. The converging evidence suggests that reduced P50 suppression is related to schizotypy, broadly defined.
1.2 Cocaine-induced psychosis
Cocaine-induced psychosis is rare compared to the frequency of cannabis- and amphetamine-induced psychoses (Thirthalli and Benegal, 2006). Schuckit (Schuckit, 2006) reported that although stimulant-induced psychoses typically clear within 30 days of abstinence from the psychoactive substance, some psychotic symptoms may persist beyond 4–6 weeks of abstinence in up to 15% of patients with stimulant-induced psychoses. Currently, it is unclear whether the persisting symptoms in this subgroup of patients reflect a precipitation of longer-term psychosis in predisposed individuals. Alternatively, the heavy use of stimulants might cause neurochemical changes associated with long-term psychotic disorders in individuals even if they were no predisposed to psychosis (Philgren and Boutros, 2007). Prior work indicates that cocaine-dependent patients display abnormal P50 suppression relative to healthy controls (Boutros et al., 2000; Fein et al., 1996). Additionally, preliminary work from our lab (Boutros et al., 2002) suggests that in cocaine-dependent individuals, none of whom had first-degree relatives with psychotic disorders, deficient P50 sensory gating may be associated with psychosis-proneness.
Psychosis proneness is a hypothesized construct indicating elevated risk for any form of psychosis. Theoretically, individuals with elevated psychosis-proneness may later manifest a number of psychotic disorders, including schizophrenia, schizoaffective disorder, bipolar disorder, delusional disorder, and drug-induced psychoses. Psychosis proneness has been extensively studied using psychometric indicators of risk such as scales developed by Loren and Jean Chapman. Longitudinal investigation reveals that the Perceptual Aberration Scale (Chapman et al., 1994) identifies a subgroup of psychosis-prone individuals, some of whom eventually manifest a psychotic disorder. These individuals had a heterogeneous outcome, with a heightened prevalence of psychotic disorders including, but not limited to schizophrenia (i.e., some individuals developed nonschizophrenia-related psychotic disorders such as delusional disorders and psychotic mood disorders). Analysis of the Chapmans' longitudinal study of psychosis-prone individuals (Kwapil, 1998) as well as a more recent longitudinal study utilizing an independent sample (Gooding et al., 2005) indicate that individuals with aberrantly high scores on the revised Social Anhedonia Scale are at heightened risk for the later development of schizophrenia-spectrum disorders. Thus, the Social Anhedonia Scale, unlike the Perceptual Aberration Scale, may identify individuals at specific risk for the development of schizophrenia-spectrum disorders. To date, the psychosis-proneness scales developed by Chapman and their associates remain the sole psychosis-proneness scales to be longitudinally validated to predict the later development of schizophrenia (Chapman et al., 1994; Kwapil, 1998) and/or schizophrenia-spectrum disorders (Gooding et al., 2005, 2007; Kwapil, 1998).
1.3 The Present Study
The goal of the present investigation were twofold: first, to investigate the clinical correlates of sensory gating indices derived from an early mid-latency auditory evoked potential believed to reflect pre-attentive processes (the P50) and the N100/P200 MLAER components believed to be more under the influence of attentive processes, in healthy controls and in abstinent cocaine-dependent patients. By investigating P50, N100, and P200 in healthy controls, we intended to examine factors related to the clinical correlates of sensory gating in general. Through our study of P50, N100, and P200 in abstinent cocaine-dependent patients using longitudinally validated measures of psychosis-proneness, we sought to examine possible underlying factors relating to the development of psychosis in the context of cocaine use. It is not currently known whether cocaine-dependence exposes an underlying psychosis diathesis, or whether psychotic symptoms are induced by stimulant use. At least one aspect of psychosis-proneness, namely, schizotypy, is thought to be trait-based (see, for example, (Meehl, 1962, 1989). If cocaine-dependence exposes an underlying psychosis diathesis, one might expect associations between psychosis-proneness and sensory gating, similar to the relationships observed among individuals with schizophrenia-related psychoses, all of whom possess schizotypy. Alternatively, if the psychotic symptoms are induced by stimulant use, we would not expect a relationship between measures of psychosis-proneness and sensory gating, but rather we would expect higher endorsements of psychotic-like experiences namely, perceptual aberrations and magical ideations, in patients with longer duration and/or greater severity of stimulant use. As such, given the goal to explore the relationship between psychometric indicators of schizotypy and electrophysiological markers of brain functioning in individuals with and without cocaine-induced psychosis, it was advantageous to use measures with documented predictive validity. We had two a priori hypotheses, namely, that deficient sensory gating in cocaine-dependent subjects would extend beyond the P50 stage of information processing, and secondly, that across both groups, P50 sensory gating deficits would be associated with psychometric indicators of schizotypy, namely, social anhedonia.
2. METHODS
2.1 Participants
2.1.1. Patients
The sample consisted of 61 participants (23 patients and 38 healthy controls). The patients (10 males and 13 females; a mean age of 36.52 years) met DSM-IV (APA, 1994) criteria for cocaine dependence during the past year and had been free of cocaine use for at least 3 weeks (documented by toxicology screens). Their mean length of abstinence was 78.52 days (SD = 102.1, range, 21 – 420). Diagnoses were established based on a clinical interview by a board-certified psychiatrist (NNB). None of the subjects reported current psychotic symptoms or met any other Axis-I disorders. All cocaine-dependent subjects were recruited from drug-rehabilitation or residential drug-treatment facilities where random urine screening for drugs of abuse is routinely performed. The Cocaine Experience Questionnaire (CEQ; (Satel and Edell, 1991)) was administered to rule out the presence of psychotic symptoms judged to be independent of cocaine use. When such psychotic symptoms were identified, subjects were excluded. Patients with a past history of treatment for depression or anxiety who were no longer under treatment were allowed.
Persons meeting criteria for dependence on other substances in addition to cocaine were not allowed, i.e., individuals with polysubstance dependence were excluded from the study. Typically, subjects used other drugs like alcohol or marijuana to ease or moderate the effects of cocaine, with cocaine being the drug of choice without the use of other drugs reaching the level of dependence. Data were obtained independently during the clinical interview then via the CEQ and results were compared. Use of the above strict criteria resulted in excluding 4 of every 5 subjects interviewed for the purpose of this study. All psychiatric histories were verified with the treating clinicians. All cocaine-dependent patients denied use of alcohol during the three weeks preceding recording of the evoked potentials. Toxicology screens confirmed that all subjects were free of cocaine, marijuana, opiates, PCP, and benzodiazepines at the time of the study. None were receiving psychotropic medications at the time of the study. The mean age when these patients first used cocaine was 20 (range, 11 to 42) years. The average age when they began regular use, operationally defined as weekly use for at least two months, was 23 (range, 11 to 42) years. Mean duration of use was 13 (range, less than 1 to 22) years.
2.1.2. Controls
Thirty-eight healthy control participants (19 males and 19 females; a mean age of 30.39 years) were also examined. The control group also had to have negative urine screening results for study inclusion. For both groups, exclusion criteria included any first-degree relatives with major psychiatric disorders (other than drug and alcohol abuse). At the time of testing, audition was tested clinically, and all subjects were able to hear finger rubbing and a hand watch clearly and equally on both sides. All subjects denied having any hearing problems. Any individual with a history of hearing difficulty was excluded from participation. Table 1 provides demographic characteristics of the two groups. The groups did not differ significantly in terms of their proportion of males, χ(1) = 0.24, n.s.
Table 1.
Demographic characteristics of the subject groups
|
Cocaine-Dependent (n = 23) |
Healthy Control (n = 38) |
|
|---|---|---|
| Age (years) | 36.52 ± 7.8 | 30.24 ± 9.9 |
| Gender (M/F) | 10/13 | 19/19 |
| Ethnicity | ||
| Caucasian | 2 | 25 |
| African-American | 16 | 6 |
| Latino | 2 | 3 |
| Other | 3 | 4 |
| Smoking status | ||
| Smoker | 15 | 8 |
| Nonsmoker | 8 | 30 |
2.2 Measures
2.2.1 Evoked Potential Recording
The evoked related potentials were elicited during a paired-stimulus paradigm. In the paired-stimulus paradigm, repeated presentations of two identical auditory stimuli (S1 and S2) separated by 500 msec were given, with an inter-pair interval of 8 seconds (Zouridakis and Boutros, 1992). These binaural stimuli were brief tones (1000 Hz) of 4 msec duration and one msec rise and fall times and 90 db SPL as measured at the ear using a measure-and-hold digital sound meter (Tandy Corp.). We collected 40 artifact-free pairs of responses to generate two grand averages (one for S1 responses and one for S2 responses). The durations of the periods from which the P50s were taken were therefore similar. The variables of interest were three components of a mid-latency auditory evoked response (MLAER), namely, the P50, N100, and P200 components.
Recordings were made from silver/silver chloride disk electrodes applied at the Fz, Cz, Pz, Oz, F7, F8, T3, T4, P5, and P6 locations and referenced to linked ears; a forehead ground was used. In order to be consistent with the majority of the extant gating literature, P50, N100, and P200 measurements were made from the Cz electrode1. Other midline electrodes were used to verify the evoked potential components, i.e., the evoked potential component had to be visible in more than one midline electrode site in order to be considered valid. One channel was devoted to detecting eye movement artifacts recorded from a supraorbital electrode to the outer canthus. On-line EEG artifact rejection allowed for trial rejection when activity in any channel exceeded 75 µV (Neuroscan Software, Herndon, Virginia). All electrodes had impedances below 5 kΩ. Electrical signals with a band pass of 0.05 Hz to 300 Hz were amplified 20,000 by Grass amplifiers and digitized at a sampling rate of 1000 Hz. In order to increase the signal to noise ratio, we further refiltered the EEG data between 10 and 50 Hz when examining the P50 component (Clementz et al., 1997; Jerger et al., 1992). A 1– 30 Hz band-pass was used to examine N100 and P200 components.
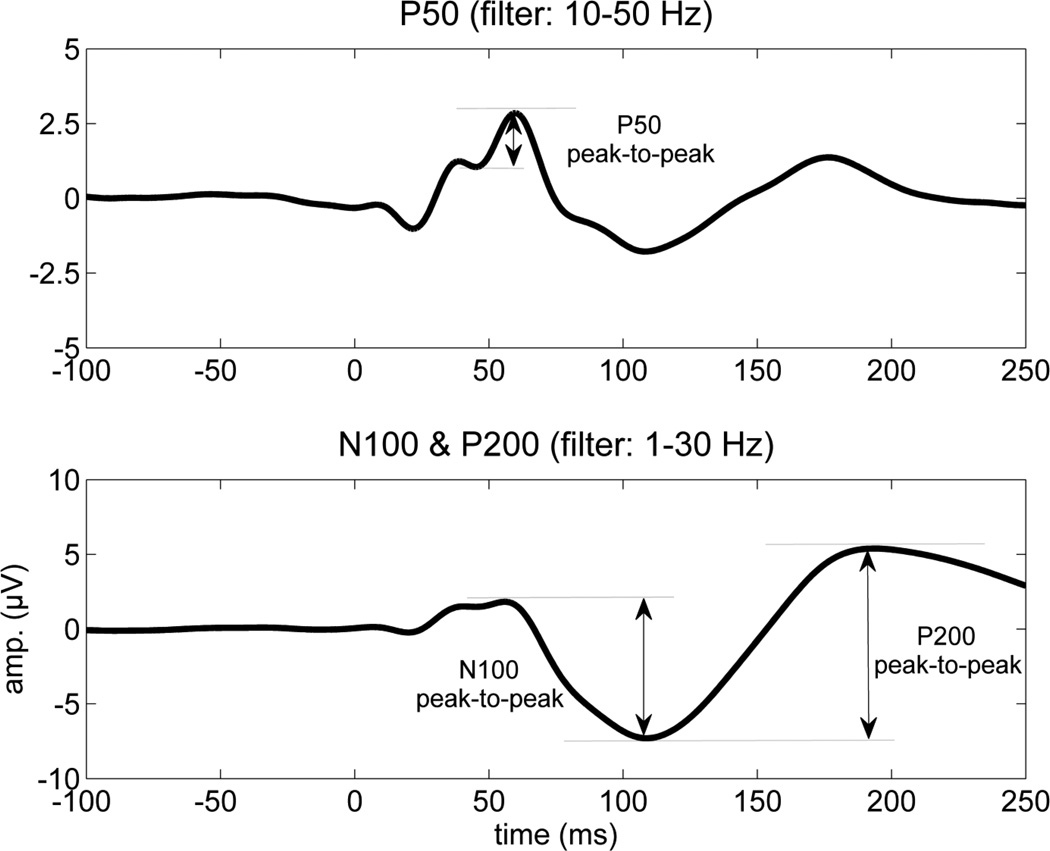
The components were identified and measured by two of the authors (SB & NB) who were naïve to the subject group. EEG epochs started 50 ms prior to stimulation and extended to 300 msec. EEG epochs were subsequently averaged together. The amplitudes of three components were measured from peak to preceding peak from the averages. Figure one shows the points from where measures were taken. The criteria used to identify the MLAER components have been described previously (Boutros et al., 2004b). Briefly, the P50 component was operationally defined as the second major positive component between 30 and 80 msec post-stimulation. The largest negative deflection between 80 and 150 msec was identified as the N100, and the largest positive deflection between 150 and 250 msec was identified as the P200. Sixty-one participants had P50 data. Data from one (healthy control) subject’s later (N100 and P200) components were removed from analysis due to the data being more than two SDs beyond the group mean.
Figure 1.
Example of the P50, N100, P200 MLAER components depicting the points from which they were measured. The top auditory evoked potential was filtered between 10 and 50 Hz to optimize scoring of the P50 component, whereas the bottom average was filtered between 1 and 30 Hz to optimize scoring of the N100 and P200.
We derived sensory gating indices for the averaged P50, N100, and P200 evoked potentials. Sensory gating was operationally defined as the percent ratio of the component potential amplitude following the second stimulus (e.g. second P50) to the component potential amplitude following the first stimulus (e.g. first P50). Smaller ratios indicate better central nervous system gating capacity (Boutros et al., 1991).
2.2.2. Cocaine Experiences Questionnaire
The 58-item Cocaine Experience questionnaire (CEQ) was administered by a trained rater. The CEQ, a semi-structured interview, is an established instrument that is frequently used for the identification of cocaine-induced paranoia. In addition to collecting data regarding basic drug use history, the CEQ is used to determine whether a subject experienced paranoid symptoms during cocaine use as well as to assess the nature of the experience. The CEQ has high concurrent validity with other interview-based measures of cocaine-induced psychotic symptoms (Boutros et al., 2002).
The CEQ was used to determine the presence or absence of cocaine-induced paranoid (i.e.. persecutory or referential) ideation. The study participants were instructed to distinguish between adaptive hypervigilance or anxiety in high-risk situations (e.g., making drug deals, engaging in illicit activities) and completely irrational beliefs. The CEQ has three subscales: paranoia (a dichotomous yes/no scale), severity (0–6 scale), and insight (0 – 3 scale). The paranoia scale assessed the nature of the experience of paranoia during cocaine use to differentiate between suspiciousness and apprehension and psychotic experiences including delusions and hallucinations. The behavioral severity subscale assessed the behavior associated with paranoid ideation and ranges from simple feelings of fear (score of 1) to attacking others in perceived need for self-defense in the absence of any real danger (score of 6). The insight subscale assessed subjects’ insight during cocaine intoxication and ranges from 0 (full insight) to 3 (total lack of insight).
2.2.3 Chapman Psychosis-Proneness Scales
We assessed psychosis-proneness and schizotypy using a 179-item true-false self-report questionnaire called the “Survey of Attitudes and Experiences”. Items from the Chapman Psychosis-proneness scales, namely, Perceptual Aberration, Magical Ideation, revised Physical Anhedonia, and revised Social Anhedonia scales as well as the Chapman Infrequency Scale, were interspersed in a single questionnaire (Chapman and Chapman, 1983; Chapman et al., 1976; Eckblad et al., 1983). Details regarding the psychometric properties of these psychosis-proneness scales can be found elsewhere (Chapman et al., 1995; Kwapil et al., 2008). Briefly, the Perceptual Aberration Scale consists of 35 items that measure aberrant bodily perceptions. The 30-item Magical Ideation Scale assesses sub-clinical delusions and beliefs in forms of causation that are not shared with the general population. The revised Social Anhedonia Scale contains 40 items designed to capture schizoid withdrawal, such as a lack of social pleasure and indifference to others. The revised Physical Anhedonia Scale includes 61 items that measure an inability to experience pleasure from typically enjoyable stimuli.
To control for acquiescent or negative response biases and/or random responding, items from the Chapman Infrequency Scale (Chapman and Chapman, 1983) were included in the survey. Individuals with scores of three or above on the Infrequency Scale were excluded from the sample2. Participants were instructed to endorse the items only if they had the experience at times when they were not using drugs. The questionnaires were scored by the first author (DCG) who was naïve to the respondents’ group status and sensory gating performance.
2.4 Procedure
After the study was explained and all questions were answered, participants signed a written informed consent form. The study was approved by the Human Investigation Committees of Yale University School of Medicine (where the data were collected) and the Institutional Review Board of the University of Wisconsin-Madison. Subjects were allowed to smoke until they arrived at the laboratory. At least 1 hour was spent in the laboratory before evoked potential recordings began.
2.5 Statistical Analyses
Independent-samples T-tests compared the two participant groups in terms of gating ratios and psychosis-proneness scale scores. When Levene’s tests for equality of variances indicated that equal variances could not be assumed, degrees of freedom were adjusted. Bonferroni correction was applied for multiple tests of the equality of means (T-tests; α = 0.007, or 0.05/7). Chi-square analyses were performed for comparisons of categorical variables, with Yates’ correction used when expected frequencies were less than five. The association between the ERP measures of sensory gating and the clinical measures (namely, the psychosis proneness scale scores and CEQ scores) was analyzed by Pearson product moment correlations. Unless we had a priori hypotheses regarding the nature of these relationships, a Bonferroni correction was applied to the significance levels for tests of the associations. To further explore relationships between the psychosis proneness scale scores and the sensory gating ratios, follow-up stepwise regression analyses were performed. All statistical analyses were conducted using SPSS for Macintosh, Version 20 (IBM, 2012).
3. RESULTS
3.1. Demographic characteristics
Demographic characteristics of the two groups can be found in Table 1. The cocaine-dependent group was significantly older than the control group, t(54) = 2.96, p < 0.01. The patient group was significantly more likely to include nonCaucasian individuals, χ(1) = 16.69, p < 0.001. Subjects were classified as smokers if they smoked at all, i.e., no distinction was made between light vs. heavy smokers, or social vs. daily smokers. The patient group was also more likely to include smokers, χ(1) = 11.90, p = 0.001.
3.2 Comparisons between cocaine-dependent patients and controls
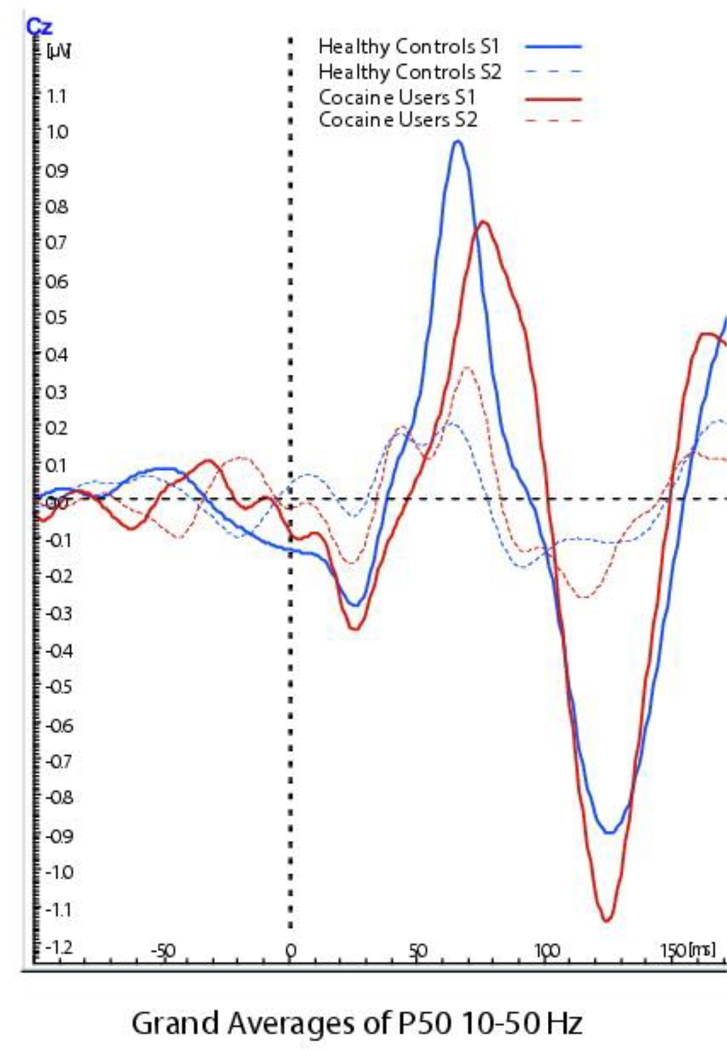
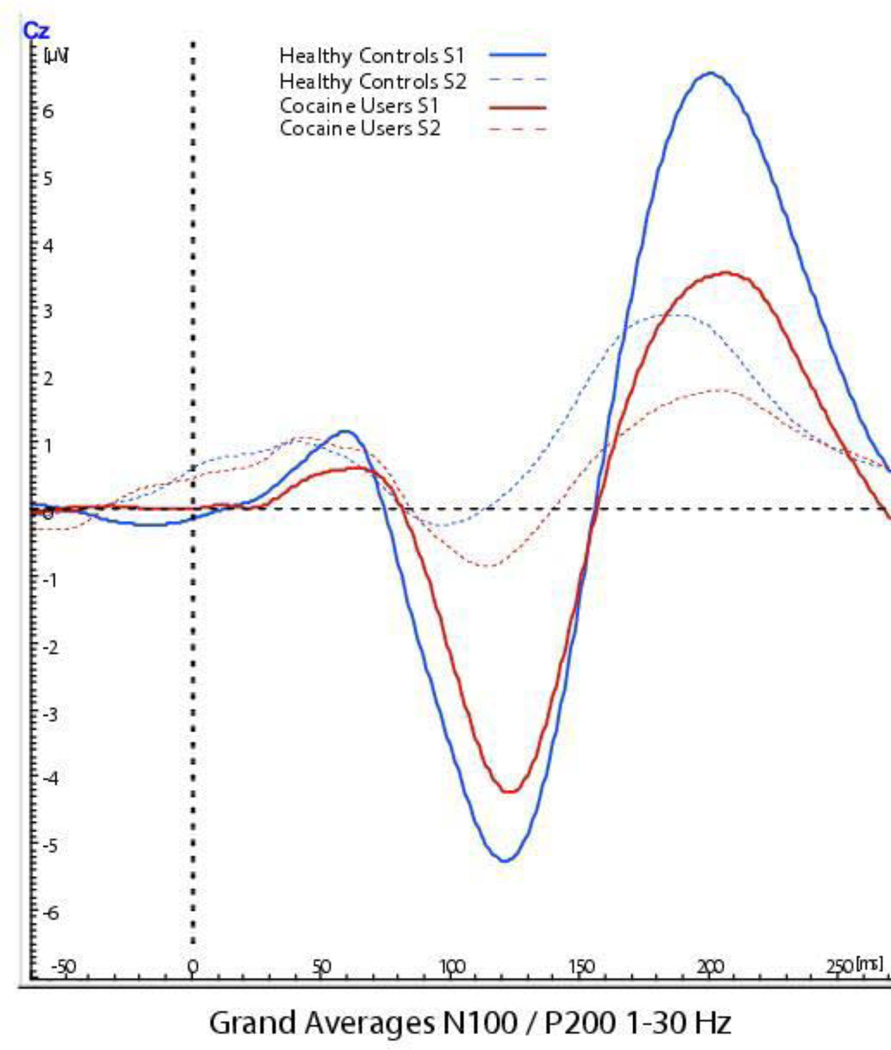
Figures 2 and 3 provide the grand averages of the MLAERS for the two groups3. We computed Pearson product moment correlations between the MLAERS separately for each group, in order to determine whether the later components might be reflecting downstream effects of P50 sensory gating, as opposed to reflecting independent processes4. In the healthy control group, P50 sensory gating was not associated with N100 sensory gating (r = 0.23, p = 0.18), though it was positively associated with P200 sensory gating (r = 0.40, p = 0.02). We observed a different pattern of associations in the cocaine-dependent patient group. In the patients, P50 ratios were not correlated with either N100 ratios (r = − 0.09, p = 0.70) or P200 ratios (r = − 0.04, p = 0.88). However, N100 and P200 gating were highly and significantly correlated in the patient group, r = 0.87, p < 0.001.
Figure 2.
Grand averages of P50 component of the ERP for the two groups. Graphed depiction of grand averages of the P50 component for the healthy controls (in blue) and cocaine users (in red). Evoked responses to the conditioning stimulus (S1) for both groups is depicted by the solid lines, whereas the evoked responses to the testing stimulus (S2) is depicted by the dashed lines.
Figure 3.
Grand averages of N100/P200 component of the ERP for the two groups. Graphed depiction of grand averages of the N100/P200 components for the healthy controls (in blue) and cocaine users (in red). Evoked responses to the conditioning stimulus (S1) for both groups is depicted by the solid lines, whereas the evoked responses to the testing stimulus (S2) is depicted by the dashed lines.
Table 2 provides the means and standard deviations for the sensory gating measures, and Chapman psychosis-proneness scale scores. Due to the performance of multiple comparisons, we used a more stringent cutoff significance value (p-value set at 0.007, or 0.05/7). As predicted, the cocaine-dependent patients displayed weaker sensory gating than the healthy control subjects. The patient group had significantly higher S2/S1 ratios for the P50 component (i.e., worse sensory gating) than the controls, t(26) = 3.14, p = 0.004. However, the gating measures for the N100 component [t(25) = 1.37, p = 0.18] and the P200 component [t(24) = 2.03, p = 0.05] were not significantly different for the two groups. The two groups differed significantly in terms of their scores on the Perceptual Aberration scale [t(26) = 3.10, p = 0.05], Magical Ideation scale [t((59) = 3.66, p = 0.001], Social Anhedonia scale [t((59) = 4.84), p < 0.001, and Physical Anhedonia scale [t((59) = 3.74, p < 0.001].
Table 2.
Evoked Potentials and Clinical Scale scores
| Cocaine-Dependent (n = 38) |
Healthy Control (n = 23) | |
|---|---|---|
| Sensory Gating Componenta | ||
| P50 | ||
| Ratio | 72.15 ± 42.3 | 43.27 ± 16.0 |
| Difference score | 0.56 ± 1.2 | 1.16 ± 1.5 |
| N100 | ||
| Ratio | 68.01 ± 47.7 | 53.97 ± 16.0 |
| Difference score | 2.01 ± 2.8 | 5.52 ± 11.9 |
| P200 | ||
| Ratio | 58.82 ± 51.1 | 36.71 ± 14.8 |
| Difference score | 5.36 ± 10.9 | 10.10 ± 13.9 |
| Chapman psychosis-proneness scales | ||
| Perceptual Aberration | 6.61 ± 6.3 | 2.34 ± 2.5 |
| Magical Ideation | 10.43 ± 5.8 | 5.39 ± 4.9 |
| Social Anhedonia | 14.74 ± 4.9 | 8.97 ± 4.3 |
| Physical Anhedonia | 13.83 ± 6.2 | 7.68 ± 6.3 |
Sensory gating ratios were calculated as S2/S1 × 102, with smaller ratios indicating better gating capacity. A higher percent is indicative of a decrease in sensory gating, i.e., there is a larger response to the second stimulus than normal.
Difference (S1amplitude – S2 amplitude) scores are an alternative way of assessing the strength of sensory gating; a higher difference score indicates stronger sensory gating.
3.3 Clinical correlates of sensory gating
Table 3 provides the intercorrelations of the various psychometric measures. As expected based on prior research, the Chapman psychosis-proneness scales were significantly associated with each other. Consistent with other samples of healthy controls as well as patient groups (e.g., (Chapman et al., 1995), the Perceptual Aberration scale and Magical Ideation scale were moderately highly correlated (r = 0.63, p < 0.01). Similarly, the two measures of anhedonia were moderately associated with each other (r = 0.47, p < 0.01). Pearson’s product moment correlations between ERP measurements and psychometric assessments are provided in Table 4 for the entire sample, the control group, and the cocaine dependent patients, respectively.
Table 3.
Intercorrelations among Psychometric Measures
| PerAb | MagId | SocAnh | PhysAnh | |
|---|---|---|---|---|
| PerAb | _____ | |||
| MagId | .63** | _____ | ||
| SocAnh | .52** | .28* | _____ | |
| PhysAnh | .48** | .50** | .47** | _____ |
Chapman Psychosis Proneness scales: Perceptual Aberration (PerAb) Scale; Magical Ideation (MagId) Scale; revised Social Anhedonia (SocAnh) scale; and revised Physical Anhedonia (PhysAnh) scale.
p < 0.05
p < 0.01
Table 4.
Correlations between ERP components and psychometric variablesa
| Entire Sample (N=61) | ||||
| PerAb | MagId | SocAnh | PhysAnh | |
| P50b | 0.22 | 0.24 | 0.35** | 0.27* |
| N100c | 0.38** | 0.18 | 0.19 | 0.14 |
| P200c | 0.51** | 0.30* | 0.22 | 0.31* |
| Control Group (N = 38) | ||||
| PerAb | MagId | SocAnh | PhysAnh | |
| P50d | −0.19 | 0.01 | 0.46** | 0.12 |
| N100e | 0.13 | 0.12 | 0.08 | 0.03 |
| P200e | 0.03 | −0.01 | 0.07 | 0.17 |
| Cocaine-Dependent Group (N=23) | ||||
| PerAb | MagId | SocAnh | PhysAnh | |
| P50 | 0.09 | 0.07 | 0.00 | 0.06 |
| N100 | 0.37 | 0.09 | 0.12 | 0.06 |
| P200 | 0.51* | 0.28 | 0.08 | 0.25 |
Psychometric scales: Chapman psychosis-proneness scales, including Perceptual Aberration (PerAb), Magical Ideation (MagId), Social Anhedonia (SocAnh), and Physical Anhedonia (PhysAnh).
Gating ratio (S2/S1) based on 61 participants.
Gating ratio (S2/S1) based on 60 participants.
Gating ratio based on 38 participants.
Gating ratio based on 37 participants.
p < 0.05
p < 0.01
P50
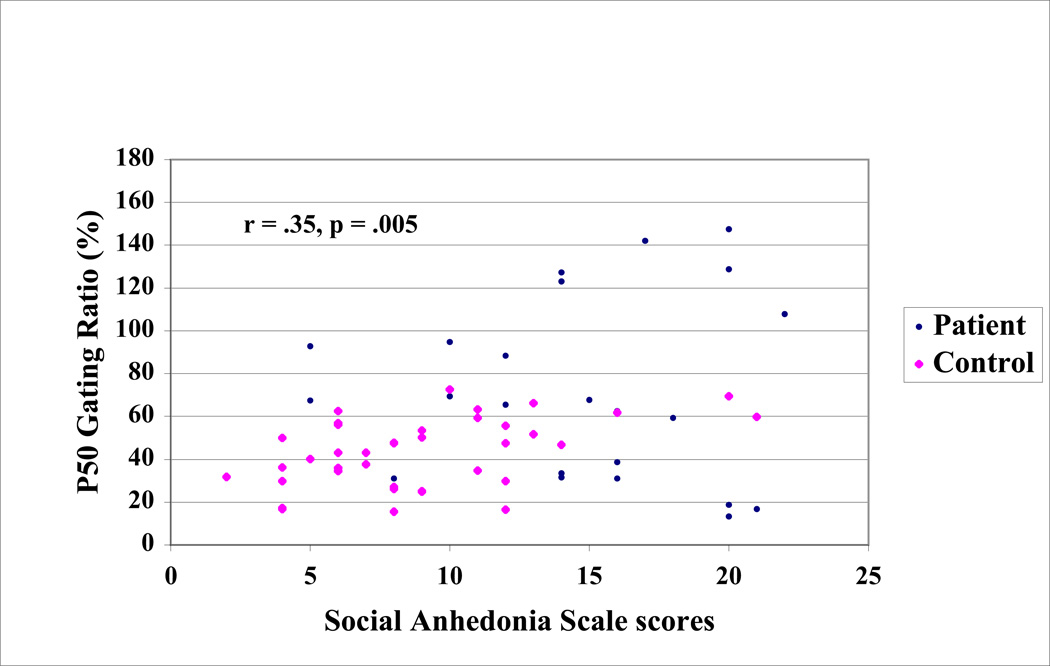
Within the control group, scores on the Social Anhedonia Scale were significantly associated with the magnitude of sensory gating, r = 0.46, p = 0.003, i.e., higher ratios reflecting weaker gating correlated with higher scores on the Social Anhedonia Scale. The association between the Social Anhedonia Scale scores and P50 ratios within the patient group did not reach statistical significance. Omnibus correlations based upon the entire sample revealed associations between the P50 gating ratios and the Chapman psychosis proneness scales. Poorer P50 sensory gating, as indexed by a higher P50 S2/S1 ratio, was significantly and positively associated with scores on the Social Anhedonia scale [r = 0. 35, p = 0.005], and the Physical Anhedonia scale [r = 0.27, p = 0.037], i.e., higher ratios indicative of worse gating correlated with higher scale scores. We also observed trends whereby poorer P50 suppression was associated with higher scores on the Perceptual Aberration Scale [r = 0.22, p = 0.08] and higher scores on the Magical Ideation Scale [r = 0.24, p = 0.066]. In a stepwise regression of Chapman scale scores onto the P50 ratio, the Social Anhedonia scale score was entered first, indicating that it accounted for the largest proportion of variance in the P50 ratio [R2 = 0.13, F(l, 59) = 8.42, p < 0.01]. The relationship between social anhedonia and P50 sensory gating is depicted in Figure 4. No other variables accounted for a significant increment in the squared multiple correlation.
Figure 4.
The association between P50 sensory gating and social anhedonia for the sample. P50 sensory gating is depicted in terms of the ratio of S2/S1 amplitude. Social anhedonia was measured using the revised Social Anhedonia Scale (Eckblad, Chapman, Chapman, & Mishlove, 1982).
N100
Across the entire sample, the ratio of S2/S1 amplitude for the N100 component was associated with the Perceptual Aberration scale scores [r = 0.38, p = 0.003]; the higher the self-reported frequency of perceptual anomalies, the poorer the N100 sensory gating (i.e., the higher the N100 S2/S1 ratio). No other significant correlations were detected between the N100 gating ratios and the other psychometric measures (r’s ranged from 0.14 to 0.19, n.s.).
P200
The ratio of P200 S2/S1 amplitude demonstrated a significant correlation with Perceptual Aberration [r = 0.51, p < 0.001], Magical Ideation [r = 0.30, p = 0.021], and Physical Anhedonia [r = 0.31, p = 0.016] scale scores. The association between self-reported social anhedonia and P200 sensory gating failed to reach statistical significance [r = 0.22, p = 0.087]. A follow-up stepwise regression analysis revealed that only the Perceptual Aberration scores accounted for the largest significant proportion of variance in the P200 gating ratios, [R2 = 0.28, F(1, 58) = 20.87, p < 0.001]. None of the other Chapman scale scores were significant predictors of the sensory gating as indexed by the P200 ratio.
3.4 Clinical description of cocaine-dependent patients
Within the cocaine-dependent group, frequency of perceptual abnormalities, as measured by the Chapman scale, was correlated with their ability to suppress neural responding to the second of two repeated stimuli. Scores on the Perceptual Aberration Scale were significantly associated with the P200 gating ratio [r = 0.51, p = 0.01], and somewhat associated with the N100 gating ratio [r = 0.37, p = 0.09]. No other correlations between indices of sensory gating and the psychometric measures neared significance. There was no significant association between scores on the Social Anhedonia scale and P50 gating ratios within the patient group, presumably due to restriction of range. We observed a trend across all cocaine-dependent patients, whereby better P50 suppression was associated with longer duration of abstinence from cocaine, r = 0.39, p = 0.068. There was no relationship between duration of cocaine abstinence and either N100 gating [r = 0.18, p = 0.42] or P200 gating [r = 0.19, p = 0.39].
Additional clinical description of the cocaine-dependent patients is provided in Table 5. The patients with cocaine-induced paranoia5 (CIP) were significantly younger when they first used cocaine [t((21) = 2.92, p < 0.01], and had a significantly longer total duration of cocaine use [t((21) = 2.56, p < 0.05], than the other cocaine-dependent patients who did not experience paranoia. This observation supports our prior finding (Floyd et al., 2006). However, the two patient groups did not differ significantly in terms of their duration of abstinence from cocaine at the time of testing [t ((21) = 0.90, p = 0.38]. When comparing the sensory gating measures of the seven subjects denying any paranoid feelings to the 16 subjects endorsing paranoid symptoms, no significant differences or trends were noted. The two patient groups did not differ significantly in terms of their scores on any of the Chapman scales of psychosis proneness.
Table 5.
Comparison of Cocaine-Dependent Patients with and without Paranoia
| Cocaine-Dependent Patientsa | ||
|---|---|---|
| With CIPb (n = 16) | Without CIP (n = 7) | |
| Age (years) | 36.63 ± 7.34 | 36.29 ± 9.43 |
| Gender (M/F) | 8/8 | 2/5 |
| Age at time of first use | 18.06 ± 2.57 | 25.57 ± 9.83 |
| Age at time of regular use | 21.63 ± 5.02 | 27.14 ± 10.1 |
| Duration of use (years) | 18.56 ± 6.90 | 10.71 ± 6.40 |
| Duration of abstinence (days) | 65.81 ± 105 | 107.57 ± 95.24 |
| Chapman psychosis-proneness scales | ||
| Perceptual Aberration | 6.81 ± 7.3 | 6.14 ± 3.3 |
| Magical Ideation | 11.13 ± 6.4 | 8.86 ± 3.7 |
| Social Anhedonia | 14.25 ± 5.6 | 15.86 ± 2.7 |
| Physical Anhedonia | 17.94 ± 6.7 | 15.00 ± 5.1 |
| Sensory gating measure | ||
| P50 ratio | 72.49 ± 42.1 | 71.35 ± 46.1 |
| N100 ratio | 65.77 ± 41.7 | 73.14 ± 62.9 |
| P200 ratio | 56.62 ± 47.65 | 63.86 ± 61.9 |
Means ± SD
CIP = cocaine-induced paranoia
3.5 Relationship between Cocaine Experiences Questionnaire and sensory gating measures
Sixteen ((16) of the 23 cocaine-dependent patients ((70%) indicated experiencing psychotic symptoms during cocaine use. After removing the 7 cocaine-dependent patients who did not experience CIP, we observed that most of the patients (nearly 63%) reportedly became paranoid within five minutes of continuous use. Overall, the CIP group displayed a moderate level of insight (e.g. had awareness that the fear was excessive approximately 50% of the time). They reported considerable distress from their cocaine induced psychotic experiences and found that it significantly detracted from their high [a median rating of 3.5 on the CEQ severity subscale that ranged from 0 to 6]. Within the CIP group, there were no significant associations between their CEQ ratings and measures of sensory gating (r’ s ranged from −0.23 to 0.24, n.s.).
4. DISCUSSION
The primary goal of this study was to investigate the clinical correlates of sensory gating, as measured in a repetition suppression paradigm of the mid-latency auditory evoked responses. This study replicated earlier findings of P50 gating deficits in cocaine-dependent patients compared to healthy controls (Boutros et al., 2002; Boutros et al., 2000; Fein et al., 1996). We hypothesized that greater psychosis proneness would be associated with weaker neuronal inhibition, as indicated by greater S2/S1 gating ratios. The data supported these predictions. In a mixed sample of cocaine-dependent patients and healthy controls, higher self-reported social and physical anhedonia were associated with poorer sensory gating, as measured by the P50 S2/S1 ratio. We also observed that within the healthy control group, social and anhedonia was positively correlated with P50 ratios (i.e., higher gating ratios indicative of poorer gating correlate with elevated social anhedonia scores). Both of these results indicate an inverse relationship negative schizotypy and early sensory gating, consistent with the earlier findings of Wang et al. (Wang et al., 2004).
We had also hypothesized that different MLAER components would be differentially associated with different aspects of psychosis proneness and/or schizotypy. We based this hypothesis on the assumption that the different MLAER components reflect different, albeit related, underlying mechanisms and functions (Boutros et al., 2004a; Brockhaus-Dumke et al., 2008; Crowley and Colrain, 2004; Lijffijt et al., 2012; Lijffijt et al., 2009). Sensory gating is a multistage process, and the different MLAER gating indices are not redundant measures of sensory gating mechanisms. Indeed, P50 is thought to reflect pre-attentional processes, N100 is associated with attentional triggering, particularly to irrelevant information, and P200 is associated with orienting and allocation of attention (Lijffijt et al., 2012; Náátánen, 1992). We found that the correlates of P50 gating were not the same as the correlates of N100 and P200 gating. Positive schizotypy, specifically, perceptual aberration, was associated with poorer N100 gating and poorer P200 gating. Perceptual Aberration Scale scores accounted for a significant proportion of variance in P200 gating across the entire sample. The association between self-reported perceptual aberrations and sensory gating in the attentive phase of information processing is intriguing. It is possible that incoming stimuli are not being filtered or gated properly and in turn, are misinterpreted, giving rise to perceptual distortions.
Moreover, we observed that in the healthy controls, P50 gating and P200 gating were significantly albeit only moderately correlated. It is unlikely that the P200 gating performance of the control participants in the present study reflected a downstream effect of P50 sensory gating, however, given the differential associations with the clinical self-report measures. It is also unlikely that the P200 gating of the patients reflected a downstream effect of P50 sensory gating because that would have rendered it more likely that we would have observed a robust group difference in terms of P200 gating. This was not the case. We observed that the N100 and P200 gating performances of the patients were highly correlated. Future investigations of sensory gating in cocaine-dependent patients would be enhanced by including a measure of impulsivity, to examine whether impulsivity was associated with sensory gating in this population.
One strength of the present study was our reliance upon a well-validated psychometric probe of psychosis-proneness, namely, the Chapman (Perceptual Aberration, Magical Ideation, Social Anhedonia, Physical Anhedonia) scales. This permitted us to confirm the suggestive findings of previous studies (Croft et al., 2004; Croft et al., 2001; Evans et al., 2007; Wang et al., 2004) indicating an association between schizotypy and gating deficits. Moreover, we extended the work to examine the clinical correlates of other MLAERs, namely N100, and P200. As discussed above, different aspects of psychosis-proneness differ in terms of their specificity and/or trait significance for the schizophrenia-spectrum. Social anhedonia is hypothesized to be a core aspect of schizotypy, the latent personality construct underlying schizophrenia and schizophrenia-spectrum disorders (Meehl, 1962). Social anhedonia has been observed to be a trait characteristic of schizophrenia though not of mood disorders (Blanchard et al., 2001). It is noteworthy that only social anhedonia accounted for a significant proportion of the variance in P50 gating ratios, given the association between P50 deficits and vulnerability to schizophrenia. It is intriguing to consider the relationship between schizotypal traits and gating deficits among nonclinical individuals in this context.
We also extended the findings regarding clinical correlates of sensory gating to a sample of cocaine-dependent patients. In contrast to our earlier finding (Boutros et al., 2002), we found no association between CEQ ratings and strength of P50 sensory gating in the cocaine-dependent patients. We attribute this to a significant difference in sample characteristics, namely, the duration of abstinence. The patients in the current study were allowed to have abstinences up to 18 months, whereas in the previous study only cocaine patients with abstinences no greater than 12 months were included in the study. The current study also included (23%) fewer patients. On the other hand, the discrepant findings may be related to yet unidentified variables related to the subjects or their cocaine use.
A limitation of the present study is the relatively small sample size. The small size of our subgroups may have contributed to the largely negative findings (of correlations between sensory gating and psychosis-proneness scale scores) in the subgroups when considered separately. Confirmation of our present findings in studies with larger samples is necessary.
The two participant groups differed in terms of their smoking history. The group difference in terms of percentage of smokers may have affected some of the study findings, i.e., group differences in gating performance, or perhaps differences in strength of relationships between the psychometric measures and sensory gating measures. However, we do not think this is likely, for several reasons: 1) earlier findings (Adler et al., 1993) suggest that nicotine effects on P50 suppression are negligible after 30 minutes and at least 60 minutes elapsed between participants’ last ingestion of nicotine and their psychophysiological testing; and 2) studies suggest that nicotine normalizes sensory gating performance, which would have served to minimize the group differences in P50 gating performance (Adler et al., 1993; Wan et al., 2008).
Stimulant drugs can lead to drug-induced psychoses that may result in deficient gating of sensory and cognitive information, similar to that observed in natural psychoses such as schizophrenia (Boutros et al., 2002; Boutros et al., 2006; Vollenweider and Geyer, 2001). While sensory gating deficits are an indicator of risk for schizophrenia-spectrum disorders, and therefore are present prior to the onset of manifest disorder, it is possible that in the case of cocaine-dependence, the observed sensory gating deficits are the sequelae of cocaine use. However, it is also possible that the P50 sensory gating deficit may not be a nonspecific consequence of cocaine use. Rather, the P50 sensory gating deficit may reflect a premorbid, genetic diathesis that plays a contributory, perhaps potentiating or facilitating role in psychomimetic effects of agents such as cocaine. Thus, while it appears that sensory gating deficits are seen in both schizophrenia patients and cocaine-dependent patients, it is unclear whether the anomalies reflect similar etiological pathways. According to equifinality, different combinations of genetic and environmental factors could lead to a common central nervous system dysfunction, which in this case would be weakened inhibition of incoming stimuli.
An important issue in need of clarification regarding the data presented in this paper is the omission of providing the S1 and S2 amplitude data. The main issue is how to interpret abnormalities of the P50 amplitude (S1) and its influence on the gating function. A full discussion and meta-analysis of this issue recently appeared (see (Chang et al., 2011). The meta-analysis clearly points to the two abnormalities (i.e., decreased amplitude of S1 and decreased attenuation of S2) being independent but with some association. As is well known, association does not indicate causation. A low amplitude S1 does not necessarily predict weak attenuation and a large amplitude S1 does not necessarily predict strong attenuation. To date, there is no formula that allows the prediction of S2 amplitude based on S1 amplitude and there has not been any evidence for a floor effect6. If sensory gating is healthy, the amplitudes of S2 responses should be significantly attenuated (thus resulting in lowS2/S1 ratios) regardless of the amplitudes of the S1 responses (Shan et al., 2010). It should also be acknowledged, however, that the association between sensory gating, as measured by the S2/S1 P50 ratio, and schizotypy, as indexed by the social anhedonia scale scores, accounted for only 13% of the variance. This indicates that there are other factors that contribute to the individuals’ sensory gating deficits that have not yet been identified.
To date, there have not been large series of chronic cocaine-dependent individuals with emergent psychotic disorders. One of the advantages of the present study is that it can provide insights regarding the relationship between cocaine-induced psychoses and the schizophrenia spectrum. However, a limitation of this study is its cross-sectional nature. The role of stimulant drugs in terms of inducing chronic psychosis can be best elucidated through longitudinal follow-up of large, well-characterized samples. Clearly, further study is needed in order to address the relationship between cocaine-induced psychosis and schizophrenia. By providing a cross-sectional snapshot utilizing a set of well-validated psychometric scales, the present investigation can help guide further longitudinal work in this important area. In summary, the present study indicates that sensory gating measured using different ERP components was associated differentially with the Chapman psychosis proneness scale scores in a sample of cocaine-dependent patients and healthy controls.
ACKNOWLEDGMENT
This work was supported by a NARSAD Independent Investigators Award, K24 DA00520, and partially by R01 DA019055 to Dr. Boutros and a Vilas Associates Award to Dr. Gooding. The authors thank Dr. Fidias Sarmiento for his feedback on an earlier draft of this paper. Portions of these data were presented at the meeting of the Society for Research in Psychopathology, Iowa City, IA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Also, P50 is largest at the Cz site.
This was a rare occurrence and resulted in 3 subjects (2 cocaine dependent patients and 1 healthy control) being excluded from the sample.
Due to the filtering, there are separate waveforms for the P50 and N100/P200 results.
The authors are indebted to an anonymous reviewer for this suggestion.
The term cocaine-induced paranoia refers to paranoia at the delusional level.
It is unfortunate that many earlier (and some recent) papers continue to mix these two commonly associated, albeit independent, abnormalities. For example, an S1 of 5µV and S2 of 3 µV or an S1 of 3 µV and S2 of 2 µV would indicate defective gating while an S1 of 4 µV and S2 of 2 µV or S1 of 2 µV and S2 of 0.5 µV would indicate healthier gating. We have thus elected to present the data related to responding to the first stimuli under a separate cover not related to investigating sensory gating, which is the sole focus of this manuscript. We thus contend that if the S2/S1 is elevated, this simply indicates difficulty attenuating the S2 response.
References
- Adler LE, Hofter LLD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. A. J. Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 4th edition ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Blanchard J, Horan WP, Brown SA. Diagnostic differences in social anhedonia: A longitudinal study of schizophrenia and major depressive disorder. J. Abnorm. Psychol. 2001;110:363–371. doi: 10.1037//0021-843x.110.3.363. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Belger A, Campbell D, D'Souza CJK. Comparisoin of four components of sensory gating in schizophrenia and normal subjects: a preliminary report. Psychiatry Res. 1999;88:119–130. doi: 10.1016/s0165-1781(99)00074-8. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Brockhaus-Dumke AKG, Vedenlapin A, Elfakahni M, Burroughs S, Keshavan MS. Sensory-gating deficit of the N100 mid-latency auditory evoked potential in medicated schizophrenia patients. Schizophr. Res. 2009;113:339–346. doi: 10.1016/j.schres.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros NN, Gelernter J, Gooding DC, Cubells J, Young A, Krystal JH, Kosten T. Sensory gating and psychosis vulnerability in cocaine-dependent individuals: Preliminary data. Biol. Psychiatry. 2002;51:683–686. doi: 10.1016/s0006-3223(01)01237-9. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Gooding D, Sundaresan K, Burroughs S, Johanson C-E. Cocaine-dependence and cocaine-induced paranoia and mid-latency auditory evoked responses and sensory gating. Psychiatry Res. 2006;145:147–154. doi: 10.1016/j.psychres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Korzyukov O, Jansen B, Feingold A, Bell M. Sensory-gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Res. 2004a;126:203–215. doi: 10.1016/j.psychres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Korzyukov O, Oliwa G, Feingold A, Campbell D, McClain-Furmanski D, Struve F, Jansen BH. Morphological and latency abnormalities of the mid-latency auditory evoked responses in schizophrenia: a preliminary report. Schizophr. Res. 2004b;70:303–313. doi: 10.1016/j.schres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Overall J, Zouridakis G. Test-retest reliability of the P50 mid-latency auditory evoked response. Psychiatry Res. 1991;39:181–192. doi: 10.1016/0165-1781(91)90086-5. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Petrakis I, Krystal J, Campbell D, Caporele M, Kosten T. The effects of cocaine use on the mid-latency auditory evoked responses. Psychiatry Res. 2000;88:119–130. doi: 10.1016/s0165-1781(00)00207-9. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Mueller RUF, Klosterkoetter J. Sensory gating revisited: relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophr. Res. 2008;99:238–249. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Light GA, Geyer MA, Braff DL. Sensory gating deficits assessed by the P50 event-related potential in subjects with schizotypal personality disorder. A. J. Psychiatry. 2000;157:55–59. doi: 10.1176/ajp.157.1.55. [DOI] [PubMed] [Google Scholar]
- Chang WP, Arfken C, Boutros NN. Probing the relative contribution of the first and second responses to sensory gating indices: A meta-analysis. Psychophysiology. 2011;48:180–192. doi: 10.1111/j.1469-8986.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Infrequency scale. Madison, Wisconsin: University of Wisconsin-Madison; 1983. [Google Scholar]
- Chapman LJ, Chapman JP, Kwapil TR. Scales for the measurement of schizotypy. In: Raine A, Lencz T, Mednick SA, editors. Schizotypal personality. New York, NY: Cambridge University Press; 1995. pp. 79–106. [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J. Abnorm. Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, JP C, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. J. Abnorm. Psychol. 1994;103:171–183. doi: 10.1037//0021-843x.103.2.171. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp. Brain Res. 2001;139:377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Dzau JR, Blumenfeld LD, Mathews S, Kissler J. Ear of stimulation determines schizophrenia-normal brain activity differences in an auditory paired-stimuli paradigm. Eur. J. Neurosci. 2003;18:2853–2858. doi: 10.1111/j.1460-9568.2003.03027.x. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Dimoska A, Gonsalvez C, Clarke AR. Suppression of P50 evoked potential component, schizotypal beliefs, and smoking. Psychiatry Res. 2004;128:53–62. doi: 10.1016/j.psychres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Lee A, Bertolot J, Gruzelier JH. Associations of P50 suppression and desensitization with perceptual and cognitive features of "unreality" in schizotypy. Biol. Psychiatry. 2001:441–446. doi: 10.1016/s0006-3223(01)01082-4. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep, and modality. Clin. Neurophysiol. 2004;115:732–744. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Eckblad ML, Chapman LJ, Chapman JP. Magical ideation as an indicator of schizotypy. J. Consult. Clin. Psychol. 1983;51:215–225. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- Evans LH, Gray NS, Snowden RJ. Reduced P50 suppression is associated with the cognitive disorganisation dimension of schizotypy. Schizophr. Res. 2007;97:152–162. doi: 10.1016/j.schres.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Fein G, Biggins C, MacKay S. Cocaine abusers have reduced auditory P50 amplitude and suppression compared to both normal controls and alcoholics. Biol. Psychiatry. 1996;39:955–965. doi: 10.1016/0006-3223(95)00299-5. [DOI] [PubMed] [Google Scholar]
- Floyd AG, Boutros NN, Struve FA, Wolf E, Oliwa GM. Risk factors for experiencing psychosis during cocaine use: A preliminary report. J. Psychiatr. Res. 2006;40:178–182. doi: 10.1016/j.jpsychires.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA, Matts CW. Clinical status of at-risk individuals 5 years later: Further validation of the psychometric high-risk strategy. J. Abnorm. Psychol. 2005;114:170–175. doi: 10.1037/0021-843X.114.1.170. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA, Matts CW. Rates of avoidant, schizotypal, schizoid and paranoid personality disorders in psychometric high-risk groups at 5-year follow-up. Schizophr. Res. 2007;94:373–374. doi: 10.1016/j.schres.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruzelier J, Burgess A, Stygall J, Irving G, Raine A. Patterns of cognitive asymmetry and syndromes of schizotypal personality. Psychiatry Res. 1995;56:71–79. doi: 10.1016/0165-1781(94)02564-y. [DOI] [PubMed] [Google Scholar]
- IBM I. SPSS 20.0 for Mac OS X. 2012 [Google Scholar]
- Jerger K, Biggins C, Fein G. P50 suppression is not affected by attentional manipulations. Biol. Psychiatry. 1992;31:365–377. doi: 10.1016/0006-3223(92)90230-w. [DOI] [PubMed] [Google Scholar]
- Kwapil TR. Social anhedonia as a predictor of the development of schizophrenia-spectrum disorders. J. Abnorm. Psychol. 1998;107:558–565. doi: 10.1037//0021-843x.107.4.558. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Barrantes-Vidal N, Silvia PJ. The dimensional structure of the Wisconsin schizotypy scales: Factor identification and construct validity. Schizophr. Bull. 2008;34:444–457. doi: 10.1093/schbul/sbm098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Cox B, Acas MD, Lane SD, Moeller FG, Swann AC. Differential relationships of impulsivity or antisocial symptoms on P50, N100, or P200 auditory sensory gating in controls and antisocial personality disorder. J. Psychiatr. Res. 2012;46:743–750. doi: 10.1016/j.jpsychires.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Lane SD, Meier SL, Boutros NN, Burroughs S, Steinberg JL, Moeller FG, Swann AC. P50, N100, and P200 sensory gating: Relationships with behavioral inhibition, attention, and working memory. Psychophysiology. 2009;46 doi: 10.1111/j.1469-8986.2009.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason O, Claridge G, Jackson M. New scales for the assessment of schizotypy. Personality and Individual Differences. 1995;18:7–13. [Google Scholar]
- Meehl PE. Schizotaxia, schizotypy, schizophrenia. Am. Psychol. 1962;17:827–838. [Google Scholar]
- Meehl PE. Schizotaxia revisited. Archives of General Psychiatry. 1989;46:935–944. doi: 10.1001/archpsyc.1989.01810100077015. [DOI] [PubMed] [Google Scholar]
- Náátánen R. Attention and brain function. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- Philgren EM, Boutros NN. Psychostimulant-induced chronic schizophrenia-like disorder. Clinical schizophrenia and related psychoses. 2007;1:54–63. [Google Scholar]
- Raine A. The SPQ: A scale for the assessment of schizotypal personality based on the DSM-III-R criteria. Schizophr. Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Satel SL, Edell WS. Cocaine-induced paranoia and psychosis proneness. A. J. Psychiatry. 1991;148:1708–1711. doi: 10.1176/ajp.148.12.1708. [DOI] [PubMed] [Google Scholar]
- Schuckit M. Coorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101(Supplement 1):76–88. doi: 10.1111/j.1360-0443.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- Siegel C, Waldo M, Mizner G, Adler LE, Freedman R. Deficits in sensory gating in schizophrenic patients and their relatives. Evidence obtained with auditory evoked responses. Arch. Gen. Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- Smith AK, Edgar JC, Huang M, Lu BY, Thoma RJ, Hanlon FM, McHaffie G, Jones AP, Paz RD, Miller GA, Canive JM. Cognitive abilities and 50- and 100-msec paired-click processes in schizophrenia. American Journal of Psychiatry. 2010;167:1264–1275. doi: 10.1176/appi.ajp.2010.09071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirthalli J, Benegal V. Psychosis among substance users. Current Opinions in Psychiatry. 2006;19:239–245. doi: 10.1097/01.yco.0000218593.08313.fd. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Bilker WB, Siegel SJ, Kohler CG, Gur RE. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Res. 2009;165:27–37. doi: 10.1016/j.psychres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables P. Input dysfunction in schizophrenia. In: Maher BA, editor. Progress in Experimental and Personality Research. 1964. pp. 1–47. [PubMed] [Google Scholar]
- Vollenweider FX, Geyer MA. A systems model of altered consciousness: Integrating natural and drug-induced psychoses. British Research Bulletin. 2001;56:495–507. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- Waldo MC, Adler LE, Freedman R. Defects in auditory sensory gating and their apparent compensation in relatives of schizophrenics. Schizophr. Res. 1988;1:19–24. doi: 10.1016/0920-9964(88)90035-7. [DOI] [PubMed] [Google Scholar]
- Wan L, Friedman BH, Boutros NN. P50 sensory gating and attentional performance. Int. J. Psychophysiol. 2008;67:91–100. doi: 10.1016/j.ijpsycho.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Miyazato H, Hokama H, Hiramatsu K-I, Kondo T. Correlation between P50 suppression and psychometric schizotypy among non-clinical Japanese subjects. Int. J. Psychophysiol. 2004;52:147–157. doi: 10.1016/j.ijpsycho.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Zouridakis G, Boutros NN. Stimulus parameter effects on the P50 evoked response (Brief Report) Biol. Psychiatry. 1992;32:839–841. doi: 10.1016/0006-3223(92)90088-h. [DOI] [PubMed] [Google Scholar]