Abstract
The purpose of this project was to assess anterior and posterior corneal stromal elasticity after corneal collagen cross linking (CXL) treatment in human cadaver eyes using Atomic Force Microscopy (AFM) through indentation. Twenty four human cadaver eyes (12 pairs) were included in this study and divided into 2 groups (6 pairs per group). In both groups, the left eye (OS) served as a control (no riboflavin or CXL treatment was performed) and the right eye (OD) underwent CXL treatment (30 minutes of riboflavin pretreatment followed by 30 minutes of exposure to 3mW/cm2 of ultraviolet light). In group 1, the anterior stroma was exposed by manual delamination of approximately 50μm of the corneal stroma including Bowman’s membrane. In group 2, the posterior stroma was exposed by delamination of the anterior 50% of the corneal stroma including Bowman’s membrane. Delamination was performed after crosslinking treatment in the case of the treated eyes. In all eyes, the stromal elasticity was quantified using AFM through indentation. Young’s modulus of elasticity for the anterior cornea (group 1) was 245.9±209.1kPa (range: 82.3 - 530.8 kPa) for the untreated control eyes, and 467.8±373.2kPa (range: 157.4 – 1126 kPa) for the CXL treated eyes. Young’s modulus for the posterior cornea (group 2) was 100.2±61.9kPa (range: 28.1 - 162.6 kPa) for the untreated control eyes and 66.0±31.8kPa (range: 31.3 - 101.7 kPa) for the CXL treated eyes. Young’s modulus of the anterior stroma significantly increased after CXL treatment (p=0.024), whereas the posterior stroma did not demonstrate a significant difference in Young’s modulus after CXL treatment (p=0.170). The anterior stroma was stiffer than the posterior stroma for both the control and CXL treatment groups (p=0.077 and p=0.023, respectively). Our findings demonstrate that stiffness of the anterior corneal stroma after CXL treatment seems to increase significantly, while the posterior stroma does not seem to be affected by CXL.
Keywords: cornea, crosslinking, keratoconus, ectasia, mechanical properties
INTRODUCTION
Corneal collagen cross linking (CXL) treatment is based on a photo-chemical reaction between ultraviolet light-A (UV-A) and riboflavin (Wollensak et al. 2003a, Wollensak et al. 2003b, Wollensak. 2006). This minimally invasive procedure increases the mechanical strength of the cornea, thereby stabilizing corneal ectatic disorders such as keratoconus and iatrogenic keratectasia (after corneal refractive surgery) (Wollensak et al. 2003a, Wollensak et al. 2003b, Wollensak. 2006). The introduction of CXL in routine clinical practice has changed the management of the above entities; furthermore, it provides a ‘true’ treatment, by inhibiting the progression of keratoconus and corneal ectasia. Prior to CXL, all interventions (glasses, contact lenses and intra-corneal ring segment implantation (Kymionis et al. 2007)) were used to improve visual function of patients, while they did not treat the underlying pathophysiology of the corneal tissue.
Theoretically, the CXL treatment’s ability to increase corneal mechanical strength, and therefore inhibit the progression of corneal ectatic disorders, stems from the treatment’s formation of additional crosslinks within the cornea’s stromal ultrastructure. Comprehensive knowledge of the precise molecular mechanisms associated with CXL and how it attributes to corneal mechanical strengthening remain unknown (Wollensak. 2006; Hayes et al. 2013; Meek and Hayes. 2013). Studies have shown that the CXL process is dependent upon the presence of carbonyl groups within cornea (McCall et al. 2010; Brummer et al. 2011). In addition, a recent study conducted by Hayes et al (2013) demonstrated that the creation of crosslinks produced from CXL most likely occurs at four particular locations, opposing the initial notion of being between collagen fibrils. The four locations are within and between collagen molecules located at the surface of the collagen fibrils as well as within and between proteoglycan core proteins (Hayes et al. 2013). Although such advancements have been made, the exact molecules involved and their reaction pathways have yet to be identified.
Many studies have been published demonstrating CXLs efficacy indirectly, mostly based on clinical findings, such as steady keratometric values and refraction (Kymionis et al. 2012; Vinciguerra et al. 2013). Investigations conducted ex vivo have been able to directly quantify the efficacy of CXL by the measurement of corneal mechanical strength (Kling et al. 2010; Kohlhaas et al. 2006, Lanchares et al. 2011, Schumacher et al. 2011, Spoerl et al. 1998, Spoerl and Seiler. 1999, Wollensak et al. 2003b, Wollensak and Iomdina. 2009a, Wollensak and Iomdina. 2009b). A land mark report by Wollensak et al (2003) demonstrates significant increase in corneal stiffness (increased by 328.9%) and in Young’s modulus (increased 4.5 times) after CXL assessed using stress-strain measurements (Wollensak et al. 2003b). Ever since then, numerous ex vivo studies have been conducted, using various corneal characterization techniques, to confirm such increase in corneal mechanical strength after CXL (Kling et al. 2010; Kohlhaas et al. 2006; Lanchares et al. 2011; Schumacher et al. 2011; Wollensak and Iomdina. 2009a; Wollensak and Iomdina. 2009b; Scarcelli et al. 2013).
Ultraviolet light absorption in the cornea is governed by the Lambert-Beer law of absorption – there is an exponential decrease in UV intensity with increasing depth in the cornea. For this reason, it would be expected that the induction of additional crosslinking bonds within the stroma is not homogeneous, but rather depth-dependent (Kohlhaas et al. 2006). Thus, it is likely that mechanical stiffening induced by crosslinking is much more pronounced in the anterior stromal region (Kohlhaas et al. 2006). In fact, this phenomenon has been demonstrated in the porcine model using strip extensiometry (Kohlhaas et al. 2006) and Brillouin microscopy (Scarcelli et al. 2013). These same conclusions were predicted by an inverse computational model that used in vivo data from patients as inputs (Roy et al. 2013). The purpose of this study was to build upon previous studies and quantify the anterior and posterior stromal elasticity in human cadaver eyes after crosslinking using Atomic Force Microscopy (AFM). Through the principle of nanoindentation and its ability to implement low indentation depths, AFM can independently characterize the distinct layers of corneal samples and perform depth-dependent characterization studies with proper hydration.
MATERIALS AND METHODS
Tissue acquisition
Experiments were conducted on 12 pairs of human cadaver eyes (age range: 39-88 years) retrieved from the Florida Lions Eye Bank (Miami, Florida). The human eye globes arrived from the eye bank in sealed vials placed in Styrofoam containers filled with ice. Upon arrival in the laboratory, the corneal epithelium was removed using a cotton-tipped applicator and the whole globes were placed, cornea side down, in 20% Dextran solution for 24 hours in the refrigerator at 4°C to restore corneal thickness to physiological levels (Borja et al. 2004, Duffey et al. 1989, Swinger and Kornmehl. 1985). The mean postmortem time of the eyes at the time of receipt was 4.58±1.31 days (range: 2 – 7 days), but the actual experiments were performed 24 hours later to enable this initial pretreatment with 20% Dextran. Pachymetry measurements were taken after the pretreatment (DGH 55 Pachmate, DGH Technology Inc., Exton, PA) to ensure the restoration of the corneal thickness within the physiological range of 400-600μm. All human eyes were obtained and used in compliance with the guidelines of the Declaration of Helsinki for research involving the use of human tissue.
Corneal collagen cross linking treatment
For this study, the right eyes (OD) were treated using the standard CXL Dresden protocol and the left eyes (OS) served as untreated controls (with no riboflavin pretreatment). Since the epithelium was removed in for swelling restoration purposes, instillation of 0.1% riboflavin solution (10mg riboflavin-5-phosphate in 10mL Dextran 20% solution) was applied on the bare corneal stroma one drop every 5 minutes for 30 minutes. The cornea was then irradiated using UVA light at 378nm wavelength and with an intensity of 3mW/cm2. The irradiance was performed for 30 minutes, corresponding to a total surface dose of 5.4 J/cm2. During UVA irradiation, riboflavin solution was applied every 5 minutes to maintain corneal saturation with riboflavin.
Six pairs of eyes (n=12) were prepared for anterior stroma measurements (Group 1) and six pairs (n=12) were prepared for posterior stroma measurements (Group 2). For anterior stroma measurements, a 4mm surgical blade (Alcon, Texas, USA) was used to remove approximately 50μm of corneal tissue from the intact cornea, thereby removing Bowman’s membrane (17.7μm thickness; Tao et al. 2011) to expose the anterior stromal region, but leaving it connected to the remaining stromal layer and Descemet’s membrane. To expose a lower plane in the cornea, approximately 50% of the restored corneal thickness was removed using the same surgical blade; this plane in the stroma was defined as the posterior stroma. Pachymetric measurements were taken incrementally to monitor the amount of stroma removed. The corneas were then excised from the whole globes, leaving a generous rim of sclera and placed in a custom cornea holder with 15% Dextran solution to maintain corneal hydration during mechanical testing.
Elasticity assessment
Mechanical property measurements were performed using a custom-built atomic force microscopy (AFM) system. The AFM system and experimental procedure have been described in detail previously (Ziebarth et al. 2011; Dias and Ziebarth. 2013). Briefly, tip-less AFM cantilevers (nominal spring constant: 4.5 N/m, NSC12 series, Mikromasch, San Jose, CA) were modified with glass microspheres (59-74μm diameter, 15926-100, Polysciences Inc). The modified tip was then calibrated to determine its spring constant using a reference force calibration cantilever (nominal spring constant: 10.4 N/m, CLFC-NOBO, Bruker, Camarillo, CA) manufactured specifically for the calibration of other probes. The modified cantilever tips were lowered onto the corneal samples using a piezoelectric mechanism (60μm maximal expansion, P-841.40, Physik Instrumente, Germany) with an approach speed of 15μm/s. When the maximal indentation force of 1000V (<20nN, which corresponds to <6μm indentation) was reached, the cantilever was immediately retracted at the same speed. The voltage detected at the photodiode due to deflection of the cantilever was recorded as a function of piezoelectric displacement. These recordings were used to derive the sample’s force-indentation curves, after factoring out the cantilever deflection on a hard surface and incorporating the measured spring constant. With the use of custom MATLAB programs, the indentation force-indentation depth curves were analyzed using the Hertz model for a spherical indenter (Hertz H. 1881):
where F [N] is the measured force (N), E [N/m2] is Young’s modulus (Pa), ν is Poisson’s ratio (ν=0.49 for the cornea (Cabrera Fernandez et al. 2005, Knox Cartwright et al. 2011)), R [m] is the radius of the spherical indenter (m), and D is the measured indentation. These recordings were repeated at least 15 times per sample. All experiments were performed at room temperature. The accuracy of the curve fits was visually verified.
Statistical analysis
Statistical analysis of data was performed by a custom made data base in Excel (Microsoft Office). All parameters followed normal distribution and a paired Student’s t-test was used to analyze the Young’s modulus of the control versus treated corneas. A p value less than 0.05 was considered to be statistically significant.
RESULTS
Mean central corneal thickness (CCT) of all eyes was 526±62μm (range: 403 - 612μm) after epithelial removal and dextran pretreatment. The eyes in group 1 (defined as anterior stroma) had an average of 41±15μm of tissue removed by manual delamination (range: 17 - 64μm), while the eyes in group 2 (defined as posterior stroma) had an average of 53±9% of their thickness removed (range: 40 - 69%) (Table 1).
Table.
The age, post-mortem time (PMT), and central corneal thickness (CCT) of human cadaver eyes used in each group. The values reported are average ± standard deviation (range).
| Group | Age (years) | PMT at Time of Experimentation (Days) | CCT after Dextran Pretreatment (μm) | Manual Delamination (μm) | Tissue Removed (percentage) | |
|---|---|---|---|---|---|---|
| Anterior Corneal Stroma | Treated | 70 ± 19 (39 – 87) | 6.3 ± 0.8 (6 – 8) | 509 ± 83 (403 – 612) | 33 ± 7 (26 – 43) | 10.3 ± 2.3 (7.3 – 14.1) |
| Untreated | 528 ± 61 (430 – 599) | 50 ± 17 (17 – 64) | 9.7 ± 3.7 (2.9 – 12.5) | |||
| Posterior Corneal Stroma | Treated | 78 ± 14 (56 – 88) | 4.8 ± 1.3 (3 – 6) | 535 ± 59 (480 – 595) | 182 ± 46 (131 – 238) | 53.4 ± 11.4 (40.2 – 69.8) |
| Untreated | 531 ± 55 (466 – 591) | 282 ± 52 (229 – 354) | 53.0 ± 5.9 (48.8 – 56.4) | |||
Young’s modulus of elasticity for the anterior stroma (group 1) was 245.9±209.1kPa (range: 82.3 - 530.8kPa) for the untreated control eyes and 467.8±373.2kPa (range: 157.4 – 1126kPa) for the CXL treated eyes (Figure 1). Young’s modulus for the posterior stroma (group 2) was 100.2±61.9kPa (range: 28.1 – 162.6kPa) for the untreated control eyes and 66.0±31.8kPa (range: 31.3 - 101.7kPa) for the CXL treated eyes (Figure 1). The variability of repeated mechanical property measurements on the same corneal sample (standard deviation divided by average) was 15±7%. For each individual eye pair, the elastic modulus of the CXL treated eye was consistently higher than its untreated counterpart for the anterior stroma measurements; however, the posterior stroma was stiffer after CXL treatment in only 3 out of 6 pairs. Young’s modulus of the anterior stroma significantly increased after CXL treatment (p=0.024), whereas the posterior stroma did not demonstrate a significant difference in Young’s modulus after CXL treatment (p=0.170). The anterior stroma was stiffer than the posterior stroma for both the control and CXL treatment groups (p=0.077 and p=0.023, respectively).
Figure 1.
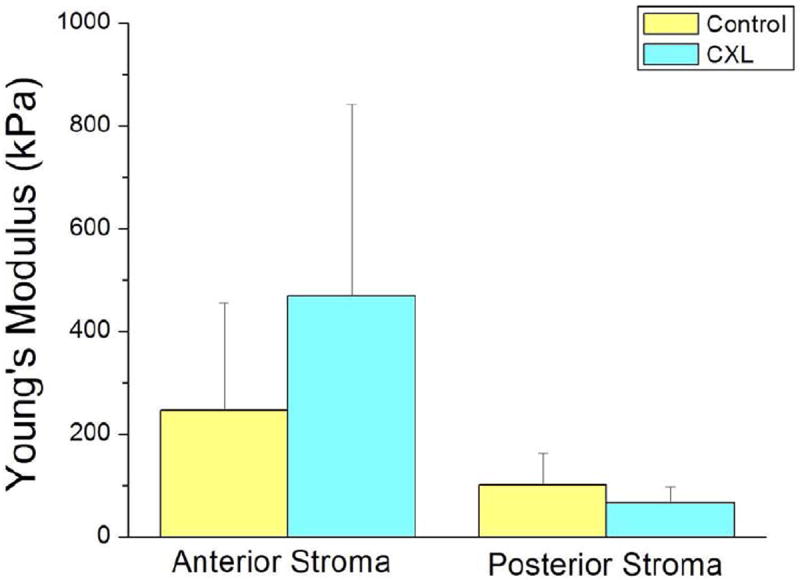
Bar graph of Young’s modulus expressed in kilo Pascals for corneas that underwent corneal collagen cross linking treatment (CXL) compared to control corneas (no CXL), both for anterior and posterior corneal stroma.
DISCUSSION
CXL treatment is based on a photo-chemical reaction between UVA and riboflavin. Riboflavin, also known as vitamin B2 (belonging to the flavin group), is a photo-sensitizer that is excited into its triplet state when irradiated, generating reactive oxygen species (ROS) (Wollensak. 2006). The ROS can react further with various molecules inducing chemical covalent bonds bridging amino groups of collagen fibrils (type II photochemical reaction), inducing cross links which increase corneal stiffness (Wollensak. 2006). The precise molecular interactions of such ROS within the corneal stroma is not yet fully understood, although some progress has been made to improve insight into the effect of CXL on the cornea at the molecular level (Hayes et al. 2013; Meek and Hayes. 2013; McCall et al. 2010; Brummer et al. 2011). There has been evidence of increase in the area, density, and diameter of corneal stromal collagen; such structural alterations may attribute to the increased corneal stiffness (Choi et al. 2012).
Efficacy of CXL treatment has been generally based on indirect clinical outcomes (Kymionis et al. 2012, Vinciguerra et al. 2013). Most studies enroll patients with progression of their ectatic disorder proven by means of corneal topography and alteration of keratometric values over a 6 month time interval. Efficacy of the treatment has therefore been linked with stable keratometric values and stable refraction over time. Long-term results from European clinical trials indicate a steady and continuous improvement in corneal irregularities up to 24 months postoperatively (Kymionis et al. 2012, Vinciguerra et al. 2013); while the trials supervised by the Food and Drug Administration in the United States have also shown great promise (Greenstein et al. 2011, Hersh et al. 2011). In addition to the clinical outcomes, there have been ex vivo laboratory studies based mainly on stress-strain tests that have demonstrated an increase in corneal stiffness after treatment in both animal models and human corneas (Kling et al. 2010, Kohlhaas et al. 2006, Lanchares et al. 2011, Schumacher et al. 2011, Spoerl et al. 1998, Spoerl and Seiler. 1999, Wollensak et al. 2003b, Wollensak and Iomdina. 2009a, Wollensak and Iomdina. 2009b).
In our study, we found that corneal collagen crosslinking treatment significantly increases corneal stiffness in the anterior corneal stromal region only (increased by ~1.9 times when compared to the untreated corneas) while the posterior corneal stroma (as described in our study as approximately 50% of the total CCT) was not significantly affected by CXL. Since riboflavin serves as the photosensitizing agent to produce ROS, the ability to produce a significant biomechanical CXL effect within the stroma is dependent upon the concentration of the riboflavin that is able to diffuse through the stroma (Spoerl et al. 2007;Raiskup and Spoerl. 2013). Studies have investigated the absorption properties of riboflavin and showed that the riboflavin concentration decreases with stromal depth (Wollensak et al. 2010; Spoerl et al.2010; Sondergaard et al. 2010). Governing the absorption of UV light in the corneal tissue, the Lambert-Beer law states that there is an exponential decrease in UV intensity with increasing depth in the cornea. With the combined effect of riboflavin concentration and the Lambert-Beer law, it would be expected that the induction of additional crosslinking bonds within the stroma is not homogeneous, but rather depth-dependent (Kohlhaas et al. 2006).
Therefore, the results of our study support the theory that the mechanical stiffening induced by crosslinking is much more pronounced in the anterior stromal region than the posterior. Such findings are similar to the qualitative trends found in the studies of Kohlhaas et al (2006) and Scarcelli et al (2013), both of which characterized the anterior and posterior stromal regions of crosslinked and normal porcine corneas. In addition, the same trend was predicted by the finite-element computational model of Roy et al (2013). All studies agree with this present one that the stiffening effect of UV crosslinking is depth-dependent and that the majority of the stiffening occurs in the anterior stromal region rather than in the posterior stroma (Kohlhaas et al. 2006; Scarcelli et al. 2013).
Our study assessed the elasticity of normal and crosslinked human cadaver corneas using a custom Atomic Force Microscopy (AFM) system. Conceptually developed by Binnig et al (1881), AFM has become an established characterization technique in the arenas of non-biological and biological mechanical testing. AFM technology enables localized mechanical testing of samples in aqueous solutions (Karrasch et al. 1994, Lal and John. 1994) and is a suitable characterization technique to conduct studies on distinct layers within the corneal stroma for depth-dependent analysis. Using this technique, standard mechanical properties like Young’s modulus can be derived by the application of a controlled vertical compressive force. The force applies stress to the sample, which will indent as an indication of the strain experienced. The Young’s modulus obtained from these measurements is therefore defined as the ratio of stress (force) to strain (indentation), along the same vertical axis. Although the versatility of AFM enables the determination of time-dependent properties associated with viscoelasticity and poroelasticity, these measurements were not performed in the current study. All experiments were performed with the exact same experimental parameter settings (ie, indentation rate and maximal voltage) and encompassed the application and immediate retraction of maximal force unto the sample. In this particular study, we used spherical indenters to probe network layers of the corneal stroma, as opposed to individual collagen fibers. With the spherical indenter geometry used, the Hertz model was used to calculate Young’s modulus of elasticity from the force and indentation information. However, this model assumes that the sample is isotropic, homogeneous, linearly elastic, and infinitely thick, none of which accurately describe the cornea because of its heterogeneous, viscoelastic, and anisotropic nature. Therefore, the use of the Hertz model limits our results to only be linear elastic modulus estimates, as opposed to reflecting the absolute elastic modulus values.
It is possible that several experimental constraints may have affected the biomechanical measurements obtained. The variation of corneal edema between the samples resulted in a different initial total corneal thickness for each sample, despite the 24-hour dextran treatment. Although all samples had a thickness within the normal physiological range, these slight differences in thickness may account for some of the measurement variability between samples. Furthermore, the use of manual delamination to access the corneal stromal regions restricted the creation of reproducible corneal tissue excisions. Consequently, there lied the inability to perform elasticity assessments on the exact same anterior stromal depths for all the corneas, which can be evidenced by the large range of the amount of stroma removed to expose the anterior stroma (17-64μm). Previous studies have shown structural differences in the collagen fiber interconnectivity at increasing depths within the anterior stromal region (Winkler et al. 2011). Although no significant trend was found between anterior stromal elastic modulus and stromal depth, such structural differences may have contributed to the variation of elastic modulus values obtained. Other limitations of this study include the small sample size of eye pairs used as well as the ex vivo experimental model, since ex vivo human corneas are affected by factors such as post mortem, collagen autolysis, stromal swelling, and lack of healing response. The effect that these experimental constraints had on the results can be evidenced by the high between sample variability, since the standard deviation of the moduli between samples was on the same order as the mean. Despite the between sample variability, the results for each eye pair consistently showed that the stiffening effect of CXL was significant in the anterior region but not in the posterior region.
In conclusion, our study validates previous experimental evidence of increased corneal stiffness after CXL treatment, using the characterization technique of AFM. The results of this study describe an anterior-posterior stromal depth dependency concerning CXL efficacy.
Highlights.
We measured human stroma elasticity after UV-B2 crosslinking using AFM.
There is a significant increase in anterior stroma stiffness after crosslinking.
There is no significant change in posterior stroma stiffness after crosslinking
Acknowledgments
Grant support NIH Initiative for Maximizing Student Diversity Graduate Fellowship (JD); NIH National Research Service Award Individual Predoctoral Fellowship (1F31EY021714-01, JD).
Donor human eyes were provided by the Florida Lions Eye Bank.
Footnotes
The authors do not have any proprietary or financial interest in any of the devices presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Borja D, Manns F, Lamar P, Rosen A, Fernandez V, Parel JM. Preparation and hydration control of corneal tissue strips for experimental use. Cornea. 2004;23:61–66. doi: 10.1097/00003226-200401000-00010. [DOI] [PubMed] [Google Scholar]
- Brummer G, Littlechild S, McCall S, Zhang Y, Conrad GW. The Role of Nonenzymatic Glycation and Carbonyls in Collagen Cross-Linking for the Treatment of Keratoconus. Invest Ophthalmol Vis Sci. 2011;52:6363–6369. doi: 10.1167/iovs.11-7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera Fernandez D, Niazy AM, Kurtz RM, Djotyan GP, Juhasz T. Finite element analysis applied to cornea reshaping. J Biomed Opt. 2005;10:064018. doi: 10.1117/1.2136149. [DOI] [PubMed] [Google Scholar]
- Choi S, Lee SC, Lee HJ, Cheong Y, Jung GB, Jin KH, Park HK. Structural response of human corneal and scleral tissues to collagen cross-linking treatment with riboflavin and ultraviolet A light. Lasers Med Sci. 2012:1–8. doi: 10.1007/s10103-012-1237-6. [DOI] [PubMed] [Google Scholar]
- Dias J, Ziebarth NM. Anterior and posterior corneal stroma elasticity assessed using nanoindentation. Exp Eye Res. 2013 doi: 10.1016/j.exer.2013.06.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffey RJ, Tchah H, Lindstrom RL. Human cadaver corneal thinning for experimental refractive surgery. Refract Corneal Surg. 1989;5:41–42. [PubMed] [Google Scholar]
- Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37:691–700. doi: 10.1016/j.jcrs.2010.10.052. [DOI] [PubMed] [Google Scholar]
- Hayes S, Kamma-Lorger CS, Boote C, Young RD, Quantock AJ, Rost A, Khatib K, Harris J, Yagi N, Terrill N, Meek KM. The Effect of Riboflavin/UVA Collagen Cross-linking Therapy on the Structure and Hydrodynamic Behaviour of the Ungulate and Rabbit Corneal Stroma. PLoS ONE. 2013;8:e52860. doi: 10.1371/journal.pone.0052860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:149–160. doi: 10.1016/j.jcrs.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Hertz H. The contact of elastic solids. Journal Für Die Reine Und Angewandte Mathematik. 1881;92:156. [Google Scholar]
- Karrasch S, Hegerl R, Hoh JH, Baumeister W, Engel A. Atomic force microscopy produces faithful high-resolution images of protein surfaces in an aqueous environment. Proc Natl Acad Sci U S A. 1994;91:836–838. doi: 10.1073/pnas.91.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling S, Remon L, Perez-Escudero A, Merayo-Lloves J, Marcos S. Corneal biomechanical changes after collagen cross-linking from porcine eye inflation experiments. Invest Ophthalmol Vis Sci. 2010;51:3961–3968. doi: 10.1167/iovs.09-4536. [DOI] [PubMed] [Google Scholar]
- Knox Cartwright NE, Tyrer JR, Marshall J. Age-related differences in the elasticity of the human cornea. Invest Ophthalmol Vis Sci. 2011;52:4324–4329. doi: 10.1167/iovs.09-4798. [DOI] [PubMed] [Google Scholar]
- Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32:279–283. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- Kymionis GD, Grentzelos MA, Kounis GA, Diakonis VF, Limnopoulou AN, Panagopoulou SI. Combined transepithelial phototherapeutic keratectomy and corneal collagen cross-linking for progressive keratoconus. Ophthalmology. 2012;119:1777–1784. doi: 10.1016/j.ophtha.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Kymionis GD, Siganos CS, Tsiklis NS, Anastasakis A, Yoo SH, Pallikaris AI, Astyrakakis N, Pallikaris IG. Long-term follow-up of Intacs in keratoconus. Am J Ophthalmol. 2007;143:236–244. doi: 10.1016/j.ajo.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Lal R, John SA. Biological applications of atomic force microscopy. Am J Physiol. 1994;266:C1–21. doi: 10.1152/ajpcell.1994.266.1.C1. [DOI] [PubMed] [Google Scholar]
- Lanchares E, del Buey MA, Cristobal JA, Lavilla L, Calvo B. Biomechanical property analysis after corneal collagen cross-linking in relation to ultraviolet A irradiation time. Graefes Arch Clin Exp Ophthalmol. 2011;249:1223–1227. doi: 10.1007/s00417-011-1674-0. [DOI] [PubMed] [Google Scholar]
- McCall AS, Kraft S, Edelhauser Hf, Kidder GW, Lundquist RR, Bradshaw HE, Dedeic Z, Dionne MJC, Clement EM, Conrad GW. Mechanisms of Corneal Tissue Cross-Linking in Response to Treatment with Topical Riboflavin and Long Wavelength Ultraviolet Radiation (UVA) Invest Ophthalmol Vis Sci. 2010;51:129–138. doi: 10.1167/iovs.09-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, Hayes S. Corneal cross-linking – a review. Ophthalmic Physiol Opt. 2013;33:78–93. doi: 10.1111/opo.12032. [DOI] [PubMed] [Google Scholar]
- Raiskup F, Spoerl E. Corneal crosslinking with riboflavin and ultraviolet A. I. Principles Ocul Surf. 2013;11:65–74. doi: 10.1016/j.jtos.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Roy AS, Rocha KM, Randleman JB, Stulting RD, Dupps WJ. Inverse computational analysis of in vivo corneal elastic modulus change after collagen crosslinking for keratoconus. Exp Eye Res. 2013 doi: 10.1016/j.exer.2013.04.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarcelli G, Kling S, Quijano E, Pineda R, Marcos S, Yun SH. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest Ophthalmol Vis Sci. 2013;54:1418–1425. doi: 10.1167/iovs.12-11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher S, Oeftiger L, Mrochen M. Equivalence of biomechanical changes induced by rapid and standard corneal cross-linking, using riboflavin and ultraviolet radiation. Invest Ophthalmol Vis Sci. 2011;52:9048–9052. doi: 10.1167/iovs.11-7818. [DOI] [PubMed] [Google Scholar]
- Sondergaard AP, Hjortdal J, Breitenbach T, Ivarsen A. Corneal Distribution of Riboflavin prior to Collagen Cross-Linking. Curr Eye Res. 2010;35:116–121. doi: 10.3109/02713680903431847. [DOI] [PubMed] [Google Scholar]
- Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- Spoerl E, Seiler T. Techniques for stiffening the cornea. J Refract Surg. 1999;15:711–713. doi: 10.3928/1081-597X-19991101-21. [DOI] [PubMed] [Google Scholar]
- Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-Riboflavin Cross-Linking of the Cornea. Cornea. 2007;26:385–389. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- Spoerl E, Raiskup F, Kampik D, Geerling G. Correlation between UV Absorption and Riboflavin Concentration in Different Depths of the Cornea in CXL. Curr Eye Res. 2010;35:1040–1041. doi: 10.3109/02713683.2010.506969. [DOI] [PubMed] [Google Scholar]
- Swinger CA, Kornmehl EW. Dehydration of post-mortem eyes for practice and experimental surgery. Ophthalmic Surg. 1985;16:182–183. [PubMed] [Google Scholar]
- Tao A, Wang J, Chen Q, Shen M, Lu F, Dubovy SR, Shousha MA. Topographic Thickness of Bowman’s Layer Determined by Ultra-High Resolution Spectral Domain–Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2011;52:3901–3907. doi: 10.1167/iovs.09-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinciguerra R, Romano MR, Camesasca FI, Azzolini C, Trazza S, Morenghi E, Vinciguerra P. Corneal Cross-Linking as a Treatment for Keratoconus: Four-Year Morphologic and Clinical Outcomes with Respect to Patient Age. Ophthalmology. 2013;120:908–916. doi: 10.1016/j.ophtha.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Winkler M, Chai D, Kriling S, Nien CJ, Brown DJ, Jester B, Juhasz T, Jester JV. Nonlinear optical macroscopic assessment of 3-d corneal collagen organization and axial biomechanics. Invest Ophthalmol Vis Sci. 2011;52:8818–8827. doi: 10.1167/iovs.11-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollensak G, Aurich H, Wirbelauer C, Sel S. Significance of the riboflavin film in corneal collagen crosslinking. J Cataract Refract Surg. 2010;36:114–120. doi: 10.1016/j.jcrs.2009.07.044. [DOI] [PubMed] [Google Scholar]
- Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol. 2006;17:356–360. doi: 10.1097/01.icu.0000233954.86723.25. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Iomdina E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. J Cataract Refract Surg. 2009a;35:540–546. doi: 10.1016/j.jcrs.2008.11.036. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Iomdina E. Long-term biomechanical properties of rabbit cornea after photodynamic collagen crosslinking. Acta Ophthalmol. 2009b;87:48–51. doi: 10.1111/j.1755-3768.2008.01190.x. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003a;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003b;29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- Ziebarth NM, Arrieta E, Feuer WJ, Moy VT, Manns F, Parel J. Primate lens capsule elasticity assessed using Atomic Force Microscopy. Exp Eye Res. 2011;92:490–494. doi: 10.1016/j.exer.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


