Abstract
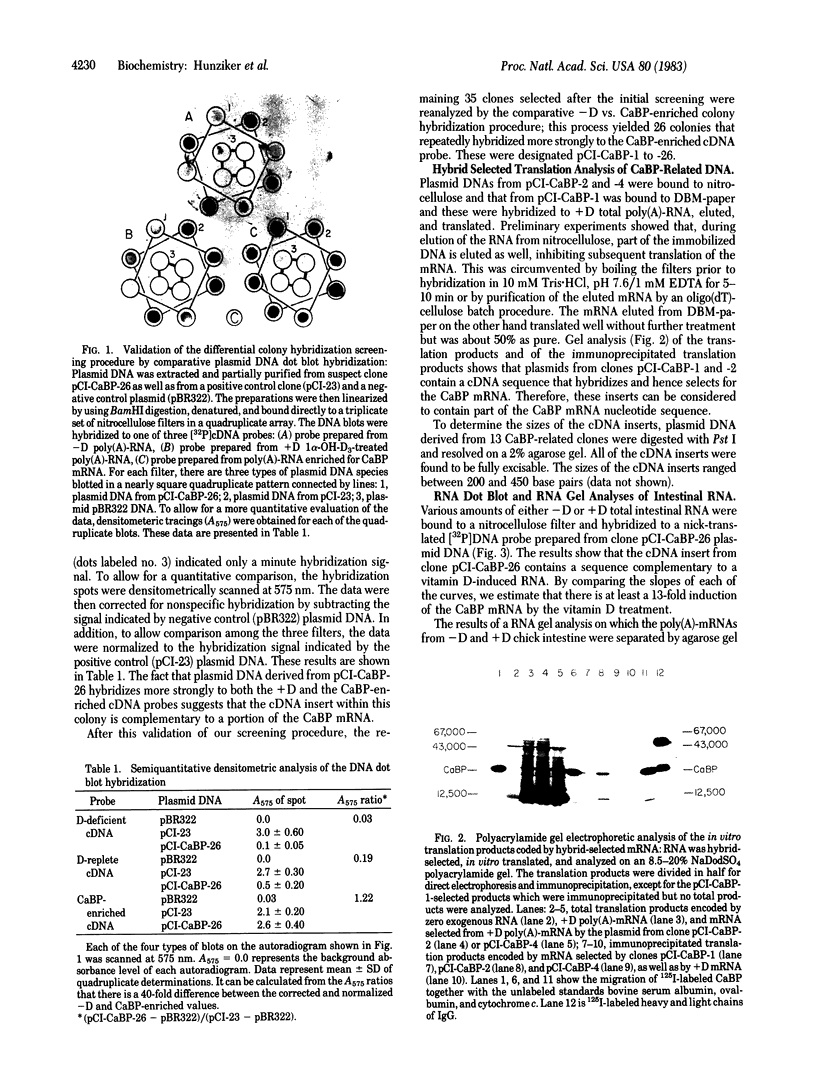
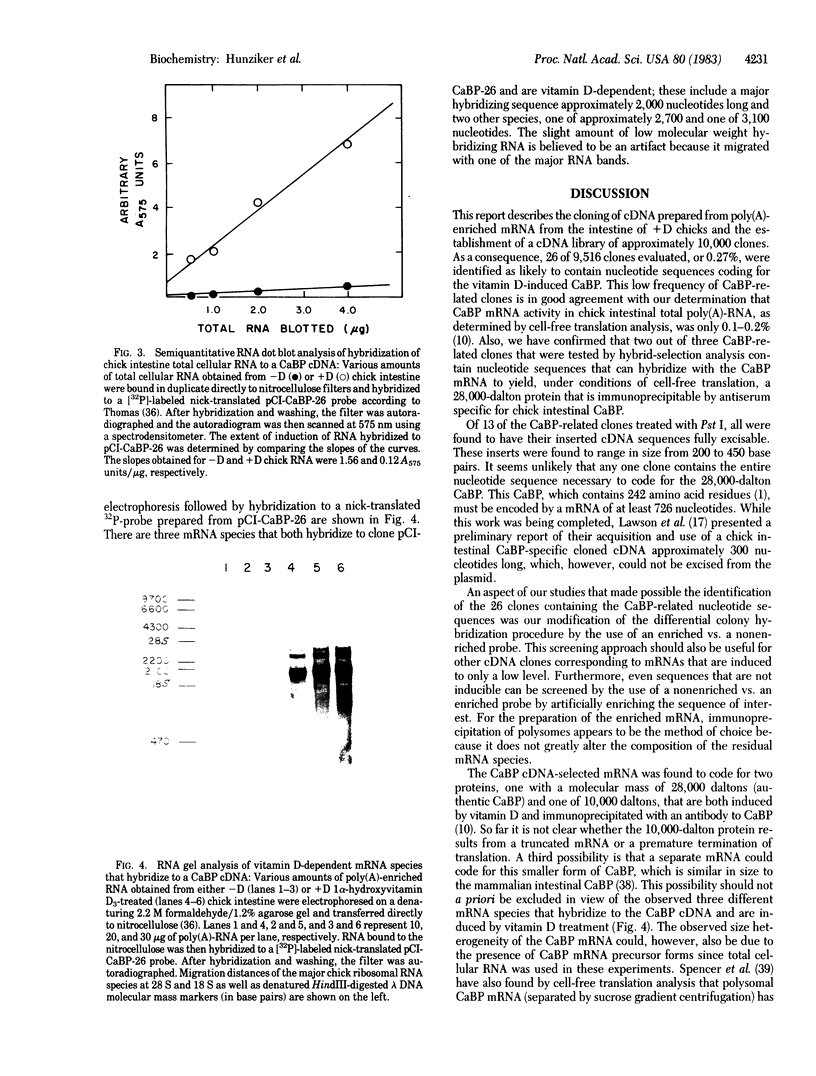
We have constructed a recombinant cDNA library to facilitate study of the genomic actions of vitamin D3 and its hormonally active metabolite 1,25-dihydroxyvitamin D3 in initiation of the de novo biosynthesis of a 28,000-dalton vitamin D-dependent calcium binding protein (CaBP) present in chick intestine. The recombinant plasmids were prepared by the homopolymeric tailing and hybridization method using as a starting template poly(A)-enriched mRNA obtained from the intestinal mucosa of vitamin D3-replete (+D) chicks. Screening of 9,516 clones in this library was effected by using a comparative in situ colony hybridization technique with two [32P]cDNA probes; these probes were prepared from total poly(A)-RNA from chick intestinal mucosa of vitamin D-deficient (-D) chicks and a poly(A)-RNA specifically enriched for chick intestinal CaBP mRNA by immunoprecipitation of polysomes derived from vitamin D-replete (+D) chicks. We identified 26 clones that consistently displayed a significantly increased hybridization signal when comparing the -D vs. CaBP-enriched probe. Further evaluation of these clones by hybrid-selected translation showed the presence of CaBP-specific sequences. By "RNA gel" analysis of poly(A)-RNA, three independent mRNA species were found to hybridize to a CaBP clone; none of these RNA species were found in -D poly(A)-RNA. With this comparative colony hybridization procedure, we were able to identify CaBP-specific clones corresponding to a mRNA that is 0.1% of the total poly(A)-mRNA. The differential colony hybridization procedure using an enriched vs. a nonenriched probe should be of value in screening for other cDNA clones complementary to rare mRNA species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles M. A., Martial J., Zolock D., Morrissey R., Baxter J. D. Regulation of calcium-binding protein messenger RNA by 1,25-dihydroxycholecalciferol. Calcif Tissue Int. 1981;33(1):15–18. doi: 10.1007/BF02409407. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christakos S., Friedlander E. J., Frandsen B. R., Norman A. W. Studies on the mode of action of calciferol. XIII. Development of a radioimmunoassay for vitamin D-dependent chick intestinal calcium-binding protein and tissue distribution. Endocrinology. 1979 May;104(5):1495–1503. doi: 10.1210/endo-104-5-1495. [DOI] [PubMed] [Google Scholar]
- Christakos S., Norman A. W. Vitamin D-dependent calcium-binding protein synthesis by chick kidney and duodenal polysomes. Arch Biochem Biophys. 1980 Sep;203(2):809–815. doi: 10.1016/0003-9861(80)90242-8. [DOI] [PubMed] [Google Scholar]
- Christakos S., Norman A. W. Vitamin D3-induced calcium binding protein in bone tissue. Science. 1978 Oct 6;202(4363):70–71. doi: 10.1126/science.211584. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradino R. A., Wasserman R. H. Actinomycin D inhibition of Vitamin D-3-induced calcium-binding protein (CaBP) formation in chick duodenal mucosa. Arch Biochem Biophys. 1968 Sep 10;126(3):957–960. doi: 10.1016/0003-9861(68)90491-8. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Emtage J. S., Lawson D. E., Kodicek E. Vitamin D-induced synthesis of mRNA for calcium-binding protein. Nature. 1973 Nov 9;246(5428):100–101. doi: 10.1038/246100a0. [DOI] [PubMed] [Google Scholar]
- Fullmer C. S., Wasserman R. H. The amino acid sequence of bovine intestinal calcium-binding protein. J Biol Chem. 1981 Jun 10;256(11):5669–5674. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hobden A. N., Harding M., Lawson D. E. 1,25-Dihydroxycholecalciferol stimulation of a mitochondrial protein in chick intestinal cells. Nature. 1980 Dec 25;288(5792):718–720. doi: 10.1038/288718a0. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Walters M. R., Bishop J. E., Norman A. W. Effect of vitamin D status on the equilibrium between occupied and unoccupied 1,25-dihydroxyvitamin D intestinal receptors in the chick. J Clin Invest. 1982 Apr;69(4):826–833. doi: 10.1172/JCI110522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick N. C., Barr C. R., Moriarity D., DeLuca H. F. Effect of vitamin D deficiency on in vitro labeling of chick intestinal proteins: analysis by two-dimensional electrophoresis. Biochemistry. 1981 Sep 1;20(18):5288–5294. doi: 10.1021/bi00521a030. [DOI] [PubMed] [Google Scholar]
- Laouari D., Pavlovitch H., Deceneux G., Balsan S. A vitamin D-dependent calcium-binding protein in rat skin. FEBS Lett. 1980 Mar 10;111(2):285–289. doi: 10.1016/0014-5793(80)80811-8. [DOI] [PubMed] [Google Scholar]
- Law S., Tamoaki T., Kreuzaler F., Dugaiczyk A. Molecular cloning of DNA complementary to a mouse alpha-fetoprotein mRNA sequence. Gene. 1980 Jun;10(1):53–61. doi: 10.1016/0378-1119(80)90143-2. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marche P., Pradelles P., Gros C., Thomasset M. Radioimmunoassay for a vitamin-D dependent calcium-binding protein in rat duodenal mucosa. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1020–1026. doi: 10.1016/0006-291x(77)90958-5. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Wong R. G. Biological activity of the vitamin D metabolite 1,25-dihydroxycholecalciferol in chickens and rats. J Nutr. 1972 Dec;102(12):1709–1718. doi: 10.1093/jn/102.12.1709. [DOI] [PubMed] [Google Scholar]
- Price P. A., Baukol S. A. 1,25-Dihydroxyvitamin D3 increases synthesis of the vitamin K-dependent bone protein by osteosarcoma cells. J Biol Chem. 1980 Dec 25;255(24):11660–11663. [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. Z., Young J. R. An immunochemical method for mRNA purification. Application to messenger RNA encoding trypanosome variable surface antigen. J Biol Chem. 1981 Feb 25;256(4):1495–1498. [PubMed] [Google Scholar]
- Siebert P., Hunziker W., Norman A. W. Cell-free translation analysis of the vitamin D-dependent calcium binding protein mRNA activity present in total RNA and polysomal extracts from chick intestine. Arch Biochem Biophys. 1982 Dec;219(2):286–296. doi: 10.1016/0003-9861(82)90159-x. [DOI] [PubMed] [Google Scholar]
- Spencer R., Charman M., Lawson D. E., Emtage J. S. Production and properties of vitamin-D-induced mRNA for chick calcium-binding protein. Eur J Biochem. 1976 Dec 11;71(2):399–409. doi: 10.1111/j.1432-1033.1976.tb11127.x. [DOI] [PubMed] [Google Scholar]
- Spencer R., Charman M., Lawson D. E., Emtage J. S. Production and properties of vitamin-D-induced mRNA for chick calcium-binding protein. Eur J Biochem. 1976 Dec 11;71(2):399–409. doi: 10.1111/j.1432-1033.1976.tb11127.x. [DOI] [PubMed] [Google Scholar]
- Spencer R., Charman M., Lawson D. E. Stimulation of intestinal calcium-binding-protein mRNA synthesis in the nucleus of vitamin D-deficient chicks by 1,25-dihydroxycholecalciferol. Biochem J. 1978 Dec 1;175(3):1089–1094. doi: 10.1042/bj1751089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H. C., Norman A. W. Studies on calciferol metabolism. 8. Evidence for a cytoplasmic receptor for 1,25-dihydroxy-vitamin D3 in the intestinal mucosa. J Biol Chem. 1973 Sep 10;248(17):5967–5975. [PubMed] [Google Scholar]
- Tsai H. C., Norman A. W. Studies on the mode of action of calciferol. VI. Effect of 1,25-dihydroxy-vitamin D3 on RNA synthesis in the intestinal mucosa. Biochem Biophys Res Commun. 1973 Sep 18;54(2):622–627. doi: 10.1016/0006-291x(73)91468-x. [DOI] [PubMed] [Google Scholar]
- Tsai H. C., Wong R. G., Norman A. W. Studies on calciferol metabolism. IV. Subcellular localization of 1,25-dihydroxy-vitamin D 3 in intestinal mucosa and correlation with increased calcium transport. J Biol Chem. 1972 Sep 10;247(17):5511–5519. [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Vitamin d3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966 May 6;152(3723):791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- Wilson P. W., Lawson D. E. Incorporation of [3H]leucine into an actin-like protein in response to 1,25-dihydroxycholecalciferol in chick intestinal brush borders. Biochem J. 1978 Aug 1;173(2):627–631. doi: 10.1042/bj1730627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerwekh J. E., Haussler M. R., Lindell T. J. Rapid enhancement of chick intestinal DNA-dependent RNA polymerase II activity by 1 alpha, 25-dihydroxyvitamin D3, in vivo. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2337–2341. doi: 10.1073/pnas.71.6.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]