Abstract
Endocan is a secreted proteoglycan that has been shown to indicate angiogenic activity: remodeling in several tumor types in humans and mice. Serum endocan levels also indicate prognosis and has been proposed as a biomarker for certain cancers. Recently, monoclonal antibodies directed against mouse endocan have been developed allowing for further characterization of endocan function and potentially as a marker for angiogenesis through immunoreactivity in endothelial tip cells. The results of the current study show that endocan immunoreactivity in the mouse brain is present in blood vascular networks including but not limited to the cortex, hippocampus and paraventricular nucleus of the hypothalamus in C57BL/6J and FVB/N mice. Endocan immunoreactivity did not vary during postnatal development or by sex. Interestingly, after vascular perfusion with fluorescein isothiocyanate (FITC), endothelial cells positive for FITC were immunonegative for endocan suggesting FITC interference with the immunohistochemistry. A small number of FITC-negative blood vessels were endocan immunoreactive suggesting the identification of new blood vessels that are not yet functional. The current study shows that endocan is normally present in the mouse brain and prior vascular perfusion with FITC may provide a useful tool for identify newly forming blood vessels.
Keywords: endocan, Fluorescein isothiocyanate, brain
1. Introduction
Endocan, previously known as endothelial cell-specific molecule-1 (ESM-1), was identified with its localization restricted to endothelial cells (Lassalle et al., 1996). Endocan is a secreted dermatan sulfate proteoglycan that has been suggested to promote angiogenesis (Chen et al., 2012). Elevated levels of endocan mRNA negatively correlate with cancer survival rates and overexpression of endocan leads to tumor formation (Scherpereel et al., 2003; Depontieu et al, 2012). High endocan mRNA levels in human tumor tissue correlates with prognosis and is proposed to serve as a biomarker for inflammatory disorders and cancer development and continues to be investigated as a target for cancer therapy (Sarrazin et al., 2006). Increased levels of endocan have been detected in the serum of sepsis patients (Sarrazin et al., 2006; Sarrazin et al., 2010; Scherpereel et al., 2003). Overall, endocan has shown promise as an indicator of angiogenesis and disease progression.
Endocan has also been studied in activated endothelial cells referred to as tip cells, which indicate newly forming blood vessels (Sarrazin et al., 2010, Del Toro et al. 2010). Endocan mRNA is upregulated on tumor-associated blood vessels and it is proposed that modification of endocan interactions with vascular endothelial growth factor receptors may inhibit tumor angiogenesis (Roudnicky et al., 2013). However, beyond its detection in endothelial cells undergoing angiogenesis, the role of endocan is not well understood.
To visualize and characterize endocan distributions, monoclonal antibodies were generated to study roles in angiogenesis, cancer, and other diseases. Antibody clones MEP14 and MEP19 were generated against the C-terminus of human endocan and recognize both rat and mouse endocan. (Depontieu et al, 2012). The generation of these antibodies may provide a useful tool to characterize changes in the distribution or levels of immunoreactive endocan under normal or disease states, and potentially its function.
The goal of the current study was to use selective antibodies directed against endocan to study the developing blood vessel network within the paraventricular nucleus of the hypothalamus (PVN). The PVN develops an unusually dense vasculature following a postnatal angiogenic period that occurs between postnatal (P) days 8–12 in the mouse (Frahm et al., 2012). The current study examined endocan as a potential marker for angiogenesis, which within the PVN may be coordinated with the postnatal angiogenic period.
In examining blood vessels and blood-brain barrier competency, several studies have utilized the small molecule dye fluorescein isothiocyanate (FITC). When perfused through the vasculature, FITC accumulates in endothelial cell nuclei and binds covalently to cellular components (Miyata & Morita, 2011). This allows for visualization of functional blood vessels and the ability to double or triple label for other proteins of interest in relevant vasculature. Extravascular FITC leakages can also indicate a compromised blood-brain barrier (Miyata & Morita, 2011). Mouse brains were processed for endocan with or without prior vasculature perfusion of FITC.
Overall, the current experiments demonstrated that immunoreactive endocan is present in a pattern that mirrors the vasculature throughout the brain only in certain mouse strains. Prior vascular perfusion with FITC prevented detection of immunoreactive endocan. Therefore, the use of FITC may provide a novel method to identify non-functional blood vessels using immunoreactive endocan as a marker.
2. Materials and Methods
2.1. Animals
Male and female mice were on a mixed C57BL/6JxS129xCBA background (Solomon et al., 2012), or pure bred C57BL/6J or FVB/N backgrounds. The day of birth was designated P0. For tissue collection, mice were anesthetized by ketamine (80 mg/kg) and xylazine (8 mg/kg) and transcardially perfused with heparanized PBS with or without FITC (ThermoFisher Scientific, MW 389.38) followed by 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4; modified from Miyata & Morita, 2011). Brains were removed, post fixed overnight, then changed into 0.1M phosphate buffer for storage at 4°C.
Mice were maintained in plastic cages with aspen bedding (autoclaved Sani-chips, Harlan Teklad, Madison, WI, USA) in the Painter Building of Laboratory Animal Resources at Colorado State University. Food (#8640, Harlan Teklad, Madison, WI, USA) with filtered tap water and environmental enrichment was provided ad libitum in a 14/10h light/dark cycle. Animal care and handling was in accordance with the Colorado State University Animal Care and Use Committee guidelines.
2.2 Immunohistochemistry
Tissue was processed as previously described (Frahm et. al., 2012) in an antigen retrieval immunohistochemical protocol. Briefly, brains were embedded in 5% agarose and cut coronally into 50μm sections using a vibrating microtome (Leica VT1000S). Free-floating serial sections were collected in 0.05M phosphate-buffered saline (PBS), pH 7.5. Excess unreacted aldehydes were neutralized in 0.1M glycine followed by 0.5% sodium borohydride. Sections were washed in room temperature PBS then were washed in sodium citrate (0.05 M, pH 8.6). The sections were then placed into sodium citrate buffer preheated to 80°C to promote antigen retrieval (Dellovade et al., 2001). They were allowed to slowly come back to room temperature after which they were returned to PBS for additional washes. Sections were washed in PBS then incubated in a PBS blocking solution (5% normal goat serum (NGS), 0.5% Triton X-100 (Tx), and 1% hydrogen peroxide). Sections were then incubated in primary monoclonal antibodies directed against endocan (either clone MEP14 or MEP19, Lunginnov, Lille, France) or platelet endothelial cell adhesion molecule (PECAM also known as CD31, 1:30; BD Biosciences, San Jose, CA, USA). All sections were incubated at 4°C overnight in primary antibodies. Sections were then washed in room temperature with 1% NGS and 0.02% Tx in PBS. Sections were incubated with the appropriate secondary antibodies for either biotin conjugated donkey anti-mouse antibodies (1:2500; Jackson Immunoresearch, West Grove, PA), biotin conjugated donkey anti-mouse (1:1000; Jackson Immunoresearch) or Cy3 conjugated anti-mouse (1:200, Jackson Immunoresearch) in PBS containing 1% NGS and 0.32% Tx. For brightfield, sections were incubated in a Vectastain reagent (3μl/ml solutions A and B - Vectastain ABC Elite kit; Vector Laboratories, Burlingame, CA) at room temperature. After washing in Tris-buffered saline (pH 7.5), reaction product was developed in Tris-buffered saline containing 0.025% diaminobenzidine, 0.02% nickel, and 0.02% hydrogen peroxide.
2.3. Data Collection
Brightfield images were acquired using an Olympus BH2 microscope with an Insight QE digital camera in Spot Advanced Software. Fluorescent images were acquired on a Zeiss 510-Meta laser-scanning confocal microscope. FITC fluorescence was imaged using a 488/543 nm bandpass filter and emission detected using a 505/530 nm bandpass emission filter. Cy3 fluorescence indicating endocan was imaged using a 488/543 nm bandpass filter and emission detected using a 585/615 nm bandpass emission filter. Z-stacks were taken with 6 layers every 3μm obtained at 40x magnification using oil immersion objectives.
3. Results
3.1. Endocan immunoreactivity at postnatal day 12
Brains at P12 were examined for immunoreactive endocan during the postnatal angiogenic period specific to the PVN (Frahm et al., 2012). The distribution of immunoreactive endocan resembled the normal blood vessel pattern in the PVN, cortex (CTX) and Hippocampus (figure 1). Previous studies examining tumors showed that endocan mRNA correlated with newly forming blood vessels (Sarrazin et al., 2010, Del Toro et al. 2010). However, the current findings suggest immunoreactive endocan is detectable within virtually all of the vasculature of the brain at P12 in FVB/N mice.
Figure 1.
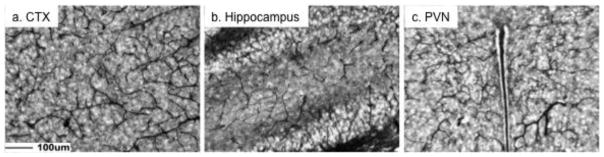
Immunoreactive endocan was distributed throughout the mouse brain at postnatal day 12. There was a global blood vessel pattern of immunoreactive endocan that is exemplified by images from the cortex (a. CTX), hippocampus (b), and paraventricular nucleus of the hypothalamus (c. PVN). Scale bar = 100μm.
3.2. Endocan immunoreactivity at postnatal day 20
To determine if the distribution of immunoreactive endocan varied by age, brains from FVB/N (figures 2a–c in brightfield) and C57BL/6J (figures 2d–g by immunofluorescence) mice were examined at P20. These strains were selected because they are commonly utilized in our laboratory and others. The distribution pattern of immunoreactive endocan again was consistent with general blood vessel patterns. There was immunoreactive signal throughout the brain, although images are specifically provided for cortex (figure 2a,d), hippocampus (figure 2b,e) and PVN (figure 2c, f) in C57BL/6J and FVB/N background mice. There was no labeling in the absence of primary antibody in control sections (figure 2g).
Figure 2.
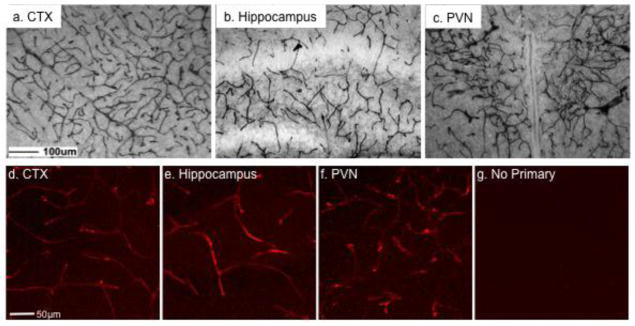
Immunoreactive endocan was distributed throughout the brain in C57BL/6J background mice on postnatal day 20. There was a global blood vessel pattern of immunoreactive endocan that is exemplified by images from the cortex (a, d; CTX), hippocampus (b, e), and paraventricular nucleus of the hypothalamus (c, f; PVN). Immunoreactivity was not detected in the absence of primary antibodies (g). Scale bar = 100μm for top images and 50μm for lower images.
3.3. Endocan immunoreactivity in FVB/N and C57BL/6J xS129xCBA mixed background mice at postnatal day 20
We next determined endocan immunoreactivity at P20 in a C57BL/6J xS129xCBA mixed background mouse used in unrelated experiments due to a specific gene disruption (Solomon et al., 2012). C57BL/6JxS129xCBA mice not containing the altered allele were examined alongside FVB/N mice. The distribution of immunoreactive endocan was similar in the cortex, hippocampus and PVN in FVB/N mice (figure 3a–c) compared to C57BL/6J mice (figure 1a–c). Matched sections demonstrate there was no endocan immunoreactivity in the cortex, hippocampus or PVN (3d–f) of C57BL/6J xS129xCBA mice using MEP19.
Figure 3.
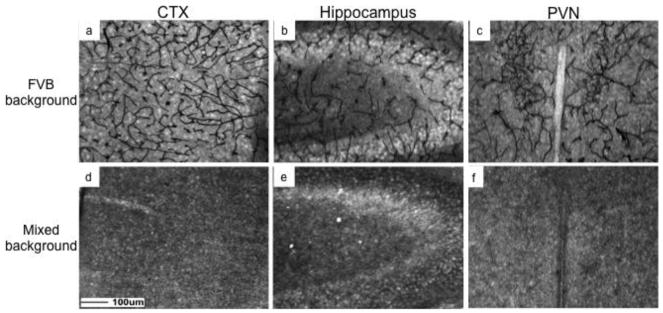
Immunoreactive endocan was distributed throughout the brain in FVB/N but not mixed C57BL/6J/S129/CBA background mice on postnatal day 20. There was a global blood vessel pattern of immunoreactive endocan in the cortex (a. CTX), hippocampus (b), and paraventricular nucleus of the hypothalamus (c. PVN) of FVB/N, but no immunoreactivity in C57BL/6J/S129/CBA background mice (d–f). Scale bar = 100μm.
3.4. Prior vascular perfusion of FITC blocked Endocan immunoreactivity
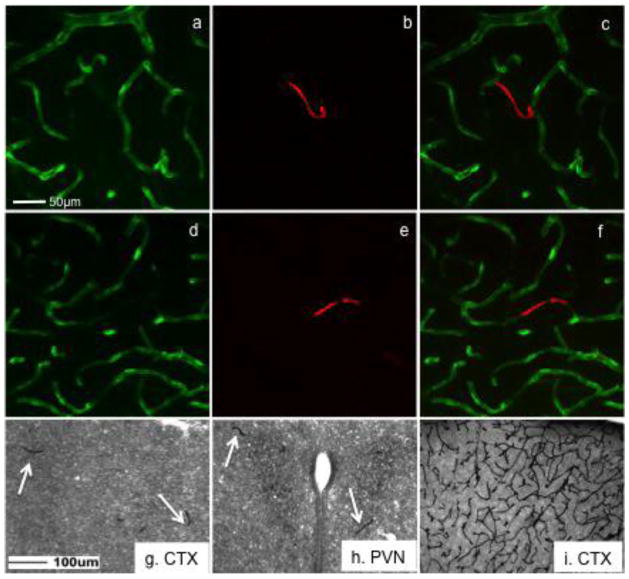
The perfusion of FITC allows for visualization of blood vessels and the localization of its extravascular leakage that likely indicates compromise of blood-brain barrier function (Miyata & Morita, 2011). However, in C57BL/6J and FVB/N mice, vascular perfusion of FITC prior to application of antibodies directed against endocan blocked endocan immunoreactivity (figure 4a, 4g). Images in figure 4a and 4d show blood vessels labeled with FITC indicating functional blood vessels. Only restricted blood vessels or blood vessels devoid of FITC contained endocan immunoreactivity (figure 4b, e). Confocal microscopy showed no overlap or colocalization between FITC and endocan (figure 4c, f). Brightfield images also showed immunoreactive endocan in a much more restricted number of blood vessels (figure 4g, h). Labeling with antibodies against platelet endothelial cell adhesion molecule (PECAM), a protein also present in endothelial cells, was not impacted due to prior FITC perfusion (figure 4i). These studies suggest that vascular perfusion of FITC prior to endocan processing might identify developing blood vessels that are not yet functional.
Figure 4.
Vascular perfusion of FITC allowed identification of cerebral vasculature, but blocked detection of endocan immunoreactivity. Images of FITC labeled blood vessels (a,d) show lack of colocalization with endocan (c,f). Immunoreactive endocan was only found in blood vessels devoid of FITC (b,e). Vascular perfusion of FITC did not block detection of immunoreactive platelet endothelial cell adhesion molecule (i). This procedure may reveal prefunctional blood vessels in the brain (g, h). Scale bar = 50μm for top images and 100μm for lower images.
4. Discussion
Elevated endocan has been observed in tumor endothelial cells in human glioblastomas arising from cell types such as astrocytes and oligodendrocytes (Maurage et al., 2009) and pituitary adenomas (Cornelius et al., 2012). Endocan immunoreactivity has also been found in normal human pituitary tissue specifically in endocrine cells, demonstrating that endocan is not limited to endothelial cells (Cornelius et al., 2012). Previous studies have also identified endocan as a potential marker for angiogenesis (Sarrazin et al., 2010, Roudnicky et al., 2013). However, the recent production of antibodies directed against endocan allows a greater sensitivity for visualizing its presence and distribution, particularly in the mouse brain. Instead of being in a tip cell distribution, immunoreactive endocan was visualized in a global blood vessel pattern at P12 and P20 in brains from C57BL/6J and FVB/N mice. This identification of endocan immunoreactivity in mouse brain endothelium calls for further examination of its potential function in mouse vasculature. The presence of immunoreactive endocan in the endothelial cells of the brain vasculature may provide insight into the potential source of endocan detected in the serum of healthy humans and mice (Depontieu et al, 2012).
Prior studies using northern blot and in situ hybridization analyses of mouse brain (Abid et al., 2006) or human tissue (Lassalle et al., 1996) were unable to detect endocan. There are at least 2 potential reasons for lack of mRNA when immunoreactive protein is found. First, the sensitivity of the mRNA methods may have been lower than the sensitivity of the immunohistochemistry with the new monoclonal antibodies used in the current study. Secondly, it is possible that since endocan is a secreted proteoglycan, that immunoreactive endocan in brain is accumulated from the circulation after synthesis in peripheral sites. However, the detection of immunoreactive endocan in vessels that likely were not exposed to vascular perfusion makes this less probable. In addition, endocan present in the brain vasculature may provide a source for releasable endocan that may diffuse away and regulate other distant processes (Sarrazin et al., 2010) opening a new avenue for investigation.
The antibodies used in this study generated against endocan have been previously shown to detect immunoreactivity in C57BL/6J and 129Sv mice, but not BALB/c mice (Depontieu et al, 2012). In the current study, there was a lack of endocan immunoreactivity in C57BL/6JxS129xCBA background mice. Clearly, strain background will be important for future studies examining endocan immunoreactivity. The MEP14 monoclonal antibody used in this study recognizes the 6 last C-terminus amino acids, identical in human, mouse and rats (Depontieu et al., 2012). This antibody was selected because it has been shown that in the absence or mutation of these 6 amino acids MEP14 did not recognize the peptide while other clones did not show this specificity. For both strains in which immunoreactive endocan was seen it was globally maintained in mouse endothelial cells within the brain. Western blots further confirmed the specificity of this antibody because immunoreactive endocan was detectable in total brain but not in the pituitary (data not shown). A previous study showed a dissimilar pattern to the findings presented in this study for endocan immunoreactivity. In human brain tissue endocan was localized in neurons and not neuroglia or blood vessels (Zhang et al., 2012). The antibodies utilized were generated by injecting purified recombinant human endocan protein into BALB/c mice, and does not indicate the specificity of their antibody. This suggests that these recently commercially available antibodies may be useful to further investigating the normal and disease state expression of endocan in a variety of tissues and cells types.
In addition to visualizing endocan immunoreactivity in numerous contexts, endocan labeling in brains taken from mice perfused with FITC may provide a tool to visualize newly forming blood vessels. There was an unexpected immunoreactive pattern in brains following vascular perfusion with FITC. Initially after finding poor immunolabeling, additional sections were retested with new antibody lots (graciously donated by Lunginnov). Upon further experiments it became clear that only brains either from the C57BL/6J xS129xCBA background or perfused with FITC had this appearance. Experiments comparing FVB/N and C57BL/6J xS129xCBA with or without FITC perfusion confirmed these findings. Only non-FITC perfused brains from C57BL/6J or FVB/N mice showed immunoreactive endocan. A preliminary experiment attempted to block endocan labeling by adding FITC to the blocking step of a western blot. Although there was a decrease, it did not result in a total loss of labeling (data not shown). To determine if FITC perfusion impacted the immunoreactivity of other proteins in endothelial cells, FITC brains were processed for immunoreactive PECAM. The labeling of antibodies against PECAM was not altered due to FITC perfusion. Although the exact mechanism for the lack of endocan immunolabeling in FITC positive vasculature remains unknown, there is a strong potential benefit of this reliable finding. FITC labels and identifies blood vessels in which blood/perfusate can flow. Only if the dye reaches the endothelial cell will they be stained. Endocan antibodies can recognize the proteoglycan, regardless of whether it is part of a functional blood vessel. Images acquired using confocal microscopy showed endocan-positive blood vessels connecting with FITC-positive blood vessels, with no colocalization. Therefore, vascular perfusion with FITC followed by examination of immunoreactive endocan may provide a tool for viewing nonfunctional blood vessels.
Overall, immunoreactive endocan was detected abundantly in a pattern consistent with the majority of cerebral vasculature in the mouse brain. At P12 and P20, C57BL/6J and FVB/N mice showed this distribution pattern, while immunoreactive endocan was absent in a C57BL/6J xS129xCBA mixed background. Brains perfused with FITC only had endocan immunoreactivity where FITC was not present, perhaps indicating locations where blood vessels are not yet functional. In conclusion, immunoreactive endocan provides another tool for examining cerebral vasculature and in the presence of FITC perfusion may also provide a tool to visualize newly forming blood vessels.
Acknowledgments
We thank Lunginnov for their generous provision of additional antibodies. We thank Circe McDonald for technical support and Drs. Jill Goldstein and Robert Handa for helpful comments throughout these experiments. This work was supported by NIH grant ORWH-NIMH P50-MH 082679 (SAT, JG, RH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abid MR, Yi X, Yano K, Shih SC, Aird WC. Vascular endocan is preferentially expressed in tumor endothelium. Microvasc Res. 2006;72:136–145. doi: 10.1016/j.mvr.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Chen LY, Liu X, Wang SL, Qin CY. Over-expression of the Endocan Gene in Endothelial Cells from Hepatocellular Carcinoma is Associated with Angiogenesis and Tumour Invasion. J Int Med Res. 2012;38:498–510. doi: 10.1177/147323001003800213. [DOI] [PubMed] [Google Scholar]
- Cornelius A, Cortet-Rudelli C, Assaker R, Kerdraon O, Gevaert MH, Prevot V, Lassalle P, Trouillas J, Delhedde M, Mauragr CA. Endothelial expression of endocan is strongly associated with tumor progression in pituitary adenoma. Brain Pathol. 2012;22:757–764. doi: 10.1111/j.1750-3639.2012.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Toro R, Prahst C, Mathivet T, Siegfried G, Kaminker JS, Larrivee B, Breant C, Duarte A, Takakura N, Fukamizu A, Penninger J, Eichmann A. Identification and functional analysis of endothelial tip cell-enriched genes. Blood. 2010;116:4025–4033. doi: 10.1182/blood-2010-02-270819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellovade TL, Davis AM, Ferguson C, Sieghart W, Homanics GE, Tobet SA. GABA influences the development of the ventromedial nucleus of the hypothalamus. J Neurobiol. 2001;49:264–276. doi: 10.1002/neu.10011. [DOI] [PubMed] [Google Scholar]
- Depontieu F, de Freitas Caires N, Gourcerol D, Giordano J, Grigoriu B, Delehedde M, Lassalle P. Development of monoclonal antibodies and ELISA specific for the mouse vascular endocan. J Immunol Methods. 2012;378:88–94. doi: 10.1016/j.jim.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Frahm KA, Schow MJ, Tobet SA. The vasculature within the paraventricular nucleus of the hypothalamus in mice varies as a function of development, subnuclear location, and GABA signaling. Horm Metab Res. 2012;44:1–6. doi: 10.1055/s-0032-1304624. [DOI] [PubMed] [Google Scholar]
- Lassalle P, Molet S, Janin A, Heyden JV, Tavernier J, Fiers W, Devos R, Tonnel AB. ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem. 1996;271:20458–20464. doi: 10.1074/jbc.271.34.20458. [DOI] [PubMed] [Google Scholar]
- Maurage CA, Adam E, Mineo JF, Sarrazin S, Debunne M, Siminski RM, Baroncini M, Blond S, Delehedde M. Endocan expression and localization in human glioblastomas. J Neuropathol Exp Neurol. 2009;68:633–641. doi: 10.1097/NEN.0b013e3181a52a7f. [DOI] [PubMed] [Google Scholar]
- Miyata S, Morita S. A new method for visualization of endothelial cells and extravascular leakage in adult mouse brain using fluorescein isothiocyanate. J Neurosci Methods. 2011;202:9–16. doi: 10.1016/j.jneumeth.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Roudnicky F, Poyet C, Wild P, Krampitz S, Negrini F, Huggenberger R, Rogler A, Strohr A, Hartmann A, Provenzano M, Otto VI, Detmar M. Endocan is upregulated on tumor vessels in invasive bladder cancer where it mediates VEGF-A-induced angiogenesis. Cancer Res. 2013;73:1097–1106. doi: 10.1158/0008-5472.CAN-12-1855. [DOI] [PubMed] [Google Scholar]
- Scherpereel A, Gentina T, Grigoriu B, Sénéchal S, Janin A, Tsicopoulos A, Plénat F, Béchard D, Tonnel AB, Lassalle P. Overexpression of Endocan Induces Tumor Formation. Cancer Res. 2003;63:6064–6089. [PubMed] [Google Scholar]
- Sarrazin S, Adam E, Lyon M, Depontieu F, Motte V, Landolfi C, Lortat-Jacob H, Bechard D, Lassalle P, Delehedde M. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta. 2006;1765:25–37. doi: 10.1016/j.bbcan.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Sarrazin S, Lyon M, Deakin JA, Guerrini M, Lassalle P, Delehedde M, Lortat-Jacob H. Characterization and binding activity of the chondroitin/dermatan sulfate chain from Endocan, a soluble endothelial proteoglycan. Glycobiology. 2010;20:1380–1388. doi: 10.1093/glycob/cwq100. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, Wulsin AC, Herman JP. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–43. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SM, Zuo L, Zhou Q, Gui SY, Shi R, Wu Q, Wei W, Wang Expression and distribution of endocan in human tissue. Biotech Histochem. 2012;87:172–178. doi: 10.3109/10520295.2011.577754. [DOI] [PubMed] [Google Scholar]