Abstract
Purpose
To evaluate the ability of cone-beam computed tomography (CBCT) acquired directly after TACE to assess lipiodol deposition in hepatocellular carcinoma (HCC) and compare it to unenhanced MDCT.
Materials and methods
Fifteen HCC patients were treated with conventional TACE, and CBCT was used to assess the lipiodol deposition directly after TACE. Unenhanced MDCT was performed 24 hours after TACE. Four patients were excluded because the margin of tumor or area of lipiodol deposition was not clear. The image enhancement density of the entire tumor (T) and liver parenchyma (L) was measured by ImageJ software, and tumor-to-liver contrast (TLC) was calculated. In addition, volumetric measurement of tumor and lipiodol was performed by semiautomatic 3D volume segmentation and compared using linear regression to evaluate consistency between two imaging modalities.
Results
The mean value of TLC on CBCT was not significantly different from that on MDCT (337.7±233.5 HU versus 283.0±152.1 HU, P=0.103). The average volume of the whole tumor and of only the lipiodol deposited regions, and the calculated average percentage of lipiodol retention on CBCT were not significantly different when compared to MDCT (tumor volume: 9.6±11.8 cm3 versus 10.8±14.2 cm3, P=0.142; lipiodol volume: 6.3±7.7 cm3 versus 7.0±8.1 cm3, P=0.214; percentage of lipiodol retention: 68.9%±24.0% versus 72.2%±23.1%, P=0.578). Additionally, there was a high correlation in the volume of tumor and lipiodol between CBCT and MDCT (R2=0.919 and 0.903, respectively).
Conclusions
The quantitative image enhancement and volume analyses demonstrate that CBCT is similar to MDCT in assessing the lipiodol deposition in HCC after TACE.
INTRODUCTION
Transarterial chemoembolization (TACE) is the mainstay treatment for unresectable hepatocellular carcinoma (HCC)(1). The degree of intratumoral lipiodol deposition has been shown to correlate with tumor necrosis and prognosis of HCC patients after conventional TACE (2–4) Fluoroscopic imaging is widely used to assess the lipiodol accumulation during and immediately after drug administration. However, fluoroscopic imaging may show only partial enhancement from lipiodol retention in the tumor because it cannot provide volumetric information like cross-sectional imaging (5). Therefore, as an alternative approach, unenhanced multi-detector computed tomography (MDCT) is frequently used to evaluate the lipiodol deposition after TACE (6) .
Cone-beam computed tomography (CBCT) provides soft-tissue cross-sectional imaging with a flat-panel detector has emerged as a useful tool during TACE procedures (7). CBCT is performed intra-procedurally and offers volumetric information about tumor location, number, vascularity, and can be used for real-time image guidance and predicting tumor response (8,9). The hypothesis of the current study was that CBCT could be used to assess lipiodol deposition intra-procedurally during TACE. This could help in accelerating and optimizing patient care workflow (no need to transfer patients for MDCT) and in minimizing radiation dose (10). Iwazawa et al (11) compared the efficacy of CBCT with that of MDCT to detect the incomplete lipiodol accumulation judged by the observers using a semi-quantitative visual grading score. Here, we expand on their work by quantitatively measuring the image enhancement and the volume of enhancement to more directly compare the capability of assessing lipiodol deposition between CBCT and MDCT. Therefore, the goal of this study was to evaluate the ability of CBCT acquired directly after TACE to assess lipiodol deposition in HCC and compare it to unenhanced MDCT.
MATERIALS AND METHODS
To ensure consistency in reporting of results, this article follows the Society of Interventional Radiology (SIR) Reporting Standards on transcatheter therapy for hepatic malignancy (12).
Patient Selection
This study was compliant with the Health Insurance Portability and Accountability Act and was approved by the Institutional Review Board. Requirement for patient informed consent was waived for this retrospective study. Diagnosis of HCC was confirmed by liver biopsy or the lesion presented with typical features on dynamic contrast-enhanced CT or MR cross-sectional imaging and α-fetoprotein level of 200 ng/ml or greater.
Our study group included only patients who had undergone conventional TACE and received CBCT scanning at the end of TACE. Unenhanced MDCT was performed 24 hours after TACE before the patient was discharged from the hospital the next day. From June, 2012 to December, 2012, 15 patients fulfilled the inclusion criteria. The selection criteria of the treated target lesion included that the lipiodol remained in the tumor and the tumor margin was conspicuous on imaging. Four patients were excluded because the margin of tumors was not conspicuous (2 cases) and the lipiodol deposition was diffuse (2 cases) in the liver on both CBCT and MDCT imaging, resulting in 11 patients with 1–5 target lesions per patient being included in this study. There were 10 men and 1 woman with a mean age of 57.8± 6.0 years (range 49 to 71). In total, 31 target tumors (mean per patient 2.8, range 1 to 5; a mean diameter 2.3±1.4 cm; range 0.7 to 4.7) were evaluated in the analysis. Table 1 lists baseline characteristics of this 11-patient cohort. More than half of patients had multifocal HCC with preserved underlying liver function (Child-Pugh class A), and 54.5% were classified as Barcelona Clinic Liver Cancer (BCLC) grade B (A/B/C = 4/6/1, respectively).
Table 1.
Baseline Characteristics of Patients with HCC
| Parameter | Value |
|---|---|
| No. of patients | 11 |
| No. of target tumors evaluated | 31 |
| Age(y)* | 57.8± 6.0 |
| Sex | |
| Male | 10 |
| Female | 1 |
| Etiology | |
| Hepatitis B virus | 2 |
| Hepatitis C virus | 9 |
| Diagnosis | |
| Clinical | 8 |
| Pathological | 3 |
| Tumor size(cm)* | 2.3±1.4 |
| Disease pattern | |
| Unifocal | 2 |
| Bifocal | 3 |
| Multifocal | 6 |
| Child-Pugh class | |
| A | 11 |
| Barcelona Clinic Liver Cancer stage | |
| A | 4 |
| B | 6 |
| C | 1 |
| Laboratory values* | |
| Basal AFP level (ng/mL) | 2376.7±7033.4 |
| Albumin (g/dL) | 3.6±0.7 |
| Total bilirubin (mg/dL) | 1.4±1.1 |
| International normalized ratio | 1.3±0.2 |
Data were expressed as means ± standard deviations.
Conventional TACE was performed according to our standard institutional protocol and all procedures were performed by an interventional radiologist (XX) with 15 years of experience in hepatic interventions. With the Seldinger technique and fluoroscopic guidance, a 5-F glide Simmons-1 catheter was advanced into the desired hepatic artery over the guide wire. Using a 3-F Renegade High-Flo catheter coaxially over a 0.014-inch wire, selective catheterization and chemoembolization were performed. HCC patients in our study were treated in a selective fashion (lobar or segmental chemoembolization). A solution containing 50 mg of doxorubicin, and 10mg of mitomycin C in a 1:1 mixture with lipiodol (Lipiodol; Guerbet, Paris, France) was infused and followed by administration of 100–300-um-diameter microspheres until arterial inflow was retarded as seen on fluoroscopy.
CBCT was performed using a commercially available angiographic system (Allura Xper FD20, Philips Healthcare, Best, The Netherlands) with the XperCT option, enabling CBCT acquisition and volumetric image reconstruction (Feldkamp back projection). Over 5 seconds, 310 projection images (60 frames per second) were acquired with the motorized C-arm covering a 220° clockwise arc under a fixed 120kVp, 50mA, 3ms setting. The two dimensional projection images were reconstructed using Feldkamp back projection into three dimensional (3D) volumetric images for a 250×250×194mm field of view (matrix size 384×384×296) with a voxel size of 0.6mm3. The patients were instructed to be at end-expiration apnea during the CBCT scanning.
Unenhanced MDCT was done with a multi-slice CT scanner (Sensation 64; Siemens Medical Solutions, Erlangen, Germany) using a standard abdominal scan protocol. The scanning parameters were the following: tube voltage, 120 kVp; current, 545 mA; scan speed, 0.33s/revolution; detector collimation, 0.6 mm/row; helical pitch factor, 0.575/revolution. Images were then reconstructed using body kernel B30f, with a 400×400×220mm field of view (matrix size512×512×300) with a voxel size of 0.78 mm3.
Tumor-to-Liver Contrast (TLC)
The conspicuity of a lesion was expressed by the attenuation difference of the tumor and the surrounding liver parenchyma as described previously (13). Both CBCT and MDCT imaging were evaluated using ImageJ software (National Institutes of Health, Bethesda, MD) to measure the attenuation of the target lesion in Hounsfield Unit (HU). For CBCT, the signal intensity was calibrated to HU using a linear transformation based on internal Philips C-arm calibrations. The mean HU values were measured in the entire tumor (Region of Interest - ROI tumor) and in the same-sized ROI of surrounding liver parenchyma located at least 1 cm distance from the tumor lesion and avoiding blood vessels and the hepatic hilum (ROI liver). ROIs were drawn on one axial slice where the tumor presented with the largest axial diameter. The TLC was calculated for each target lesion using the formula: TLC = ROI tumor-ROI liver by an experienced interventional radiology (XX with 10 years of experience).
Tumor and Lipiodol Segmentation
The volume measurements of the entire tumor and the lipiodol retention were performed by the same experienced interventional radiologist who measured the TLC. A semiautomatic 3D volume segmentation software based on non-Euclidean radial basis functions (Medisys, Philips Research, Suresnes, France) was used to independently segment the entire tumor and then only the lipiodol retention regions. The volume was directly calculated based on the segmentation. Specifically, the number of voxels in the segmentation was multiplied by the voxel dimensions. Special care was taken to avoid regions with pronounced necrosis and non-lipiodol deposition for the lipiodol volume retention segmentation. This method can accurately segment in three dimensions and provides excellent reproducibility as previously reported (14,15). The percentage of the lipiodol retention was calculated by dividing the lipiodol volume by the tumor volume.
Statistics
Data analysis was performed using SPSS 15.0 (SPSS, Chicago, IL). The difference between CBCT and MDCT was compared using the two-tailed Student’s t-test for the paired data and using linear regression to evaluate consistency including the R2 value of correlation. A two-sided P value ≤ 0.05 was considered statistically significant.
RESULTS
Lipiodol Conspicuity in the Tumor
Thirty-one target tumors with lipiodol deposition in 11 patients were present on both CBCT and MDCT. Tumor-to-liver contrast (TLC) is considered to be an indicator of tumor conspicuity on the imaging (13) and obtained by comparing the difference attenuation of the tumor and the surrounding liver (Fig 1). For 31 target lesions, the mean value of TLC on CBCT imaging was not significantly different from the one on MDCT (mean±SD; 337.7± 233.5 HU versus 283.0±152.1 HU, P=0.103).
Figure 1.
Calculation of TLC on a representative case with CBCT and MDCT images. (a) CBCT image obtained intraprocedurally during TACE. Arrow indicates the targeted tumor with lipiodol deposition in the liver. (b) MDCT image at 24h after TACE. Arrow indicates the targeted tumor. (c) ROI drawn on the whole tumor at the largest axial slice (red circle) and same-sized ROI in the surrounding liver tissue (black circle) on CBCT. (d) ROI of the tumor (red circle) and surrounding liver tissue (black circle) on MDCT at corresponding slice level as CBCT. The attenuation values of tumor and liver measured on CBCT were 525.0HU and 254.6HU, respectively. The TLC on CBCT was 270.4HU. The attenuation values of tumor and liver measured on MDCT were 287.7HU and 59.3 HU, and TLC=228.4HU.
Tumor Volume and Lipiodol Retention
The volumes of the entire tumor and only the lipiodol retention on CBCT and MDCT were measured using the 3D segmentation software (Fig 2, Fig 3). As shown in Table 2, the average volume of the whole tumor and the lipiodol retention, and the calculated average percentage of lipiodol retention in the target tumors on CBCT were not significantly different when compared to MDCT. Additionally, the high correlation in both the tumor volume and the lipiodol volume between CBCT and MDCT imaging was evidenced by the linear regression analysis shown in Figure 2. The R2 value for the tumor volume and the lipiodol volume were 0.919 and 0.903 respectively (Fig 4), indicating the strong agreement between CBCT and MDCT.
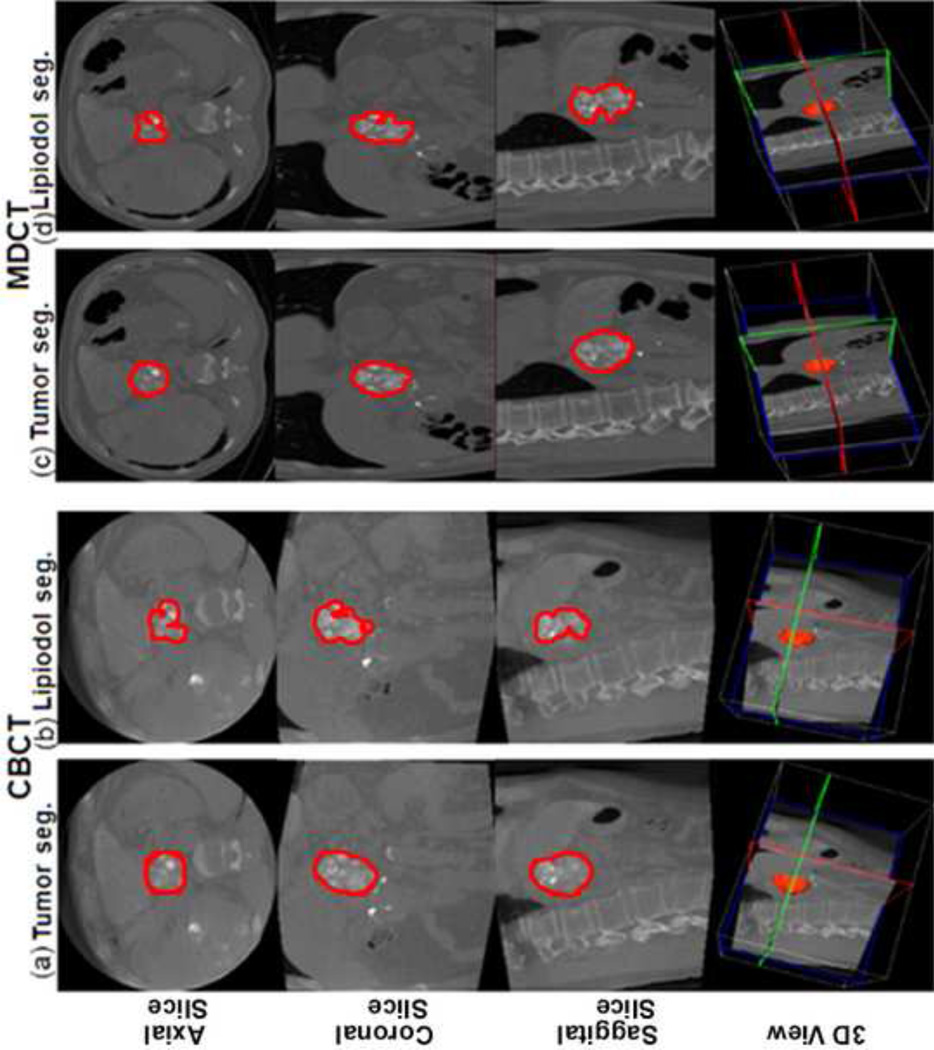
Figure 2.
Tumor and lipiodol segmentation on CBCT and MDCT images (the same case as in Figure 1). The top row shows axial slices, the second row coronal slices, the third row sagittal slices and the bottom row the three-dimensional (3D) projected volume. (a) The tumor segmentation on CBCT images. Tumor volume= 44.5cm3. (b) The lipiodol segmentation on CBCT images. Lipiodol volume=31.9cm3. The calculated percent of lipiodol retention on CBCT=71.7%. (c) The tumor segmentation on MDCT images. Tumor volume=42.1cm3. (d) The lipiodol segmentation on MDCT images. Lipiodol volume=27.2cm3. The percent of lipiodol retention on MDCT=64.6%. Note the special care to avoid areas with pronounced necrosis and non-lipiodol deposition during lipiodol segmentation. Seg=segmentation.
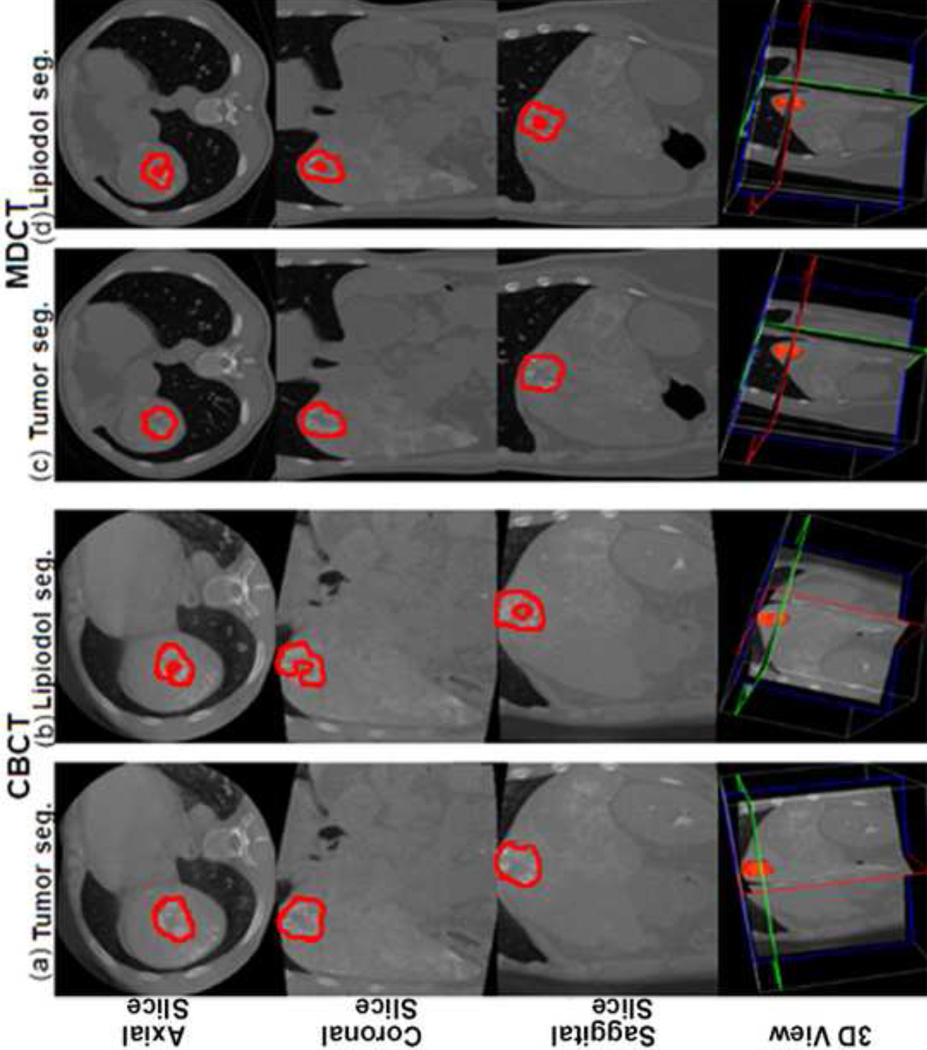
Figure 3.
Tumor and lipiodol segmentation on CBCT and MDCT images in another patient case. (a) The tumor segmentation on CBCT images. Tumor volume=26.7cm3. (b) The lipiodol segmentation on CBCT images. Lipiodol volume=13.9cm3. The calculated percent of lipiodol retention on CBCT=52.1%. (c) The tumor segmentation on MDCT images. Tumor volume=32.8cm3. (d) The lipiodol segmentation on MDCT images. Lipiodol volume=13.7cm3. The percent of lipiodol retention on MDCT=41.8%.
Table 2.
The mean volume of tumor and lipiodol retention measured on CBCT and MDCT
| CBCT | MDCT | P-value | |
|---|---|---|---|
| Tumor volume(cm3) | 9.6±11.8 | 10.8±14.2 | 0.142 |
| Lipiodol volume(cm3) | 6.3±7.7 | 7.0±8.1 | 0.214 |
| Percentage of lipiodol retention(%) | 68.9%±24.0% | 72.2%±23.1% | 0.578 |
Data were expressed as means ± standard deviations.
Figure 4.
Linear regression curve for the volume of tumor measured from CBCT and MDCT (a) and for the volume of lipiodol between CBCT and MDCT (b).
DISCUSSION
Our study demonstrates that CBCT imaging directly acquired intraprocedurally during TACE has a similar capability to assess lipiodol deposition when compared to post-procedure MDCT. CBCT is not only superior to fluoroscopic imaging for assessing the lipiodol deposition in small HCC directly after TACE (5), but can also modify intra-procedural treatment decision-making (16). Iwazawa et al (11) reported that CBCT is nearly equivalent to MDCT in terms of detecting incomplete lipiodol accumulation after TACE. However, only a semi-quantitative visual grading method was used.
In this study, we applied quantitative methods to compare the two imaging modalities, clearly showing that CBCT and MDCT have the same capability to assess lipiodol deposition in two aspects. Firstly, lipiodol conspicuity is similar between CBCT and MDCT imaging, indicated by tumor-to-liver contrast calculated from the attenuation difference of the tumor and the surrounding liver parenchyma (13). The attenuation difference has been used to calculate the intratumoral lipiodol concentration after TACE (17). The absolute HU value of the tumor and the liver tissue on CBCT was higher than that on MDCT. This can be explained by these reasons. CBCT is more sensitive to motion than MDCT. Also, there is only partial projection angle sampling for CBCT (220 degrees instead of 360 degrees). These factors combined increase the noise level on CBCT. In addition, the CBCT imaging was done intra-procedurally when there is still small amount of contrast medium present. While some contrast medium may remain 24 hours later, a portion of the contrast medium has already washed out by the time of performing MDCT. Secondly, quantitative 3D volume measurements of tumor and lipiodol show strong consistency across both imaging modalities. This volumetric segmentation method is proven to be effective and reproducible as previously described (14,15). The trend of increased volume of the tumor and the lipiodol found on MDCT obtained at 24 hours after TACE compared to CBCT can be explained by the slightly increased tissue edema after TACE.
Compared with MDCT, CBCT performed intraprocedurally to evaluate lipiodol retention has clinical advantages and disadvantages. It is convenient and time-saving to directly assess lipiodol deposition after TACE without the need of transferring the patient for MDCT. Furthermore, assessing lipiodol deposition at time of TACE allows for intra-procedural feedback for modification of delivery endpoint. In addition, CBCT has lower radiation exposure than MDCT (10). On the other hand, the image quality of CBCT is more sensitive to patient motion and typically has more image artifacts than MDCT (18). Additionally, CBCT has a smaller field of view compared with MDCT. This could result in peripheral tumors that could be partially included or missed. In our study, the CBCT image quality for all cases were acceptable by clearly instructing breath hold control to patients to reduce motion artifacts and the proper positioning of the treated region within the field of view during CBCT scanning.
There were some limitations to this study. First, the total number of patients was relatively small, although many had multiple lesions and each lesion was considered individually. Nevertheless, our results need be further validated in a larger cohort of patients. Second, there was potential bias from a single observer, non-blinded reviewing or learning bias from reviewing the two modalities. However, to minimize the bias, we repeated the segmentation six times and randomized the order of segmentation. In addition, the semiautomatic 3D volume segmentation software provides excellent reproducibility as we had previously shown. These will reduce the bias. Third, this study was limited to HCC. As TACE is being extended to treating non-HCC tumors (i.e. metastatic disease) in the liver, our conclusion also needs to be investigated for different liver tumors.
CONCLUSIONS
Our study demonstrates that there is a quantitative correlation between CBCT imaging acquired intraprocedurally and MDCT imaging acquired post-procedurally after TACE in assessing lipiodol deposition. This information could modify the clinical work-flow of using CBCT in place of MDCT for treatment evaluation during or immediately after lipiodol administration.
Acknowledgments
Support for this work was provided by NIH/NCI R01 CA160771, P30 CA006973, Philips Research North America, Briarcliff Manor, NY, USA and the French Society of Radiology (SFR).
ABBREVIATIONS
- HCC
Hepatocellular carcinoma
- TACE
Transarterial chemoembolization
- CBCT
cone-beam computed tomography
- MDCT
multi-detector computed tomography
- ROI
regions of interest
- TLC
Tumor-to-liver contrast
- HU
Hounsfield unit
- Seg
segmentation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 2.Monsky WL, Kim I, Loh S, et al. Semiautomated segmentation for volumetric analysis of intratumoral ethiodol uptake and subsequent tumor necrosis after chemoembolization. AJR. 2010;195:1220–1230. doi: 10.2214/AJR.09.3964. [DOI] [PubMed] [Google Scholar]
- 3.Takayasu K, Muramatsu Y, Maeda T, et al. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR. 2001;176:681–688. doi: 10.2214/ajr.176.3.1760681. [DOI] [PubMed] [Google Scholar]
- 4.Lee HS, Kim KM, Yoon JH, et al. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus endemic area: a prospective cohort study. J Clin Oncol. 2002;20:4459–4465. doi: 10.1200/JCO.2002.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Jeon UB, Lee JW, Choo KS, et al. Iodized oil uptake assessment with cone-beam CT in chemoembolization of small hepatocellular carcinomas. World J Gastroenterol. 2009;15:5833–5837. doi: 10.3748/wjg.15.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi BI, Kim HC, Han JK, et al. Therapeutic effect of transcatheter oily chemoembolization therapy for encapsulated nodular hepatocellular carcinoma: CT and pathologic findings. Radiology. 1992;182(3):709–713. doi: 10.1148/radiology.182.3.1311116. [DOI] [PubMed] [Google Scholar]
- 7.Tognolini A, Louie JD, Hwang GL, Hofmann LV, Sze DY, Kothary N. Utility of C-arm CT in patients with hepatocellular carcinoma undergoing transhepatic arterial chemoembolization. J Vasc Interv Radiol. 2010;21:339–347. doi: 10.1016/j.jvir.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Higashihara H, Osuga K, Onishi H, et al. Diagnostic accuracy of C-arm CT during selective transcatheter angiography for hepatocellular carcinoma: comparison with intravenous contrast-enhanced, biphasic, dynamic MDCT. Eur Radiol. 2012;22:872–879. doi: 10.1007/s00330-011-2324-y. [DOI] [PubMed] [Google Scholar]
- 9.Loffroy R, Lin M, Yenokyan G, et al. Intraprocedural C-arm dual-phase cone-beam CT: can it be used to predict short-term response to TACE with drug-eluting beads in patients with hepatocellular carcinoma? Radiology. 2013;266:636–648. doi: 10.1148/radiol.12112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S, Yoshizumi TT, Toncheva G, Yoo S, Yin FF. Comparison of radiation doses between cone beam CT and multi detector CT: TLD measurements. Radiat Prot Dosimetry. 2008;132:339–345. doi: 10.1093/rpd/ncn305. [DOI] [PubMed] [Google Scholar]
- 11.Iwazawa J, Ohue S, Kitayama T, Sassa S, Mitani T. C-arm CT for assessing initial failure of iodized oil accumulation in chemoembolization of hepatocellular carcinoma. AJR. 2011;197:W337–W342. doi: 10.2214/AJR.10.5614. [DOI] [PubMed] [Google Scholar]
- 12.Brown DB, Gould JE, Gervais DA, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S425–S434. doi: 10.1016/j.jvir.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Sultana S, Awai K, Nakayama Y, et al. Hypervascular hepatocellular carcinomas: bolus tracking with a 40-detector CT scanner to time arterial phase imaging. Radiology. 2007;243:140–147. doi: 10.1148/radiol.2431060069. [DOI] [PubMed] [Google Scholar]
- 14.Tacher V, Lin M, Chao M, et al. Semiautomatic Volumetric Tumor Segmentation for Hepatocellular Carcinoma: Comparison between C-arm Cone Beam Computed Tomography and MRI. Acad Radiol. 2013;20(4):446–452. doi: 10.1016/j.acra.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellerin O, Lin M, Bhagat N, Ardon R, Mory B, Geschwind JF. Comparison of semi-automatic volumetric VX2 hepatic tumor segmentation from cone beam CT and multi-detector CT with histology in rabbit models. Acad Radiol. 2013;20(1):115–121. doi: 10.1016/j.acra.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun JH, Wang LG, Bao HW, et al. Usefulness of C-arm angiographic computed tomography for detecting iodized oil retention during transcatheter arterial chemoembolization of hepatocellular carcinoma. J Int Med Res. 2010;38:1259–1265. doi: 10.1177/147323001003800407. [DOI] [PubMed] [Google Scholar]
- 17.Taguchi K, Funama Y, Zhang M, Fishman EK, Geschwind JF. Quantitative measurement of iodine concentration in the liver using abdominal C-arm computed tomography. Acad Radiol. 2009;16:200–208. doi: 10.1016/j.acra.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Akpek S, Brunner T, Benndorf G, Strother C. Three-dimensional imaging and cone beam volume CT in C-arm angiography with flat panel detector. Diagn Interv Radiol. 2005;11:10–13. [PubMed] [Google Scholar]