Abstract
Postpartum women have highly disturbed sleep, also known as sleep fragmentation. Fragmentation extends their total sleep period, also disrupting sleep timing. A stable and earlier sleep period among non-postpartum populations are related to better performance, physical health, and mental health. However, sleep timing has not been examined among postpartum women who are also vulnerable to daytime impairment. The study objective was to examine how the timing and regularity of sleep during the early postpartum period are related to daytime functioning across postpartum weeks 2-13. In this field-based study, 71 primiparous women wore an actigraph, a small wrist-worn device that monitors sleep and sleep timing, for the 12-week study period. Mothers self-administered a 5-minute psychomotor vigilance test (PVT) each morning to evaluate the number of >500ms response lapses. They also completed a Morningness-Eveningness scale at the beginning of the study to identify chronotype. After controlling for maternal age, earlier sleep timing was associated with significantly fewer PVT lapses at postpartum weeks 9,12; a more stable sleep midpoint was associated with significantly fewer PVT lapses at postpartum weeks 2,5-13. Earlier sleep midpoints were related to more stable sleep midpoints at postpartum week 2 and a morning-type chronotype. An earlier sleep midpoint was also associated with a reduced slope of worsening PVT lapses across weeks. Across the first 12 postpartum weeks, women with earlier or more stable sleep periods had less daytime impairment than women with later or more variable sleep midpoints. Postpartum women with earlier sleep midpoints also showed less severe decrements in performance across time, which has been attributed to cumulative impacts of sleep disturbance. These data suggest the sleep period, in addition to sleep duration and fragmentation, should be more closely examined, particularly among vulnerable women, as it may affect the neurobehavioral performance of new mothers.
Keywords: individual differences, maternal, postpartum, psychomotor vigilance, sleep midpoint, sleep period
1. Introduction
Sleep disruptions can impair overall health [1], and neurobehavioral performance [2]. Postpartum mothers experience frequent nocturnal awakenings that result in fragmented sleep, a type of sleep disruption whereby the quality of sleep is reduced. While sleep fragmentation and sleep deprivation can co-occur, this is not the case among postpartum women whose total sleep time is preserved [3-5]. The fragmented sleep experienced by postpartum women appears to have similar detrimental consequences as sleep deprivation on their daytime sleepiness and functioning. Analyzing the relations between PVT and sleep, including the implications of accumulated sleep debt, was the overarching purpose of the study and those results have been published previously. Among the current study's sample, the women's total sleep time did not significantly change throughout the study, but their sleep efficiency gradually improved across weeks [5]. Interestingly, despite improvements in sleep efficiency across the first 12 postpartum weeks, attention lapse frequency increases across the same period [6]. While experimental studies support the notion that this effect may be due to the cumulative effects of sleep disturbance [7], it is not clear what other factors might play a role in recovery of daytime functioning during the postpartum period.
There are likely multiple behaviors that help facilitate recovery from the effects of sleep disturbance; one of these may be the timing and regularity of the nocturnal sleep period. The timing and amount of sleep is influenced by two major processes: Process S and Process C [8]. Process S is the homeostatic sleep drive and increases with time spent awake due to sleep pressure. Process C is the endogenous circadian timing system, and can be influenced by environmental time cues (zeitgebers), the strongest of which is light [9]. Processes S and C interact to determine sleep periods in a way that normally facilitates sleep during the night and wake during the day. However, in some circumstances these two processes become misaligned, commonly seen in jet lag and shift work, and can lead to consequences including impaired mood, daytime functioning, and physical health [10-12]. Process C is also strongly associated with an individuals’ chronotype, or whether they are considered a morning-type or evening-type person [13,14]. An individual's chronotype correlates well with the actual timing of sleep [15]. During the postpartum period, the timing of sleep may be further influenced by (1) the infant, who is not born with an established circadian rhythm [16]; and (2) the potential buildup of sleep debt among this population [6] both of which may impact the homeostatic sleep drive. However, the timing of sleep periods or their relation to daytime functioning has not been examined among postpartum women. This is important given that this may be a source of poorer health and functioning.
Among healthy adults and clinical populations, the impact of sleep timing on mental and physical health is contradictory. In some instances, evening-types were more likely to report poor general health than morning types [17], increased depressive symptoms [18], and more psychological and somatic disorders [19]. However, morning-types are not always reported to fare any better than evening-types. Contrary to the above data, the morning “larks” (people who went to bed before 11pm) did not differ significantly from evening “owls” (people who went to bed after 11pm) in income, cognitive function, state of health, and mortality during a follow-up assessment [20, 21]. Given the potential role of timing of sleep in health and functioning, and the known sleep-related impairments among postpartum women, assessing sleep timing among this population may give insight into their recovery from postpartum sleep disturbance.
Additionally, regularity of the nocturnal sleep period has not been assessed among postpartum women. Among healthy adults, maintaining a consistent sleep schedule is associated with shorter reaction times, better affect, and more time spent in slow wave and rapid eye movement sleep than healthy adults who maintain an irregular sleep schedule [22]. When sleep-wake schedules were experimentally regulated among a population of healthy university students with highly irregular sleep-wake habits, improvements in mood and reductions in daytime parasympathetic activity were noted [23]. This association between a stable sleep schedule and better heath and performance suggests these relations might also account for some variance in postpartum daytime functioning.
While research exists on the many impacts of postpartum sleep disturbances [24-26] no study has assessed how the timing and regularity of sleep among postpartum women could be related to daytime functioning. The midpoint of sleep is the time halfway between sleep onset and wake time, and is more closely related to dim-light melatonin onset (DLMO; a measure of circadian phase) [27,28] and chronotype than either sleep onset or wake time [15]. No study has assessed sleep midpoint among a population that does not obtain a consolidated nocturnal sleep period, such as postpartum women. The current study examined both the timing of postpartum sleep and sleep period regularity using the sleep midpoint, and the relation of these measures to daytime functioning across the first three months postpartum. It also examined how chronotype was associated with sleep midpoint among this population. The current study tested the hypotheses that earlier and more stable sleep periods, a modifiable behavior that influences and is influenced by underlying circadian physiology, would be associated with improved daytime functioning during this period of potential vulnerability.
2. Materials and Methods
The study used pre-existing data from a longitudinal field-based descriptive study of maternal postpartum sleep disturbance that was conducted in Morgantown, West Virginia and surrounding areas from 2007-2010 [5]. West Virginia University's Institutional Review Board approved the study and informed consent and Health Insurance Portability and Accountability Act (HIPAA) authorization were administered to all participants.
2.1 Participants
Recruitment of first-time (primiparous) healthy mothers occurred prenatally via community advertisements, obstetric and midwifery clinics, childbirth classes, and offices that administer the Women, Infant, and Children Supplemental Nutrition Program (WIC). Women were excluded from the study based on premature delivery, pregnancy with multiples, infant admission into the neonatal intensive care unit, discharge from the hospital after the standard 2 (for vaginal delivery) to 3 (for surgical delivery) days postpartum, any major medical conditions, and/or history, current diagnosis, or high risk for major depressive disorder defined as a score of >16 on the 20-item Center for Epidemiologic Studies Depression Scale [29].
2.2 Procedure
Participants contributed 12 continuous weeks of data from the beginning of the second postpartum week. Each night sleep was actigraphically-estimated and corroborated with a self-reported electronic sleep diary on a personal digital assistant (PDA). Every morning participants self-administered a 5-minute psychomotor vigilance reaction time test on their PDA within 2 hours after awakening. Participants were visited in their home once a week by a member of the research team to replace their actigraph and PDA.
2.3 Measures
2.3.1 Actigraphy
Nonintrusive, continuous sleep/wake monitoring was recorded using Actigraphy (Mini Mitter Actiwatch-64, Philips Respironics, Bend, Oregon). Use of the Actiwatch in detection of adult sleep-wake patterns has been validated [30-32] and previously used among postpartum women [33,34]. The Actiwatch records motion via an accelerometric sensor that uses digital integration. The device resembles a wrist watch and is worn on a strap around the woman's nondominant wrist. The highest resolution (15-second epochs) and default (medium) sensitivity setting were used to identify periods of sleep and wake within diary-defined sleep periods. Actiware Software version 5.5 (Mini Mitter Actiwatch-64, Philips Respironics, Bend, Oregon) was used to manage, analyze, and store actigraphy data. The software's algorithm scores each epoch as either sleep or wake by comparison to a wake threshold value (default settings ≥ 40 = wake). The Actiwatch allows for measurement of normative sleep patterns in the field and has been correlated with polysomnography for differentiating sleep from wake, with 91% agreement rates [35].
2.3.2 Personal Digital Assistant (PDA)
Participants also filled out a sleep diary continuously for the duration of the study using a Palm Zire 72 handheld PDA with customized software (Bruner Consulting, Inc. Longmont, Colorado). The program includes a sleep diary, actigraph on/off diary, and psychomotor vigilance test. Sleep onset and offset times were participant-identified in real time and retrospective sleep and wake times were flagged. PDA-based participant sleep diaries were corroborated with actigraphy data to define nocturnal sleep periods. Sleep onset was identified as the beginning of the first 2 minute period of immobility proceeding reported bedtime. Sleep offset was identified as the end of the last 2 minute period of immobility preceding reported rise time.
2.3.3 Sleep Midpoint
Sleep midpoint was calculated each study night as the halfway point between sleep onset and sleep offset. Each participant's nightly sleep midpoints were then averaged within each postpartum week to create a more robust Average Sleep Midpoint variable. In order to calculate variability in sleep midpoints for each woman, her standard deviation of nightly midpoints was calculated within each postpartum week, creating the Variation of Sleep Midpoint variable. The main nocturnal sleep period was defined as the period between initial sleep onset and final morning awakening (subjectively identified by participants, and objectively corroborated with actigraphy). Thus, the sleep midpoint of these women includes the time they were awake at night. The advantage of using the sleep midpoint is it takes into consideration the entire sleep period, and is more closely related to the gold-standard biological marker of circadian phase, the dim light melatonin onset, than is sleep onset or wake [27,28]. Sleep midpoint has never previously been used among a population without a consolidated sleep period.
2.3.4 Chronotype
The Morningness-Eveningness scale was administered to participants during the recruitment visit. The trait assessment is a 7-question survey adapted from Östberg and Horne's 19 question scale [13]. The survey has been validated and found to be associated with peak evening temperatures and bed and rise times [13]. Questions on the survey are about sleep-wake habits and time of day preferences for engaging in a variety of activities. Higher scores are associated with morning-types and lower scores with evening-types. This survey has previously been used among a postpartum population [36].
2.3.5 Psychomotor Vigilance Test (PVT)
The psychomotor vigilance test (PVT) was self-administered using the PDA each morning within two hours after awakening and prior to the consumption of any caffeine, to prevent possible caffeine-related or circadian alerting effects [37]. Each test lasted 5 minutes, during which bull's eye stimuli were presented at random inter-stimulus intervals and the latency to button press was recorded. The use of this 5-minute test is support by a validation of PVTs less than 10 minutes in duration [38]. Reaction times were recorded for response to each individual stimulus, and number of lapses (reaction times ≥500ms) were identified. The frequency of lapses was used as the index of performance in the present study because of its sensitivity in detecting sleep disturbance among adults and its high operational validity [37,39]. Each participant's frequency of PVT lapses was averaged within each postpartum week. To protect against environmental distractions that may occur because it is a field-based study, all individual response times that exceeded the group mean by greater than two times the group standard deviation were excluded from analyses.
2.4 Statistical Analyses
SPSS version 18.0 was used for analyses and a p<.05 was considered statistically significant. Repeated measures Analysis of Covariances (ANCOVAs) were used to compare within-subject changes in midpoint variables and PVT lapses across study weeks accounting for maternal age. There was no significant relation between the latency of postpartum women's sleep midpoint time to completion of the PVT and number of lapses on the PVT at postpartum week 2 among this sample, indicating sleep inertia was likely not impacting performance in the current study. Partial correlations were used to account for maternal age when assessing the relations between sleep midpoint variables and PVT performance outcomes because significant relations in the current dataset between younger women and later sleep midpoints (r=−0.41, p<0.001) and between younger women and more variable sleep midpoints (r=−0.28, p=0.02) were found. This finding is consistent with the known delayed sleep phase among adolescents [40-42]. This phase delay does not shift to the characteristic adult circadian phase until on average the early 20s. Linear slopes of each participant's weekly average PVT performance lapses across study weeks were calculated using linear trend analyses. Missing data was handled using pairwise deletion.
3. Results
Seventy-one primiparous women contributed data to the current analyses. Each week had a varying number of participants contributing data (N range=52-70) due to equipment malfunctioning, participant dropout, or lack of participant adherence to the protocol that resulted in fewer than a minimum of 3 days of data in any given week. Each woman contributed an average of 10.5 weeks (SD=1.9) of data. The sample's demographic characteristics are described in Table 1.
Table 1.
Participant Demographics
| Variable | Mean/Percentage | Standard Deviation | Range |
|---|---|---|---|
| Age | 26.3 | 18.4-35.5 | |
| Annual Household Income | $61,349 | $35,211 | $5,000-$150,000 |
| Years of Education | 15.7 | 2.9 | 9-22 |
| Ethnicity | |||
| White | 90.1% | ||
| White (Hispanic) | 1.4% | ||
| Black or African American | 1.4% | ||
| American Indian | 1.4% | ||
| More than one race | 2.8% | ||
| Marital Status | |||
| Married/Cohabitating | 81.7% | ||
| Divorced/Separated | 1.4% | ||
| Single | 16.9% | ||
| Feeding Method (at postpartum week 2) | |||
| Exclusively breast milk | 69.0% | ||
| Exclusively formula | 12.7% | ||
| Both breast milk and formula | 18.3% | ||
| Delivery | |||
| Vaginal | 69.0% | ||
| C-section | 29.6% (81.0% emergency) | ||
| Gestational Age | 39.4 | 2.0 | 26-41.5 |
| Infant's Birth Weight | 7 lbs 8 oz | 1 lb 9 oz | 6 lbs 2 oz-11 lbs 1oz |
*All variables assessed at the beginning of the study
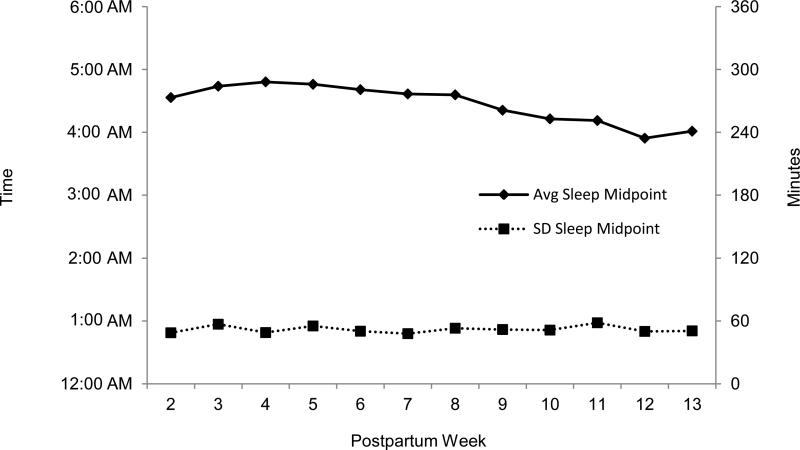
Sleep midpoint values at postpartum week 2 and standard deviation of sleep midpoint values at postpartum week 2 were used for the correlation analyses with weekly PVT measures as the outcome. Given the effects of cumulative sleep disturbance on PVT performance among this sample [6], the earliest recorded sleep data were initially chosen for the sleep midpoint analyses. Interestingly, follow-up repeated-measures ANCOVAs (controlling for maternal age) conducted across postpartum weeks 2-12 revealed that neither average weekly sleep midpoint (F=1.19, p=0.31) nor weekly standard deviation of sleep midpoint (F=1.58, p=0.17) changed significantly over time within individuals (see Figure 1 for graphical representation of sleep midpoint averages and standard deviations). The stability of the sleep midpoint suggests similar relations with neurobehavioral performance could be obtained if sleep midpoint at another week was chosen. However, week 2 was used for the sleep midpoint and standard deviation of sleep midpoint values for the correlation analyses because of the study's initial interest in the early postpartum period. Repeated measures ANOVAs across weeks for PVT lapses revealed a significant increase in lapses over time (F=4.57, p<0.001) (see Table 2), as has been previously reported for this sample [6]. This worsening in performance provided the basis for analyzing PVT lapses at each of the postpartum weeks.
Figure 1. Average weekly sleep midpoint (minutes from midnight) and average weekly standard deviation of sleep midpoint plotted separately to illustrate stability of variance compared to timing.
Figure 1 illustrates descriptive values of the average sleep midpoint and standard deviation of sleep midpoint values of the sample at each postpartum week. Neither of these variables changes significantly across the study period.
Table 2.
Average Number of PVT Lapses across Study Weeks
| W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 | W10 | W11 | W12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 5.6 | 7.7 | 8.7 | 9.3 | 9.3 | 9.5 | 9.6 | 9.6 | 9.2 | 10.1 | 9.3 | 10.2 |
| SD | 4.7 | 5.1 | 5.8 | 6.1 | 6.3 | 6.4 | 6.6 | 6.5 | 6.5 | 6.3 | 6.1 | 6.2 |
| Range | 0.2-20.3 | 0.6-22.5 | 0-21.5 | 0.7-31.5 | 0-26.0 | 0.4-24.0 | 0-27.0 | 1.2-31.2 | 0.4-28.2 | 0.2-27.8 | 0.1-27.3 | 1.3-28.7 |
*These descriptive data have been published in Insana et al., 2013
3.1 Average Sleep Midpoint
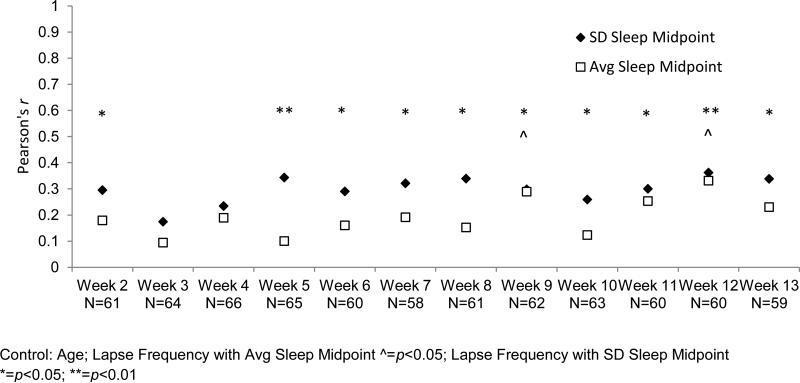
After adjusting for the influence of maternal age, an earlier sleep midpoint at postpartum week 2 was associated with fewer PVT lapses at each study week. These associations were statistically significant at postpartum weeks 9 and 12 (see Figure 2). Sleep midpoint at postpartum week 2 was also significantly associated with the within-subjects change in number of lapses on the PVT across weeks. The slope of increasing PVT lapses across weeks was significantly reduced among women with an earlier sleep midpoint (r=0.26, p=0.03). Finally, the sleep midpoint at postpartum week 2 was significantly associated with more morning-type on the morningness-eveningness scale (r=−0.62, p<.001) even after accounting for age.
Figure 2. Partial correlations between week 2 average sleep midpoint and week 2 SD sleep midpoint and number of lapses at each postpartum week.
Figure 2 illustrates the Pearson's r values of correlations between lapse frequency and both average sleep midpoint and standard deviation of sleep midpoint at each postpartum week.
3.2 Variation of Sleep Midpoint
A more stable sleep midpoint at postpartum week 2 was associated with fewer lapses at each study week after accounting for maternal age. These associations were statistically significant at postpartum weeks 2,5,6,7,8,9,10,11,12 and 13 (see Figure 2). Additionally, average sleep midpoint and standard deviation of sleep midpoint at postpartum week 2 were significantly, positively correlated (r=0.43, p<0.001). However, standard deviation of sleep midpoint at postpartum week 2 was not associated with slope of change in frequency of PVT lapses over time (r=0.04, p=0.75).
3.3 Marital Status
ANCOVAs (controlling for maternal age) were conducted between marital status and PVT lapses at each week, week 2 average sleep midpoint, and week 2 sleep midpoint variation. The difference between marital status and average sleep midpoint approached statistical significance: single women had a later sleep midpoint (M=4:45am, SD=53.4 mins) than married/cohabitating women (M=4:31am, SD=74.4 mins) (F=3.08, p=0.08). Single women also trended toward a larger variation in sleep midpoint (64.0 mins) than married/cohabitating women (45.9 mins), (F=2.29, p=.13). While none of these comparisons were statistically significant, the relatively small number of single women may have precluded sufficient statistical power.
4. Discussion
In this study of healthy postpartum women, those with an earlier or more stable sleep midpoint at postpartum week 2 performed better on daily psychomotor vigilance tests across the early postpartum period than women with a later or more variable sleep midpoint. These findings persisted after accounting for variance in age, as the younger women were more likely to have a later and more variable sleep midpoint. Additionally, timing of sleep midpoint was associated with stability of sleep midpoint; women with an earlier sleep midpoint were more likely to have a stable sleep midpoint. An earlier sleep midpoint was associated with a more morningness chronotype among this sample. This finding suggests women have some control over their sleep period, even with the changing sleep schedules necessitated by nocturnal infant caregiving. In addition, sleep midpoint is a measure that has not previously been used among a population without a consolidated nocturnal sleep period, yet the current study's associations with performance and chronotype suggest it may be a measure that warrants consideration in these populations (including perhaps those with sleep-maintenance insomnia).
Despite the positive association between sleep timing and stability, and between each of these measures and performance, timing, but not stability, of sleep midpoint was related to individual differences in changes in psychomotor vigilance test performance over time. The current study found a reduced slope of worsening performance over time was associated with an earlier sleep midpoint. These data suggest women who go to bed earlier have less severe performance decrements compared to women who go to bed later across the early postpartum period. The importance of this finding is emphasized by the PVT's ability to predict sleepiness and its directly applicability to likelihood of falling asleep while driving [37,39].
These findings align with previous studies among healthy and clinical populations suggesting benefits of a consistent sleep schedule. Healthy adults on a more consistent sleep schedule have improved sleep quality [22,43], performance [22], and mood [23]. Among insomniacs, stabilizing the sleep period has also lead to improved sleep [44,45]. The current study also provides more information about the benefits of an earlier sleep period, a scarcely researched topic with contradicting evidence. The associations between better performance and an earlier sleep period support and add to the literature that suggests benefits of an earlier sleep period on mental [18,19] and physical health [17,19].
These findings add to previous research examining perinatal changes of sleep timing. Postpartum women report later rise times compared to pregnancy (Swain et al., 1997; Wolfson et al., 2003), indicating a tendency towards delayed sleep timing during the postpartum period. This is further emphasized by an average 42 minute phase delay of the dim light melatonin onset (DLMO) from 33 weeks gestation to 6 weeks postpartum, however, with large individual differences in phase changes (Sharkey et al., 2013). There is also evidence to suggest later sleep and circadian phase postpartum is associated with increased depressive symptoms (Wolfson et al., 2003; Sharkey et al., 2013). Among peri- and post-menopausal women, a later DLMO and sleep time has been associated with increased depressive symptoms, appetite, and BMI (Meliska et al 2011). The current study adds to this literature by describing neurobehavioral performance in relation to sleep timing among postpartum women.
If sleep timing is determined to be a modifiable risk factor, the potential significance of these data for informing postpartum interventions is particularly important given postpartum interventions have been largely ineffective [50]. Current studies have focused on behavioral-educational interventions settings informing postpartum women about the importance of sleep, specifically focused on increasing postpartum total sleep time [50,51]. More recent research has discovered mothers’ total sleep time is preserved, but their sleep is highly fragmented [5], suggesting it is the fragmentation leading to sleep-related health and daytime deficits. This indicates that increasing postpartum total sleep time by “sleeping when your baby sleeps” may not be as effective as previously thought. The current study suggests the daytime performance of new mothers may be improved by manipulating and stabilizing the timing of sleep, which could be done through the utilization of environmental cues such as lighting. However, these modifications are based on speculation, and require further research before they should be suggested as interventions.
4.1 Limitations
The main study limitation is the inability to determine causation, and thus future studies should evaluate whether establishing an earlier or more stable sleep period could improve daytime impairment among postpartum women, as well as determine what specific factors may be modifying these associations, including work status and social support. While information about marital status was collected, the relatively small number of single women may have precluded sufficiently powered comparisons. These trends toward earlier and more stable sleep midpoints among married/cohabitating women suggest that marital status, and the differences in women's lives due to marital status, may account for sufficient variance that this may be an interesting avenue for future research. It would also be of interest to examine sleep timing among postpartum fathers, who also exhibit daytime sleepiness [52] and fatigue that has been associated with compromised work safety [53,54]. The use of actigraphy over the gold standard polysomnography in measuring sleep can be seen as another limitation, as actigraphy measures movement as estimates of sleep, and thus cannot identify sleep stages and precise onset of sleep. However, the benefits of using actigraphy to measure normative sleep in the participants’ home environments among this study's vulnerable population outweighs the limitations because polysomnography has an impact on sleep [55,56]. Finally, while our sample was representative of the demographics in West Virginia [57], there was a low representation of minority groups (90.1% White) suggesting caution should be taken in generalizing these findings to minority groups.
4.2 Conclusion
In sum, postpartum women with earlier or more stable sleep periods showed less daytime impairment than women with later or more variable sleep periods. Postpartum women with earlier sleep midpoints also showed less severe decrements in performance across time. The sleep period should be examined more closely during the postpartum period, and potentially considered when directing sleep interventions for new mothers.
Highlights.
Earlier sleep midpoints were associated with better performance postpartum
More stable sleep midpoints were associated with better performance postpartum
Chronotype was related to sleep midpoint among postpartum women
Sleep periods should be more thoroughly examined postpartum
Sleep midpoints should be researched in populations without consolidated sleep
Acknowledgements
We thank the participating families. Salvatore Insana, PhD, Megan Clegg-Kraynok, PhD, and Eleanor Santy, BS assisted with data collection and processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alvarez GG, Ayas NT. The impact of daily sleep duration on health: A review of the literature. Prog Cardiovasc Nurs. 2004;19:56–9. doi: 10.1111/j.0889-7204.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 2.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29(4):320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gay CL, Lee K, Lee SY. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5:311–8. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter LP, Rychnovsky JD, Yount SM. A selective review of maternal sleep characteristics in the postpartum period. J Obstet Gynecol Neonatal Nurs. 2009;38:60–8. doi: 10.1111/j.1552-6909.2008.00309.x. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery-Downs HE, Insana SP, Clegg-Kraynok MM, Mancini LM. Normative longitudinal maternal sleep: the first 4 postpartum months. Am J Obstet Gynecol. 2010;203:465, e1–7. doi: 10.1016/j.ajog.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insana SP, Williams KB, Montgomery-Downs HE. Sleep disturbance and neurobehavioral performance among postpartum women. Sleep. 2013;36:73–81. doi: 10.5665/sleep.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 8.Borbély AA. Two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 9.Roenneberg T, Daan S, Merrow M. The art of entrainment. J Biol Rhythms. 2003;18:183–94. doi: 10.1177/0748730403018003001. [DOI] [PubMed] [Google Scholar]
- 10.Mendlewicz J. Disruptions of the circadian timing systems: Molecular mechanisms in mood disorders. CNS Drugs. 2009;23:15–26. doi: 10.2165/11318630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Mosendane T, Mosendane T, Raal FJ. Shift work and its effects on the cardiovascular system. Cardiovasc J Afr. 2008;19:210–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Reid KJ. Zee PC Circadian rhythm disorders. Semin Neurol. 2009;29:393–405. doi: 10.1055/s-0029-1237120. [DOI] [PubMed] [Google Scholar]
- 13.Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 14.Taillard J, Philip P, Coste O, Sagaspe P, Bioulac B. The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. J Sleep Res. 2003;12:275–82. doi: 10.1046/j.0962-1105.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- 15.Zavada A, Gordijn MCM, Beersma DGM, Daan S, Roenneberg T. Comparison of the Munich Chronotype Questionnaire with the Horne-Östberg's morningness-eveningness score. Chronobiol Int. 2005;22:267–78. doi: 10.1081/cbi-200053536. [DOI] [PubMed] [Google Scholar]
- 16.Kleitman N, Engelmann TG. Sleep characteristics of infants. J Appl Physiol. 1953;6:269–82. doi: 10.1152/jappl.1953.6.5.269. [DOI] [PubMed] [Google Scholar]
- 17.Paine SJ, Gander PH, Travier N. The epidemiology of morningness/eveningness: Influence of age, gender, ethnicity, and socioeconomic factors in adults (30–49 years). J Biol Rhythms. 2006;21:68–76. doi: 10.1177/0748730405283154. [DOI] [PubMed] [Google Scholar]
- 18.Chelminski I, Ferraro FR, Petros TV, Plaud JJ. An analysis of the “eveningness-morningness” dimension in “depressive” college students. J Affect Disorders. 1999;52:19–29. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- 19.Mecacci L, Rocchetti G. Morning and evening types : stress-related personality aspects. Pers Indiv Differ. 1998;25:537–42. [Google Scholar]
- 20.Gale C, Martyn C. Larks and owls and health, wealth, and wisdom. Br Med J. 1998;317:1675–7. doi: 10.1136/bmj.317.7174.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukamal KJ, Wellenius GA, Mittleman MA. Early to bed and early to rise: Does it matter? Can Med Assoc J. 2006;175:1560–3. doi: 10.1503/cmaj.060745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taub JM. Behavioral and psychophysiological corelates of irregularity in chronic sleep routines. Biol Psychol. 1978;7:37–53. doi: 10.1016/0301-0511(78)90041-8. [DOI] [PubMed] [Google Scholar]
- 23.Takasu NN, Takenaka Y, Fujiwara M, Toichi M. Effects of regularizing sleep-wake schedules on daytime autonomic functions and psychological states in healthy university students with irregular sleep-wake habits. Sleep Biol Rhythms. 2012;10:84–93. [Google Scholar]
- 24.Dørheim SK, Bondevik GT, Eberhard-Gran M, Bjorvatn B. Sleep and depression in postpartum women: a population-based study. Sleep. 2009;32:847–55. doi: 10.1093/sleep/32.7.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posmontier B. Sleep quality in women with and without postpartum depression. J Obstet Gynecol Neonatal Nurs. 2008;37:722–35. doi: 10.1111/j.1552-6909.2008.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross LE, Murray BJ, Steiner M. Examen critique Sleep and perinatal mood disorders : a critical review. J Psychiatry Neurosci. 2005;30:247–57. [PMC free article] [PubMed] [Google Scholar]
- 27.Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19:695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- 28.Terman JS, Terman M, Lo E- S, Cooper TB. Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiat. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- 29.Radloff LS. Center for Epidemiological studies depression scale. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 30.Edinger JD, Means MK, Stechuchak KM, Olsen MK, Edinger JD, Olsen MK. A Pilot Study of Inexpensive Sleep-Assessment Devices A Pilot Study of Inexpensive Sleep-Assessment Devices. Behav Sleep Med. 2004;2:41–9. doi: 10.1207/s15402010bsm0201_4. [DOI] [PubMed] [Google Scholar]
- 31.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 32.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Bei B, Coo Calcagni S, Milgrom J, Trinder J. Day-to-day alteration of 24-hour sleep pattern immediately before and after giving birth. Sleep Biol Rhythms. 2012;10:212–21. [Google Scholar]
- 34.Goyal D, Gay C, Lee K. Fragmented maternal sleep is more strongly correlated with depressive symptoms than infant temperament at three months postpartum. Arch Womens Ment Health. 2009;12:229–37. doi: 10.1007/s00737-009-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26:81–5. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki A, Lee KA, Kennedy HP, Weiss SJ. Parent chrontypes and sleeping and crying/fussing in 4-5 week infants. Sleep Biol Rhythms. 2005;3:158–61. [Google Scholar]
- 37.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann NY Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 38.Loh S, Lamond N, Dorrian J, Roach G, Dawson D. The validity of psychomotor vigilance tasks of less than 10-minute duration. Behav Res Meth Ins C. 2004;36:339–46. doi: 10.3758/bf03195580. [DOI] [PubMed] [Google Scholar]
- 39.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8:602–12. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, et al. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–9. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 42.Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, Gislason TH, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res. 2002;53:529–37. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 43.Soehner AM, Kennedy KS, Monk TH. Circadian preference and sleep-wake regularity: associations with self-report sleep parameters in daytime-working adults. Chronobiol Int. 2011;28:802–9. doi: 10.3109/07420528.2011.613137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finley CL, Cowley BJ. The effects of a consistent sleep schedule on time taken to achieve sleep. Clin Case Stud. 2005;4:304–11. [Google Scholar]
- 45.Suh S, Nowakowski S, Bernert RA, Ong JC, Siebern AT, Dowdle CL, et al. Clinical significance of night-to-night sleep variability in insomnia. Sleep Med. 2012;13:469–75. doi: 10.1016/j.sleep.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swain AM, O'Hara MW, Starr KR, Gorman LL. A prospective study of sleep, mood, and cognitive function in postpartum and nonpostpartum women. Obstet Gynecol. 1997;90:381–6. doi: 10.1016/s0029-7844(97)89252-6. [DOI] [PubMed] [Google Scholar]
- 47.Wolfson AR, Crowley SJ, Anwer U, Bassett JL. Changes in sleep patterns and depressive symptoms in first-time mothers: Last trimester to 1-year postpartum. Behav Sleep Med. 2003;1:54–67. doi: 10.1207/S15402010BSM0101_6. [DOI] [PubMed] [Google Scholar]
- 48.Sharkey KM, Pearlstein TB, Carskadon MA. Circadian phase shifts and mood acress the perinatal period in women with a history of major depressive disorder. In Press. J Affect Disorders. doi: 10.1016/j.jad.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meliska CJ, Martínez LF, López AM, Sorenson DL, Nowakowski S, Parry BL. Relationship of morningness-eveningness questionnaire score to melatonin and sleep timing, body mass index and atypical depressive symptoms in peri- and post-menopausal women. Psychiatry Res. 2011;188:88–95. doi: 10.1016/j.psychres.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stremler R, Hodnett E, Lee K, MacMillan S, Mill C, Ongcangco L, et al. A behavioral-educational intervention to promote maternal and infant sleep: a pilot randomized, controlled trial. Sleep. 2006;29:1609–15. doi: 10.1093/sleep/29.12.1609. [DOI] [PubMed] [Google Scholar]
- 51.Meltzer LJ, Mindell JA. Nonpharmalogical treatment for pediatric sleeplessness. Pediatr Clin N Am. 2004;51:135–51. doi: 10.1016/s0031-3955(03)00178-0. [DOI] [PubMed] [Google Scholar]
- 52.Insana SP, Montgomery-Downs HE. Sleep and sleepiness among first-time postpartum parents: A field- and laboratory-based multimethod assessment. Dev Psychobiol. 2012 May; doi: 10.1002/dev.21040. Online ahead of pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mellor G, St John W. Work safety during early fatherhood. AAOHN J. 2010;58:297–301. doi: 10.3928/08910162-20100616-01. [DOI] [PubMed] [Google Scholar]
- 54.Mellor G, St. John W. Fatigue and work safety behavior in men during early fatherhood. Am J Mens Health. 2012;6:80–7. doi: 10.1177/1557988311423723. [DOI] [PubMed] [Google Scholar]
- 55.Agnew HW, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiol. 1966;2:263–6. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 56.Le Bon O, Staner L, Hoffmann G, Dramaix M, San Sebastian I, Murphy JR, et al. The first-night effect may last more than one night. J Psychiatr Res. 2001;35:165–172. doi: 10.1016/s0022-3956(01)00019-x. [DOI] [PubMed] [Google Scholar]
- 57.US Census Bureau [February 25, 2012];State and County Quick Facts: Monongalia County, W.V. 2010 from: http://quickfacts.census.gov/qfd/states/54/54061.html.