Abstract
Rationale
Response inhibition abnormalities contribute to several maladaptive behaviors commonly observed during adolescence, including heavy drinking.
Objectives
The present study aimed to determine whether abnormalities in brain response during response inhibition predate or follow adolescents' transition into heavy drinking, which is pivotal in identifying the neural antecedents and consequences of adolescent alcohol use.
Methods
Longitudinal functional magnetic resonance imaging (fMRI) acquired during a response inhibition task was collected on adolescents before the onset of heavy drinking, and then again on the same scanner approximately 3 years later. Adolescents who transitioned into heavy drinking (n=20) were matched to continuously nondrinking adolescents (n=20) on baseline and follow-up demographic and developmental variables.
Results
During no-go relative to go trials, participants showed responses common to inhibitory circuitry: frontal (e.g., pre-supplementary motor area), temporal, and parietal regions. A repeated measures analysis of covariance revealed that adolescents who later transitioned into heavy drinking showed less fMRI response contrast at baseline than continuous nondrinkers in frontal, parietal, subcortical, and cerebellar regions (p < .01, clusters > 756 microliters), then increased activation after the onset of heavy drinking in frontal, parietal, and cerebellar areas.
Conclusions
Future heavy drinkers showed less activation of inhibitory circuitry before the onset of heavy drinking. After transitioning into heavy drinking, they showed more activation during response inhibition than nondrinking controls. These results contribute to the growing literature suggesting that neural vulnerabilities exist prior to the onset of substance use, and the initiation of heavy drinking may lead to additional alterations in brain functioning.
Keywords: Alcohol, inhibition, neuroimaging, heavy drinking
Introduction
Adolescence is a developmental period between childhood and adulthood characterized by marked physiological, psychological, and behavioral changes. Adolescents experience rapid physical growth, sexual maturation, and advances in cognitive and emotional processing (Forbes and Dahl, 2010). These changes coincide with increases in substance use, with alcohol being the most widely used illegal substance among adolescents (Windle et al, 2008; Witt, 2010). National survey data indicate that 33% of 8th grade students have tried alcohol, and this percentage increases to 70% among 12th graders (Johnston et al, 2012). Of greater concern is the increase in heavy episodic drinking (i.e., consuming 5 of more drinks in a row during the previous two weeks) where prevalence rates increase from 6% to 22% for 8th and 12th grades, respectively (Johnston et al, 2012), as heavy episodic drinking during adolescence is associated with numerous negative effects on adolescent health and well-being, including risky sexual behaviors (Quinn and Fromme, 2010), hazardous driving (Lee et al, 2011), and alterations in adolescent brain development (Squeglia et al, 2009a).
During adolescence, the brain undergoes significant changes, and a recent longitudinal neuroimaging study suggests that heavy episodic drinking during this developmental period alters brain functioning (Squeglia et al, 2012a). Squeglia and colleagues (2012a) examined the effects of heavy episodic drinking on brain function during a visual working memory task, comparing brain activity in adolescents at baseline and again at follow-up (approximately 3 years later) to compare brain activity in those who transitioned into heavy drinking during adolescence (i.e., ages 13-19) to demographically matched adolescents who remained nondrinkers. Adolescents who initiated heavy drinking exhibited increasing brain activity in frontal and parietal brain regions during a visual working memory task compared to adolescents who remained nondrinkers through follow-up, who showed decreasing frontal activation, consistent with studies in typical development (Luna et al, 2010; Luna and Sweeney, 2004). Thus, adolescent heavy episodic drinking may alter brain functioning involved in working memory; however, additional longitudinal studies are needed to explore the effects of alcohol on neural correlates of other vital cognitive processes, such as response inhibition.
Response inhibition refers to the ability to withhold a prepotent response in order to select a more appropriate, goal-directed response (Luna et al, 2010; Stevens et al, 2007). The neural circuitry underlying response inhibition develops during adolescence (e.g., Durston et al, 2006; Velanova et al, 2008), and as such, brain response during inhibition changes during adolescence (Luna et al, 2010). Briefly, cross-sectional research indicates that brain activation during response inhibition transitions from diffuse prefrontal and parietal activation to localized prefrontal activation (Luna et al, 2010; Luna and Sweeney, 2004). Longitudinal studies report that atypical brain responses during response inhibition, despite comparable performance, is predictive of later alcohol use (Norman et al, 2011), substance use and dependence symptoms (Mahmood et al, 2012), and alcohol-related consequences (Wetherill et al, 2012b). Together, these findings indicate that neural substrates associated with response inhibition change over time and abnormalities in development may contribute to later substance use.
To this end, the current longitudinal fMRI study examined the effects of initiating heavy drinking during adolescence on brain activity during response inhibition. We examined blood oxygen level dependent (BOLD) response during a go/no-go response inhibition task prior to alcohol initiation (ages 11.7-16.7), then again on the same scanner approximately 3 years later, after some adolescents had transitioned into heavy drinking. Based on our previous findings (Squeglia et al, 2012a), we hypothesized that adolescents who transition into heavy drinking would show reduced BOLD response during response inhibition prior to initiating heavy drinking (baseline time point) followed by increased activation after the onset of heavy episodic drinking, as compared to adolescents who remained non-users. By identifying potential neurobiological antecedents and consequences of heavy episodic drinking, this study will extend previous research on the effects of alcohol on brain function and point to risk factors for heavy episodic drinking during adolescence.
Methods
Participants
Participants in the current prospective study (N=40) were identified from a sample of 296 adolescents participating in a neuroimaging study of neurocognition and neural functioning in youth at-risk for substance use disorders (Squeglia et al, 2011; (Squeglia et al, 2012b; Squeglia et al, 2009b; Wetherill et al, 2012a). Adolescents were recruited through flyers distributed to households of students attending local public schools and had minimal, if any, substance use at project entry (Bava et al, 2011; Pulido et al, 2009; Squeglia et al, 2009b). Parents and adolescents completed phone screens, and those eligible each privately completed semi-structured diagnostic interviews to confirm eligibility. Participants provided informed assent and parents provided informed consent, as approved by the University of California, San Diego Human Research Protections Program.
Baseline exclusion criteria that helped rule out potential confounds included parental history of bipolar, psychotic, or antisocial personality disorder; any indication of in utero alcohol, tobacco, or illicit drug exposure; complicated or premature birth (<34 weeks gestation); left-handedness; history of chronic medical illness, any neurological or Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV, American Psychiatric Association, 1994) Axis I disorder; traumatic brain injury with loss of consciousness > 2 minutes or learning disabilities; use of psychoactive medications; more than 6 lifetime alcohol, cigarette, marijuana, or other illicit drug uses, or more than 1 drink per drinking occasion; MRI contraindications (e.g., braces, positive pregnancy test); sensory impairments; and non-fluency in English. The current sample at baseline was 55% Caucasian, 20% Latino/a, 15% multiracial, 5% Asian, and 5% African American, and participants had ≤1 total lifetime drinks, ≤1 lifetime uses of marijuana, ≤1 lifetime cigarette uses, and no history of other illicit substance use. At approximately the 3-year follow-up, 20 adolescents were categorized as Heavy Drinkers, and 20 continuously non-drinking Controls (see Figure 1) were matched based on demographics (see Table 1). Of this sample, fMRI data from participants were reported on previously – 9 in Norman et al, 2011, 25 in Squeglia et al, 2012a, and 21 in Wetherill et al, 2012b.
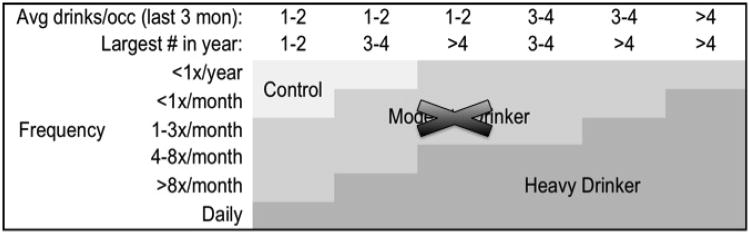
Figure 1.
Drinking classification for heavy drinking adolescents, based on Cahalan et al., 1969 and Squeglia et al., 2009. Note that Moderate Drinkers were not included in this study.
Table 1. Baseline and follow-up demographic characteristics.
| Controls (n = 20) M (SD) | Heavy Drinkers (n = 20) M (SD) | ||
|---|---|---|---|
| Baseline | Age (range 11.7 to 16.7) | 14.1 (1.2) | 14.7 (1.1) |
| % female | 45% | 45% | |
| Hollingshead SESa | 26.2 (18.0) | 24.1 (17.9) | |
| Family history density | 0.8 (0.8) | 0.8 (0.8) | |
| Achenbach Externalizing T-score | 39.8 (6.6) | 43.1 (8.3) | |
| Achenbach Internalizing T-score | 38.5 (9.3) | 42.3 (10.7) | |
| Reading standard score | 110.1 (13.0) | 113.0 (6.2) | |
| Vocabulary T-score | 53.7 (11.8) | 58.3 (6.5) | |
| Pubertal stage | 2.6 (0.6) | 2.9 (0.6) | |
| Body mass index | 21.0 (5.2) | 20.2 (2.8) | |
| Inhibitions percent correct (%) | 77.4 (1.1) | 83.3 (1.1) | |
| D-prime (D′) | 3.1 (0.6) | 3.1 (0.5) | |
| Response bias (β) | 0.2 (0.2) | 0.2 (0.1) | |
| Follow-up | Age (range 14.7 to 20.6) | 17.6 (1.2) | 18.5 (1.8) |
| Years between baseline and follow-up | 3.4 (0.8) | 3.3 (1.2) | |
| Achenbach Externalizing T-score | 37.6 (3.9) | 41.6 (13.0) | |
| Achenbach Internalizing T-score | 34.8 (7.2) | 38.9 (11.4) | |
| Past year drinking days per month*** | 0.0 (0.1) | 2.8 (1.8) | |
| Past year average drinks/episode*** | 0.2 (0.4) | 4.2 (2.1) | |
| Past year bingeb episodes*** | 0.0 (0.0) | 9.0 (13.8) | |
| Past year peak drinks/episode*** | 0.0 (0.0) | 10.5 (11.3) | |
| Lifetime number of drinks*** | 0.5 (0.9) | 92.3 (94.5) | |
| Lifetime number of cigarettes*** | 0.0 (0.0) | 0.5 (0.2) | |
| Lifetime cannabis use episodes* | 0.3 (1.1) | 4.8 (9.1) | |
| Lifetime other drug use episodes*** | 0.0 (0.0) | 0.2 (0.5) | |
| Days since last alcohol binge episodec | N/A | 70 | |
| Days since last cannabis usec | 138 | 116 | |
| Days since last other drug usec | N/A | 131 | |
| Inhibitions percent correct (%) | 86.4 (1.0) | 90.6 (0.7) | |
| D-prime (D′) | 3.3 (1.0) | 3.8 (0.5) | |
| Response bias (β) | 0.2 (0.1) | 0.3 (0.1) |
p < 0.05
p < 0.01
p < 0.001
Hollingshead SES scores typically range from 11-77, with lower scores indicating higher SES.
Binge episode = 4+ drinks for females or 5+ drinks for males.
Of those individuals who reported alcohol, cannabis, and/or other drug use, average number of days between date of last use and scan date.
Measures
Substance use
The Customary Drinking and Drug Use Record (CDDR) (Brown et al, 1998) assessed lifetime and past year alcohol, tobacco, and other drug use, DSM-IV abuse and dependence criteria, and other substance-related consequences. The Timeline Followback (Sobell and Sobell, 1992) assessed the participants' substance use quantity and frequency for the 30 days prior to scan sessions. Breathalyzers, urine toxicology, and parent reports on youth substance use verified adolescent self-report data.
Family background
The Hollingshead scale provided a measure of socioeconomic status that incorporates level of education and occupation of each parent (Hollingshead, 1965). The Family History Assessment Module (FHAM) (Rice et al, 1995) assessed DSM-IV criteria of alcohol and other drug abuse and dependence in first and second-degree relatives. Family history density was ascertained by adding 0.5 for each biological parent and 0.25 per biological grandparent endorsed by either youth or parent as having AUD or SUD (Zucker et al, 1994).
Development
The Pubertal Development Scale (Petersen et al, 1988) ascertained current level of pubertal development with five sex-specific items. Body mass index (BMI) was calculated using the standard formula incorporating height and weight. [BMI = (weight (lbs)/(height (in)2)) * 703]
Neurocognition
At baseline, the Vocabulary subtest of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) estimated verbal IQ, and the Wide Range Achievement Test, 3rd Edition (Wilkinson, 1993) determined reading skill level.
Psychopathology
At baseline, the Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3) assessed DSM-IV Axis I diagnoses (Shaffer et al, 1996). At baseline and follow-ups until age 18, the Child Behavior Checklist (CBCL) (Achenbach and Rescorla, 2001) provided a level of adolescent psychopathological syndromes (e.g., internalizing and externalizing behaviors). Similar self-report data were obtained with the Youth Self-Report (YSR) for those age 18 and under, and the Adult Self-Report (ASR) after turning 18 (Achenbach and Rescorla, 2003) to provide quantitative measures of psychopathological syndromes including externalizing and internalizing behaviors.
Experimental paradigm
An event-related go/no-go paradigm (Norman et al, 2011; Wetherill et al, 2012b) measured response inhibition during fMRI scanning. Participants viewed a serial presentation of blue shapes on a screen, comprised of 64 large circles, 16 small circles, 43 large squares, and 57 small squares with each stimulus appearing for 200 msec. Participants were instructed to press a button each time a shape appeared (go stimuli) but not press when shown the small square (no-go stimulus, 32% of trials). The intertrial interval was 1500 msec. Total task duration was 6 minutes and 24 seconds. Primary analyses contrasted BOLD response to the no-go (response inhibition) trials relative to the go (response selection) trials.
Procedures
Eligible youth completed baseline (i.e., prior to onset of substance use) interviews, urine toxicology, Breathalyzer screens, neuropsychological testing, and neuroimaging, and parents provided collateral reports of youth substance use, general life functioning, and behavior. Quarterly thereafter, participants completed phone interviews assessing substance use and psychological functioning. Annually after the baseline assessment, youth who transitioned into heavy drinking and a demographically matched nondrinking control completed a full follow-up assessment (see Measures) and a neuroimaging session on the same 3T scanner as used at baseline. Follow-up rates exceeded 95% at each annual time point in this project.
MR acquisition
Imaging data at baseline and follow-up were collected on the same 3.0-Tesla General Electric Excite MR system with an 8-channel phase-array head coil (General Electric Medical System, Milwaukee, WI, USA) at the UCSD Keck Center for Functional MRI. Scan sessions involved a 10-second scout scan and a sagittally acquired high-resolution three-dimensional T1-weighted anatomical image (field of view = 24 cm, 256 × 256 × 192 matrix, 0.94 × 0.94 × 1 mm voxels, 176 slices; repetition time=8 ms, echo time=3 ms, flip angle 12°, 7:19 minutes). Whole-brain echo planar images were collected axially (field of view=24 cm, 64 × 64 matrix, 3.75 × 3.75 × 3.8 mm voxels, repetition time = 2000 ms, echo time = 30 ms, 90º flip angle, 32 slices with no gap, 6:24 minutes).
Data processing and analysis
Baseline and follow-up imaging data were processed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996) (http://afni.nimh.nih.gov/). Small movements were corrected by registering image repetitions to a selected base volume using the AFNI 3D volume registration program (Cox and Jesmanowicz, 1999). Two trained raters visually inspected time series data and removed repetitions containing visible head motions. All data sets included in analyses retained >85% of repetitions.
Separate hemodynamic task response functions for inhibitions and response selection trials were calculated using deconvolution based on each participant's behavioral data, while accounting for hemodynamic delays (Bandettini et al, 1993) and covarying for motion and linear trends. This yielded fit coefficients representing the BOLD response across behavioral conditions. Voxel-wise fit-coefficient data were transformed to standard coordinates (Talairach and Tournoux, 1988), resampled into 3 mm3 isotropic voxels, and smoothed spatially with a 5 mm full width at half-maximum (FWHM) Gaussian filter.
Statistical analyses
For non-imaging data, t-tests compared group differences in continuous demographic, substance use, and task performance variables, and chi-square tests compared groups on categorical variables. Go/no-go task performance measures were percent correct on inhibitory trials, d′ (a measure of accuracy in discriminating between go and no-go stimuli)1, and β (a measure of response bias)2 (Green and Swets, 1966).
For imaging data, two separate analyses were conducted. First, we examined BOLD response contrast between no-go trials and go trials on whole-brain fMRI data using a single-sample t-test to examine activation at baseline. Second, a repeated measures ANCOVA (AFNI 3dANOVA3) with time (baseline and follow-up) as the within-subjects factor and group (Heavy Drinker n=20 versus Control n = 20) as the between-subjects factor determines main effects of drinking group and time and their interaction, for no-go relative to go trial BOLD response contrast on whole-brain fMRI data. Lifetime marijuana, nicotine, and other illicit drug use differed between groups, and as such, these variables were composited into a lifetime substance use variable and included as a covariate. Given potential sex differences in both substance use and neural activity, sex was also included as a covariate. To control against Type I error, Monte Carlo simulations using the AFNI 3dClustSim program were used and only clusters with a voxel threshold of p <.01 exceeding 756 μl, equal to 28 contiguous significant (α <.05) 3 mm3 voxels were considered significant. Data representing no-go relative to go BOLD response contrasts in significant clusters for each participant were exported to PASW 18.0 (Chicago, IL) for follow-up analyses.
Post hoc analyses examined whether BOLD response change over time correlated with subsequent alcohol involvement in Heavy Drinkers (i.e., follow-up lifetime number of drinks, past year drinking days per month, past year drinks per drinking occasion, and past year maximum number of drinks during a drinking occasion). Follow-up hierarchical linear regressions assessed whether baseline activation within clusters showing significant correlations predicted follow-up alcohol consumption, after statistically controlling for baseline alcohol use, biological sex, and follow-up age.
Results
Table 1 provides baseline and follow-up descriptive information for Heavy Drinkers and Controls. Of note, the ability to inhibit prepotent responses improved with age (p < 0.001) with no differences in this improvement between groups (Group × Time: p > 0.20).
The no-go versus go contrast at baseline revealed activations consistent with meta-analyses of response inhibition (Criaud and Boulinguez, 2013; Swick et al, 2011) showing significant clusters of activation in inferior, superior, and medial frontal gyri, and in parietal, temporal, cerebellar, and subcortical areas (p < 0.01, cluster > 756 μl, see Online Resource 2).
Because Heavy Drinkers reported significantly more substance use than Controls at follow-up, a lifetime substance use composite (excluded alcohol use) and biological sex were included as covariates. A repeated measures ANCOVA revealed significant group × time interactions in 5 regions: the bilateral middle frontal gyri, right inferior parietal lobule, left putamen, and left cerebellar tonsil (see Table 2 and Figure 2). Independent samples t-tests probed significant group × time interactions within these 5 clusters. At baseline, Heavy Drinkers showed significantly less no-go BOLD contrast than Controls in all 5 clusters (p's < 0.05). Across adolescence, Heavy Drinkers exhibited increasing response inhibition BOLD contrast, and Controls showed attenuated response in clusters. At follow-up, Heavy Drinkers showed significantly greater response inhibition activity than Controls in 4 brain regions (p's < 0.05): bilateral middle frontal gyri, right inferior parietal lobule, and left cerebellar tonsil.
Table 2. Drinking group × time interactions for BOLD response during inhibitory processing(N = 40).
| Structure | BA | Volume (μl) | Peak Coordinatesc | Group by Timea Effect Size η2 | t-test at Baselineb | t-test at Follow-Upb | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| x | y | z | ||||||
| R middle frontal gyrus | 10 | 1,755 | 24 | 46 | 8 | 0.19** | C>HD* | HD>C** |
| L middle frontal gyrus | 10 | 972 | −25 | 38 | 6 | 0.18** | C>HD** | HD>C* |
| R inferior parietal lobule | 40 | 1,458 | 35 | −35 | 48 | 0.30*** | C>HD** | HD>C* |
| L putamen | 1,107 | −29 | −14 | 6 | 0.19** | C>HD** | ns | |
| L cerebellar tonsil | 1,944 | −26 | −38 | −34 | 0.24** | C>HD* | HD>C** | |
η2 from repeated measures ANCOVA with biological sex and lifetime substance use as covariates.
t-values from independent samples t-tests.
Peak coordinates are in Talairach & Tournoux atlas (Talairach & Tournoux, 1988).
p < 0.05
p < 0.01
p < 0.001
Abbreviations. BA: Brodmann's area, C: Controls, HD: Heavy Drinkers, Ns: non-significant.
Figure 2.
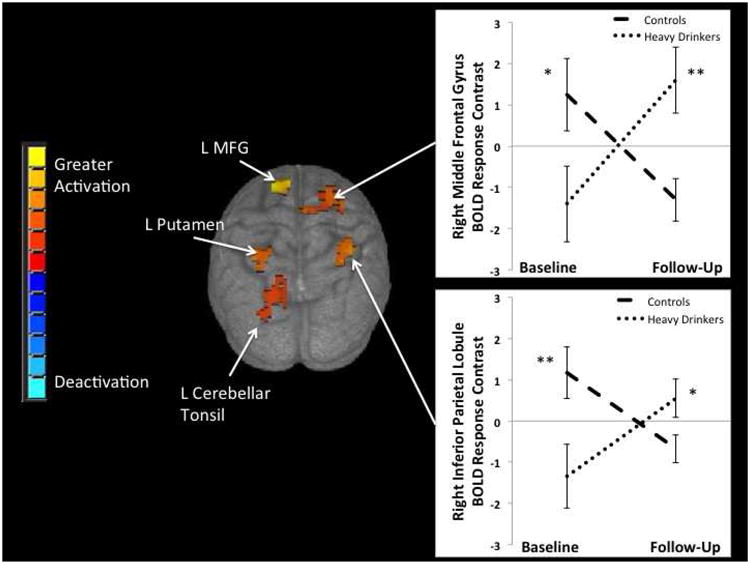
Significant (p < 0.01, cluster > 756 microliters) group × time interactions in BOLD response contrast to no-go relative to go trials were seen in 5 clusters in frontal, parietal, cerebellar, and subcortical brain regions. Graphs are shown for two of these regions: right middle frontal gyrus and right inferior parietal lobule. Future heavy drinkers showed less BOLD response at baseline in several brain regions, but greater BOLD response at follow-up after the initiation of heavy drinking, compared to continuous non-drinking controls. MFG = Middle Frontal Gyrus, L = Left, * p <.05, ** p <.01.
Exploratory post-hoc analyses examined whether BOLD response contrast change over time correlated with subsequent alcohol involvement in Heavy Drinkers (i.e., follow-up lifetime number of drinks, past year drinking days per month, past year drinks per drinking occasion, and past year maximum number of drinks during a drinking occasion). BOLD response contrast during no-go relative to go trials over time in the right middle frontal gyrus positively correlated with follow-up lifetime number of drinks (n =20, r = 0.65, p = 0.001) (Type I error controlled with α = 0.05/20 = 0.0025). Follow-up hierarchical linear regressions revealed BOLD response contrast at baseline did not predict follow-up alcohol consumption after controlling for baseline alcohol, biological sex, and follow-up age at our conservative, corrected threshold (p < 0.001).
Discussion
The present longitudinal neuroimaging study examined the effects of initiating heavy drinking during adolescence on brain responses during response inhibition. We hypothesized, based on previous findings (Squeglia et al, 2012a), that adolescents who transition into heavy drinking would show reduced BOLD response during response inhibition prior to initiating heavy drinking (baseline time point) followed by increased activation after the onset of heavy episodic drinking, as compared to adolescents who remained non-drinkers. Examining a longitudinal neuroimaging sample of youth both pre- and post-alcohol use initiation allowed us to address the etiology of neural pattern differences. Although group × time effect sizes were small, our findings suggest that differential neural activity patterns predate alcohol initiation and also arise as a consequence of heavy drinking.
We found significant drinking status × time interactions in a number of distinct and reproducible brain regions commonly associated with response inhibition. Prior to initiating substance use, adolescents who initiated heavy use showed less BOLD activation during inhibitory trials in frontal regions, including the bilateral middle frontal gyri, and non-frontal regions, including the right inferior parietal lobule, putamen, and cerebellar tonsil, compared with those who continued to abstain from alcohol use. This pattern of hypoactivity among youth who later initiated heavy drinking during response inhibition is consistent with studies showing decreased activity during response inhibition predicts later alcohol use (Norman et al, 2011) and substance use (Mahmood et al, 2012). Indeed, change in BOLD response contrast over time in the right middle frontal gyrus was associated with lifetime alcohol drinks at follow-up. Together, these findings provide additional evidence for the utility of fMRI in identifying neural vulnerabilities to substance use even when no behavioral differences are apparent.
At follow up, adolescents who transitioned into heavy drinking showed increasing brain activation in the bilateral middle frontal gyri, right inferior parietal lobule, and left cerebellar tonsil during inhibition; whereas, non-drinking controls exhibited decreasing brain activation in these brain regions. These regions have been implicated in processes of stimulus recognition, working memory, and response selection (Criaud and Boulinguez, 2013; Noonan et al, 2011; Rubia et al, 2006), all of which are critical to successful response inhibition. Indeed, neuroanatomical models of inhibitory control highlight the importance of frontoparietal attentional control and working memory networks (Corbetta and Shulman, 2002, 2011; Munakata et al, 2012). These models posit that inhibition and cognitive control involve frontoparietal brain regions (i.e., connections between the middle frontal gyrus extending posteriorly to the inferior parietal lobule) when detecting and responding to behaviorally relevant stimuli. Thus, findings suggest that heavy drinkers recruit greater activity in these neural networks in order to successfully inhibit prepotent responses.
Given the longitudinal nature of the current study, it is important to consider our findings in the context of typical adolescent neural maturation. During typical neural maturation, adolescents exhibit less activation over time, as neural networks become more refined and efficient (Luna et al, 2010). This typical pattern of neural maturation occurred among adolescents who remained nondrinkers. Adolescents who transitioned into heavy drinking showed the opposite pattern – increasing activation despite similar performance, suggesting that alcohol consumption may alter typical neural development.
The current findings should be considered in light of possible limitations. Although heavy drinking and non-drinking youth groups were matched on several baseline and follow-up measures, heavy drinking youth reported more cannabis, nicotine, and other illicit drug use at follow-up. Differential activation remained significant after statistically controlling for lifetime substance use and such differences may contribute to our findings. Further, simultaneous substance use (i.e., use of marijuana and/or tobacco with alcohol during the same episode) might be associated with these results. Future research should explore the effects polysubstance use during the same episode compared to the effects of heavy drinking on neural responses. It is also important to note that adolescence is a period of significant inter-individual differences in neural development, and as such, we matched self-reported pubertal development and age at baseline and follow-up to address this issue. For the current sample, histograms of age distributions at baseline and follow-up are provided in Online Resource 1. Again, our groups were well matched on these variables; however, additional longitudinal research to examine the effects puberty and hormonal changes on neural functioning and response inhibition are needed.
In summary, the current data suggest that pre-existing differences in brain activity during response inhibition increase the likelihood of initiating heavy drinking, and initiating heavy alcohol consumption leads to differential neural activity associated with response inhibition. These findings make a significant contribution to the developmental and addictive behaviors fields, as this is the first study to examine neural responses differences during response inhibition prior to and following the transition into heavy drinking among developing adolescents. Further, we provide additional support for the utility of fMRI in identifying neural vulnerabilities to substance use even when no behavioral differences are apparent. Identifying such neural vulnerabilities before associated behaviors (e.g., substance use) emerge provides an additional tool for selecting and applying targeted prevention programs. Given that primary prevention approaches among youth have not been widely effective, it is possible that targeted prevention programs for youth who are at greatest neurobiological risk could be a novel, effective approach. As such, our findings provide important information for improving primary prevention programs, as well as answering the question of whether neural differences predate alcohol initiation or whether differences arise as a consequence of alcohol use.
Supplementary Material
Acknowledgments
This research was made possible by funding from the National Institute on Alcohol Abuse and Alcoholism (R01 AA13419, T32 AA013525).
Footnotes
The authors declare no conflicts of interest.
d′ = Φ−1 (H) − Φ−1 (F). Φ−1 = z score, (H) = hit rate, (F) = false-alarm rate
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA Adult Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2003. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2011;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011;34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Criaud M, Boulinguez P. Have we been asking the right questions when assessing resp.onse inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci Biobehav Rev. 2013;37:11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal-detection theory and psychophysics. Wiley; New York: 1966. [Google Scholar]
- Hollingshead AB. Two-factor index of social position. Yale University; New Haven, CT: 1965. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2009: Volume I Secondary school students. Institute for Social Research; Ann Arbor: 2012. [Google Scholar]
- Lee CM, Maggs JL, Neighbors C, Patrick ME. Positive and negative alcohol-related consequences: associations with past drinking. J Adolesc. 2011;34:87–94. doi: 10.1016/j.adolescence.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Mahmood OM, Goldenberg D, Thayer R, Migliorini R, Simmons AN, Tapert SF. Adolescents' fMRI activation to a response inhibition task predicts future substance use. Addict Behav. 2012;38:1435–1441. doi: 10.1016/j.addbeh.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, Snyder HR, Chatham CH. Developing cognitive control: Three key transitions. Curr Dir Psychol Sci. 2012;21:71–77. doi: 10.1177/0963721412436807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Mars RB, Rushworth MF. Distinct roles of three frontal cortical areas in reward-guided behavior. J Neurosci. 2011;31:14399–14412. doi: 10.1523/JNEUROSCI.6456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pulido C, Anderson KG, Armstead AG, Brown SA, Tapert SF. Family history of alcohol-use disorders and spatial working memory: effects on adolescent alcohol expectancies. J Stud Alcohol Drugs. 2009;70:87–91. doi: 10.15288/jsad.2009.70.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Self-regulation as a protective factor against risky drinking and sexual behavior. Psychol Addict Behav. 2010;24:376–385. doi: 10.1037/a0018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Dulcan MK, Davies M, Piacentini J, Schwab-Stone ME, et al. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): description, acceptability, prevalence rates, and performance in the MECA Study. Methods for the Epidemiology of Child and Adolescent Mental Disorders Study. J Am Acad Child Adolesc Psychiatry. 1996;35:865–877. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009a;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. J Stud Alcohol Drugs. 2012a;73:749–760. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res. 2011;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology. 2012b;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009b;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme Medical Publishers; New York: 1998. [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, Yang TT, et al. Frontoparietal connectivity in substance-naive youth with and without a family history of alcoholism. Brain Res. 2012a;1432:66–73. doi: 10.1016/j.brainres.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Castro N, Squeglia LM, Tapert SF. Atypical neural activity during inhibitory processing in substance-naive youth who later experience alcohol-induced blackouts. Drug Alcohol Depend. 2012b;128:243–249. doi: 10.1016/j.drugalcdep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. The Wide Range Achievement Test-3 Administration Manual. Jastak Associates; Wilmington, DE: 1993. [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, et al. Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age. Pediatrics. 2008;121(Suppl 4):S273–289. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED. Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol. 2010;44:119–124. doi: 10.1016/j.alcohol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE. Developmental evidence for at least two alcoholisms. I. Biopsychosocial variation among pathways into symptomatic difficulty. Ann N Y Acad Sci. 1994;708:134–146. doi: 10.1111/j.1749-6632.1994.tb24706.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.