Abstract
Given the often chronic nature of substance use disorders, patients sometimes receive less intensive continuing care following an initial period of more intensive treatment. This meta-analysis estimated the effect of continuing care and formally tested several proposed moderators (intervention duration, intensity, modality, setting) of that effect. A systematic search identified 33 controlled trials of continuing care; 19 included a no/minimal treatment condition and were analyzed to assess the overall effect of continuing care versus control. Continuing care had a small, but significant, positive effect size, both at the end of the continuing care interventions (g=0.187, p<0.001) and at follow-up (g=0.271, p<0.01). Limited by a small number of studies, analyses did not identify any significant moderators of overall effects. These results show that continuing care can provide at least modest benefit after initial treatment. We discuss study characteristics that may have reduced the magnitude of the overall continuing care effect estimate.
Keywords: continuing care, aftercare, substance use disorders, meta-analysis, treatment
1. Introduction
More than half of patients in treatment for substance use disorders relapse within the first year after entering treatment, and they remain at heightened risk of relapse throughout the early years of recovery (De Soto, O’Donnell, & De Soto, 1989; Hunt, Barnett, & Branch, 1971; Jin, Rourke, Patterson, Taylor, & Grant, 1998; Miller & Hester, 1986). Continuing care, a period of less intensive treatment following a more intensive initial treatment episode, has been utilized in an effort to extend and reinforce an initial period of recovery and is recommended in several guidelines for the treatment of substance use disorders - e.g., U.S. Department of Veterans Affairs and U.S. Department of Defense (2009), and American Psychiatric Association (2006).
Although, intuitively, continuing care would seem to be helpful and well-matched to the chronic nature of some individuals’ substance use disorders, studies testing the efficacy of continuing care have produced mixed results. For example, McKay (2009) conducted a systematic review of 20 comparative trials of continuing care. He classified studies as either having “positive results” (i.e., at least one significant group difference on a primary substance use outcome favoring continuing care) or “negative results” (i.e., no significant difference or a significant effect favoring the control group). Only half of the studies (n = 10) had positive results. To further investigate those mixed effects, McKay examined different trial characteristics qualitatively. Positive trials tended to have a longer planned duration of care, more intensive continuing care, and more active efforts to deliver continuing care to patients. Negative trials tended to have smaller sample sizes and to include a comparison condition with some continuing care, rather than no treatment. The type of continuing care (cognitive-behavioral therapy [CBT] versus other treatments) was not associated with positive or negative results.
The review by McKay (2009) underscored the mixed effects of continuing care and highlighted several potential moderators that might account for variation in the results of existing studies. However, his “box-score” review was limited by reliance on significance tests, which can be influenced by a number of factors (e.g., sample size and associated statistical power, number of tests for treatment effects conducted), and to perusal of results rather than statistical tests to try to identify study features that were associated with stronger continuing care effects. The current review builds on that of McKay in three ways: First, it is a meta-analysis of effect sizes of continuing care, which has the advantage of producing an estimate of the magnitude of the overall continuing care effect based on weighted study estimates of effects, including under-powered studies which are less likely to yield “positive results” in a review based purely on significance tests. Second, it formally tests several treatment characteristics identified in McKay’s (2009) review as potential moderators of the effect of continuing care, including (a) intensity and duration of the treatment, (b) treatment orientation (CBT versus others), and (c) method of treatment delivery (outpatient, telephone, home visits). Finally, it uses an updated and expanded sample of continuing care studies by including those published through 2011.
1.1. Intensity and duration
What is the optimal length and intensity of continuing care? The duration (i.e., the total amount of time over which the intervention is provided) and the intensity (i.e., how often sessions are provided and how long each one lasts) of continuing care interventions vary widely across existing studies (McKay, 2009). Effective short or low-intensity treatments typically would be more cost-effective than longer or higher intensity ones, but it is unclear whether differences in duration or intensity are associated with variation in effectiveness. An observational study (Ritsher, Moos, & Finney, 2002) and an earlier review by McKay (2005) provided support for the hypothesis that a longer duration of continuing care is beneficial, whereas a meta-analysis of psychosocial interventions for substance use disorders (including both studies of initial treatment as well as continuing care) found a negative effect of treatment duration (Dutra et al., 2008). Regarding intensity, neither Ritsher et al.’s (2002) observational study nor Dutra et al.’s (2008) meta-analysis provided support for a significant influence of treatment intensity on outcome. Considering these mixed results, we hypothesized that, within continuing care interventions, a longer planned duration is associated with greater positive effects on substance use outcomes, but that planned intensity is not significantly associated with outcomes.
1.2. Type of treatment
CBT has been shown to be efficacious in studies of both initial treatment and continuing care for substance use disorders (Bennett et al., 2005; Maude-Griffin et al., 1998; O’Farrell, Choquette, Cutter, Brown, & McCourt, 1993; Rohsenow et al., 2001). However, one meta-analysis found that the effect was small when CBT was compared to another active treatment (Magill & Ray, 2009). Therefore, we hypothesized that CBT-based continuing care interventions have a significant overall effect compared to control conditions, but that CBT-based interventions do not have a significantly larger effect when compared to non-CBT-based active conditions.
1.3. Method of treatment delivery
Continuing care has been provided in a range of settings (e.g., outpatient visits, home visits, telephone sessions) and various techniques have been used to “[take] the intervention to the patient” (McKay, 2009). Such techniques aim to increase patients’ participation in treatment by making it easier for them to receive care. They include telephone counseling, home visits, and other actions to remind patients of appointments and assertively follow-up with patients after missed sessions. Some of these approaches (e.g., telephone counseling) also may cost less. We hypothesized that continuing care provided through telephone sessions, home visits, or outpatient counseling with some additional active elements (such as appointment prompts and active follow-up after missed sessions) is associated with larger positive effect sizes on substance use outcomes compared to exclusively outpatient continuing care without other active elements to increase participation.
1.4. Summary
This meta-analysis adds to the previous research on continuing care in several ways. First, we use and describe an updated sample of 33 controlled continuing care trials. Second, we assess the magnitude and significance of the overall effect of continuing care on substance use outcomes at the end of treatment, as well as at specific follow-up points, in the subgroup of 19 studies including a no- or minimal-treatment comparison group. This information can be used by patients and treatment providers to judge the usefulness of continuing care. Third, in different subsamples of studies, we evaluate the influence of certain treatment and study design characteristics, using formal moderator analyses to help identify “what matters” in continuing care treatment and to attempt to generate information for those making decisions about what kind of continuing care (if any) to provide.
2. Materials and methods
2.1. Eligibility criteria
This meta-analysis included controlled trials (i.e., randomized or using some other form of assignment to groups involving chance, such as sequential cohorts) of one or more continuing care interventions for persons with alcohol and/or other drug use disorders. Other eligibility criteria included publication since 1988, assignment of at least five participants to each condition, and inclusion of at least one substance use-related outcome.
2.2. Information sources
We began our sample with the 20 relevant studies identified by McKay (2009), who reviewed comparative studies of continuing care published from 1988 to 2006. That sample was updated and expanded by searching the PubMed database, as well as scanning the reference lists in relevant articles and reviews. PubMed was searched using the substance use disorder keywords “alcohol*,” “drug,” and “substance,” along with the continuing care-related keywords “continuing care,” “continued care,” and “aftercare.” The search built on McKay’s literature review, so it overlapped with only the last two years examined by McKay. Thus, our search was limited to articles published from 2004 through the end of 2011 (last searched on March 6, 2012). All resulting citations and abstracts were examined for relevance and full articles additionally were checked where necessary to assess fulfillment of our inclusion criteria.
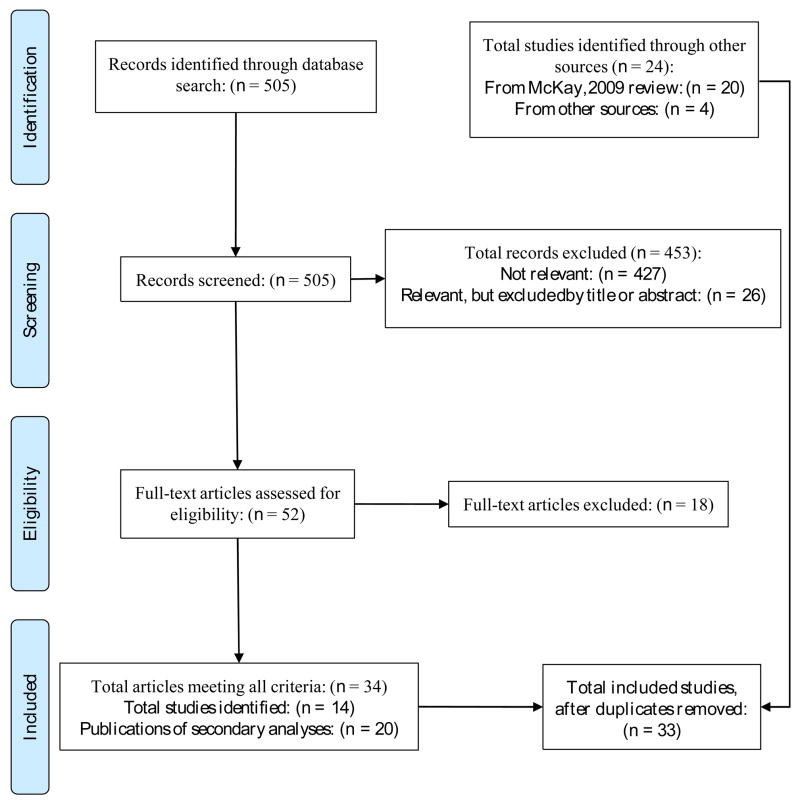
In all, 33 studies meeting our criteria were identified (see Figure 1), 19 of which compared a continuing care condition to a no- or minimal-treatment control condition. The full sample includes the 20 studies reviewed by McKay (2009), plus 10 additional studies published after the cutoff point for McKay’s review (i.e., published between 2006 and 2011), and three additional studies that were not included in McKay’s review (Brown, Seraganian, Tremblay, & Annis, 2002; Dennis, Scott, & Funk, 2003; Lash, Petersen, O’Connor, & Lehmann, 2001). Two of those studies (Dennis et al., 2003; Lash et al., 2001) were cited in McKay’s review, although it was not specified why they were not included in the box score ratings. The studies tested interventions aimed at encouraging participants to re-enter more intensive treatment as needed (Dennis et al., 2003), and increasing continuing care attendance (Lash et al., 2001), so the studies may not have met McKay’s criteria for a continuing care intervention. Brown et al. (2002) compared outpatient Relapse Prevention and Twelve-Step Facilitation continuing care interventions provided after residential treatment. This study was identified in searching reference lists of other study reports; it is unclear why it was not included in McKay’s review.
Fig. 1.
Literature search flow diagram.
2.3. Coding of studies
A coding form was used to extract study information. Two of the authors (JB and IF) trained on approximately 10% of the studies and then independently coded all included studies. Consensus was reached on all discrepancies with a third author (NM) when agreement could not be reached by the two coders. Additional information was requested from authors when necessary.
2.3.1. Study design characteristics
We coded study design characteristics, such as whether participants were randomly allocated, whether there was a control condition that received no- or minimal-treatment (e.g., basic referrals or assistance provided only as needed and requested), and the type of sample for which data were analyzed and results published (e.g., the full randomized sample, the sample that was successfully followed up, the sample that completed the planned treatment).
2.3.2. Moderator coding
Potential moderators were coded separately for the randomized (distinctive) elements of each treatment group and for any common elements received by participants in all of the conditions within a study. Duration was defined as the number of months of planned continuing care, whereas intensity was defined as the number of planned sessions per week. Active psychotherapy treatments either had a primary orientation (CBT, 12-step focused, Motivational Enhancement Therapy, process groups) or they were coded as general/unspecified counseling. Treatments with a CBT orientation have been studied often and use specific cognitive and behavioral therapy techniques to help participants systematically change their thoughts and behaviors to be supportive of their recovery. Such treatments vary in how structured and in depth they are. Therefore, according to McKay’s (2009) distinctions, CBT-based treatments were coded as being CBT (i.e., a full “variant of CBT,” such as the manual-guided CBT intervention used in the Matching Alcoholism Treatments to Client Heterogeneity trial; Project MATCH Research Group, 1997) or as “CBT-like” (i.e., “contain[ing] elements of CBT,” such as skills training, problem solving approaches, contracting, or incentives, but not constituting comprehensive CBT).
For each treatment, we also coded the setting and method of delivery, including whether continuing care was provided by telephone, home visits, or outpatient sessions at a clinical/treatment center, and whether there were active efforts to improve participation, such as brief reminder phone calls, behavior contracting or social reinforcement.
2.4. Outcome measures
We calculated effect sizes for any outcomes that measured some facet of substance use. We included only outcomes relevant to the entire study sample, not those assessed or reported for only subgroups of participants. To capture short-term effects, we calculated effect sizes at the end of the planned continuing care period. More extended effects were captured by calculating effect sizes from substance use outcome data at the longest follow-up after the end of the planned continuing care period. To prevent confusion in presenting results, each study is exclusively identified by the first author and year of the primary publication, which we defined as the earliest publication of results for each study. Effect sizes extracted from data presented in secondary publications may have been used in analyses of end of treatment or follow-up effects. Further details are available from the authors.
Two coders calculated effect sizes and double-checked them for accuracy. For continuous outcomes, we calculated the standardized mean difference using the formula for Cohen’s d (Cohen, 1988) in the computer program ES: A Computer Program for Effect Size Calculation (Shadish, Robinson, & Lu, 1999). We then applied Hedges g correction for small sample bias (Borenstein, 2009; Hedges & Olkin, 1985). For dichotomous outcomes, we calculated the odds ratio for 2 × 2 tables (Haddock, Rindskopf, & Shadish, 1998) and then converted them to g’s for comparison purposes (Borenstein, 2009). When proportions or means and standard deviations were not presented, an F-test statistic or p-value, if provided, was transformed into a standardized mean difference (Borenstein, 2009; Shadish & Haddock, 2009). In the absence of other data, if the results were presented only as statistically “non-significant,” we assigned the effect size a value of zero (Rosenthal, 1995). Scores on “negative” outcomes (e.g., drinking problems) were reversed, so that a positive effect size always indicates that the focal continuing care group had a better outcome than the control group.
In order to obtain one effect size per study, we combined all effect sizes within each study using the aggregation procedures in MAd (Del Re & Hoyt, 2010) and RcmdrPlugin.MAd (Del Re, 2010). Our meta-analysis of naltrexone and acamprosate for alcohol use disorders (Maisel, Blodgett, Wilbourne, Humphreys, & Finney, 2013) has additional information about sample size decisions and procedures for collapsing across stochastically dependent effect sizes. Effect size values of 0.20 were considered small, values of 0.50 were considered medium, and values of 0.80 were considered large (Cohen, 1988).
2.5. Meta-analyses
We calculated overall effect sizes using a random-effects model, given our goal of generalizability and our assumption of heterogeneity of effects (Raudenbush, 2009). Because we were interested in moderators that might explain variability in effect sizes across studies, we calculated the Q-statistic, a measure of the heterogeneity of effect sizes, with a significant p-value suggesting that effect sizes varied more across studies than expected from sampling fluctuations (Borenstein & Hedges, 2009, p. 113). We also calculated the I2 statistic, which estimates the percentage of variability in effect sizes across the studies that is due to heterogeneity (Borenstein & Hedges, 2009, p. 118). Conventions are that 0% represents no observed heterogeneity, 25% is low heterogeneity, 50% is moderate heterogeneity, and 75% is high heterogeneity (Higgins, Thompson, Deeks, & Altman, 2003). We considered an effect to be heterogeneous if the I2 indicated at least low to moderate heterogeneity (>35%) and the Q-statistic was significant (e.g., Cuijpers, van Straten, Bohlmeijer, Hollon, & Andersson, 2009).
Moderator analyses were conducted using Comprehensive Meta-Analysis (CMA) version 2.2.048 and an R routine (Viechtbauer, 2011). For categorical moderators, we conducted univariate mixed-effects tests of subgroups in CMA in order to present the aggregate effect size for each subgroup (Cuijpers et al., 2009; Lipsey & Wilson, 2000). The mixed-effects method allows for calculation of random-effects model effect sizes within subgroups and fixed-effect model effect sizes across the subgroups (Overton, 1998). For continuous moderators, we used the restricted maximum likelihood meta-regression in R’s metafor program (Viechtbauer, 2011).
3. Results
3.1 Descriptive information
Descriptive information on study characteristics and the moderators of interest for each of the 33 identified continuing care studies is presented in Table 1.
Table 1.
Continuing care studies included in meta-analysis
| First author (year) | Number of randomized participants | Disorder of focus | Duration of continuing care | Common interventiona | Randomized interventions (Modality codeb) | Randomized intervention settingc (number of planned sessions) | Follow-up pointsd (Time since end at the longest follow-up) |
|---|---|---|---|---|---|---|---|
| Gilbert (1988) | 96 | Alcohol | 12 monthse | Traditional aftercare (after randomized period) | Home visit (2) Case-manager (2) Traditional aftercare (2) |
Home visits (18) Outpatient+ (prompting) (18) Outpatient (18) |
A |
| Ito (1988) | 45 | Alcohol | 6 monthsf | Usual aftercare (after randomized period) | Relapse Prevention (1) Interpersonal Process groups (5) |
Outpatient (18) Outpatient (8) |
B (6 months) |
| McLatchie (1988) | 155 | Alcohol | 3 months | - | Mandatory aftercare (6) Voluntary aftercare (8) No aftercare (8) |
Outpatient+ (contracting) (4) Outpatient (N/A) N/A (N/A) |
A, B (9 months) |
| Hawkins (1989) | 130 | Drug | Phase 1: 10 weeks Phase 2: 6 months |
Participants remained in initial Therapeutic Community for at least 10 weeks | Project skills (2) Control (8) |
Outpatient (>20) N/A (N/A) |
B (6 months) |
| Kadden (1989)g | 118 | Alcohol | 6 months | - | Coping skills (1) Interactional group therapy (5) |
Outpatient (26) Outpatient (26) |
A, B (18 months) |
| McAuliffe (1990) | 168 | Drug | 6 months | - | Recovery Training and Self- Help (6) Control (8) |
Outpatient (52) Outpatient (N/A) |
A, B (6 months) |
| Foote (1991) | 325 | Alcohol and/or Drug | 1 year | - | Special follow-up (6) Regular Care (8) |
Outpatient+ (active follow-up) (13–20) Outpatient (N/A) |
A |
| Patterson (1991)h | 127 | Alcohol | 1 year | - | Community Psychiatric Nurse (6) Control (8) |
Home visits (16) Outpatient (N/A) |
A, B (4 years) |
| Connors (1992) | 63 | Alcohol | 6 months | - | Group aftercare (1) Telephone aftercare (1) No aftercare (8) |
Outpatient (8) Telephone (8) N/A (N/A) |
A, B (1 year) |
| O’Farrell (1993)i | 64 | Alcohol | 1 year | - | Couples Relapse Prevention (1) Control (8) |
Outpatient (15) N/A (N/A) |
A, B (18 months) |
| Graham (1996) | 216 | Alcohol and/or Drug | 3 months | - | Relapse Prevention (1) Relapse Prevention (1) |
Outpatient (12) Outpatient (12) |
B (9 months) |
| Project MATCH (1997) | 774 | Alcohol | 3 months | - | Cognitive Behavioral Coping Skills (1) Motivational Enhancement Therapy (4) Twelve-step Facilitation (3) |
Outpatient (12) Outpatient (4) Outpatient (12) |
A, B (1 year) |
| Schmitz (1997) | 47 | Drug | 8 weeks | - | Relapse Prevention (1) Relapse Prevention (1) |
Outpatient+ (incentives for attendance) (12) Outpatient+ (incentives for attendance) (12) |
A, B (6 months) |
| McKay (1999) | 132 | Drug | 5–6 months | - | Standard treatment (3) Relapse Prevention (1) |
Outpatient (48) Outpatient (48) |
A, B (18 months) |
| Brown (2001)j | 170 | Drug | 6 months | - | Aftercare (2) Control (8) |
Outpatient+ (frequent phone calls to maintain contact) (schedule negotiated by client and staff) N/A (N/A) |
A, B (6 months) |
| Lash (2001) | 81 | Alcohol and/or Drug | 8 weeks | Standard treatment with contracting and prompting | Social Reinforcement (2) No additional treatment (8) |
Outpatient+ (social reinforcement) (N/A) N/A (N/A) |
B (4 months) |
| Brown (2002) | 266 | Alcohol and/or Drug | 10 weeks | - | Twelve-step Facilitation (3) Relapse Prevention (1) |
Outpatient (10) Outpatient (10) |
B (4 months) |
| Sannibale (2003) | 77 | Alcohol and/or Drug | 6 months | - | Structured Aftercare (1) Unstructured Aftercare (8) |
Outpatient+ (prompting) (9) Outpatient (N/A) |
A, B (6 months) |
| Dennis (2003) | 448 | Alcohol and/or Drug | 24 months | - | Recovery Management Check-ups (4) Control (8) |
Outpatient+ (prompting, linkage assistance) (6) N/A (N/A) |
A |
| Horng (2004) | 77 | Alcohol | 3 months | - | Telephone aftercare (6) Control (8) |
Telephone (5) N/A (N/A) |
A |
| McKay (2004) | 359 | Alcohol and/or Drug | 3 months | - | Standard group counseling (3) Relapse Prevention (1) Telephone counseling (2) |
Outpatient (24) Outpatient (24) Telephone (16) |
A, B (21 months) |
| Bennett (2005) | 124 | Alcohol | 15 weeks | Aftercare as Usual | Early Warning Signs Relapse Prevention Training (1) No additional treatment (8) |
Outpatient (15) N/A (N/A) |
A, B (9 months) |
| Mundt (2006) | 60 | Alcohol | 6 months | Standard referrals | Prompted IVR (2) ad libitum IVR (2) No IVR |
Telephone (180) Telephone (180) N/A (N/A) |
A |
| Godley (2007) | 183 | Alcohol and/or Drug | 3 months | Usual continuing care | Assertive Continuing Care (1) No additional treatment (8) |
Home visits (13) N/A (N/A) |
A, B (6 months) |
| Hubbard (2007) | 339 | Alcohol and/or Drug | 12 weeks | Standard Continuing Care plan | Telephone call group (6) No additional treatment (8) |
Telephone (7) N/A (N/A) |
A |
| Jason (2007) | 150 | Alcohol and/or Drug | Open-ended | - | Oxford House (7) Usual Care (8) |
Residential (N/A) Outpatient (N/A) |
open-ended treatment, last follow-up at 2 years from start of continuing care period |
| Lash (2007) | 150 | Alcohol and/or Drug | 11 months | Standard aftercare | Contracting, Prompting, Reinforcement (2) Standard treatment (8) |
Outpatient+ (contracting, prompting, reinforcement) (2) Outpatient (N/A) |
A |
| Kaminer (2008) | 144 | Alcohol | 3 months | - | In-person aftercare (1) Brief telephone aftercare (1) No-active aftercare (8) |
Outpatient (5) Telephone (5k) N/A (N/A) |
A, B (6 months) |
| Bowen (2009) | 168 | Alcohol and/or Drug | 8 weeks | - | Mindfulness-Based Relapse Prevention (1) Treatment as Usual (6) |
Outpatient (8) Outpatient (12) |
A, B (4 months) |
| Scott (2009) | 446 | Alcohol and/or Drug | 4 years | - | Recovery Management Check-ups (4) Control (8) |
Outpatient+ (linkage assistance) (16) N/A (N/A) |
A |
| Godley (2010) | 104 | Alcohol and/or Drug | 3 months | Usual continuing care | Telephone Continuing Care (6) No additional treatment (8) |
Telephone (8) N/A (N/A) |
A, B (3 months) |
| Godley (2010) | 320 | Alcohol and/or Drug | 3 months | - | Assertive Continuing Care (1) No Assertive Continuing Care (8) |
Home visits (13) N/A (N/A) |
A, B (6 months) |
| McKay (2010) | 252 | Alcohol | 18 months | Treatment as Usualk | Telephone Monitoring and Counseling (1,4) Telephone Monitoring (8) No additional treatment (8) |
Telephone (37) N/A (N/A) N/A (N/A) |
A, B (6 months) |
Note. CBT = cognitive-behavioral therapy, MET/MI = Motivational Enhancement Therapy/Motivational Interviewing.
Concurrent with randomized intervention unless noted.
1 = CBT; 2 = CBT-like; 3 = 12-step; 4 = MET/MI; 5 = Process groups; 6 = General/unspecified counseling; 7 = Residential mutual self-help; 8 = Control.
Outpatient+ = Outpatient treatment, plus active efforts to improve participation.
A = end of continuing care; B = any follow-up after end of continuing care.
Six months of randomized intervention, followed by 6 months of common intervention.
Eight weeks of randomized intervention, followed by 4 months of common intervention.
Cited in McKay (2009) as Cooney, Kadden, Litt, & Getter (1991), we used the earlier publication as our primary source.
Cited in McKay (2009) as D. G. Patterson, Macpherson, & Brady (1997), we used the earlier publication as our primary source.
Cited in McKay (2009) as O’Farrell, Choquette, & Cutter (1998), we used the earlier publication as our primary source.
Cited in McKay (2009) as B. S. Brown, O’Grady, Battjes, & Farrell (2004), we used the earlier publication as our primary source.
Participants remained in Treatment as Usual for a limited period during the randomized intervention (generally less than three months).
To examine the overall effect of continuing care, we restricted the analysis to the 19 studies that compared a continuing care condition to a no- or minimal-treatment control condition. Summary descriptive information on this sample of studies is presented in Table 2. The 19 studies included a total of 3,542 participants. At the study level, the mean age of participants across the studies in this sample was 35 years, less than one-third of the participants were women, and there was wide variation in racial/ethnic representation. The most common setting of the initial treatment episode was inpatient, and nearly half of the studies were focused on treatment for alcohol use disorders. More than half of the studies tested CBT-based continuing care interventions, and none of the studies tested an intervention explicitly described as 12-step based (although some interventions classified as “general/unspecified counseling” may have used this approach).
Table 2.
Descriptive information about continuing care studies included in the analysis of the main effect of continuing care compared to a no- or minimal-treatment control condition (total N = 19 studies).
| Variable | N studies (%where specified) | Mean | SD | Range | Median |
|---|---|---|---|---|---|
| Participant descriptive | |||||
|
| |||||
| Sample size (total: 3,542) | 19 | 194.3 | 125.1 | 60–448 | 155.0 |
| Age | 18 | 34.7 | 9.2 | 15.9–44.4 | 37.0 |
| % Women | 18 | 28.9 | 17.8 | 0–62.0 | 30.4 |
| % Whitea | 9 | 55.1 | 37.5 | 8–100 | 73.0 |
| % Blacka | 11 | 50.1 | 36.4 | 4.2–95.8 | 50.0 |
| % Latinoa | 6 | 6.0 | 4.3 | 2.0–13.2 | 5.0 |
|
| |||||
| Study descriptive | |||||
|
| |||||
| Initial treatment setting | |||||
| All received inpatient | 42.1% | N/A | N/A | N/A | N/A |
| All received outpatient | 36.8% | N/A | N/A | N/A | N/A |
| Mixed inpatient/outpatient | 21.1% | N/A | N/A | N/A | N/A |
| Disorder of focus | |||||
| Alcohol use disorders | 47.4% | N/A | N/A | N/A | N/A |
| Drug use disorders | 10.5% | N/A | N/A | N/A | N/A |
| Substance (alcohol and/or drug) use disorders | 42.1% | N/A | N/A | N/A | N/A |
| Modality | |||||
| CBT | 42.1% | N/A | N/A | N/A | N/A |
| CBT or CBT-like | 52.6% | N/A | N/A | N/A | N/A |
| MET/MI | 10.5% | N/A | N/A | N/A | N/A |
| General/unspecified counseling | 36.8% | N/A | N/A | N/A | N/A |
| Longest follow-up point after end of continuing care (months since the end of continuing care) | 12 | 12.0 | 12.0 | 6.0–48.0 | 7.3 |
| Planned continuing care length (months) | 18 | 9.7 | 11.0 | 2.8–48 | 6.0 |
| Planned sessions/week | 17 | 0.9 | 1.6 | 0.1–7.0 | 0.3 |
Studies may have presented no race information or any combination of one or more categories, so these values represent different samples of studies. Of the 12 studies that presented any race information, 1 study presented only % white, 3 studies presented only % black, 2 studies presented % white and % black, 1 study presented % black and % Latino, and 5 studies presented % white, % black, and % Latino. Only one study specified % Asian participants, at 47.6%.
3.2. Overall effect of continuing care
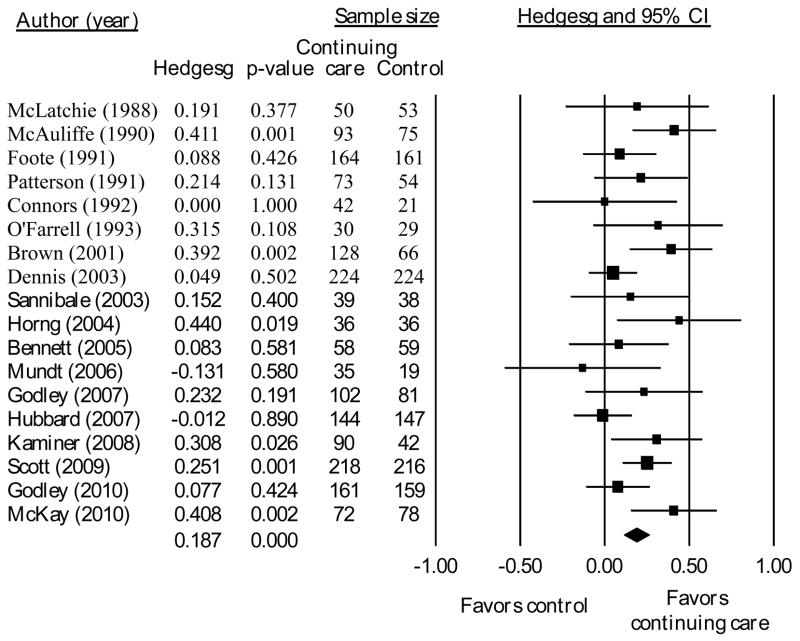
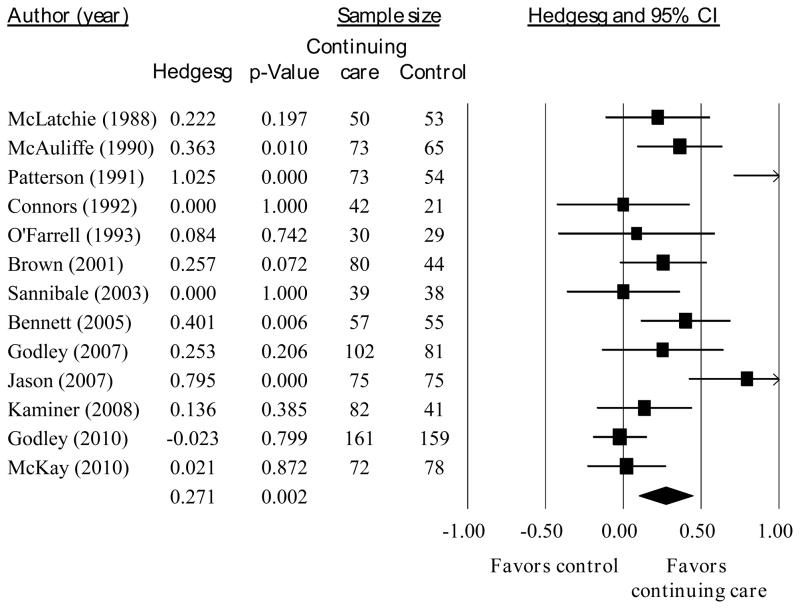
From the total sample of 19 studies including a no- or minimal-treatment control condition, the sample size of studies included in each analysis below varies depending on the assessment points for which data were available for each study. Across all outcomes at the end of treatment, a significant, but small, effect favored continuing care over control (g = 0.187, p < 0.001, n = 18 studies, see Figure 2). Heterogeneity in the effect sizes approached significance (I2 = 35%, Q = 26.1, p = 0.07). At the last follow-up point after the end of continuing care, the overall effect size was larger than at the end of continuing care (g = 0.271, p < 0.01, n = 13, see Figure 3). However, there was significant heterogeneity in those results (I2 = 76%, Q = 49.2, p < 0.001).
Fig. 2.
Effect sizes and 95% confidence interval for continuing care compared to no- or minimal-treatment at the end of the planned continuing care intervention.
Fig. 3.
Effect sizes and 95% confidence interval for continuing care compared to no- or minimal-treatment at the longest follow-up point after the end of the planned continuing care intervention.
3.3. Moderators
Intervention duration, intensity, CBT focus and method of treatment delivery were tested as potential moderators of continuing care effects, although the relevant samples varied across analyses. We examined the first two moderators (duration and intensity) in the sample of 19 studies comparing a continuing care treatment to a no- or minimal-treatment control condition. Duration and intensity were not significantly correlated with each other (r = −0.203, p = 0.43).
3.3.1. Duration
In the studies that specified a desired duration, months of planned continuing care was not significantly associated with effect size at the end of continuing care (b = 0.001, p = 0.66, n = 17) or at the last follow-up point after the end of continuing care (b = 0.008, p = 0.67 n = 12). Thus, studies with longer planned treatments did not have larger effect sizes than studies with shorter prescribed periods of treatment.
3.3.2. Intensity
In the studies that had a protocol-specified number of intervention sessions, planned sessions per week was not significantly associated with the effect of continuing care compared to control at the end of treatment (b = −0.027, p = 0.46, n = 17). This effect remained non-significant for outcomes assessed at the last follow-up (b = −0.062, p = 0.74, n = 12).
3.3.3. CBT
To assess the impact of CBT-based interventions, we analyzed the 13 studies that compared a CBT treatment condition to a non-CBT condition (either another type of treatment, such as general supportive counseling, or a no-treatment control condition). The overall effect of CBT at the end of treatment was small, but significant and positive (g = 0.120, p = 0.01, I2 = 39%, n = 13).
A moderator analysis showed a larger effect when CBT was compared to a control group (g = 0.195, p < 0.001, n = 8) than when compared to another active intervention (g = 0.023, p = 0.74, n = 5), and this difference was significant (Q = 4.01, p = 0.05). When three additional studies that included a “CBT-like” intervention were included, the results did not change in direction or significance for the overall effect (g = 0.124, p < 0.01, I2 = 38%, n = 16), or the moderator analysis (Q = 6.11, p = 0.01). In an analysis of outcomes at the last follow-up point after the end of the continuing care period, the effect size of CBT compared to other conditions was very small and not significant (g = 0.035, p = 0.27, I2 = 0%, n = 15). Although this effect remained small, it was significant when three CBT-like treatments were included (g = 0.060, p = 0.05, I2 = 0%, n = 18).
3.3.4. Method of treatment delivery
Our sample included three studies that compared an outpatient continuing care treatment with some additional active effort to encourage and maintain participation to the same outpatient continuing care treatment without additional efforts (Gilbert, 1988; Godley, Coleman-Cowger, Titus, Funk, & Orndorff, 2010; Lash et al., 2007). The active efforts in all three studies included telephone calls to encourage and support continuing care involvement, whereas one study additionally included contracting and social reinforcement (Lash et al., 2007). Our analysis did not find a significant positive effect of adding active efforts to an outpatient treatment at the end of treatment (g = 0.086, p = 0.47, I2 = 14%, n = 3), although the power to detect a significant difference was very low, given the small number of studies.
Additionally, several studies of CBT-based continuing care compared a similar intervention provided in different settings. Across three studies, there was no effect of telephone administration versus in-person administration at the end of continuing care (g = 0.013, p = 0.88, I2 = 0%). Similarly, in the one study that directly compared outpatient to home visit administration (Gilbert, 1988), there was a small, but non-significant, effect favoring outpatient administration over home visits (g = 0.284, p = 0.20).
Finally, two of the identified continuing care studies did not fit into any of the above analyses (Graham, Annis, Brett, & Venesoen, 1996; Schmitz et al., 1997). Both studies compared individual to group administration of CBT continuing care. We were unable to synthesize these results because one of the publications (Graham et al., 1996) did not provide sufficient information to calculate effect sizes, stating only that there was no significant difference in substance use outcomes for group versus individual administration nine months after the end of continuing care. In the other study (Schmitz et al., 1997), there was a moderate and significant effect favoring group administration at the end of continuing care (g = 0.636, p = 0.01), but the effect was small, and non-significant, six months later (g = 0.202, p = 0.42).
3.4. Publication bias
To assess the possibility that the published studies included in the meta-analysis were not representative of the total population of studies that have been conducted (“file-drawer problem”; Rosenthal, 1979), we inspected the funnel plot for the analysis of the overall effect of continuing care. In addition, we used Duval and Tweedie’s trim-and-fill procedure (Duval & Tweedie, 2000), which plots the relationship between effect sizes and a measure of sample size (the standard error). If bias were present, there would be an asymmetrical plot, such that smaller studies will be more likely to have been published if they showed large effects rather than smaller ones (i.e., smaller samples with smaller effects would be missing from the plot). Although inspection of a (not shown) funnel plot did not seem to indicate publication bias in the sample of 18 studies, nevertheless, the trim-and-fill procedure trimmed and filled one study and the point estimate at the end of continuing care changed from g = 0.187 to a still significant g = 0.178 (p < 0.05).
3.5. Sensitivity analyses
To test for outliers’ exerting undue influence, we removed one study at a time and examined the overall effect size without that study. The overall effect of continuing care remained small, but significant, in each of these analyses (g’s varying from 0.171 to 0.205 at the end of continuing care). We also examined the overall effect size excluding two studies that did not formally randomize participants (Horng & Chueh, 2004; Patterson, McCourt, & Shiels, 1991). Excluding these studies, as well as additionally removing a unique study that examined daily Interactive Voice Response participation and had our largest negative effect size (Mundt, Moore, & Bean, 2006), did not significantly change the magnitude, direction or significance of our results (e.g., at the end of continuing care the effect size changed from g = 0.187 (p < 0.001, n = 18) to g = 0.185 (p < 0.001, n = 15).
In our main analyses, dichotomous outcomes were adjusted to assume that participants who were not followed-up had a negative outcome. We carried out a sensitivity analysis to examine our results using the outcomes as presented in individual studies (i.e., only with data on persons who were followed-up). The overall effect size across all available outcomes remained small, but significant, in this analysis, e.g., at the end of continuing care the effect size changed from g = 0.187 (p < 0.001, n = 18) to g = 0.206 (p < 0.001, n = 18).
Finally, some of the studies in our sample included at least one treatment group that received a group-based treatment. Interactions between participants within these group treatments may lead to violations of the assumption of independent observations of outcomes. Although the literature dealing with this issue is still developing, we carried out an adjustment on the aggregate effect size for each study that included a group-based treatment (Rooney & Murray, 1996). The final adjustments did not significantly affect our results. As expected, the overall g went down slightly, but it remained significant (e.g., at the end of continuing care, assuming a conservative intraclass correlation within each group of 0.30: g = 0.166, p < 0.001, I2 = 7%, n = 18).
4. Discussion
4.1. Main effect of continuing care
Gauged across a wide variety of treatments and at different time points, continuing care has a positive, although limited, impact on substance use outcomes. The overall effect of continuing care from this meta-analysis, while positive and significant, may seem quite small in light of the large body of observational studies finding associations between amount of continuing care and positive outcomes. However, in observational studies, those who remain longer in treatment may do so, at least in part, due to individual characteristics, such as level of motivation or family support. Those characteristics also may cause those patients to be more likely to do well, regardless of the length of continuing care. Patients who are assigned to attend more treatment in randomized trials will not possess or benefit from such individual characteristics more than persons in the control condition. On the other hand, participants in the control group may benefit, for example, from family or mutual-help group support, even if they are not offered a continuing care intervention.
In addition, not only did the interventions in these studies vary greatly, but it often was unclear exactly how much outside treatment participants were receiving. For example, in the reports of many studies that included a “no treatment” control group, it was acknowledged that all participants (including control participants) were still given standard referrals to outside mutual-help groups and/or continuing care providers. In some cases, participants were told that they could access certain resources on demand or as needed. Details on both what was offered and what was actually accessed often were not provided in published reports. Thus, we assume that unknown proportions of participants in all treatment conditions likely accessed these resources with widely varying intensity. Although research indicates that, in general, less than half of patients completing treatment use continuing care services (e.g., Fortney, Booth, Blow, & Bunn, 1995; Moos, Finney, Ouimette, & Suchinsky, 1999; Peterson, Swindle, Phibbs, Recine, & Moos, 1994), receipt of any other treatment or mutual-help services would have diluted the effects of the continuing care treatment being studied.
4.2. Moderators of the effects of continuing care
The effects of continuing care varied across studies, particularly when outcomes were assessed at follow-ups occurring after the end of treatment. With respect to moderators of continuing care effects, for the studies that included the most “straightforward” and interpretable comparisons between a specific continuing care treatment and a no- or minimal-treatment control condition, we did not find an association between either longer planned duration or treatments of greater prescribed intensity and better outcomes. The absence of a relationship between longer prescribed continuing care and better outcomes may reflect attrition that occurred during the treatment period, with actual treatment falling well short of the planned duration or intensity. Both persons who are functioning well and those who are functioning poorly may be more likely to discontinue treatment, rendering the average duration of continuing care received less lengthy than that prescribed. Attrition would be greater with longer prescribed durations of continuing care.
We also examined whether CBT or CBT-like treatments were more or less effective than other treatment approaches. Nearly three-quarters of the studies included at least one group receiving a CBT or CBT-like intervention. Among the studies that compared a CBT or CBT-like intervention to a control condition or to a non-CBT-based intervention, CBT-based continuing care generally had better outcomes than the comparison conditions at the end of treatment, but this effect was smaller at post-treatment follow-ups. We did not find a significant difference in efficacy in studies comparing CBT-based interventions to active interventions with other orientations. We thought it might be possible that CBT-based continuing care following initial treatment that used another orientation (e.g., 12-step treatment) would be less effective than if it followed initial CBT or some other “compatible” treatment. In a small sample of studies (n = 11), although the findings were in the expected direction, there was no significant difference between studies in which continuing care CBT was consistent with the initial treatment and those in which it was not (moderator test p = 0.29).
Other moderators tapped different methods and different settings for delivering continuing care. In very limited samples of studies, we did not find a significant impact of adding active efforts to improve participation to outpatient continuing care, or of providing treatment by telephone or through home visits. Additionally, two studies did not show a consistent difference in CBT-based continuing care provided in a group versus an individual outpatient setting. These null findings may reflect one of the limitations (see below) of our meta-analysis.
4.3. Limitations
Although we identified a sample of 33 continuing care studies, the number of studies included in any single analysis was much lower, particularly in the analyses examining the impact of different treatment settings and methods of delivery. Consequently, our power to detect significant differences was reduced. Thus, although we did not identify any significant differences of providing similar treatments in different ways (e.g., telephone or home visits compared to outpatient visits, or the addition of active elements aimed at encouraging and maintaining participation), these results should be interpreted with caution, given the small Ns of studies.
Due to variation in interventions, participants, and treatment delivery methods, it was not possible to determine whether some study features (e.g., number of sessions, specific activities) moderated continuing care effects with other study features controlled. Additionally, although we did carry out some CBT-related analyses, we were generally unable to conduct analyses on specific treatment modalities, such as Twelve-Step Facilitation, across an appropriately large sample of studies. Thus, these results mainly reflect the combined efficacy of a variety of continuing care approaches. Additional research may be able to identify the most important approaches for, and elements of, effective continuing care treatments.
Likewise, we were limited in our ability to identify samples of patients that may be more likely to benefit from continuing care. Some studies have found limited evidence that poorer prognosis patients (e.g. those who were unable to achieve abstinence early in the initial treatment period, those with low motivation to change, those with higher levels of crime and violence, and those with a younger age of first use) may be more likely to benefit from some types of continuing care (e.g., Dennis & Scott, 2012; Lynch et al., 2010; McKay et al., 1997). Many continuing care studies limited participants to those who had ‘successfully completed’ (using varying definitions) the initial treatment phase. This design feature may have excluded some participants who would have benefitted from continuing care. This exclusion may have contributed to the small overall effect size and it also limited our ability to test such participant level characteristics as moderators of continuing care effects.
A limitation of the published reports was that most presented findings from analyses based only on participants who were successfully followed-up. It is unclear how this study feature may have influenced the outcomes. Participants may drop-out and become lost to follow-up either because they are doing well and feel they no longer need formal treatment or, on the other end of the spectrum, because they have relapsed and cannot be located or do not want to reveal their condition to researchers or treatment staff. An examination of the data for the outcome of “percent days abstinent” (PDA) at the end of continuing care supports the notion that participants who are followed-up and who continue to participate in the research are likely to be doing well. Seven studies reported PDA only for those participants who were followed-up, i.e., they did not impute missing data for participants who could not be located or refused to be followed-up. The mean PDA across all followed-up participants in these studies was 90% (SD=7%). The high PDA may indicate a “ceiling effect,” leaving little room for variability due to receipt or non-receipt of continuing care. Thus, a ceiling effectmay have contributed to the small overall effect size for these intuitively helpful interventions.
4.4. Conclusion
Our results expand upon and provide a different perspective on the efficacy or effectiveness of continuing care than that provided by McKay’s (2009) box-score review. When his sample was limited to studies comparing a continuing care treatment to a control condition, 64% of studies found a significant effect favoring continuing care on at least one outcome. These results imply that the studies generally had the power to detect positive effects on at least one outcome with multiple, unadjusted statistical tests for intervention effects. However, as we have shown, when all substance use outcomes are combined, the effect is small in magnitude. Alternatively, it might be that focusing on a specific outcome across all studies would result in different results. Unfortunately, the studies we examined did not present results for a standard set of outcomes, so only by examining aggregated effect sizes across all substance use-related outcomes could we include a reasonably large number of studies and estimate a more general overall effect of continuing care.
Because box score reviews are sensitive to the statistical power of each included study, they can obscure similarities in the results of large and small studies. For example, the study by Foote and Erfurt (1991), in which 325 participants were randomized, was classified as a positive study in McKay’s review, but had a small effect size at the end of the planned continuing care (g = 0.088). On the other hand, the study by McLatchie and Lomp (1988), in which 103 participants were randomized, was classified as a negative study in McKay’s review, but had a larger, though still small, positive effect size (g = 0.191). This meta-analysis adds to the knowledge provided by previous reviews by testing the magnitude of the overall effect of continuing care across studies with varying levels of power.
Overall, our results, although tentative given the wide variety of approaches employed in a relatively small number of studies, support the general provision of continuing care. A planned period of continuing care can be an important element in supporting many patients in their recovery from substance use disorders. However, the small overall effect that we obtained may indicate the appropriateness of lower-cost interventions and of an individualized approach, such as adaptive, measurement-based care (Cacciola et al., 2013). Continuing care could be provided at different levels of intensity over different periods of time, depending on the individual client’s current functioning, other recovery resources and risk factors for relapse, which could be repeatedly assessed over time by an instrument, such as the Brief Addiction Monitor (McKay, Drapkin, Goodman, & DePhilippis, 2009). Future research also could examine the influence of specific continuing care elements on, as well as specific patient characteristics as moderators of, the effectiveness of continuing care.
Acknowledgments
Preparation of this article was supported by US National Institute on Alcohol Abuse and Alcoholism Grant No. AA008689 and the US Department of Veterans Affairs, Office of Research and Development, Health Services Research and Development Service and Substance Use Disorder Quality Enhancement Research Initiative. The views expressed are those of the authors and do not necessarily represent the views of the National Institute on Alcohol Abuse and Alcoholism, the Department of Veterans Affairs, or any other US Government entity. We thank James McKay for his helpful comments on an earlier version of this article. We are grateful to Kimberly Keller for her contributions in working on earlier versions of the coding forms and in coding studies.
Footnotes
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. American Psychiatric Association practice guidelines for the treatment of psychiatric disorders: Compendium 2006. Arlington, VA: American Psychiatric Pub; 2006. [Google Scholar]
- Bennett GA, Withers J, Thomas PW, Higgins DS, Bailey J, Parry L. A randomised trial of early warning signs relapse prevention training in the treatment of alcohol dependence. Addictive Behaviors. 2005;30:1111–1124. doi: 10.1016/j.addbeh.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Borenstein M. Effect sizes for continuous data. In: Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2. New York: Russell Sage Foundation; 2009. pp. 221–235. [Google Scholar]
- Borenstein M, Hedges LV. Introduction to meta-analysis. Hoboken, NJ: John Wiley & Sons Inc; 2009. [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Substance Abuse. 2009;30:295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BS, O’Grady K, Battjes RJ, Farrell EV. Factors associated with treatment outcomes in an aftercare population. The American Journal on Addictions. 2004;13:447–460. doi: 10.1080/10550490490512780. [DOI] [PubMed] [Google Scholar]
- Brown BS, O’Grady KE, Battjes RJ, Farrell EE, Smith NP, Nurco DN. Effectiveness of a stand-alone aftercare program for drug-involved offenders. Journal of Substance Abuse Treatment. 2001;21:185–192. doi: 10.1016/s0740-5472(01)00201-x. [DOI] [PubMed] [Google Scholar]
- Brown TG, Seraganian P, Tremblay J, Annis H. Matching substance abuse aftercare treatments to client characteristics. Addictive Behaviors. 2002;27:585–604. doi: 10.1016/s0306-4603(01)00195-2. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, DePhilippis D, Drapkin ML, Valadez JC, Fala NC. Development and initial evaluation of the Brief Addiction Monitor (BAM) Journal of Substance Abuse Treatment. 2013;44:256–263. doi: 10.1016/j.jsat.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Connors GJ, Tarbox AR, Faillace LA. Achieving and maintaining gains among problem drinkers: Process and outcome results. Behavior Therapy. 1992;23:449–474. [Google Scholar]
- Cooney NL, Kadden RM, Litt MD, Getter H. Matching alcoholics to coping skills or interactional therapies: two-year follow-up results. Journal of Consulting and Clinical Psychology. 1991;59:598–601. doi: 10.1037//0022-006x.59.4.598. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, Bohlmeijer E, Hollon SD, Andersson G. The effects of psychotherapy for adult depression are overestimated: a meta-analysis of study quality and effect size. Psychological Medicine. 2009;40:211–223. doi: 10.1017/S0033291709006114. [DOI] [PubMed] [Google Scholar]
- De Soto CB, O’Donnell WE, De Soto JL. Long-term recovery in alcoholics. Alcoholism: Clinical and Experimental Research. 1989;13:693–697. doi: 10.1111/j.1530-0277.1989.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Del Re A. RcmdrPlugin.MAd: Meta-Analysis with Mean Differences (MAd) Rcmdr Plug-in. R package version 1.0.8. 2010 Retrieved from http://cran.r-project.org/web/packages/RcmdrPlugin.MAd.
- Del Re A, Hoyt WT. MAd: Meta-Analysis with Mean Differences. R package version 0.4. 2010 Retrieved from http://CRAN.R-project.org/package=MAd.
- Dennis M, Scott CK, Funk R. An experimental evaluation of recovery management checkups (RMC) for people with chronic substance use disorders. Evaluation and Program Planning. 2003;26:339–352. doi: 10.1016/S0149-7189(03)00037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ML, Scott CK. Four-year outcomes from the Early Re-Intervention (ERI) experiment using Recovery Management Checkups (RMCs) Drug and Alcohol Dependence. 2012;121:10–17. doi: 10.1016/j.drugalcdep.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Veterans Affairs and Department of Defense. VA/DoD Clinical Practice Guideline for Management of Substance Use Disorders (SUD) Washington, DC: VA/DoD; 2009. [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Foote A, Erfurt JC. Effects of EAP follow-up on prevention of relapse among substance abuse clients. Journal of Studies on Alcohol and Drugs. 1991;52:241–248. doi: 10.15288/jsa.1991.52.241. [DOI] [PubMed] [Google Scholar]
- Fortney JC, Booth BM, Blow FC, Bunn JY. The effects of travel barriers and age on the utilization of alcoholism treatment aftercare. The American Journal of Drug and Alcohol Abuse. 1995;21:391–406. doi: 10.3109/00952999509002705. [DOI] [PubMed] [Google Scholar]
- Gilbert FS. The effect of type of aftercare follow-up on treatment outcome among alcoholics. Journal of Studies on Alcohol and Drugs. 1988;49:149–159. doi: 10.15288/jsa.1988.49.149. [DOI] [PubMed] [Google Scholar]
- Godley MD, Coleman-Cowger VH, Titus JC, Funk RR, Orndorff MG. A randomized controlled trial of telephone continuing care. Journal of Substance Abuse Treatment. 2010;38:74–82. doi: 10.1016/j.jsat.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godley MD, Godley SH, Dennis ML, Funk RR, Passetti LL. The effect of assertive continuing care on continuing care linkage, adherence and abstinence following residential treatment for adolescents with substance use disorders. Addiction. 2007;102:81–93. doi: 10.1111/j.1360-0443.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- Godley SH, Garner BR, Passetti LL, Funk RR, Dennis ML, Godley MD. Adolescent outpatient treatment and continuing care: main findings from a randomized clinical trial. Drug and Alcohol Dependence. 2010;110:44–54. doi: 10.1016/j.drugalcdep.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K, Annis HM, Brett PJ, Venesoen P. A controlled field trial of group versus individual cognitive-behavioural training for relapse prevention. Addiction. 1996;91:1127–1140. doi: 10.1046/j.1360-0443.1996.91811275.x. [DOI] [PubMed] [Google Scholar]
- Haddock CK, Rindskopf D, Shadish WR. Using odds ratios as effect sizes for meta-analysis of dichotomous data: A primer on methods and issues. Psychological Methods. 1998;3:339. [Google Scholar]
- Hawkins JD, Catalano RF, Jr, Gillmore MR, Wells EA. Skills training for drug abusers: generalization, maintenance, and effects on drug use. Journal of Consulting and Clinical Psychology. 1989;57:559–563. doi: 10.1037//0022-006x.57.4.559. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. San Diego: Academic Press; 1985. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horng FF, Chueh KH. Effectiveness of telephone follow-up and counseling in aftercare for alcoholism. The Journal of Nursing Research. 2004;12:11–20. doi: 10.1097/01.jnr.0000387484.40568.bb. [DOI] [PubMed] [Google Scholar]
- Hubbard RL, Leimberger JD, Haynes L, Patkar AA, Holter J, Liepman MR. Telephone enhancement of long-term engagement (TELE) in continuing care for substance abuse treatment: a NIDA clinical trials network (CTN) study. The American Journal on Addictions. 2007;16:495–502. doi: 10.1080/10550490701641678. [DOI] [PubMed] [Google Scholar]
- Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. Journal of Clinical Psychology. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Ito JR, Donovan DM, Hall JJ. Relapse prevention in alcohol aftercare: effects on drinking outcome, change process, and aftercare attendance. British Journal of Addiction. 1988;83:171–181. doi: 10.1111/j.1360-0443.1988.tb03978.x. [DOI] [PubMed] [Google Scholar]
- Jason LA, Olson BD, Ferrari JR, Majer JM, Alvarez J, Stout J. An examination of main and interactive effects of substance abuse recovery housing on multiple indicators of adjustment. Addiction. 2007;102:1114–1121. doi: 10.1111/j.1360-0443.2007.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Rourke SB, Patterson TL, Taylor MJ, Grant I. Predictors of relapse in long-term abstinent alcoholics. Journal of Studies on Alcohol and Drugs. 1998;59:640–646. doi: 10.15288/jsa.1998.59.640. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Cooney NL, Getter H, Litt MD. Matching alcoholics to coping skills or interactional therapies: posttreatment results. Journal of Consulting and Clinical Psychology. 1989;57:698–704. doi: 10.1037//0022-006x.57.6.698. [DOI] [PubMed] [Google Scholar]
- Kaminer Y, Burleson JA, Burke RH. Efficacy of outpatient aftercare for adolescents with alcohol use disorders: a randomized controlled study. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:1405–1412. doi: 10.1097/CHI.0b013e318189147c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash SJ, Petersen GE, O’Connor EA, Lehmann LP. Social reinforcement of substance abuse aftercare group therapy attendance. Journal of Substance Abuse Treatment. 2001;20:3–8. doi: 10.1016/s0740-5472(00)00140-9. [DOI] [PubMed] [Google Scholar]
- Lash SJ, Stephens RS, Burden JL, Grambow SC, DeMarce JM, Jones ME. Contracting, prompting, and reinforcing substance use disorder continuing care: a randomized clinical trial. Psychology of Addictive Behaviors. 2007;21:387–397. doi: 10.1037/0893-164X.21.3.387. [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical meta-analysis. Vol. 49. Thousand Oaks, CA: Sage Publications; 2000. Incorporated. [Google Scholar]
- Lynch KG, Van Horn D, Drapkin M, Ivey M, Coviello D, McKay JR. Moderators of response to telephone continuing care for alcoholism. American Journal of Health Behavior. 2010;34:788–800. doi: 10.5993/ajhb.34.6.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill M, Ray LA. Cognitive-behavioral treatment with adult alcohol and illicit drug users: a meta-analysis of randomized controlled trials. Journal of Studies on Alcohol and Drugs. 2009;70:516–527. doi: 10.15288/jsad.2009.70.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PW, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for alcohol dependence: When are these medications most helpful? Addiction. 2013;108:275–293. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude-Griffin PM, Hohenstein JM, Humfleet GL, Reilly PM, Tusel DJ, Hall SM. Superior efficacy of cognitive-behavioral therapy for urban crack cocaine abusers: Main and matching effects. Journal of Consulting and Clinical Psychology. 1998;66:832. doi: 10.1037//0022-006x.66.5.832. [DOI] [PubMed] [Google Scholar]
- McAuliffe WE. A randomized controlled trial of recovery training and self-help for opioid addicts in New England and Hong Kong. Journal of Psychoactive Drugs. 1990;22:197–209. doi: 10.1080/02791072.1990.10472544. [DOI] [PubMed] [Google Scholar]
- McKay JR. Is there a case for extended interventions for alcohol and drug use disorders? Addiction. 2005;100:1594–1610. doi: 10.1111/j.1360-0443.2005.01208.x. [DOI] [PubMed] [Google Scholar]
- McKay JR. Continuing care research: what we have learned and where we are going. Journal of Substance Abuse Treatment. 2009;36:131–145. doi: 10.1016/j.jsat.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Alterman AI, Cacciola JS, O’Brien CP, Koppenhaver JM, Shepard DS. Continuing care for cocaine dependence: comprehensive 2-year outcomes. Journal of Consulting and Clinical Psychology. 1999;67:420–427. doi: 10.1037//0022-006x.67.3.420. [DOI] [PubMed] [Google Scholar]
- McKay JR, Alterman AI, Cacciola JS, Rutherford MJ, O’Brien CP, Koppenhaver J. Group counseling versus individualized relapse prevention aftercare following intensive outpatient treatment for cocaine dependence: initial results. Journal of Consulting and Clinical Psychology. 1997;65:778–788. doi: 10.1037//0022-006x.65.5.778. [DOI] [PubMed] [Google Scholar]
- McKay JR, Drapkin ML, Goodman J, DePhilippis D. Brief Addiction Monitor (BAM): A New Performance Measure in VA SUD Care. Washington, DC: Department of Veterans Affairs; 2009. [Google Scholar]
- McKay JR, Lynch KG, Shepard DS, Ratichek S, Morrison R, Koppenhaver J. The effectiveness of telephone-based continuing care in the clinical management of alcohol and cocaine use disorders: 12-month outcomes. Journal of Consulting and Clinical Psychology. 2004;72:967–979. doi: 10.1037/0022-006X.72.6.967. [DOI] [PubMed] [Google Scholar]
- McKay JR, Van Horn DH, Oslin DW, Lynch KG, Ivey M, Ward K. A randomized trial of extended telephone-based continuing care for alcohol dependence: within-treatment substance use outcomes. Journal of Consulting and Clinical Psychology. 2010;78:912–923. doi: 10.1037/a0020700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie BH, Lomp KG. An experimental investigation of the influence of aftercare on alcoholic relapse. British Journal of Addiction. 1988;83:1045–1054. doi: 10.1111/j.1360-0443.1988.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Miller WR, Hester RK. Inpatient alcoholism treatment: Who benefits? American Psychologist. 1986;41:794. doi: 10.1037//0003-066x.41.7.794. [DOI] [PubMed] [Google Scholar]
- Moos RH, Finney JW, Ouimette PC, Suchinsky RT. A comparative evaluation of substance abuse treatment: I. Treatment orientation, amount of care, and 1-year outcomes. Alcoholism: Clinical and Experimental Research. 1999;23:529–536. [PubMed] [Google Scholar]
- Mundt JC, Moore HK, Bean P. An interactive voice response program to reduce drinking relapse: a feasibility study. Journal of Substance Abuse Treatment. 2006;30:21–29. doi: 10.1016/j.jsat.2005.08.010. [DOI] [PubMed] [Google Scholar]
- O’Farrell TJ, Choquette KA, Cutter HS. Couples relapse prevention sessions after behavioral marital therapy for male alcoholics: outcomes during the three years after starting treatment. Journal of Studies on Alcohol and Drugs. 1998;59:357–370. doi: 10.15288/jsa.1998.59.357. [DOI] [PubMed] [Google Scholar]
- O’Farrell TJ, Choquette KA, Cutter HS, Brown ED, McCourt WF. Behavioral marital therapy with and without additional couples relapse prevention sessions for alcoholics and their wives. Journal of Studies on Alcohol and Drugs. 1993;54:652–666. doi: 10.15288/jsa.1993.54.652. [DOI] [PubMed] [Google Scholar]
- Overton RC. A comparison of fixed-effects and mixed (random-effects) models for meta-analysis tests of moderator variable effects. Psychological Methods. 1998;3:354. [Google Scholar]
- Patterson DG, Macpherson J, Brady NM. Community psychiatric nurse aftercare for alcoholics: a five-year follow-up study. Addiction. 1997;92:459–468. [PubMed] [Google Scholar]
- Patterson DG, McCourt MW, Shiels JR. Community psychiatric nurse aftercare for alcoholics. Irish Journal of Psychological Medicine 1991 [Google Scholar]
- Peterson KA, Swindle RW, Phibbs CS, Recine B, Moos RH. Determinants of readmission following inpatient substance abuse treatment: a national study of VA programs. Medical Care. 1994;32:535–550. doi: 10.1097/00005650-199406000-00001. [DOI] [PubMed] [Google Scholar]
- Project MATCH Research Group. Matching Alcoholism Treatments to Client Heterogeneity: Project MATCH posttreatment drinking outcomes. Journal of Studies on Alcohol and Drugs. 1997;58:7–29. [PubMed] [Google Scholar]
- Raudenbush S. Analyzing effect sizes: Random-effects models. In: Cooper H, Hedges LV, Valentine JC, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. New York: Russell Sage Foundation; 2009. pp. 295–315. [Google Scholar]
- Ritsher JB, Moos RH, Finney JW. Relationship of treatment orientation and continuing care to remission among substance abuse patients. Psychiatric Services. 2002;53:595–601. doi: 10.1176/appi.ps.53.5.595. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA. Cue exposure with coping skills training and communication skills training for alcohol dependence: 6-and 12-month outcomes. Addiction. 2001;96:1161–1174. doi: 10.1046/j.1360-0443.2001.96811619.x. [DOI] [PubMed] [Google Scholar]
- Rooney BL, Murray DM. A meta-analysis of smoking prevention programs after adjustment for errors in the unit of analysis. Health Education & Behavior. 1996;23:48–64. doi: 10.1177/109019819602300104. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The file drawer problem and tolerance for null results. Psychological Bulletin. 1979;86:638. [Google Scholar]
- Rosenthal R. Writing meta-analytic reviews. Psychological Bulletin. 1995;118:183. [Google Scholar]
- Sannibale C, Hurkett P, van den Bossche E, O’Connor D, Zador D, Capus C. Aftercare attendance and post-treatment functioning of severely substance dependent residential treatment clients. Drug and Alcohol Review. 2003;22:181–190. doi: 10.1080/09595230100100624. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Oswald LM, Jacks SD, Rustin T, Rhoades HM, Grabowski J. Relapse prevention treatment for cocaine dependence: group vs. individual format. Addictive Behaviors. 1997;22:405–418. doi: 10.1016/s0306-4603(96)00047-0. [DOI] [PubMed] [Google Scholar]
- Scott CK, Dennis ML. Results from two randomized clinical trials evaluating the impact of quarterly recovery management checkups with adult chronic substance users. Addiction. 2009;104:959–971. doi: 10.1111/j.1360-0443.2009.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadish W, Robinson L, Lu C. ES: A computer program and manual for effect size calculation (Version 1) Minneapolis, MN: Assessment Systems; 1999. [Google Scholar]
- Shadish WR, Haddock CK. Combining estimates of effect size. In: Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2. New York: Russell Sage Foundation; 2009. pp. 257–277. [Google Scholar]
- Viechtbauer W. Metafor. Meta-Analysis Package for R. 2011 Retrieved from http://CRAN.R-project.org/package=MAd.