Abstract
In recent decades there has been marked progress in the imaging and laboratory evaluation of dizzy patients. However, detailed history taking and comprehensive bedside neurotological evaluation remain crucial for a diagnosis of dizziness. Bedside neurotological evaluation should include examinations for ocular alignment, spontaneous and gaze-evoked nystagmus, the vestibulo-ocular reflex, saccades, smooth pursuit, and balance. In patients with acute spontaneous vertigo, negative head impulse test, direction-changing nystagmus, and skew deviation mostly indicate central vestibular disorders. In contrast, patients with unilateral peripheral deafferentation invariably have a positive head impulse test and mixed horizontal-torsional nystagmus beating away from the lesion side. Since suppression by visual fixation is the rule in peripheral nystagmus and is frequent even in central nystagmus, removal of visual fixation using Frenzel glasses is required for the proper evaluation of central as well as peripheral nystagmus. Head-shaking, cranial vibration, hyperventilation, pressure to the external auditory canal, and loud sounds may disclose underlying vestibular dysfunction by inducing nystagmus or modulating the spontaneous nystagmus. In patients with positional vertigo, the diagnosis can be made by determining patterns of the nystagmus induced during various positional maneuvers that include straight head hanging, the Dix-Hallpike maneuver, supine head roll, and head turning and bending while sitting. Abnormal smooth pursuit and saccades, and severe imbalance also indicate central pathologies. Physicians should be familiar with bedside neurotological examinations and be aware of the clinical implications of the findings when evaluating dizzy patients.
Keywords: dizziness, bedside examination, nystagmus, head impulse test
Introduction
Dizziness, which is one of the most common symptoms of patients referred for neurological evaluation, comprises a variety of symptoms. Even though each symptom may have a different pathophysiologic mechanism and significance, the description is often vague and intriguing. Accordingly, understanding what is meant by "dizziness", which can manifest as presyncope, disequilibrium, oscillopsia, and vertigo, should be the first step in evaluating dizzy patients.1
Lightheadedness or presyncope is a sensation of impending loss of consciousness due to a momentary decrease in the cerebral blood flow. Patients experience presyncopal dizziness when rapidly standing up from a relaxed supine or seated position. It may occur with multiple sensory disturbances, orthostatic hypotension, and cardiac arrhythmias as well as hyperventilation syndrome and panic attacks.
Disequilibrium is an imbalance or unsteadiness experienced while standing or walking. It is caused by various factors including diminished vision, loss of vestibular function, defects in proprioception, and motor dysfunction from the central or peripheral nervous system. It also relates to nonvestibular syndromes such as visual vertigo, presyncopal faintness, or somatoform phobic postural vertigo.
Oscillopsia is the subjective illusion of visual motion. While vertigo occurs with the eyes open or closed, oscillopsia only occurs when the eyes are open. Patients with acquired nystagmus report spontaneous oscillopsia due to apparent motion of the visual scene from retinal slip. Patients with bilateral loss of the vestibulo-ocular reflex (VOR) frequently experience oscillopsia during head movements. Defects in eye movements due to inappropriate VOR cause retinal image motion during head motion.
Vertigo is the illusion of movements of oneself or the environment due to an imbalance of tonic neural activity in the vestibular-cortical pathway. Although patients usually report rotational vertigo, occasionally they describe a sensation of linear displacement or tilt. Vertigo is commonly exacerbated by head movements and accompanied by nausea and vomiting. Vertigo may be due to unilateral injury to the peripheral vestibular organs such as the labyrinth or vestibular nerve. It also results from damage to the central vestibular structures including the vestibular nuclei, vestibular thalamic nuclei, vestibular cortex, and cerebellum. While peripheral vestibular disorders are always characterized by a combination of perceptual, ocular motor, and postural symptoms and signs, central vestibular lesions may give rise to only some of them.
The primary aim of the bedside evaluation of a dizzy patient is the detection of any vestibular deficits. While the vestibular impairments can be readily determined in patients with acute vertigo, meticulous examination is required in those with chronic dizziness. When the vertigo is induced in certain circumstances, reproduction of those situations is important during the evaluation. One of the most important aims of the bedside evaluation, especially in acute vertigo, is to differentiate the central from peripheral vestibular pathologies. Peripheral vestibular disorders resulting from damage to the labyrinth or vestibular nerve are usually benign, despite the possible presence of severe dizziness. Conversely, central vestibular disorders may be fatal without prompt and proper management. The bedside neurotological examination should include evaluation both for static and dynamic vestibular imbalances. Due to an anatomical proximity, patients with dizziness often also exhibit ocular motor impairments, and so a comprehensive neurotological examination should include an evaluation of the eye movements.2 Recent studies have indicated that simple bedside examinations such as tests for head impulse, gaze-evoked nystagmus (GEN), and skew deviation can reliably predict a central pathology in patients with isolated vertigo.3,4
Head Posture and Ocular Alignment
Bedside examination of dizzy patients should begin with observation of the head posture (i.e., tilt or turn) and ocular alignment. Dysfunction of the pupil and eyelid may aid central localization of the vestibulopathy, as in lateral medullary infarction (Wallenberg syndrome) with Horner syndrome. Ocular misalignment is frequent in central vestibulopathy, and should be determined in nine cardinal positions of the gaze, along with the range of eye movements.
The ocular tilt reaction (OTR) refers to the triad of head tilt, ocular torsion, and skew deviation (Fig. 1).5 The OTR is known to reflect unilateral or asymmetric dysfunction of the graviceptive pathways from the utricle. Skew deviation indicates a vertical misalignment of the eyes due to a supranuclear pathology.2 The presence of skew deviation may be inferred by vertical diplopia and can be confirmed by the cover test. This involves covering one eye and detecting a corrective vertical movement of the other eye to fixate the target, which indicates the presence of skew deviation. As a rule, the head is tilted toward the side of the lower eye and the ocular torsion occurs in the same direction with the upper poles of the eyes rotating to the lower eye.6 Since the graviceptive pathway responsible for the OTR is known to cross the midline just above the abducens nucleus level and ascend in the contralateral medial longitudinal fasciculus (MLF),7,8 lesions below the lower pons cause ipsiversive OTR, and more rostral lesions induce contraversive OTR. Cerebellar lesions may give rise to ipsi- or contraversive OTR depending upon the structures involved.9 Although it can occur in peripheral vestibular disorders, skew deviation observed in acute isolated vertigo more commonly indicates a central vestibular lesion.3
Fig. 1.
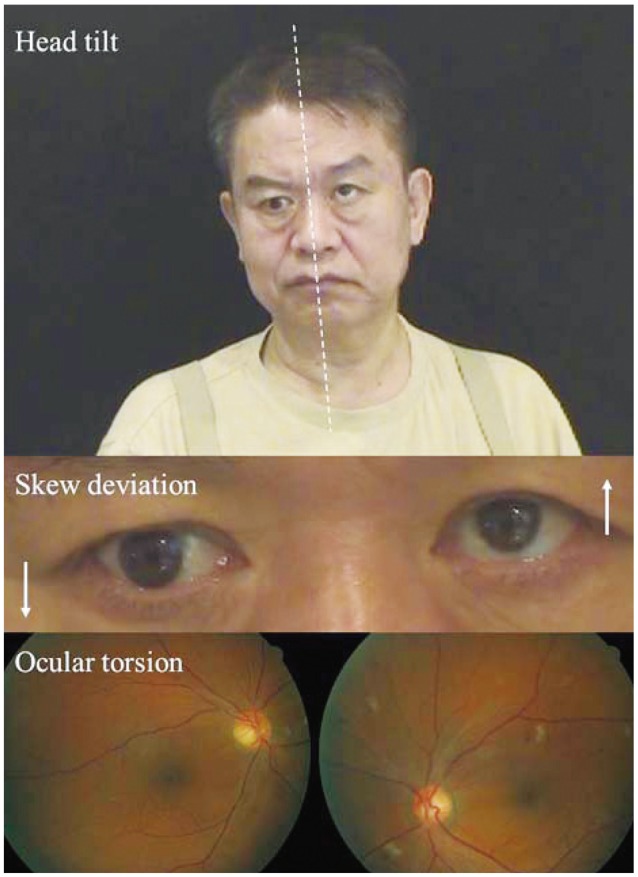
The ocular tilt reaction (OTR). The OTR refers to the head tilt, ocular torsion, and skew deviation that are ascribed to asymmetry in the otolithic pathway from the utricle. The head tilt and ocular torsion occur toward the hypotropic eye.
A tilt of the subjective visual vertical (SVV) is the most sensitive sign of vestibular tone imbalance in the roll plane.6 It may occur in lesions involving either the central or peripheral vestibular pathways. The bucket method is a simple and reliable bedside test for measuring a tilt of SVV,10 in which patients indicate the position of a bucket where the line on the bottom inside the bucket is estimated to be truly vertical while they sit upright and look into a plastic bucket. The examiner then measures the SVV tilt using an external scale as the angle that the line is off the true vertical.
Spontaneous Nystagmus and Other Involuntary Eye Movements
The patterns of spontaneous nystagmus (when present) are most informative for evaluating patients with dizziness/vertigo.11 The proper evaluation of the spontaneous nystagmus requires observation of the direction and the effects of gaze on the intensity and direction of the nystagmus. In unilateral peripheral deafferentation, the spontaneous nystagmus is mixed horizontal-torsional beating away from the lesion side (Fig. 2).12 The nystagmus typically increases during the gaze in the direction of the spontaneous nystagmus, and decreases during the gaze in the opposite direction (Alexander's law) (Fig. 2).13 Since the peripheral vestibular nystagmus is markedly suppressed by visual fixation, proper observation of the nystagmus requires the removal of visual fixation using Frenzel glasses14 that have 20-diopter convex lenses to prevent fixation and to magnify eye motion, allowing a better observation of the eyes (Fig. 3). When Frenzel glasses are unavailable, an ophthalmoscope can be used to observe nystagmus without fixation. By observing the optic disc with a direct ophthalmoscope while the other eye is covered, the nystagmus can be examined without fixation.15 However, when determining the direction of the nystagmus, it should be borne in mind that the retina (optic disc) moves in the direction opposite to the cornea for horizontal and vertical nystagmus. Since the disc also moves vertically in torsional nystagmus, motion of the surrounding retinal vessels should be considered when determining the direction of the nystagmus. In torsional nystagmus the discs move in opposing directions vertically. The distinguishing features of peripheral and central nystagmus are summarized in Table 1.
Fig. 2.
Nystagmus in left peripheral vestibulopathy. In unilateral peripheral vestibular deafferentation, mixed torsional-horizontal nystagmus beating occurs toward the intact side. The nystagmus typically increases during the gaze in the direction of nystagmus and decreases during the gaze in the opposite direction (Alexander's law), but never changes direction.
Fig. 3.
Frenzel glasses remove visual fixation using 20-diopter convex lenses, and facilitate the detection of nystagmus by magnifying the eyes.
Table 1.
Differentiation of peripheral and central nystagmus

In contrast, the direction and fixation effects are variable in central vestibular nystagmus. Accordingly, when the characteristics of nystagmus do not conform to those of peripheral vestibular nystagmus, it should be considered central.16-19 However, even unidirectional horizontal-torsional nystagmus suppressed by visual fixation should not be simply regarded as peripheral unless other findings, such as a positive head impulse test or caloric paresis, are indicative of peripheral vestibular lesions.
Induction or Modulation of Nystagmus
Various bedside maneuvers may induce nystagmus or modulate the preexisting spontaneous nystagmus. Even in patients with spontaneous nystagmus, the modulation pattern may disclose an underlying pathology or aid in diagnosis. In patients with compensated vestibulopathy, the induction of nystagmus by various maneuvers is crucial in disclosing the underlying vestibular imbalance.20
Gaze-evoked nystagmus
Gaze-evoked nystagmus refers to the nystagmus that develops when patients take eccentric eye positions. Since GEN is caused by impaired gaze-holding in those positions, which causes centripetal drift of the eyes, GEN beats in the direction of gaze.2,21 GEN is one of the most sensitive ocular motor signs for central pathologies in patients with acute vestibular syndrome.3,22 GEN is attributed to dysfunction of the common neural integrator, which converts premotor eye velocity signals into an eye position signal and holds the eyes steady at an eccentric position in the orbit, and may occur in either the horizontal or vertical plane.2 The nucleus prepositus hypoglossi and medial vestibular nuclei are the main neural integrators for horizontal eye movements,2 while the interstitial nucleus of Cajal is the main contributor to neural integration for vertical and torsional eye movements.23 The flocculus/paraflocculus also takes part in the integration of ocular motor signals; this role may depend on the feedback of eye movement signals by the cell groups of the paramedian tracts.24 The most common cause of GEN is medications, usually sedatives, tranquilizers, or anticonvulsants, or alcohol. GEN due to medication usually occurs in both the horizontal and vertical planes.2
Gaze-evoked nystagmus should be differentiated from the end-point nystagmus that may be observed in extreme gazes even in normal subjects. End-point nystagmus is mostly transient, with a low amplitude (slower drift) and frequency.25
Head-shaking nystagmus
Head-shaking nystagmus (HSN) can be assessed using either a passive (by the examiner) or active (by the patient) head-shaking maneuver. The patient's head is pitched forward by approximately 20° to bring the horizontal semicircular canals (HCs) into the plane of stimulation, and then the head is shaken horizontally in a sinusoidal fashion at a rate of about 2-3 Hz with an amplitude of 20° for 15 seconds.26 In unilateral peripheral vestibulopathy, the typical pattern of HSN initially consists of contralesional nystagmus that decays over 20 seconds and then goes through a weak reversal.27 A combination of Ewald's second law and the velocity storage mechanism provides an explanation for the mechanism of HSN. Since excitatory vestibular inputs are more effective than inhibitory ones (Ewald's second law), asymmetric vestibular inputs would be generated during horizontal head-shaking in peripheral vestibulopathies. These asymmetric vestibular inputs are believed to accumulate in the central vestibular structures (velocity storage) during head-shaking, and to discharge as contralesional nystagmus after the head-shaking. In contrast, patterns of HSN in central vestibular disorders may vary. In general, central patterns of HSN include unusually strong HSN elicited by weak head-shaking, intense HSN in patients without caloric paresis, ipsilesional HSN, HSN in the direction opposite to the spontaneous nystagmus, and perverted HSN (i.e., vertical or torsional nystagmus developing in response to horizontal head-shaking).26,28-30
Hyperventilation-induced nystagmus
Hyperventilation may elicit nystagmus [hyperventilation-induced nystagmus (HIN)] by revealing vestibular asymmetry in central as well as peripheral vestibular disorders, including compensated peripheral vestibulopathies, perilymph fistula, acoustic neuroma, lesions at the craniocervical junction, and cerebellar degeneration.31-33 To assess HIN, subjects are asked to hyperventilate for about 30 seconds while seated in the darkness, taking an average of one deep breath per second. HIN beating to the side of reduced caloric response or hearing loss may be a valuable sign for cerebellopontine-angle (CPA) tumors.34 Ipsilesional HIN, especially in patients with a small CPA tumor and less caloric asymmetry, may be ascribed to an improvement of axonal conductance in partially demyelinated vestibular nerve fibers.
Vibration-induced nystagmus
Vibration applied to the forehead or mastoid induces nystagmus [vibration-induced nystagmus (VIN)] in various vestibular disorders.35 In peripheral vestibulopathies, the direction of VIN is mostly toward the healthy side, with an exception in some patients with Ménière's disease, in which VIN may beat toward the affected side.36 It has been proposed that VIN is the result of altered proprioceptive inputs from the neck muscles or direct stimulation of the vestibular receptors in the intact labyrinth after unilateral vestibular deafferentation.36
Positional testing
Positional nystagmus refers to the nystagmus that develops in association with changes in the dependent position of the head in the direction of gravity. Positional testing is an essential part of the vestibular examination for diagnosing positional vertigo. Positional nystagmus may be either paroxysmal or persistent. Both peripheral and central vestibular disorders may produce positional nystagmus. The positional nystagmus is mostly paroxysmal in peripheral vestibular disorders, and is almost always observed in benign paroxysmal positional vertigo (BPPV), which is ascribed to otolithic debris that becomes detached from the maculae of the otolithic organs and enters one of the semicircular canals.2 The patterns of positional nystagmus in BPPV differ according to the canal involved. In rare cases the positional nystagmus is observed in patients with lesions involving the inferior cerebellar vermis.37-39 This central positional nystagmus (CPN) may be either paroxysmal or persistent. The nystagmus is mostly vertical or apogeotropic, and may change direction depending on the positional maneuver. Prominent positional nystagmus in the absence of dizziness also suggests a central pathology.40
To stimulate each of the six semicircular canals with a maximal intensity, patients usually undergo the Dix-Hallpike maneuver in either direction, with head turning to either side while supine. Various other maneuvers may be added to the routine positional test battery depending on the suspected disease and the positional changes that induces the vertigo.
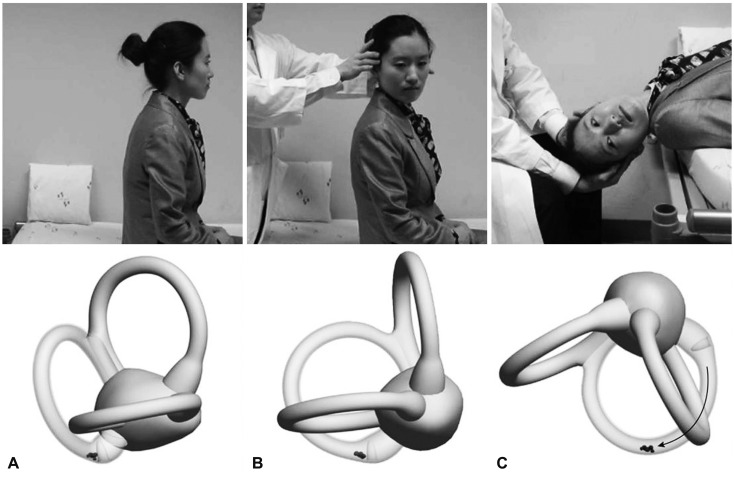
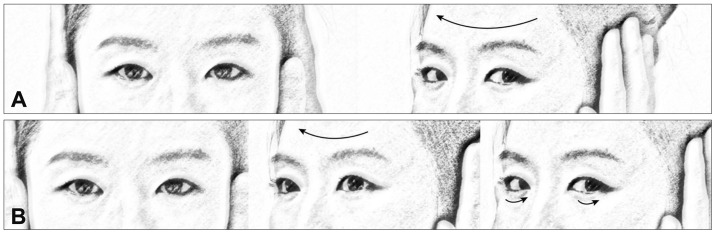
The Dix-Hallpike maneuver is the gold-standard test for a diagnosis of BPPV involving the posterior semicircular canal (PC, PC-BPPV).41,42 While seated on the examination table, the patient's head is turned 45° toward the side to be tested. The patient is then moved en bloc to a supine position, ending with the head hanging 20° below the examination table. This maneuver places the PC in the most dependent position (Fig. 4). In PC-BPPV the elicited nystagmus would be mixed upbeat and torsional with the upper pole of the eyes beating toward the lower ear. When free-floating otolithic debris is present (canalolithiasis) in the PC being tested, nystagmus usually develops with a latency of several seconds (up to 30 seconds) and resolves within 1 minute (usually within 30 seconds). It may reverse direction upon sitting and tends to habituate with repeated testing (fatigue).41 In rare cases the Dix-Hallpike maneuver is contraindicated or not possible due to the presence of a neck problem (e.g., cervical spine instability, cervical disc herniation, prior cervical spine surgery, and vascular dissection), low back pain, or obesity.43 The side-lying test may be adopted as an alternative in such cases,44 wherein the patient is quickly laid en bloc toward the side being tested after the head is turned 45° away from the side to be tested (Fig. 5).
Fig. 4.
The Dix-Hallpike maneuver for benign paroxysmal positional vertigo involving the right posterior semicircular canal (PC). After seating the patient upright (A), the head is turned 45° in the direction of the involved ear (B: right ear in this figure). The patient is then moved from the sitting to the supine position, ending with the head hanging at 20° off the end of the examination table (C). The corresponding illustrations demonstrate the orientation of the semicircular canals and location of the otolithic debris in the PC (viewed from the patient's right side).
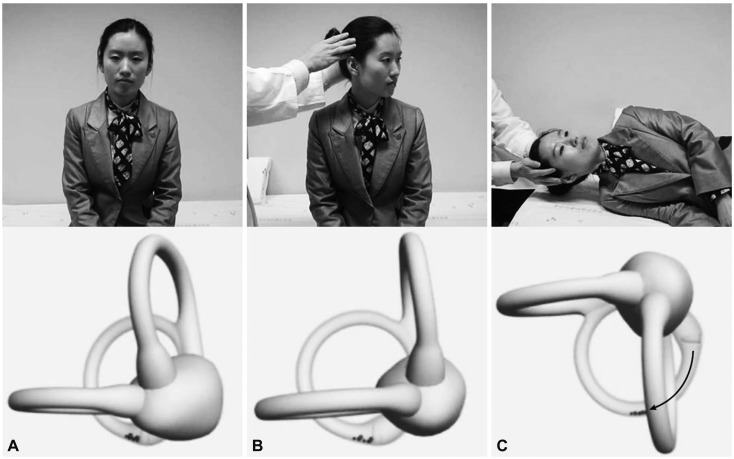
Fig. 5.
Side-lying test for diagnosis of right posterior canal benign paroxysmal positional vertigo. After seating the patient on the examination table (A), the head is turned 45° away from the involved ear (B). The patient then lies on the side of the involved ear (C). The corresponding illustrations demonstrate the orientation of the semicircular canals and location of the otolithic debris in the posterior canal (viewed from the front).
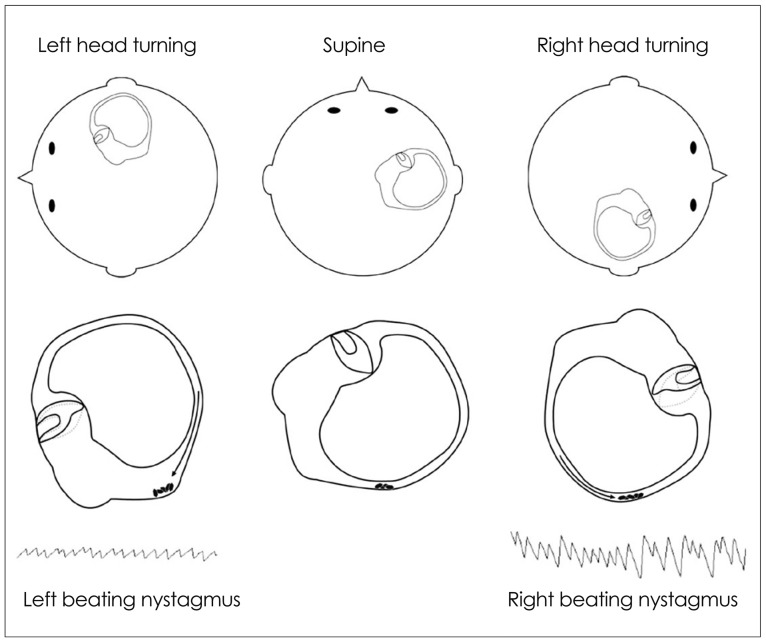
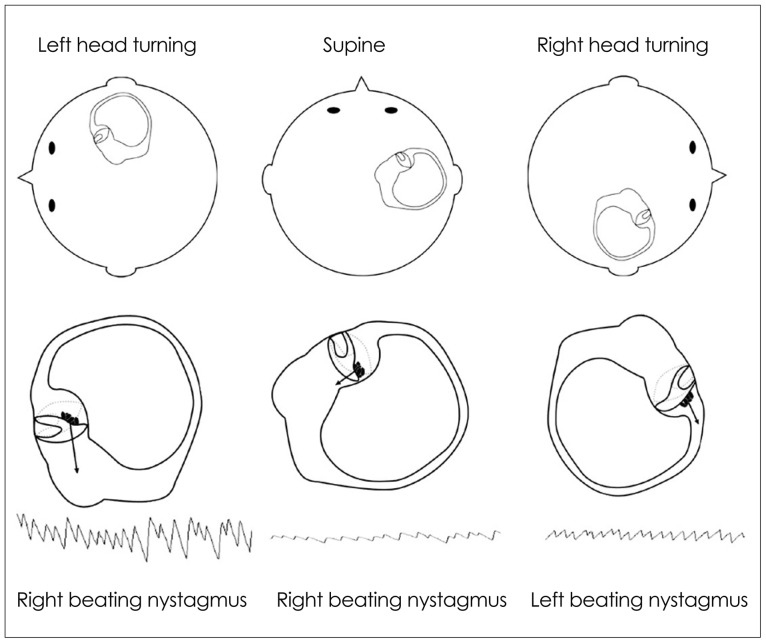
The HC can be maximally stimulated using the supine roll test, in which the patient's head is first flexed forward about 30° to align the HC with the earth vertical, and then turned about 90° to each side. Two types of nystagmus may be observed in BPPV involving the HC (HC-BPPV): geotropic nystagmus beating toward the ground (lower ear) or apogeotropic nystagmus beating toward the ceiling (upper ear). Geotropic nystagmus is paroxysmal and is attributed to the presence of free-floating otoconia (canalolithiasis) in the HC. The induced nystagmus is stronger when the affected ear is lower since the ampullopetal flow of the endolymph induces a stimulatory response in the HC (Fig. 6), and the stimulation is more effective in inducing the vestibular responses than the inhibition (according to the Ewald's second law).2,45 Apogeotropic nystagmus is usually persistent and is ascribed to otolith debris attached or near to the cupula (cupulolithiasis). In contrast to geotropic nystagmus, head turning to the healthy ear side elicits more intense nystagmus in apogeotropic HC-BPPV (Fig. 7).46
Fig. 6.
Supine roll test in geotropic benign paroxysmal positional vertigo involving the right horizontal canal. The head is turned about 90° to each side while supine. The corresponding illustrations demonstrate the migration of the otolithic debris in the horizontal canal in each position (arrows), and the direction of the induced nystagmus.
Fig. 7.
Supine roll test in apogeotropic benign paroxysmal positional vertigo involving the right horizontal canal. When the head is turned about 90° to each side while supine, deflection of the cupula due to attached otolithic debris induces apogeotropic nystagmus (arrows).
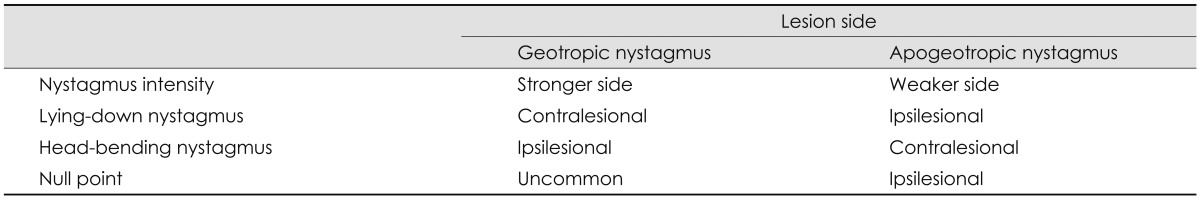
When determination of the involved side using the Ewald's second law is difficult due to rather symmetrical responses during the supine roll test, the direction of lying-down nystagmus (LDN)47 or head-bending nystagmus (HBN)48 may aid lateralization of the involved ear. In geotropic HC-BPPV, LDN beats mostly toward the intact side, while HBN is directed to the affected side. By contrast, LDN is usually ipsilesional and HBN is mostly contralesional in apogeotropic HC-BPPV. Patients with apogeotropic HC-BPPV may present a null head position in which the induced horizontal nystagmus disappears or becomes minimal. The null position is usually found when the head is turned to the affected side by 10-20°.49 At the null position, the heavy cupula is assumed to be aligned with the direction of the gravitational vector, resulting in no or minimal cupular deflection. The findings useful for lateralizing HC-BPPV are summarized in Table 2. However, CPN should be considered, especially when the repeated canalith-repositioning maneuvers fail to ameliorate the nystagmus and vertigo in apogeotropic positional nystagmus.
Table 2.
Lateralization of benign paroxysmal positional vertigo involving the horizontal canal
In the rare subtype of BPPV involving the anterior semicircular canal (AC, AC-BPPV), straight head hanging as well as the Dix-Hallpike maneuver on either side may elicit downbeat nystagmus with a small ipsitorsional component (i.e., with the upper poles of the eyes beating toward the affected side).23,24 AC-BPPV should be differentiated from CPN, especially when the torsional component is minimal and the downbeat nystagmus is persistent.
Sound stimuli, the Valsalva maneuver, and tragal compression
Vertigo and imbalance may be induced by sound stimuli to the ear. This so-called Tullio phenomenon is caused by a defect in the bone overlying the labyrinth, which is thought to create a third communication window in the wall of the labyrinth that provides a low-resistance pathway for the transmission of low-frequency sound energy through the labyrinth.50 Likewise, increased intracranial pressure during the Valsalva maneuver may produce similar symptoms and signs in patients as those produced by the Tullio phenomenon.51 During a Valsalva maneuver against a closed glottis (in which the patient is asked to "bear down"), the intracranial pressure appears to be transmitted to the labyrinth via the meninges and perilymph through the cochlear aqueduct to generate an endolymph flow in the semicircular canal.51 In contrast, the nasal Valsalva maneuver with an open glottis may directly increase the middle-ear pressure so as to induce endolymph flow.50 Pressure applied against the sealed external auditory canal (tragal compression) may also elicit vertigo in patients with a labyrinthine fistula (Hennebert's sign).51 These maneuvers should be performed in patients with vertigo, oscillopsia, or imbalance induced by loud sounds, coughing, or nose blowing.
The Vestibulo-Ocular Reflex
Unilateral loss of vestibular function leads to static imbalance due to a difference in the tonic discharges between the vestibular nuclei on the two sides, and decreased dynamic sensitivity during rotation due to deficits in the push-pull combination of the responses. Loss of dynamic sensitivity results in reduced VOR gain, which can be identified using bedside tests.
Head impulse test
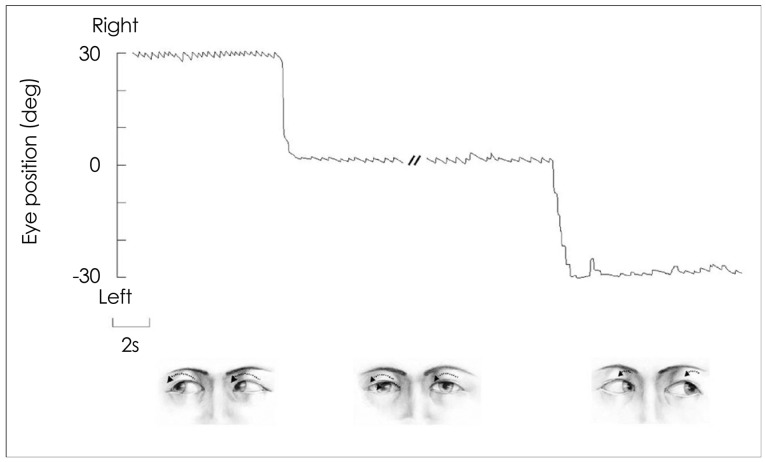
The head impulse test is the most effective method of detecting loss of vestibular function at the bedside.52 To test the horizontal VOR, the examiner asks the patient to fixate upon a target in front of the eyes and then briskly turns the patient's head horizontally. The head rotation impulse should be unpredictable with a low amplitude (10-20°) and a high acceleration (2000-4000°/second2).53 If the VOR is working normally, the head impulse will generate a compensatory eye movement in the direction opposite to the head rotation with an equal amplitude, holding the gaze steady. In contrast, the head impulse toward the side of a peripheral vestibular lesion would give rise to a refixation catch-up saccade at the end of head motion to bring the image of the target back to the fovea.
This corrective saccade (overt saccade) indicates a decreased VOR gain in patients with peripheral vestibular deficits (Fig. 8). The function of the vertical semicircular canals can also be assessed with vertical head impulses,54 in which the patient's head is rotated vertically in either the right-AC-left-PC or left-AC-right-PC plane.
Fig. 8.
Head impulse test. A: In healthy subjects, a head impulse (arrow) normally induces a rapid compensatory eye movement in the opposite direction, and steady fixation is attained. B: In patients with unilateral peripheral vestibular hypofunction, a head impulse toward the affected side (large arrow) produces a corrective saccade (small arrows) after head rotation since the eyes move with the head due to a defective vestibulo-ocular reflex, and lose the target with the head rotation.
While overt saccade is observed in most patients with acute peripheral vestibular disorders, the head impulse test is mostly normal in central vestibular lesions.55 A central pathology should therefore be suspected if a patient with acute vertigo and spontaneous nystagmus exhibits a normal head impulse test.22 A refixation saccade in the other plane (i.e., vertical catch-up saccade after horizontal rotation) also suggests a central lesion.56-58 However, a bedside head impulse test may be negative when the vestibular deficits are partial59 or the covert saccades complement the vestibular deficits.53
Visual enhancement of the VOR
For clear perception of stationary targets, the ratio of eye velocity to head velocity (VOR gain) must be approximately unity. However, in the absence of vision, the VOR gain is less than one during head motion at frequencies below 1.0 Hz.2 Vision is used to augment the VOR when viewing stationary targets [visual enhancement of the VOR (VVOR)]. This modulation of the VOR stabilizes the target image near the fovea. Visual enhancement of the VOR may be mediated by the smooth pursuit system. To test the VVOR, the subjects are instructed to maintain fixation on a stationary target presented in front of them while they are rotated passively in the chair.
Visual cancellation of the VOR
The VOR should be suppressed (VOR cancellation) in order to shift the direction of gaze while the head is in motion (eye-head tracking). At least two mechanisms have been proposed to explain this cancellation: cancellation of the VOR by smooth pursuit signal and reduction of the VOR gain.2 A bedside VOR cancellation test can be accomplished as follows. While seated on a rotating chair, subjects are asked to outstretch the arm forward with the thumb up and maintain fixation on the thumb during an en bloc rotation of the chair and hence also the subjects' body from side to side. If the VOR cancellation is impaired, the eyes will be continually taken off the target by slow phases of the VOR and corrective saccades will be made. For example, impaired smooth pursuit to the right would result in deficient cancellation of the VOR during rotation to the right. VOR cancellation is impaired in lesions involving structures that are concerned with smooth pursuit and eye-head tracking, which includes the flocculus/paraflocculus and the medial superior temporal area, frontal eye field, and dorsal pontine nuclei.
Dynamic visual acuity test
The dynamic visual acuity test is especially useful for diagnosing bilateral vestibular failure.60 To assess the dynamic visual acuity, the examiner manually oscillates the patient's head horizontally or vertically at about 2 Hz, while the patient reads a Snellen visual acuity chart. If the VOR gain is abnormal, visual acuity would deteriorate by more than two lines compared with the initial visual acuity measured when the head is still.
Head heave test
The head heave test can evaluate the translational VOR and the utricular function at the bedside.61 The examiner should move the head laterally with brief and rapid motions while the patient fixates on a target. The translational VOR would generate compensatory eye movements to keep the target stable on the retina. A corrective saccade after the heave indicates vestibular hypofunction on the side toward which the head is moved.
Saccades
Saccades are rapid eye movements that shift the line of sight between successive points of fixation. The patient is instructed to fixate alternatively on two targets, such as the tip of a pen and the examiner's nose. Saccades can be examined in both the horizontal and vertical planes. The velocity, accuracy, and conjugacy of the saccades should be determined at the bedside. Peripheral vestibular lesions do not impair saccades. Horizontal saccadic slowing is generally caused by dysfunction of the paramedian pontine reticular formation or the structures located more distally in the neural circuits for horizontal saccades,62 while vertical saccadic slowing is often due to lesions involving the rostral interstitial nucleus of the MLF or more distal structures.63 In general, lesions involving the pons cause horizontal saccadic slowing, whereas lesions affecting the rostral midbrain cause vertical saccadic slowing. Internuclear ophthalmoplegia can also cause slow saccades and lagging of the adducting eye during attempted contraversive saccades. The slow adducting saccades can be easily detected by having the patient follow an optokinetic drum or tape. Rapid phases made by the eye on the lesion side are smaller and slower. Saccades may overshoot (hypermetria) or undershoot (hypometria) the target, and corrective saccades would follow. These saccadic dysmetria are often caused by lesions involving the cerebellum (especially the dorsal vermis) or its connections.64,65 A unilateral lesion in the dorsal cerebellar vermis mostly induces contralateral saccadic hypermetria and ipsilateral hypometria, while lesions involving the fastigial nucleus generate contralateral saccadic hypometria and ipsilateral hypermetria.64,65 Patients with Wallenberg syndrome make hypermetric saccades to the lesion side (ipsipulsion) due to lesions affecting the inferior cerebellar peduncle.66
Smooth Pursuit
Smooth pursuit is a slow eye movement that holds the image of a small moving target on the fovea. The patient is asked to track a small target moving slowly in the horizontal or vertical direction with the head still. Corrective catch-up saccades occur if the pursuit movement does not match the target velocity. Since many neural structures are involved generating a smooth pursuit, and various factors including age, medications, and alertness can influence pursuit eye movements, impaired pursuit generally does not allow either topographical or etiological classification.62 However, marked asymmetry of pursuit eye movements suggests a unilateral lesion along the pursuit pathway. Since patients with horizontal nystagmus may exhibit impaired smooth pursuit in the direction of the nystagmus, the evaluation of vertical smooth pursuit may be helpful in determining smooth pursuit deficits in acute vestibulopathy.67,68
Balance
The severity of imbalance and falling direction may provide important clues as to the underlying vestibular impairments.
Romberg test
In the Romberg test the patient is asked to stand with the feet close together with the eyes open, and then to close the eyes so as to eliminate visual cues. The test result is positive when the patient is stable with the eyes open but loses balance with the eyes closed. A positive test indicates defects in the proprioceptive function, but may be found in patients with acute unilateral vestibulopathy or severe bilateral vestibular deficits. Patients with cerebellar dysfunction exhibit postural imbalance both with the eyes open and closed. When the presence of imbalance is unclear from the Romberg test, a tandem or sharpened Romberg test should be performed, in which the patient is asked to perform the same task as in Romberg test but while standing with the feet in a heel-to-toe position. In general, patients with vestibular neuritis, Wallenberg's syndrome, and cerebellar dysfunction tend to fall toward the lesion side. To-and-fro sway can be evident in patients with BPPV, bilateral vestibulopathy, and downbeat or upbeat nystagmus.69
Stepping test
The stepping test is useful for detecting vestibular deficits by disrupting proprioceptive compensation. The examiner asks the patient to march in a fixed position with the arms extended and eyes closed. A gradual turning toward the lesion side is observed in patients with a unilateral vestibular deficit.
Conclusion
A crucial aspect in the management of dizzy patients is differentiating central vestibular disorders from the more benign peripheral vestibular disorders. A thorough bedside examination and careful history taking remain the most important diagnostic tools for dizziness/vertigo. Subtle ocular signs such as GEN, skew deviation, and a normal head impulse test are useful for determining the occurrence of acute stroke in patients with acute dizziness/vertigo.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Baloh RW. Patient with dizziness. In: Baloh RW, Halmagyi GM, editors. Disorders of the Vestibular System. New York: Oxford University Press; 1996. pp. 157–170. [Google Scholar]
- 2.Leigh RJ, Zee DS. The Neurology of Eye Movements. 4th ed. New York: Oxford University Press; 2006. [Google Scholar]
- 3.Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504–3510. doi: 10.1161/STROKEAHA.109.551234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ. 2011;183:E571–E592. doi: 10.1503/cmaj.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westheimer G, Blair SM. The ocular tilt reaction--a brainstem oculomotor routine. Invest Ophthalmol. 1975;14:833–839. [PubMed] [Google Scholar]
- 6.Dieterich M, Brandt T. Ocular torsion and tilt of subjective visual vertical are sensitive brainstem signs. Ann Neurol. 1993;33:292–299. doi: 10.1002/ana.410330311. [DOI] [PubMed] [Google Scholar]
- 7.Diamond SG, Markham CH. Ocular counterrolling as an indicator of vestibular otolith function. Neurology. 1983;33:1460–1469. doi: 10.1212/wnl.33.11.1460. [DOI] [PubMed] [Google Scholar]
- 8.Halmagyi GM, Brandt T, Dieterich M, Curthoys IS, Stark RJ, Hoyt WF. Tonic contraversive ocular tilt reaction due to unilateral meso-diencephalic lesion. Neurology. 1990;40:1503–1509. doi: 10.1212/wnl.40.10.1503. [DOI] [PubMed] [Google Scholar]
- 9.Baier B, Bense S, Dieterich M. Are signs of ocular tilt reaction in patients with cerebellar lesions mediated by the dentate nucleus? Brain. 2008;131(Pt 6):1445–1454. doi: 10.1093/brain/awn086. [DOI] [PubMed] [Google Scholar]
- 10.Zwergal A, Rettinger N, Frenzel C, Dieterich M, Brandt T, Strupp M. A bucket of static vestibular function. Neurology. 2009;72:1689–1692. doi: 10.1212/WNL.0b013e3181a55ecf. [DOI] [PubMed] [Google Scholar]
- 11.Serra A, Leigh RJ. Diagnostic value of nystagmus: spontaneous and induced ocular oscillations. J Neurol Neurosurg Psychiatry. 2002;73:615–618. doi: 10.1136/jnnp.73.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baloh RW. Clinical practice. Vestibular neuritis. N Engl J Med. 2003;348:1027–1032. doi: 10.1056/NEJMcp021154. [DOI] [PubMed] [Google Scholar]
- 13.Robinson DA, Zee DS, Hain TC, Holmes A, Rosenberg LF. Alexander's law: its behavior and origin in the human vestibulo-ocular reflex. Ann Neurol. 1984;16:714–722. doi: 10.1002/ana.410160614. [DOI] [PubMed] [Google Scholar]
- 14.Hotson JR, Baloh RW. Acute vestibular syndrome. N Engl J Med. 1998;339:680–685. doi: 10.1056/NEJM199809033391007. [DOI] [PubMed] [Google Scholar]
- 15.Zee DS. Ophthalmoscopy in examination of patients with vestibular disorders. Ann Neurol. 1978;3:373–374. doi: 10.1002/ana.410030422. [DOI] [PubMed] [Google Scholar]
- 16.Baloh RW, Yee RD. Spontaneous vertical nystagmus. Rev Neurol (Paris) 1989;145:527–532. [PubMed] [Google Scholar]
- 17.Böhmer A, Straumann D. Pathomechanism of mammalian downbeat nystagmus due to cerebellar lesion: a simple hypothesis. Neurosci Lett. 1998;250:127–130. doi: 10.1016/s0304-3940(98)00450-9. [DOI] [PubMed] [Google Scholar]
- 18.Baloh RW, Spooner JW. Downbeat nystagmus: a type of central vestibular nystagmus. Neurology. 1981;31:304–310. doi: 10.1212/wnl.31.3.304. [DOI] [PubMed] [Google Scholar]
- 19.Glasauer S, Hoshi M, Kempermann U, Eggert T, Büttner U. Three-dimensional eye position and slow phase velocity in humans with downbeat nystagmus. J Neurophysiol. 2003;89:338–354. doi: 10.1152/jn.00297.2002. [DOI] [PubMed] [Google Scholar]
- 20.Choi KD, Oh SY, Kim HJ, Koo JW, Cho BM, Kim JS. Recovery of vestibular imbalances after vestibular neuritis. Laryngoscope. 2007;117:1307–1312. doi: 10.1097/MLG.0b013e31805c08ac. [DOI] [PubMed] [Google Scholar]
- 21.Büttner U, Grundei T. Gaze-evoked nystagmus and smooth pursuit deficits: their relationship studied in 52 patients. J Neurol. 1995;242:384–389. doi: 10.1007/BF00868394. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Sohn SI, Cho YW, Lee SR, Ahn BH, Park BR, et al. Cerebellar infarction presenting isolated vertigo: frequency and vascular topographical patterns. Neurology. 2006;67:1178–1183. doi: 10.1212/01.wnl.0000238500.02302.b4. [DOI] [PubMed] [Google Scholar]
- 23.Zapala DA. Down-beating nystagmus in anterior canal benign paroxysmal positional vertigo. J Am Acad Audiol. 2008;19:257–266. doi: 10.3766/jaaa.19.3.10. [DOI] [PubMed] [Google Scholar]
- 24.Brantberg K, Bergenius J. Treatment of anterior benign paroxysmal positional vertigo by canal plugging: a case report. Acta Otolaryngol. 2002;122:28–30. doi: 10.1080/00016480252775698. [DOI] [PubMed] [Google Scholar]
- 25.Shallo-Hoffmann J, Schwarze H, Simonsz HJ, Mühlendyck H. A reexamination of end-point and rebound nystagmus in normals. Invest Ophthalmol Vis Sci. 1990;31:388–392. [PubMed] [Google Scholar]
- 26.Choi KD, Oh SY, Park SH, Kim JH, Koo JW, Kim JS. Head-shaking nystagmus in lateral medullary infarction: patterns and possible mechanisms. Neurology. 2007;68:1337–1344. doi: 10.1212/01.wnl.0000260224.60943.c2. [DOI] [PubMed] [Google Scholar]
- 27.Hain TC, Fetter M, Zee DS. Head-shaking nystagmus in patients with unilateral peripheral vestibular lesions. Am J Otolaryngol. 1987;8:36–47. doi: 10.1016/s0196-0709(87)80017-0. [DOI] [PubMed] [Google Scholar]
- 28.Minagar A, Sheremata WA, Tusa RJ. Perverted head-shaking nystagmus: a possible mechanism. Neurology. 2001;57:887–889. doi: 10.1212/wnl.57.5.887. [DOI] [PubMed] [Google Scholar]
- 29.Kim JS, Ahn KW, Moon SY, Choi KD, Park SH, Koo JW. Isolated perverted head-shaking nystagmus in focal cerebellar infarction. Neurology. 2005;64:575–576. doi: 10.1212/01.WNL.0000150729.87682.79. [DOI] [PubMed] [Google Scholar]
- 30.Huh YE, Kim JS. Patterns of spontaneous and head-shaking nystagmus in cerebellar infarction: imaging correlations. Brain. 2011;134(Pt 12):3662–3671. doi: 10.1093/brain/awr269. [DOI] [PubMed] [Google Scholar]
- 31.Choi KD, Cho HJ, Koo JW, Park SH, Kim JS. Hyperventilation-induced nystagmus in vestibular schwannoma. Neurology. 2005;64:2062. doi: 10.1212/01.WNL.0000170969.19299.D7. [DOI] [PubMed] [Google Scholar]
- 32.Robichaud J, DesRoches H, Bance M. Is hyperventilation-induced nystagmus more common in retrocochlear vestibular disease than in end-organ vestibular disease? J Otolaryngol. 2002;31:140–143. doi: 10.2310/7070.2002.10796. [DOI] [PubMed] [Google Scholar]
- 33.Walker MF, Zee DS. The effect of hyperventilation on downbeat nystagmus in cerebellar disorders. Neurology. 1999;53:1576–1579. doi: 10.1212/wnl.53.7.1576. [DOI] [PubMed] [Google Scholar]
- 34.Choi KD, Kim JS, Kim HJ, Koo JW, Kim JH, Kim CY, et al. Hyperventilation-induced nystagmus in peripheral vestibulopathy and cerebellopontine angle tumor. Neurology. 2007;69:1050–1059. doi: 10.1212/01.wnl.0000271378.54381.6a. [DOI] [PubMed] [Google Scholar]
- 35.Karlberg M, Aw ST, Black RA, Todd MJ, MacDougall HG, Halmagyi GM. Vibration-induced ocular torsion and nystagmus after unilateral vestibular deafferentation. Brain. 2003;126(Pt 4):956–964. doi: 10.1093/brain/awg091. [DOI] [PubMed] [Google Scholar]
- 36.Ohki M, Murofushi T, Nakahara H, Sugasawa K. Vibration-induced nystagmus in patients with vestibular disorders. Otolaryngol Head Neck Surg. 2003;129:255–258. doi: 10.1016/S0194-5998(03)00529-1. [DOI] [PubMed] [Google Scholar]
- 37.Nam J, Kim S, Huh Y, Kim JS. Ageotropic central positional nystagmus in nodular infarction. Neurology. 2009;73:1163. doi: 10.1212/WNL.0b013e3181bacfde. [DOI] [PubMed] [Google Scholar]
- 38.Arai M, Terakawa I. Central paroxysmal positional vertigo. Neurology. 2005;64:1284. doi: 10.1212/01.WNL.0000147466.46705.41. [DOI] [PubMed] [Google Scholar]
- 39.Büttner U, Helmchen C, Brandt T. Diagnostic criteria for central versus peripheral positioning nystagmus and vertigo: a review. Acta Otolaryngol. 1999;119:1–5. doi: 10.1080/00016489950181855. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez C, Alzate R, Lindsay JR. Experimental observations on postural nystagmus. II. Lesions of the nodulus. Ann Otol Rhinol Laryngol. 1960;69:94–114. doi: 10.1177/000348946006900108. [DOI] [PubMed] [Google Scholar]
- 41.Dix MR, Hallpike CS. The pathology symptomatology and diagnosis of certain common disorders of the vestibular system. Proc R Soc Med. 1952;45:341–354. [PMC free article] [PubMed] [Google Scholar]
- 42.Baloh RW, Honrubia V, Jacobson K. Benign positional vertigo: clinical and oculographic features in 240 cases. Neurology. 1987;37:371–378. doi: 10.1212/wnl.37.3.371. [DOI] [PubMed] [Google Scholar]
- 43.Humphriss RL, Baguley DM, Sparkes V, Peerman SE, Moffat DA. Contraindications to the Dix-Hallpike manoeuvre: a multidisciplinary review. Int J Audiol. 2003;42:166–173. doi: 10.3109/14992020309090426. [DOI] [PubMed] [Google Scholar]
- 44.Cohen HS. Side-lying as an alternative to the Dix-Hallpike test of the posterior canal. Otol Neurotol. 2004;25:130–134. doi: 10.1097/00129492-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 45.McClure JA. Horizontal canal BPV. J Otolaryngol. 1985;14:30–35. [PubMed] [Google Scholar]
- 46.Baloh RW, Yue Q, Jacobson KM, Honrubia V. Persistent direction-changing positional nystagmus: another variant of benign positional nystagmus? Neurology. 1995;45:1297–1301. doi: 10.1212/wnl.45.7.1297. [DOI] [PubMed] [Google Scholar]
- 47.Koo JW, Moon IJ, Shim WS, Moon SY, Kim JS. Value of lying-down nystagmus in the lateralization of horizontal semicircular canal benign paroxysmal positional vertigo. Otol Neurotol. 2006;27:367–371. doi: 10.1097/00129492-200604000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Lee SH, Choi KD, Jeong SH, Oh YM, Koo JW, Kim JS. Nystagmus during neck flexion in the pitch plane in benign paroxysmal positional vertigo involving the horizontal canal. J Neurol Sci. 2007;256:75–80. doi: 10.1016/j.jns.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 49.Bisdorff AR, Debatisse D. Localizing signs in positional vertigo due to lateral canal cupulolithiasis. Neurology. 2001;57:1085–1088. doi: 10.1212/wnl.57.6.1085. [DOI] [PubMed] [Google Scholar]
- 50.Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249–258. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- 51.Tilikete C, Krolak-Salmon P, Truy E, Vighetto A. Pulse-synchronous eye oscillations revealing bone superior canal dehiscence. Ann Neurol. 2004;56:556–560. doi: 10.1002/ana.20231. [DOI] [PubMed] [Google Scholar]
- 52.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 53.Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Head impulse test in unilateral vestibular loss: vestibulo-ocular reflex and catch-up saccades. Neurology. 2008;70:454–463. doi: 10.1212/01.wnl.0000299117.48935.2e. [DOI] [PubMed] [Google Scholar]
- 54.Cremer PD, Halmagyi GM, Aw ST, Curthoys IS, McGarvie LA, Todd MJ, et al. Semicircular canal plane head impulses detect absent function of individual semicircular canals. Brain. 1998;121(Pt 4):699–716. doi: 10.1093/brain/121.4.699. [DOI] [PubMed] [Google Scholar]
- 55.Newman-Toker DE, Kattah JC, Alvernia JE, Wang DZ. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology. 2008;70(24 Pt 2):2378–2385. doi: 10.1212/01.wnl.0000314685.01433.0d. [DOI] [PubMed] [Google Scholar]
- 56.Walker MF, Zee DS. Directional abnormalities of vestibular and optokinetic responses in cerebellar disease. Ann N Y Acad Sci. 1999;871:205–220. doi: 10.1111/j.1749-6632.1999.tb09186.x. [DOI] [PubMed] [Google Scholar]
- 57.Walker MF, Zee DS. Cerebellar disease alters the axis of the high-acceleration vestibuloocular reflex. J Neurophysiol. 2005;94:3417–3429. doi: 10.1152/jn.00375.2005. [DOI] [PubMed] [Google Scholar]
- 58.Jeong SH, Kim JS, Baek IC, Shin JW, Jo H, Lee AY, et al. Perverted head impulse test in cerebellar ataxia. Cerebellum. 2013 doi: 10.1007/s12311-013-0480-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Perez N, Rama-Lopez J. Head-impulse and caloric tests in patients with dizziness. Otol Neurotol. 2003;24:913–917. doi: 10.1097/00129492-200311000-00016. [DOI] [PubMed] [Google Scholar]
- 60.Kim S, Oh YM, Koo JW, Kim JS. Bilateral vestibulopathy: clinical characteristics and diagnostic criteria. Otol Neurotol. 2011;32:812–817. doi: 10.1097/MAO.0b013e31821a3b7d. [DOI] [PubMed] [Google Scholar]
- 61.Ramat S, Zee DS, Minor LB. Translational vestibulo-ocular reflex evoked by a "head heave" stimulus. Ann N Y Acad Sci. 2001;942:95–113. [PubMed] [Google Scholar]
- 62.Gaymard B, Pierrot-Deseilligny C. Neurology of saccades and smooth pursuit. Curr Opin Neurol. 1999;12:13–19. doi: 10.1097/00019052-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Sharpe JA, Kim JS. Midbrain disorders of vertical gaze: a quantitative re-evaluation. Ann N Y Acad Sci. 2002;956:143–154. doi: 10.1111/j.1749-6632.2002.tb02815.x. [DOI] [PubMed] [Google Scholar]
- 64.Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol. 1993;70:1741–1758. doi: 10.1152/jn.1993.70.5.1741. [DOI] [PubMed] [Google Scholar]
- 65.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998;80:1911–1931. doi: 10.1152/jn.1998.80.4.1911. [DOI] [PubMed] [Google Scholar]
- 66.Helmchen C, Straube A, Büttner U. Saccadic lateropulsion in Wallenberg's syndrome may be caused by a functional lesion of the fastigial nucleus. J Neurol. 1994;241:421–426. doi: 10.1007/BF00900959. [DOI] [PubMed] [Google Scholar]
- 67.Chen L, Lee W, Chambers BR, Dewey HM. Diagnostic accuracy of acute vestibular syndrome at the bedside in a stroke unit. J Neurol. 2011;258:855–861. doi: 10.1007/s00415-010-5853-4. [DOI] [PubMed] [Google Scholar]
- 68.Cnyrim CD, Newman-Toker D, Karch C, Brandt T, Strupp M. Bedside differentiation of vestibular neuritis from central "vestibular pseudoneuritis". J Neurol Neurosurg Psychiatry. 2008;79:458–460. doi: 10.1136/jnnp.2007.123596. [DOI] [PubMed] [Google Scholar]
- 69.Dieterich M, Grünbauer WM, Brandt T. Direction-specific impairment of motion perception and spatial orientation in downbeat and upbeat nystagmus in humans. Neurosci Lett. 1998;245:29–32. doi: 10.1016/s0304-3940(98)00175-x. [DOI] [PubMed] [Google Scholar]